Itch in Adult Population with Type 2 Diabetes Mellitus: Clinical Profile, Pathogenesis and Disease-Related Burden in a Cross-Sectional Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design and Applied Questionnaires
2.3. Statistical Analysis
3. Results
3.1. General Demographics and Systemic Comorbidities
3.2. Clinical Characteristics of Itch
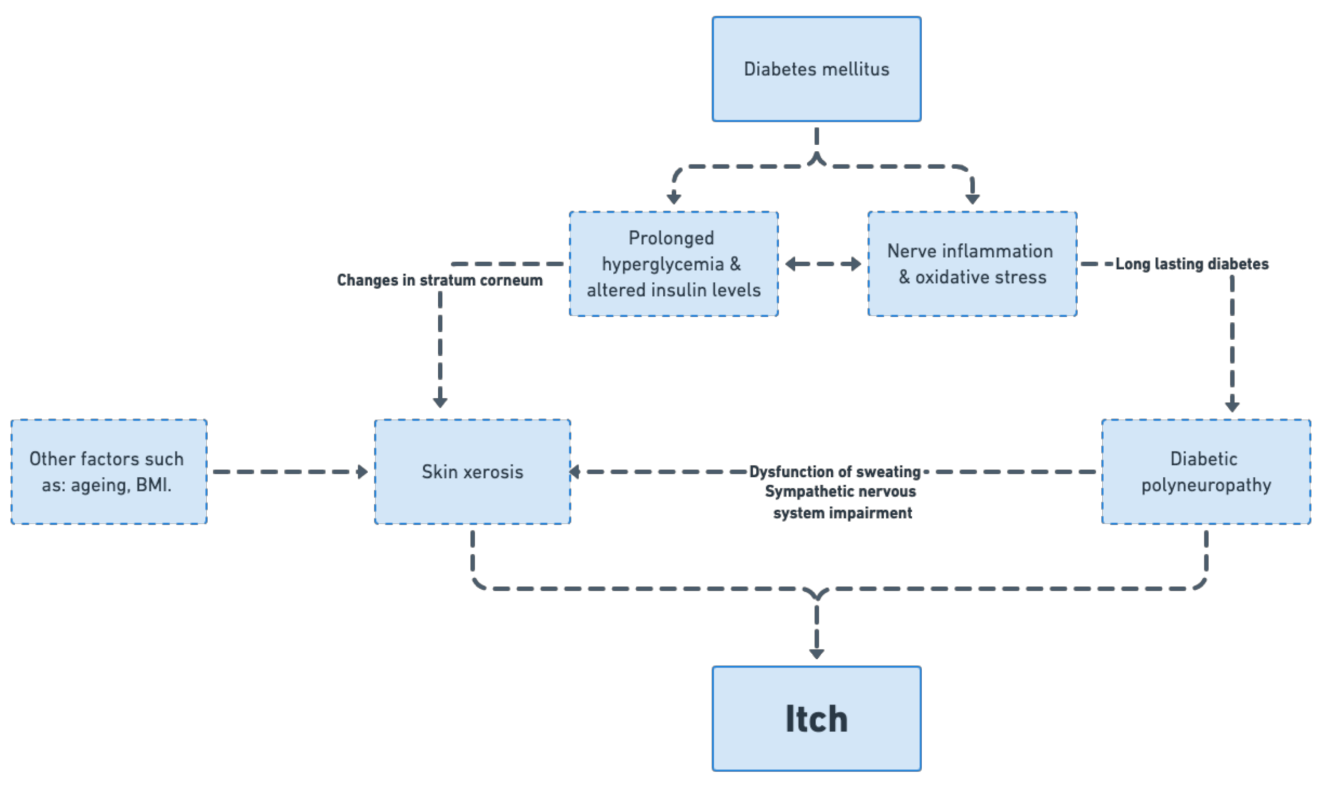
3.3. Factors Contributing to Itch in DM: Glycaemic Control, Polyneuropathy, Skin Xerosis, Treatment Used
3.4. Itch and Patients Well-Being
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baena-Díez, J.M.; Peñafiel, J.; Subirana, I.; Ramos, R.; Elosua, R.; Marín-Ibañez, A.; Guembe, M.J.; Rigo, F.; Tormo-Díaz, M.J.; Moreno-Iribas, C.; et al. Risk of cause-specific death in individuals with diabetes: A competing risks analysis. Diabetes Care 2016, 39, 1987–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarwar, N.; Gao, P.; Kondapally Seshasai, S.R.; Gobin, R.; Kaptoge, S.; Di Angelantonio, E.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; Stampfer, M.; et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [Green Version]
- Ständer, S.; Streit, M.; Darsow, U.; Niemeier, V.; Vogelgsang, M.; Ständer, H.; Gieler, U.; Gollnick, H.; Metze, D.; Weisshaar, E. Diagnostic and therapeutic procedures in chronic pruritus. J. Dtsch. Dermatol. Ges. 2006, 4, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Weisshaar, E.; Mettang, T.; Szepietowski, J.; Carstens, E.; Ikoma, A.; Bergasa, N.; Gieler, U.; Misery, L.; Wallengren, J.; et al. Clinical classification of itch: A position paper of the international forum for the study of itch. Acta Derm. Venereol. 2007, 87, 291–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, N.; Mirpour, S.H.; Golmohamadi, R.; Darjani, A.; Eftekhari, H.; Rafiei, R.; Gharaei Nejad, K.; Azimi, S.Z. Chronic generalized pruritus without primary skin lesions: A longitudinal prospective observational study. Int. J. Dermatol. 2019, 58, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Schmelz, M.; Szabó, I.L.; Oaklander, A.L. Clinical presentation, management, and pathophysiology of neuropathic itch. Lancet Neurol. 2018, 17, 709–720. [Google Scholar] [CrossRef]
