Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications
Abstract
:Simple Summary
Abstract
1. Introduction
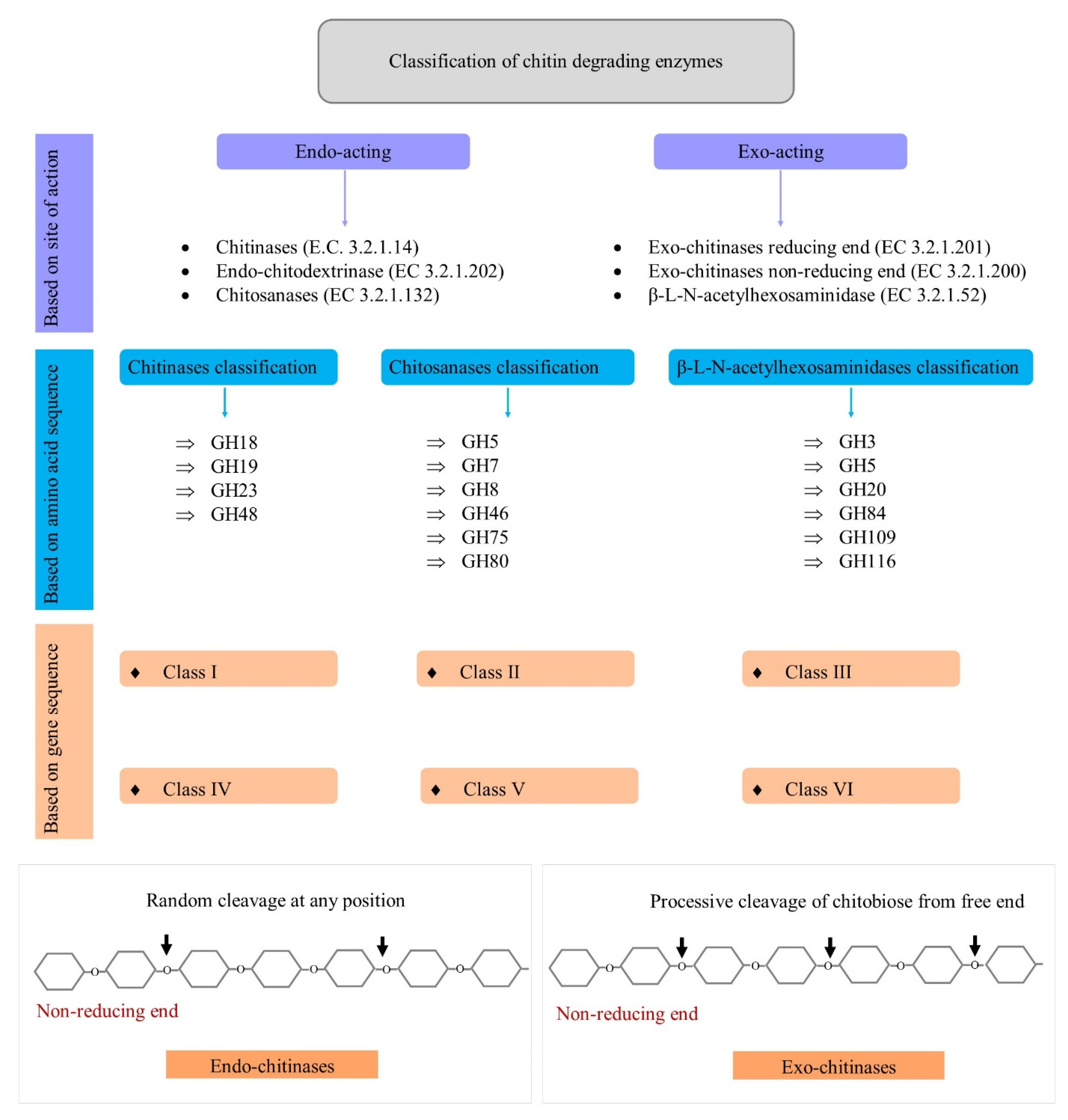
2. Classification of Chitin-Degrading Enzymes
2.1. Site of Action-Based Classification
2.2. Gene Sequence-Based Classification
2.3. Amino Acid Sequence-Based Classification
2.3.1. GH Family of Chitinases
2.3.2. GH Family of β-L-N-acetylhexosaminidases
2.3.3. GH Family of Chitosanases
3. Structural Organization of Chitinases
4. Sources of Chitinases
4.1. Plant Chitinases
4.2. Chitinases from Insects and Other Organisms
4.3. Microbial Chitinases
4.3.1. Bacteria
4.3.2. Actinomycetes
4.3.3. Fungi
5. Microbial Fermentation for Chitinase Production
5.1. Submerged Fermentation (SmF) for Chitinase Production
5.2. Solid-State Fermentation (SSF) for Chitinase Production
5.3. Factors Affecting Chitinase Production by Fermentation
5.3.1. Substrate Used
5.3.2. Temperature and pH
5.3.3. Agitation Speed
5.3.4. Fermentation Period
5.3.5. Inoculum Size
5.3.6. Carbon and Nitrogen Source
5.3.7. Metal Ions
5.4. Purification of Chitinases
5.5. Applications of Chitinases
5.5.1. Biocontrol Agent
5.5.2. Single-Cell Protein (SCP) Production
5.5.3. Protoplast Isolation
5.5.4. Biomedical Applications
5.5.5. Chitooligosaccharide (COS) Production
5.5.6. Waste Management
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.; Goyal, A. Chitin and chitinase: Role in pathogenicity, allergenicity and health. Int. J. Biol. Macromol. 2017, 97, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S.; Khajuria, A.; Ohri, P.; Kaur, R.; Kaur, R. Biocontrol potential of chitinases produced by newly isolated Chitinophaga sp. S167. World J. Microbiol. Biotechnol. 2020, 36, 90. [Google Scholar] [CrossRef]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef]
- McDonald, A.G.; Boyce, S.; Tipton, K.F. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2009, 37, D593–D597. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Khan, M.; Ahmad, M.; Ahmad, M.M.; Abdin, M.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Nath, A.K.; Chauhan, A.; Parmar, S.C.; Pandey, H.; Thakur, K. Chitinases from microbial sources, their role as biocontrol agents and other potential applications. J. Entomol. Zool. Stud. 2019, 7, 837–843. [Google Scholar]
- Bosquez-Molina, E.; Zavaleta-Avejar, L. Chapter 2—New Bioactive Biomaterials Based on Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 33–64. [Google Scholar] [CrossRef]
- Dhole, N.P.; Dar, M.A.; Pandit, R.S. Recent advances in the bioprospection and applications of chitinolytic bacteria for valorization of waste chitin. Arch. Microbiol. 2021, 203, 1953–1969. [Google Scholar] [CrossRef]
- Bibra, M.; Krishnaraj, R.N.; Sani, R.K. An Overview on Extremophilic Chitinases. In Extremophilic Enzymatic Processing of Lignocellulosic Feedstocks to Bioenergy; Sani, R.K., Krishnaraj, R.N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 225–247. [Google Scholar] [CrossRef]
- Mathew, G.M.; Madhavan, A.; Arun, K.B.; Sindhu, R.; Binod, P.; Singhania, R.R.; Sukumaran, R.K.; Pandey, A. Thermophilic Chitinases: Structural, Functional and Engineering Attributes for Industrial Applications. Appl. Biochem. Biotechnol. 2021, 193, 142–164. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.S.; Ghormade, V.; Deshpande, M.V. Chitinolytic enzymes: An exploration. Enzym. Microb. Technol. 2000, 26, 473–483. [Google Scholar] [CrossRef]
- Passarinho, P.A.; de Vries, S.C. Arabidopsis Chitinases: A Genomic Survey. Arab. Book 2002, 1, e0023. [Google Scholar] [CrossRef] [Green Version]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Aalten, D.M.; Komander, D.; Synstad, B.; Gåseidnes, S.; Peter, M.G.; Eijsink, V.G. Structural insights into the catalytic mechanism of a family 18 exo-chitinase. Proc. Natl. Acad. Sci. USA 2001, 98, 8979–8984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juárez-Hernández, E.O.; Casados-Vázquez, L.E.; Brieba, L.G.; Torres-Larios, A.; Jimenez-Sandoval, P.; Barboza-Corona, J.E. The crystal structure of the chitinase ChiA74 of Bacillus thuringiensis has a multidomain assembly. Sci. Rep. 2019, 9, 2591. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Chen, L.; Zhou, Y.; Jiang, X.; Duan, Y.; Yang, Q. Structure, Catalysis, and Inhibition of OfChi-h, the Lepidoptera-exclusive Insect Chitinase*. J. Biol. Chem. 2017, 292, 2080–2088. [Google Scholar] [CrossRef] [Green Version]
- Robertus, J.D.; Monzingo, A.F. The structure and action of chitinases. EXS 1999, 87, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Greene, L.H. Sequence and Structural Analysis of the Chitinase Insertion Domain Reveals Two Conserved Motifs Involved in Chitin-Binding. PLoS ONE 2010, 5, e8654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.; Tan, X. Comprehensive Analysis of the Chitinase Family Genes in Tomato (Solanum lycopersicum). Plants 2019, 8, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesari, P.; Patil, D.N.; Kumar, P.; Tomar, S.; Sharma, A.K.; Kumar, P. Structural and functional evolution of chitinase-like proteins from plants. Proteomics 2015, 15, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhu, Y.; Tian, Y.; Yao, J.-L.; Bian, S.; Zhang, H.; Zhang, R.; Gao, Q.; Yan, Z. MdPR4, a pathogenesis-related protein in apple, is involved in chitin recognition and resistance response to apple replant disease pathogens. J. Plant Physiol. 2021, 260, 153390. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Caldo, K.M.P.; Liang, S.; Facchini, P.J. PR10/Bet v1-like Proteins as Novel Contributors to Plant Biochemical Diversity. ChemBioChem 2021, 22, 264–287. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 265–281. [Google Scholar]
- Li, D.; Zhang, J.; Wang, Y.; Liu, X.; Ma, E.; Sun, Y.; Li, S.; Zhu, K.Y.; Zhang, J. Two chitinase 5 genes from Locusta migratoria: Molecular characteristics and functional differentiation. Insect Biochem. Mol. Biol. 2015, 58, 46–54. [Google Scholar] [CrossRef]
- Xi, Y.; Pan, P.L.; Ye, Y.X.; Yu, B.; Xu, H.J.; Zhang, C.X. Chitinase-like gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2015, 24, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Corpuz, L.; Choi, H.K.; Muthukrishnan, S. Sequence of a cDNA and expression of the gene encoding epidermal and gut chitinases of Manduca sexta. Insect Biochem. Mol. Biol. 1993, 23, 691–701. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Zuo, H.; Zheng, J.; Weng, S.; He, J.; Xu, X. A chitinase from pacific white shrimp Litopenaeus vannamei involved in immune regulation. Dev. Comp. Immunol. 2018, 85, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Osafune, T.; Tamehira, S.; Yano, K. Piglets can secrete acidic mammalian chitinase from the pre weaning stage. Sci. Rep. 2021, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017, 3, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Karthik, N.; Akanksha, K.; Binod, P.; Pandey, A. Production, purification and properties of fungal chitinases—A review. Indian J. Exp. Biol. 2014, 52, 1025–1035. [Google Scholar] [PubMed]
- Wang, X.; Zhao, Y.; Tan, H.; Chi, N.; Zhang, Q.; Du, Y.; Yin, H. Characterisation of a chitinase from Pseudoalteromonas sp. DL-6, a marine psychrophilic bacterium. Int. J. Biol. Macromol. 2014, 70, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Makhdoumi, A.; Dehghani-Joybari, Z.; Mashreghi, M.; Jamialahmadi, K.; Asoodeh, A. A novel halo-alkali-tolerant and thermo-tolerant chitinase from Pseudoalteromonas sp. DC14 isolated from the Caspian Sea. Int. J. Environ. Sci. Technol. 2015, 12, 3895–3904. [Google Scholar] [CrossRef] [Green Version]