- Tseng, H.-W.; Ger, L.-P.; Liang, C.-K.; Liou, H.-H.; Lam, H.-C. High prevalence of cutaneous manifestations in the elderly with diabetes mellitus: An institution-based cross-sectional study in Taiwan. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1631–1635. [Google Scholar] [CrossRef]
- Yamaoka, H.; Sasaki, H.; Yamasaki, H.; Ogawa, K.; Ohta, T.; Furuta, H.; Nishi, M.; Nanjo, K. Truncal pruritus of unknown origin may be a symptom of diabetic polyneuropathy. Diabetes Care 2010, 33, 150–155. [Google Scholar] [CrossRef] [Green Version]
- Stefaniak, A.; Chlebicka, I.; Szepietowski, J. Itch in diabetes: A common underestimated problem. Adv. Dermatol. Allergol. 2019, 36, 177–183. [Google Scholar] [CrossRef]
- Association, A.D. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reich, A.; Mȩdrek, K.; Szepietowski, J. Czteropunktowy kwestionariusz oceny świa̧du—Walidacja kwestionariusza. Prz. Dermatol. 2012, 99, 600–604. [Google Scholar]
- Stefaniak, A.A.; Zubkiewicz-Kucharska, A.; Matusiak, Ł.; Noczyńska, A.; Szepietowski, J.C. Itch in children with type 1 diabetes: A cross-sectional study. Dermatol. Ther. 2020, 10, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, P.K.; Olczyk, P.; Krajewska, M.; Krajewski, W.; Szepietowski, J.C. Clinical characteristics of itch in renal transplant recipients. Front. Med. 2021, 7, 615334. [Google Scholar] [CrossRef]
- Reich, A.; Chatzigeorkidis, E.; Zeidler, C.; Osada, N.; Furue, M.; Takamori, K.; Ebata, T.; Augustin, M.; Szepietowski, J.C.; Ständer, S. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm. Venereol. 2017, 97, 759–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Association, A.D. 11. microvascular complications and foot care: Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [Green Version]
- Katzenwadel, A.; Sachse, G.; Federlin, K. Measurement of vibration sensitivity and temperature discrimination in peripheral diabetic neuropathy. Akt. Endokr. Stoffwechs. 1987, 8, 155–160. [Google Scholar]
- Haupt, E.; Ledermann, H.; Köpcke, W. Benfotiamine in the treatment of diabetic polyneuropathy—A three-week randomized, controlled pilot study (BEDIP study). Int. J. Clin. Pharmacol. Ther. 2005, 43, 71–77. [Google Scholar] [CrossRef]
- Rogoziewicz, M.; Wiszniewska, M.; Wiszniewski, P.; Michalak, S.; Kozubski, W. Clinical and neurophysiological evaluation of peripheral nervous system abnormalities in patients with lymphoproliferative disorders. Neurology 2012, 78, P06.018. [Google Scholar] [CrossRef]