- Gao, C.; Zhang, A.; Chen, K.; Hao, Z.; Tong, J.; Ouyang, P. Characterization of extracellular chitinase from Chitinibacter sp. GC72 and its application in GlcNAc production from crayfish shell enzymatic degradation. Biochem. Eng. J. 2015, 97, 59–64. [Google Scholar] [CrossRef]
- Laribi-Habchi, H.; Bouanane-Darenfed, A.; Drouiche, N.; Pauss, A.; Mameri, N. Purification, Characterization, and molecular cloning of an extracellular chitinase from Bacillus licheniformis stain LHH100 isolated from wastewater samples in Algeria. Int. J. Biol. Macromol. 2015, 72, 1117–1128. [Google Scholar] [CrossRef]
- Yang, S.; Fu, X.; Yan, Q.; Guo, Y.; Liu, Z.; Jiang, Z. Cloning, expression, purification and application of a novel chitinase from a thermophilic marine bacterium Paenibacillus barengoltzii. Food Chem. 2016, 192, 1041–1048. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, S.K.; Hur, J.Y.; Kim, Y.C. Purification and Characterization of a Major Extracellular Chitinase from a Biocontrol Bacterium, Paenibacillus elgii HOA73. Plant Pathol. J. 2017, 33, 318–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouacem, K.; Laribi-Habchi, H.; Mechri, S.; Hacene, H.; Jaouadi, B.; Bouanane-Darenfed, A. Biochemical Characterization of a novel thermostable chitinase from Hydrogenophilus hirschii strain KB-DZ44. Int. J. Biol. Macromol. 2018, 106, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, Y.-S.; Choi, Y.-L. Cloning, purification, and Characterization of an organic solvent-tolerant chitinase, MtCh509, from Microbulbifer thermotolerans DAU221. Biotechnol. Biofuels 2018, 11, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, M.; Li, J.; Lv, X.; Du, G.; Liu, L. Molecular engineering of chitinase from Bacillus sp. DAU101 for enzymatic production of chitooligosaccharides. Enzym. Microb. Technol. 2019, 124, 54–62. [Google Scholar] [CrossRef]
- Li, Z.; Xia, C.; Wang, Y.; Li, X.; Qiao, Y.; Li, C.; Zhou, J.; Zhang, L.; Ye, X.; Huang, Y.; et al. Identification of an endo-chitinase from Corallococcus sp. EGB and evaluation of its antifungal properties. Int. J. Biol. Macromol. 2019, 132, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Ding, H.; Yu, Y.; Chen, B. A Cold-Adapted Chitinase-Producing Bacterium from Antarctica and Its Potential in Biocontrol of Plant Pathogenic Fungi. Mar. Drugs 2019, 17, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahiaoui, M.; Laribi-Habchi, H.; Bouacem, K.; Asmani, K.-L.; Mechri, S.; Harir, M.; Bendif, H.; Aïssani-El Fertas, R.; Jaouadi, B. Purification and biochemical Characterization of a new organic solvent-tolerant chitinase from Paenibacillus timonensis strain LK-DZ15 isolated from the Djurdjura Mountains in Kabylia, Algeria. Carbohydr. Res. 2019, 483, 107747. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.J.S.; Silva, C.F.B.; Sousa, J.S.; Monteiro, J.E.; Freire, J.E.C.; Sousa, B.L.; Lobo, M.D.P.; Monteiro-Moreira, A.C.O.; Grangeiro, T.B. A thermostable chitinase from the antagonistic Chromobacterium violaceum that inhibits the development of phytopathogenic fungi. Enzyme Microb. Technol. 2019, 126, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Xu, P.; Zong, M.; Lou, W. Purification and Characterization of alkaline chitinase from Paenibacillus pasadenensis CS0611. Chin. J. Catal. 2017, 38, 665–672. [Google Scholar] [CrossRef]
- Xu, P.; Ni, Z.-F.; Zong, M.-H.; Ou, X.-Y.; Yang, J.-G.; Lou, W.-Y. Improving the thermostability and activity of Paenibacillus pasadenensis chitinase through semi-rational design. Int. J. Biol. Macromol. 2020, 150, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bhuvanachandra, B.; Podile, A.R. A transglycosylating chitinase from Chitiniphilus shinanonensis (CsChiL) hydrolyzes chitin in a processive manner. Int. J. Biol. Macromol. 2020, 145, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, S.; Kumar, M.; Kumari, P.; Mahapatro, G.K.; Banerjee, N.; Sarin, N.B. Novel insecticidal chitinase from the insect pathogen Xenorhabdus nematophila. Int. J. Biol. Macromol. 2020, 159, 394–401. [Google Scholar] [CrossRef]
- Rani, T.S.; Madhuprakash, J.; Podile, A.R. Chitinase-E from Chitiniphilus shinanonensis generates chitobiose from chitin flakes. Int. J. Biol. Macromol. 2020, 163, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, J.; Liang, Y.; Yan, R.; Xu, X.; Lin, J. Expression and Characterization of a chitinase from Serratia marcescens. Protein Expr. Purif. 2020, 171, 105613. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Zouaoui, M.; Najjari, A.; Sadfi, N. Potentialities and Characterization of an Antifungal Chitinase Produced by a Halotolerant Bacillus licheniformis. Curr. Microbiol. 2021, 78, 513–521. [Google Scholar] [CrossRef]
- Du, J.; Duan, S.; Miao, J.; Zhai, M.; Cao, Y. Purification and characterization of chitinase from Paenibacillus sp. Biotechnol. Appl. Biochem. 2021, 68, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Karthik, N.; Binod, P.; Pandey, A. Purification and Characterisation of an acidic and antifungal chitinase produced by a Streptomyces sp. Bioresour. Technol. 2015, 188, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Nawani, N.N.; Kapadnis, B.P.; Das, A.D.; Rao, A.S.; Mahajan, S.K. Purification and characterization of a thermophilic and acidophilic chitinase from Microbispora sp. V2. J. Appl. Microbiol. 2002, 93, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Gaber, Y.; Mekasha, S.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Fraaije, M.W. Characterization of a chitinase from the cellulolytic actinomycete Thermobifida fusca. Biochim. Biophys. Acta 2016, 1864, 1253–1259. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, N.; He, J.; Li, Y.; Gao, X.; Huang, L.; Yan, X. Expression and Characterization of a novel chitinase with antifungal activity from a rare actinomycete, Saccharothrix yanglingensis Hhs.015. Protein Expr. Purif. 2018, 143, 45–51. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Secundo, F.; Gao, X.; Xue, C.; Mao, X. Cloning, Characterization and substrate degradation mode of a novel chitinase from Streptomyces albolongus ATCC 27414. Food Chem. 2018, 261, 329–336. [Google Scholar] [CrossRef]
- Ray, L.; Panda, A.N.; Mishra, S.R.; Pattanaik, A.K.; Adhya, T.K.; Suar, M.; Raina, V. Purification and Characterization of an extracellular thermo-alkali stable, metal tolerant chitinase from Streptomyces chilikensis RC1830 isolated from a brackish water lake sediment. Biotechnol. Rep. 2019, 21, e00311. [Google Scholar] [CrossRef]
- Lv, C.; Gu, T.; Ma, R.; Yao, W.; Huang, Y.; Gu, J.; Zhao, G. Biochemical Characterization of a GH19 chitinase from Streptomyces alfalfae and its applications in crystalline chitin conversion and biocontrol. Int. J. Biol. Macromol. 2021, 167, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Abd-Elnabey, H.M.; Ibrahim, H.A.H.; El-Shenawy, M. Purification, Characterization and antimicrobial activity of chitinase from marine-derived Aspergillus terreus. Egypt. J. Aquat. Res. 2016, 42, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Brar, A.; Vivekanand, V.; Pareek, N. Process optimization, purification and Characterization of a novel acidic, thermostable chitinase from Humicola grisea. Int. J. Biol. Macromol. 2018, 116, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Krolicka, M.; Hinz, S.W.A.; Koetsier, M.J.; Joosten, R.; Eggink, G.; van den Broek, L.A.M.; Boeriu, C.G. Chitinase Chi1 from Myceliophthora thermophila C1, a Thermostable Enzyme for Chitin and Chitosan Depolymerization. J. Agric. Food Chem. 2018, 66, 1658–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehata, A.N.; Abd El Aty, A.A.; Darwish, D.A.; Abdel Wahab, W.A.; Mostafa, F.A. Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated Aspergillus griseoaurantiacus KX010988. Int. J. Biol. Macromol. 2018, 107, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-J.; Shi, D.; Mao, H.-H.; Li, Z.-W.; Liang, S.; Ke, Y.; Luo, X.-C. Heterologous expression and Characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int. J. Biol. Macromol. 2019, 134, 113–121. [Google Scholar] [CrossRef]