- Stumpf, A.; Pfleiderer, B.; Fritz, F.; Osada, N.; Chen, S.C.; Ständer, S. Assessment of quality of life in chronic pruritus: Relationship between itchyQol and dermatological life quality index in 1150 patients. Acta Derm. Venereol. 2018, 98, 142–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Love, E.M.; Marrazzo, G.A.; Kini, S.; Veledar, E.; Chen, S.C. ItchyQoL bands: Pilot clinical interpretation of scores. Acta Derm. Venereol. 2015, 95, 114–115. [Google Scholar] [CrossRef]
- Lu, Y.; Duller, P.; van der Valk, P.G.M.; Evers, A.W.M. Helplessness as predictor of perceived stigmatization in patients with psoriasis and atopic dermatitis. Dermatol. Psychosom. 2003, 4, 146–150. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, A.M. A study of the skin in five hundred cases of diabetes. J. Am. Med. Assoc. 1927, 89, 774–776. [Google Scholar] [CrossRef]
- Neilly, J.B.; Martin, A.; Simpson, N.; MacCuish, A.C. Pruritus in diabetes mellitus: Investigation of prevalence and correlation with diabetes control. Diabetes Care 1986, 9, 273–275. [Google Scholar] [CrossRef]
- Ko, M.-J.; Chiu, H.-C.; Jee, S.-H.; Hu, F.-C.; Tseng, C.-H. Postprandial blood glucose is associated with generalized pruritus in patients with type 2 diabetes. Eur. J. Dermatol. 2013, 23, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.N.; Mansharmani, G.G. Diabetes mellitus in geriatric females. J. Indian Med. Assoc. 1989, 87, 138–139. [Google Scholar] [PubMed]
- Valdes-Rodriguez, R.; Mollanazar, N.; González-Muro, J.; Nattkemper, L.; Torres-Alvarez, B.; López-Esqueda, F.; Chan, Y.; Yosipovitch, G. Itch prevalence and characteristics in a Hispanic geriatric population: A comprehensive study using a standardized itch questionnaire. Acta Derm. Venereol. 2015, 95, 417–421. [Google Scholar] [CrossRef]
- Drivsholm, T.; de Fine Olivarius, N.; Nielsen, A.B.S.; Siersma, V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia 2005, 48, 210–214. [Google Scholar] [CrossRef] [Green Version]
- Wahid, Z.; Kanjee, A. Cutaneous manifestations of diabetes mellitus. J. Pak. Med. Assoc. 1998, 48, 304–305. [Google Scholar]