- Shahbaz, U.; Yu, X. Cloning, isolation, and Characterization of novel chitinase-producing bacterial strain UM01 (Myxococcus fulvus). J. Genet. Eng. Biotechnol. 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Beygmoradi, A.; Homaei, A.; Hemmati, R.; Santos-Moriano, P.; Hormigo, D.; Fernández-Lucas, J. Marine chitinolytic enzymes, a biotechnological treasure hidden in the ocean? Appl. Microbiol. Biotechnol. 2018, 102, 9937–9948. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yan, Q.; Wang, J.; Yang, S.; Jiang, Z. Purification and biochemical Characterization of novel acidic chitinase from Paenicibacillus barengoltzii. Int. J. Biol. Macromol. 2016, 91, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.; Gothwal, R.K.; Krishna Mohan, M.; Ghosh, P. Production and purification of a hyperthermostable chitinase from Brevibacillus formosus BISR-1 isolated from the Great Indian Desert soils. Extremophiles 2014, 18, 451–462. [Google Scholar] [CrossRef]
- Li, R.K.; Hu, Y.J.; He, Y.J.; Ng, T.B.; Zhou, Z.M.; Ye, X.Y. A thermophilic chitinase 1602 from the marine bacterium Microbulbifer sp. BN3 and its high-level expression in Pichia pastoris. Biotechnol. Appl. Biochem. 2020, 68, 1076–1085. [Google Scholar] [CrossRef]
- Gruber, S.; Seidl-Seiboth, V. Self versus non-self: Fungal cell wall degradation in Trichoderma. Microbiology 2012, 158, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosado, I.V.; Rey, M.; Codón, A.C.; Govantes, J.; Moreno-Mateos, M.A.; Benítez, T. QID74 Cell wall protein of Trichoderma harzianum is involved in cell protection and adherence to hydrophobic surfaces. Fungal Genet. Biol. 2007, 44, 950–964. [Google Scholar] [CrossRef]
- Dolatabad, H.K.; Javan-Nikkhah, M.; Shier, W.T. Evaluation of antifungal, phosphate solubilisation, and siderophore and chitinase release activities of endophytic fungi from Pistacia vera. Mycol. Prog. 2017, 16, 777–790. [Google Scholar] [CrossRef]
- Stoykov, Y.M.; Pavlov, A.I.; Krastanov, A.I. Chitinase biotechnology: Production, purification, and application. Eng. Life Sci. 2015, 15, 30–38. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, A.; Laksh, S.A.; Rana, M. Process optimization of extracellular chitinase production from Bacillus sp. Isolated from fish waste Dumping site. Eur. J. Pharm. Med. Res. 2017, 4, 474–480. [Google Scholar]
- Shivalee, A.; Lingappa, K.; Mahesh, D. Influence of bioprocess variables on the production of extracellular chitinase under submerged fermentation by Streptomyces pratensis strain KLSL55. J. Genet. Eng. Biotechnol. 2018, 16, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Abu-Tahon, M.A.; Isaac, G.S. Anticancer and antifungal efficiencies of purified chitinase produced from Trichoderma viride under submerged fermentation. J. Gen. Appl. Microbiol. 2020, 66, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, T.B.; de Oliveira Ornela, P.H.; de Oliveira, A.H.C.; Jorge, J.A.; Guimarães, L.H.S. Production and Characterization of a thermostable antifungal chitinase secreted by the filamentous fungus Aspergillus niveus under submerged fermentation. 3 Biotech 2018, 8, 369. [Google Scholar] [CrossRef]
- Elawati, N.E.; Pujiyanto, S.; Kusdiyantini, E. Production of extracellular chitinase Beauveria bassiana under submerged fermentation conditions. J. Phys. Conf. Ser. 2018, 1025, 012074. [Google Scholar] [CrossRef]