- Weisshaar, E.; Szepietowski, J.C.; Dalgard, F.; Garcovich, S.; Gieler, U.; Gimenez-Arnau, A.; Lambert, J.; Leslie, T.; Mettang, T.; Misery, L.; et al. European S2k guideline on chronic pruritus. Acta Derm. Venereol. 2019, 99, 469–506. [Google Scholar] [CrossRef] [Green Version]
- Wertheimer, E.; Trebicz, M.; Eldar, T.; Gartsbein, M.; Nofeh-Moses, S.; Tennenbaum, T. Differential roles of insulin receptor and insulin-like growth factor-1 receptor in differentiation of murine skin keratinocytes. J. Investig. Dermatol. 2000, 115, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagen, K.M.; Ousman, S.S. Aging and the immune response in diabetic peripheral neuropathy. J. Neuroimmunol. 2021, 355, 577574. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Baynes, J.W. Non-enzymatic glycosylation and the chronic complications of diabetes: An overview. Diabetologia 1984, 26, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, S.; Kikuchi, K.; Satoh, J.; Tagami, H.; Inoue, S. Functional properties of the stratum corneum in patients with diabetes mellitus: Similarities to senile xerosis. Br. J. Dermatol. 2005, 153, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Endo, Y.; Ozawa, N.; Sugawara, T.; Kusaka, A.; Sayo, T.; Inoue, S.; Tagami, H. Characteristics of the epidermis and stratum corneum of hairless mice with experimentally induced diabetes mellitus. J. Investig. Dermatol. 2003, 120, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, L.; Smith, T.; Havsager, A.M.; Madsen, C.; Kjeldsen, M.J.; Dalsgaard, N.J.; Gaist, D.; Schrøder, H.D.; Sindrup, S.H. Evaluation of patients with symptoms suggestive of chronic polyneuropathy. J. Clin. Neuromuscul. Dis. 2001, 3, 47–52. [Google Scholar] [CrossRef]
- Maser, R.E.; Steenkiste, A.R.; Dorman, J.S.; Nielsen, V.K.; Bass, E.B.; Manjoo, Q.; Drash, A.L.; Becker, D.J.; Kuller, L.H.; Greene, D.A.; et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh epidemiology of diabetes complications study. Diabetes 1989, 38, 1456–1461. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Cheng, H.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet 2012, 11, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Lauria, G.; Merkies, I.S.J.; Faber, C.G. Small fibre neuropathy. Curr. Opin. Neurol. 2012, 25, 542–549. [Google Scholar] [CrossRef]
- Sun, P.-C.; Chen, C.-S.; Kuo, C.-D.; Lin, H.-D.; Chan, R.-C.; Kao, M.-J.; Wei, S.-H. Impaired microvascular flow motion in subclinical diabetic feet with sudomotor dysfunction. Microvasc. Res. 2012, 83, 243–248. [Google Scholar] [CrossRef]