- Dukariya, G.; Kumar, A. Statistical optimization of chitinase production by Box–Behnken design in submerged fermentation using Bacillus cereus GS02. J. Appl. Biol. Biotechnol. 2021, 9, 60–66. [Google Scholar] [CrossRef]
- Baldoni, D.B.; Antoniolli, Z.I.; Mazutti, M.A.; Jacques, R.J.S.; Dotto, A.C.; de Oliveira Silveira, A.; Ferraz, R.C.; Soares, V.B.; de Souza, A.R.C. Chitinase production by Trichoderma koningiopsis UFSMQ40 using solid state fermentation. Braz. J. Microbiol. 2020, 51, 1897–1908. [Google Scholar] [CrossRef]
- Michael, R.Z. Production of Chitinase from Chromobacterium violaceum Using Agro Industrial Residues under Solid State Fermentation. Indian J. Pharm. Sci. 2021, 83, 39–44. [Google Scholar] [CrossRef]
- dos Reis, C.B.L.; Sobucki, L.; Mazutti, M.A.; Guedes, J.V.C.; Jacques, R.J.S. Production of Chitinase from Metarhizium anisopliae by Solid-State Fermentation Using Sugarcane Bagasse as Substrate. Ind. Biotechnol. 2018, 14, 230–234. [Google Scholar] [CrossRef]
- Chaiharn, M.; Pikomol, A.; Lumyoung, S. Purification and Characterization of an Extracellular Chitinase from Bacillus thuringiensis R 176 using Solid State Fermentation with Shrimp Shells Waste. Chiang Mai J. Sci. 2019, 46, 495–509. [Google Scholar]
- Wang, L.; Yang, S.-T. Chapter 18—Solid State Fermentation and Its Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 465–489. [Google Scholar] [CrossRef]
- Mutturi, S.; Ike, M.; Yamagishi, K.; Tokuyasu, K. Isolation, characterization, and application of thermotolerant Streptomyces sp. K5 for efficient conversion of cellobiose to chitinase using pulse- feeding strategy. Process Biochem. 2020, 94, 58–65. [Google Scholar] [CrossRef]
- Cheba, B.A.; Zaghloul, T.I.; El-Mahdy, A.R.; El-Massry, M.H. Effect of nitrogen sources and fermentation conditions on Bacillus sp. R2 chitinase production. Procedia Manuf. 2018, 22, 280–287. [Google Scholar] [CrossRef]
- Saima; Kuddus, M.; Roohi; Ahmad, I.Z. Isolation of novel chitinolytic bacteria and production optimization of extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, M.; Zhao, S.; Lin, C.; Song, J.; Yang, Q. ATP-Binding Cassette Transporter Regulates N,N′-diacetylchitobiose Transportation and Chitinase Production in Trichoderma asperellum T4. Int. J. Mol. Sci. 2019, 20, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akeed, Y.; Atrash, F.; Naffaa, W. Partial purification and Characterization of chitinase produced by Bacillus licheniformis B307. Heliyon 2020, 6, e03858. [Google Scholar] [CrossRef] [PubMed]
- Fenice, M.; Barghini, P.; Selbmann, L.; Federici, F. Combined effects of agitation and aeration on the chitinolytic enzymes production by the Antarctic fungus Lecanicillium muscarium CCFEE 5003. Microb. Cell Factories 2012, 11, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandhya, C.; Adapa, L.K.; Nampoothiri, K.M.; Binod, P.; Szakacs, G.; Pandey, A. Extracellular chitinase production by Trichoderma harzianum in submerged fermentation. J. Basic Microbiol. 2004, 44, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727–742. [Google Scholar] [CrossRef]
- Sukalkar, S.R.; Kadam, T.A.; Bhosale, H.J. Optimization of chitinase production from Streptomyces macrosporeus M1. Res. J. Life Sci. Bioinform. Pharm. Chem. Sci. 2018, 4, 106–114. [Google Scholar]
- Gomaa, E.Z. Chitinase production by Bacillus thuringiensis and Bacillus licheniformis: Their potential in antifungal biocontrol. J. Microbiol. 2012, 50, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P. (Ed.) Chapter 6—Purification of Xylanases. In Xylanolytic Enzymes; Academic Press: Amsterdam, The Netherlands, 2014; pp. 53–61. [Google Scholar] [CrossRef]
- Asmani, K.-L.; Bouacem, K.; Ouelhadj, A.; Yahiaoui, M.; Bechami, S.; Mechri, S.; Jabeur, F.; Taleb-Ait Menguellet, K.; Jaouadi, B. Biochemical and molecular Characterization of an acido-thermostable endo-chitinase from Bacillus altitudinis KA15 for industrial degradation of chitinous waste. Carbohydr. Res. 2020, 495, 108089. [Google Scholar] [CrossRef]
- Laribi-Habchi, H.; Bouacem, K.; Allala, F.; Jabeur, F.; Selama, O.; Mechri, S.; Yahiaoui, M.; Bouanane-Darenfed, A.; Jaouadi, B. Characterization of chitinase from Shewanella inventionis HE3 with bio-insecticidal effect against granary weevil, Sitophilus granarius Linnaeus (Coleoptera: Curculionidae). Process Biochem. 2020, 97, 222–233. [Google Scholar] [CrossRef]
- Kidibule, P.E.; Costa, J.; Atrei, A.; Plou, F.J.; Fernandez-Lobato, M.; Pogni, R. Production and Characterization of chitooligosaccharides by the fungal chitinase Chit42 immobilized on magnetic nanoparticles and chitosan beads: Selectivity, specificity and improved operational utility. RSC Adv. 2021, 11, 5529–5536. [Google Scholar] [CrossRef]
- Sastre, D.E.; Reis, E.A.; Marques Netto, C.G.C. Chapter 4 - Strategies to rationalize enzyme immobilization procedures. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 630, pp. 81–110. [Google Scholar] [CrossRef]
- Ismail, S.A.; Hassan, M.E.; Hashem, A.M. Single step hydrolysis of chitin using thermophilic immobilized exochitinase on carrageenan-guar gum gel beads. Biocatal. Agric. Biotechnol. 2019, 21, 101281. [Google Scholar] [CrossRef]
- Prasad, M.; Palanivelu, P. Immobilization of a thermostable, fungal recombinant chitinase on biocompatible chitosan beads and the properties of the immobilized enzyme. Biotechnol. Appl. Biochem. 2015, 62, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, R.; Agheshlouie, M.; Mahdavinia, G.R. Expression of chitinase gene in BL21 pET system and investigating the biocatalystic performance of chitinase-loaded AlgSep nanocomposite beads. Int. J. Biol. Macromol. 2017, 104, 1664–1671. [Google Scholar] [CrossRef]
- Patil, N.S.; Jadhav, J.P. Significance of Penicillium ochrochloron chitinase as a biocontrol agent against pest Helicoverpa armigera. Chemosphere 2015, 128, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.I.; Bisetty, K.; Singh, S.; Permaul, K.; Hassan, M.I. Chitinase from Thermomyces lanuginosus SSBP and its biotechnological applications. Extremophiles 2015, 19, 1055–1066. [Google Scholar] [CrossRef]
- Patil, N.; Jadhav, J. Single cell protein production using Penicillium ochrochloron chitinase and its evaluation in fish meal formulations. J. Microb. Biochem. Technol. 2014, 4, 2. [Google Scholar] [CrossRef]
- Revah-Moiseev, S.; Carroad, P.A. Conversion of the enzymatic hydrolysate of shellfish waste chitin to single-cell protein. Biotechnol. Bioeng. 1981, 23, 1067–1078. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Kulkarni, S.S.; Ghosh, J.S. Chitinase Production in Solid-State Fermentation from Oerskovia xanthineolytica NCIM 2839 and its Application in Fungal Protoplasts Formation. Curr. Microbiol. 2011, 63, 295. [Google Scholar] [CrossRef]
- Dahiya, N.; Tewari, R.; Tiwari, R.P.; Hoondal, G.S. Production of an Antifungal Chitinase from Enterobacter sp. NRG4 and its Application in Protoplast Production. World J. Microbiol. Biotechnol. 2005, 21, 1611–1616. [Google Scholar] [CrossRef]
- Vannella, K.M.; Ramalingam, T.R.; Hart, K.M.; de Queiroz Prado, R.; Sciurba, J.; Barron, L.; Borthwick, L.A.; Smith, A.D.; Mentink-Kane, M.; White, S.; et al. Acidic chitinase primes the protective immune response to gastrointestinal nematodes. Nat. Immunol. 2016, 17, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allonsius, C.N.; Vandenheuvel, D.; Oerlemans, E.F.M.; Petrova, M.I.; Donders, G.G.G.; Cos, P.; Delputte, P.; Lebeer, S. Inhibition of Candida albicans morphogenesis by chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019, 9, 2900. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Chand, M.; Sharma, H.O.; Kumar, P. Role of Chitinases as a waste management to control global crisis. Int. J. Environ. Rehabil. Conserv. 2020, 303–313. [Google Scholar] [CrossRef]