- Pereira, M.P.; Derichs, L.; Meyer Zu Hörste, G.; Agelopoulos, K.; Ständer, S. Generalized chronic itch induced by small-fibre neuropathy: Clinical profile and proposed diagnostic criteria. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1795–1802. [Google Scholar] [CrossRef]
- Pereira, M.P.; Wiegmann, H.; Agelopoulos, K.; Ständer, S. Neuropathic itch: Routes to clinical diagnosis. Front. Med. 2021, 8, 175. [Google Scholar] [CrossRef]
- Hillson, R.M.; Hockaday, T.D.; Newton, D.J.; Pim, B. Delayed diagnosis of non-insulin-dependent diabetes is associated with greater metabolic and clinical abnormality. Diabet. Med. 1985, 2, 383–386. [Google Scholar] [CrossRef]
- Carr, C.W.; Veledar, E.; Chen, S.C. Factors mediating the impact of chronic pruritus on quality of life. JAMA Dermatol. 2014, 150, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, T.; Shetty, A.K.; Badiger, S.; Chan, Y.H.; Yosipovitch, G. Prevalence and characteristics of pruritus and association with quality of life in people living with HIV: A cross-sectional study. J. Pain Symptom Manag. 2018, 55, e4–e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeidler, C.; Steinke, S.; Riepe, C.; Bruland, P.; Soto-Rey, I.; Storck, M.; Garcovich, S.; Blome, C.; Bobko, S.; Legat, F.J.; et al. Cross-European validation of the ItchyQoL in pruritic dermatoses. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lelonek, E.; Matusiak, Ł.; Wróbel, T.; Kwiatkowski, J.; Szepietowski, J.C. Burden of aquagenic pruritus in polycythaemia vera. Acta Derm. Venereol. 2018, 98, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tee, S.I.; Lim, Z.V.; Theng, C.T.; Chan, K.L.; Giam, Y.C. A prospective cross-sectional study of anxiety and depression in patients with psoriasis in Singapore. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1159–1164. [Google Scholar] [CrossRef]
- Tan, W.F.; Voo, S.Y.M.; Sulaiman, N.; Robinson, S. Psychosocial burden of patients with atopic dermatitis at two tertiary referral centres in malaysia. Med. J. Malays. 2021, 76, 643–652. [Google Scholar]
- Choi, G.S.; Nam, Y.H.; Park, C.S.; Kim, M.Y.; Jo, E.J.; Park, H.K.; Kim, H.K. Anxiety, depression, and stress in Korean patients with chronic urticaria. Korean J. Intern. Med. 2020, 35, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Tat, T.S. Higher levels of depression and anxiety in patients with chronic urticaria. Med. Sci. Monit. 2019, 25, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, C.; Pereira, M.P.; Dugas, M.; Augustin, M.; Storck, M.; Weyer-Elberich, V.; Schneider, G.; Ständer, S. The burn in chronic prurigo: Patients with chronic prurigo suffer more than patients with chronic pruritus on non-lesional skin: A comparative, retrospective, explorative statistical analysis of 4484 patients in a real-world cohort. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Altunay, İ.K.; Özkur, E.; Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; Szabo, C.; et al. Psychosocial aspects of adult acne: Data from 13 European countries. Acta Derm. Venereol. 2020, 100, adv00051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
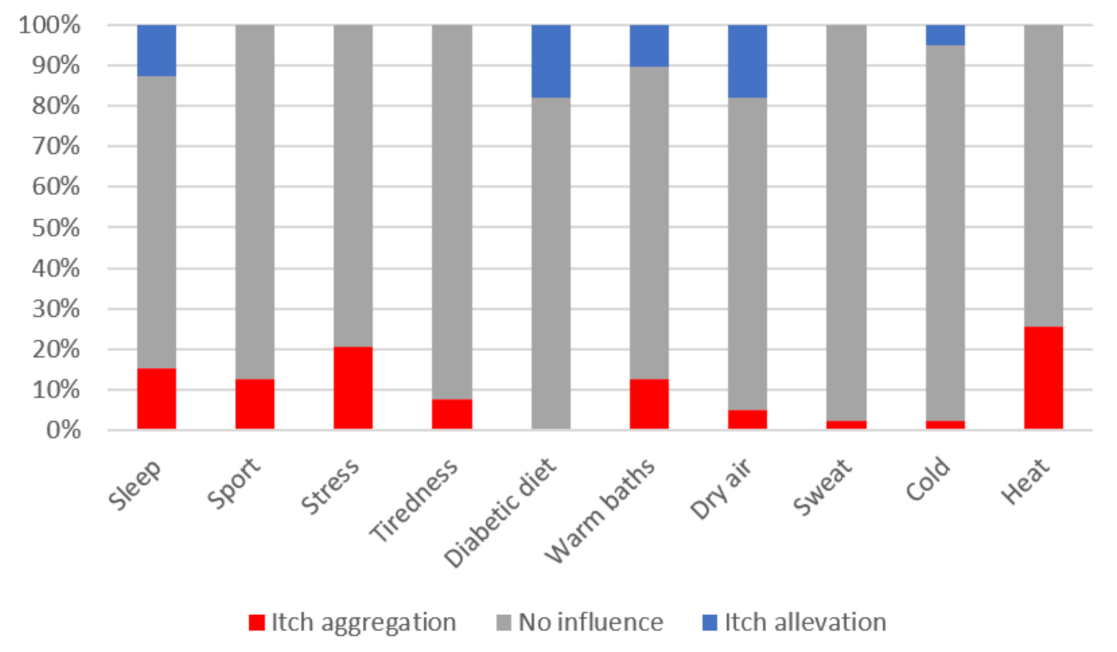
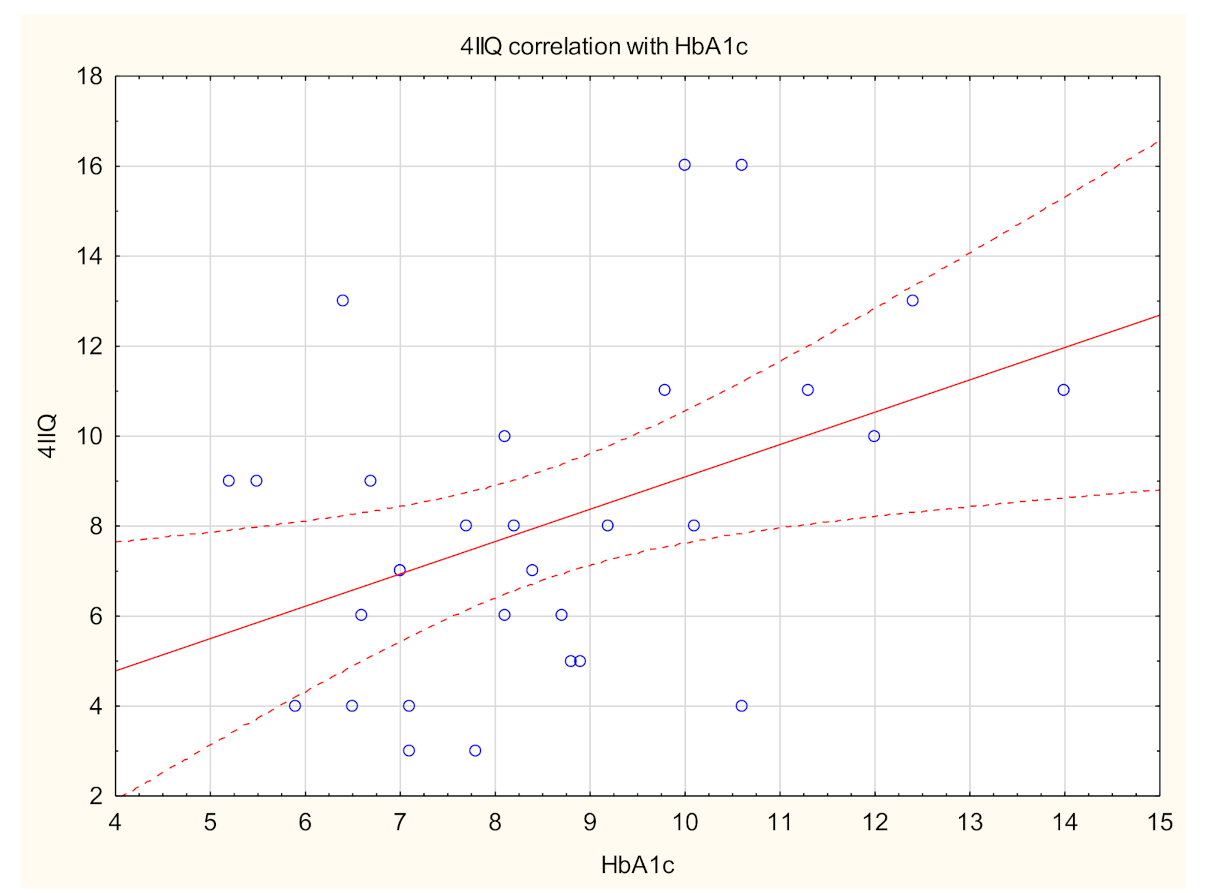
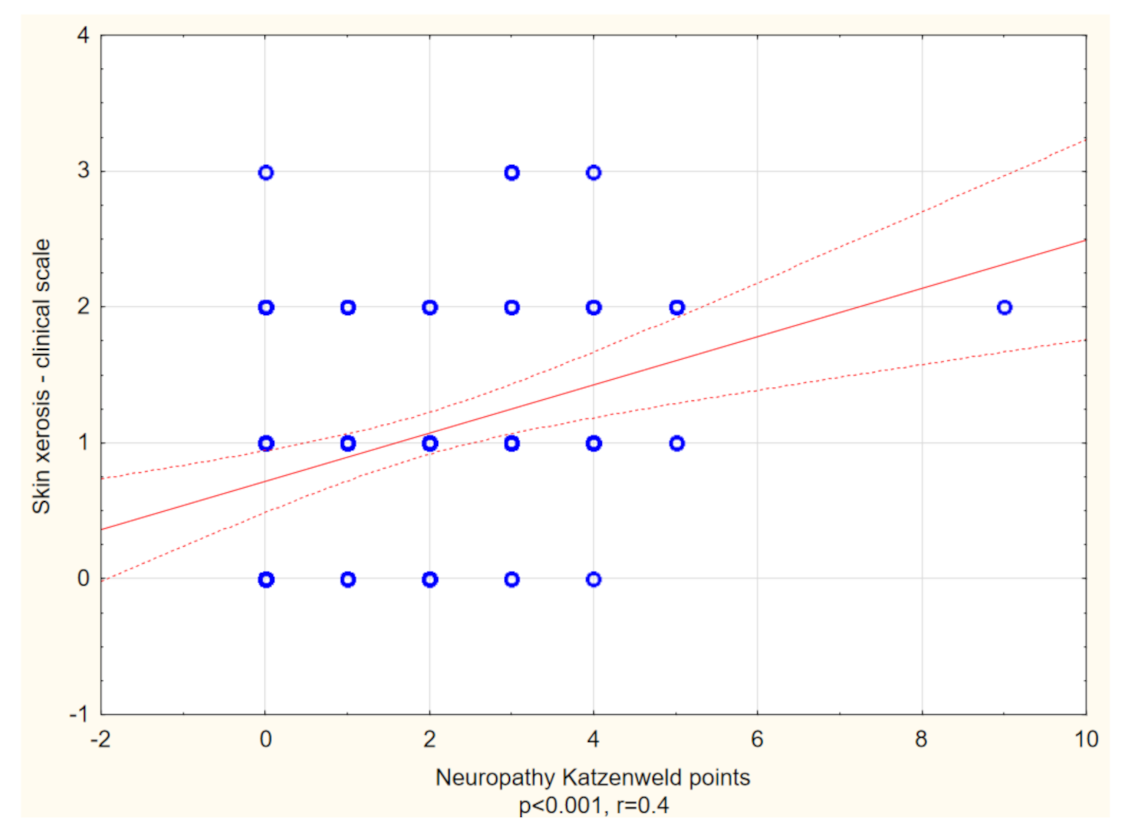
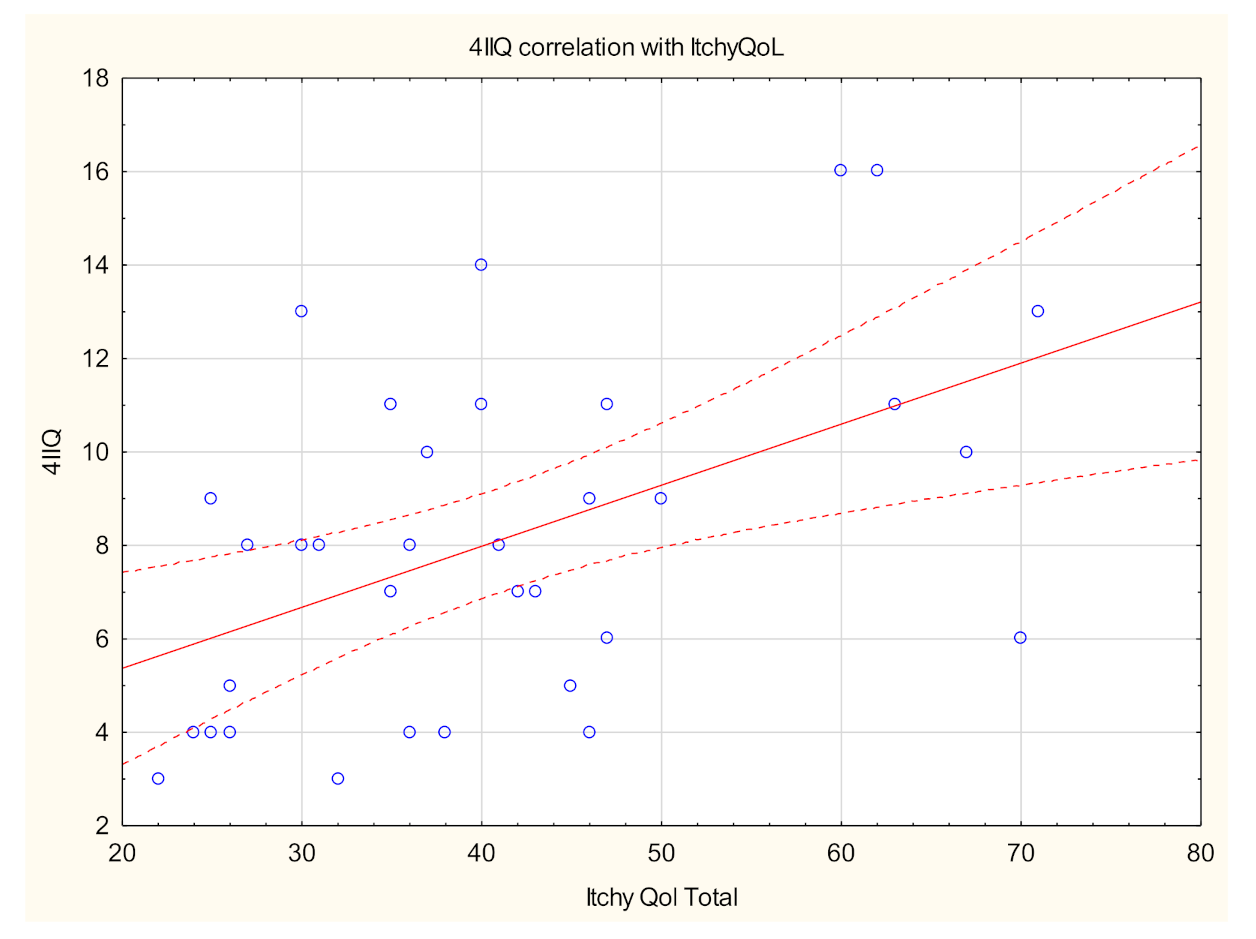
| Total | With Itch | Without Itch | p-Value * | |
|---|---|---|---|---|
| Sex (n) Female Male | 109 56 (51.4%) 53 (48.6%) | 39 24 (61.5%) 15 (38.5%) | 70 32 (45.7%) 38 (54.3%) | NS |
| Age (years), mean ± SD Range Median | 62.4 ± 14.4 (21–89) 65 | 60.3 ± 13.8 (23–83) 65 | 63.5 ± 14.7 (21–89) 65 | NS |
| BMI (kg/m2), mean ± SD Range Median | 29.4 ± 5.7 (18.7–48.4) 28.2 | 29.7 ± 6.1 (18.7–48.4) 29.1 | 29.2 ± 5.4 (19.1–46.0) 28.1 | NS |
| Duration of diabetes (years), mean ± SD Range Median | 12.7 ± 9.5 (0–45) 12 | 14 ± 8.9 (0–32) 13 | 12 ± 12.7 (0–45) 10 | NS |
| Level of education (n) Primary Secondary Higher education No data | 18 (16.5%) 49 (45%) 24 (22%) 18 (16.5%) | 7 (18%) 18 (46.1%) 9 (23.1%) 5 (12.8%) | 11 (15.7%) 31 (44.3%) 15 (21.4%) 13 (18.6%) | NS NS NS |
| Stimulant use Smoking Alcohol Drugs | 48 (44%) 30 (27.5%) 3 (2.8%) | 20 (51.3%) 13 (33.3%) 2 (5.1%) | 28 (40%) 17 (24.3%) 1 (1.4%) | NS NS NS |
| Packet years (years), mean ± SD Range Median | 19.8 ± 19.4 (0–90) 15 | 18.1 ± 15.2 (0–50) 10 | 21 ± 21.9 (0–90) 12.5 | NS |
| Comorbidities: | ||||
| Cardiovascular disorders | 69 (35.8%) | 22 (56.4%) | 47 (67.1%) | NS |
| Thyroid and parathyroid gland disease | 29 (26.6%) | 12 (30.8%) | 17 (58.6%) | NS |
| Chronic kidney disease | 13 (11.9%) | 6 (15.4%) | 7 (10%) | NS |
| Malignancies in the past and neoplasms (incl. benign prostatic hyperplasia) | 10 (9.2%) | 5 (12.8%) | 5 (7.1%) | NS |
| Joint diseases | 5 (4.6%) | 1 (2.6%) | 4 (5.7%) | NS |
| Asthma and COPD | 4 (3.7%) | 1 (2.6%) | 3 (4.3%) | NS |
| Neurological disorders | 4 (3.7%) | 1 (2.6%) | 3 (4.3%) | NS |
| Infectious diseases in the past (incl. borreliosis, syphilis, meningitidis, tuberculosis) | 4 (3.7%) | 2 (5.1%) | 2 (2.9%) | NS |
| Psychiatric disorders | 4 (3.7%) | 2 (5.1%) | 2 (2.9%) | NS |
| Gastrointestinal disorders | 3 (2.7%) | 0 (0%) | 3 (4.3%) | NS |
| Time of the Day/Frequency | Not at All | Rarely | Often | All the Time |
|---|---|---|---|---|
| Morning | 8 (20.5%) | 24 (61.6%) | 5 (12.8%) | 2 (5.1%) |
| Afternoon | 1 (2.6%) | 22 (56.4%) | 13 (33.3%) | 3 (7.7%) |
| Evening | 8 (20.5%) | 20 (51.3%) | 9 (23.1%) | 2 (5.1%) |
| Night | 9 (23.1%) | 17 (43.6%) | 12 (30.7%) | 1 (2.6%) |
| With Itch (n = 39) | Without Itch (n = 70) | p-Value | |
|---|---|---|---|
| Glycaemic control: | |||
| HbA1C (%), mean ± SD Range Median | 8.5 ± 2 (5.2–14) 8.2 | 8 ± 2.3 (5–15.1) 7.5 | NS |
| FPG (mg/dl), mean ± SD Range Median | 174.6 ± 62.3 (88–356) 164 | 148 ± 69.2 (62–510) 124 | p = 0.01 |
| Treatment of diabetes: (n) | |||
| Insulin | 17 (43.6%) | 18 (25.7%) | p = 0.03 |
| Oral treatment | 20 (51.3%) | 56 (80%) | p < 0.01 |
| Diet only | 3 (7.7%) | 1 (1.4%) | NS |
| Skin xerosis: | |||
| Skin xerosis examined clinically (points), mean ± SD Range Median | 1.3 ± 0.8 (0–3) 1 | 0.9 ± 0.9 (0–3) 1 | p < 0.01 |
| Epidermal hydration (AU), mean ± SD (median) • Forearm • Lower leg • Abdomen • Chest | 27.4 ± 10.6 (28.9) 32.1 ± 13.2 (33.3) 20.4 ± 9.4 (18.9) 39.4 ± 14.6 (38.2) | 31.2 ± 12.2 (29.9) 32.8 ± 12.4 (31.6) 25.9 ± 16.2 (21.3) 37.1 ± 18.5 (39.3) | NS NS NS NS |
| Polyneuropathy: | |||
| Other than itch sensations related to polyneuropathy (n): • Tingling • Numbness • Pain • Stinging • Burning • Hyperesthesia • Hypoesthesia | 26 (66.7%) 22 (56.4%) 8 (20.5%) 7 (17.9%) 14 (35.9%) 7 (17.9%) 4 (10.3%) | 20 (28.6%) 22 (31.4%) 8 (11.4%) 3 (4.3%) 7 (6.4%) 7 (6.4%) 2 (2.9%) | p < 0.01 p = 0.01 NS p = 0.02 p < 0.01 NS NS |
| Katzenwadel scale (points), mean ± SD Range Median | 3.0 ± 1.8 (0–9) 3 | 1.3 ± 1.4 (0–5) 1 | p < 0.01 |
| With Itch (n = 39) | Without Itch (n = 70) | p-Value | |
|---|---|---|---|
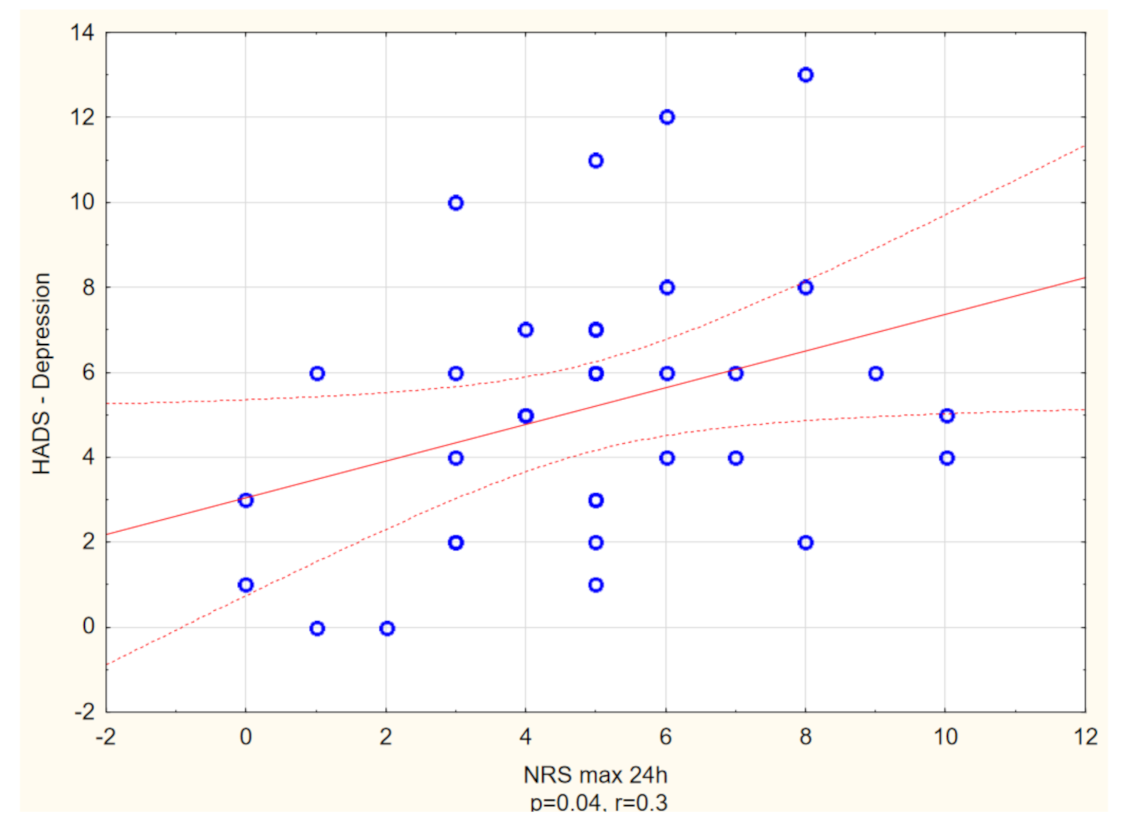
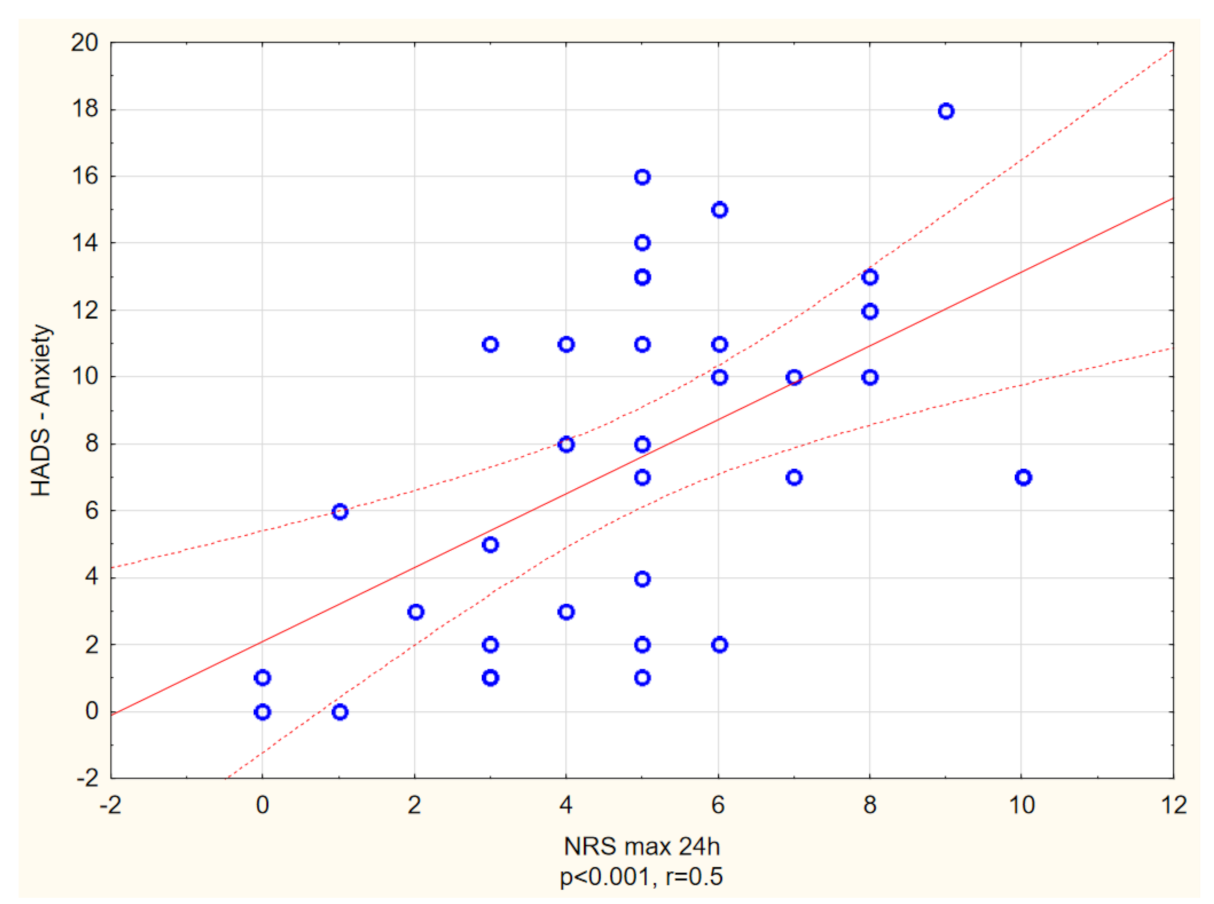
| HADS-Anxiety (points), mean ± SD Range Median | 7.6 ± 4.9 (0–18) 7 | 3.9 ± 3.9 (0–21) 3 | p < 0.01 |
| HADS-Depression (points), mean ± SD Range Median | 5.1 ± 3 (0–13) 5 | 3 ± 3.3 (0–21) 3 | p < 0.01 |
| 6-ISS, mean ± SD Range Median | 1.5 ± 1.8 (0–8) 1 | - | NA |
| ItchyQoL (raw), mean ± SD Range Median | 41.2 ± 13.4 (22–71) 39 | - | NA |
| ItchyQol Subscale Mean Scores ± SD (Median) | |
|---|---|
| Symptoms | 2.1 ± 0.8 (2.1) |
| Functioning | 1.7 ± 0.6 (1.7) |
| Emotions | 1.8 ± 0.8 (1.6) |
| Combined | 1.9 ± 0.6 (1.8) |
| NRSmax Three Days Mean ± SD | NRSmax 24 h Mean ± SD | 4IIQ Mean ± SD | |
|---|---|---|---|
| Adult population with DM2 (current study) | 6.31 ± 2.16 | 4.9 ± 2.5 | 8.1 ± 3.5 points |
| Pediatric population with DM1 (Stefaniak et al. 2020) | 6.3 ± 3.0 | 5.0 ± 3.8 | 6.7 ± 3.5 points |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefaniak, A.A.; Krajewski, P.K.; Bednarska-Chabowska, D.; Bolanowski, M.; Mazur, G.; Szepietowski, J.C. Itch in Adult Population with Type 2 Diabetes Mellitus: Clinical Profile, Pathogenesis and Disease-Related Burden in a Cross-Sectional Study. Biology 2021, 10, 1332. https://doi.org/10.3390/biology10121332
Stefaniak AA, Krajewski PK, Bednarska-Chabowska D, Bolanowski M, Mazur G, Szepietowski JC. Itch in Adult Population with Type 2 Diabetes Mellitus: Clinical Profile, Pathogenesis and Disease-Related Burden in a Cross-Sectional Study. Biology. 2021; 10(12):1332. https://doi.org/10.3390/biology10121332
Chicago/Turabian StyleStefaniak, Aleksandra A., Piotr K. Krajewski, Dorota Bednarska-Chabowska, Marek Bolanowski, Grzegorz Mazur, and Jacek C. Szepietowski. 2021. "Itch in Adult Population with Type 2 Diabetes Mellitus: Clinical Profile, Pathogenesis and Disease-Related Burden in a Cross-Sectional Study" Biology 10, no. 12: 1332. https://doi.org/10.3390/biology10121332
APA StyleStefaniak, A. A., Krajewski, P. K., Bednarska-Chabowska, D., Bolanowski, M., Mazur, G., & Szepietowski, J. C. (2021). Itch in Adult Population with Type 2 Diabetes Mellitus: Clinical Profile, Pathogenesis and Disease-Related Burden in a Cross-Sectional Study. Biology, 10(12), 1332. https://doi.org/10.3390/biology10121332