- Wang, D.; Li, A.; Han, H.; Liu, T.; Yang, Q. A potent chitinase from Bacillus subtilis for the efficient bioconversion of chitin-containing wastes. Int. J. Biol. Macromol. 2018, 116, 863–868. [Google Scholar] [CrossRef] [PubMed]
| GH Family | Clan | Mechanism | Catalytic Domain 3D Structure | Catalytic Nucleophile/Base | Catalytic Proton Donor | Enzyme Name |
|---|---|---|---|---|---|---|
| GH3 | - | Retaining | - | Aspartate | Glutamate (for hydrolases)/Histidine (for phosphorylases) | β-L-N-acetylhexosaminidase |
| GH5 | GH-A | Retaining | (β/α)8-barrel | Glutamate | Glutamate | Chitosanase, β-L-N-acetylhexosaminidase |
| GH7 | GH-B | Retaining | β-jelly roll | Glutamate | Glutamate | Chitosanase |
| GH8 | GH-M | Inverting | (α/α)6 | Aspartate | Glutamate | Chitosanase |
| GH18 | GH-K | Retaining | (β/α)8-barrel | Carbonyl oxygen of C-2 acetamido group of the substrate | Glutamate | Chitinase |
| GH19 | - | Inverting | - | - | - | Chitinase |
| GH20 | GH-K | Retaining | (β/α)8 | Carbonyl oxygen of C-2 acetamido group of the substrate | Glutamate | β-L-N-acetylhexosaminidase |
| GH23 | - | - | - | - | Glutamate | Chitinase |
| GH46 | - | Inverting | - | Probably aspartate | Probably glutamate | Chitosanase |
| GH48 | GH-M | Inverting | (α/α)6-barrel | - | Glutamate | Chitinase |
| GH75 | - | Inverting | - | Probably aspartate | Probably glutamate | Chitosanase |
| GH80 | GH-I | Inverting | α+β | - | - | Chitosanase |
| GH84 | - | Retaining | (β/α)8-barrel | Carbonyl oxygen of C-2 acetamido group of the substrate | Aspartate | β-L-N-acetylhexosaminidase |
| GH109 | - | Retaining | - | Not applicable | None | β-L-N-acetylhexosaminidase |
| GH116 | GH-O | Retaining | (α/α)6-barrel | Glutamate | Aspartate | β-L-N-acetylhexosaminidase |
| S.No. | Organism Name | Class of Enzyme | Molecular Weight (kDa) | Optimum pH/Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| 1. | Pseudoalteromonas sp. DL-6 | GH18 | 113.5 | 8.0/20 | Wang et al. [31] |
| 2. | Pseudoalteromonas sp. DC14, | - | 65 | 9.0/40 | Makhdoumi et al. [32] |
| 3. | Chitinibacter sp. GC72 | - | 65 | 6.8/40 | Gao et al. [33] |
| 4. | Bacillus licheniformis LHH100 | - | 65 | 4.0/75 | Laribi-Habchi et al. [34] |
| 5. | Paenibacillus barengoltzii | GH18 | 70 | 5.5/55 | Yang et al. [35] |
| 6. | Paenibacillus elgii HOA73 | - | 68 | 7.0/50 | Kim et al. [36] |
| 7. | Hydrogenophilus hirschii | - | 59 | 5.0/85 | Bouacem et al. [37] |
| 8. | Microbulbifer thermotolerans DAU221 | GH18 | 60 | 4.6/55 | Lee et al. [38] |
| 9. | Bacillus subtilis WB600 | GH18 | 62 | 5.0/60 | Pan et al. [39] |
| 10. | Corallococcus sp. EGB | GH18 | 52.9 | 6.0/50 | Li et al. [40] |
| 11. | Pseudomonas | - | - | 4.5/35 | Liu et al. [41] |
| 12. | Paenibacillus timonensis LK-DZ15 | GH18 | 70 | 4.5/80 | Yahiaoui et al. [42] |
| 13. | Chromobacterium violaceum | GH18 | 46 | 5.0/60 | Sousa et al. [43] |
| 14. | Paenibacillus pasadenensis CS0611 | GH18 | 69 | 5.0/50 | Guo et al. [44] and Xu et al. [45] |
| 15. | Chitiniphilus shinanonensis | GH18 | 45.3 | 6.0/50 | Bhuvanachandra and Podile [46] |
| 16. | Xenorhabdus nematophila | GH18 | 76 | - | Mahmood et al. [47] |
| 17. | Chitiniphilus shinanonensis | GH18 | 58.87 | 7.0/50 | Rani et al. [48] |
| 18. | Serratia marcescens | - | 55.6 | 6.0/55 | Li et al. [49] |
| 19. | Bacillus licheniformis J24 | GH18 | - | 7.0/70 | Essghaier et al. [50] |
| 20. | Paenibacillus sp. | - | 30 | 4.5/50 | Du et al. [51] |
| Actinomycetes | |||||
| 1. | Streptomyces sp. | - | 40 | 2&6/50 | Karthik et al. [52] |
| 2. | Microbispora sp. V2 | - | 35 | 3.0/60 | Nawani et al. [53] |
| 3. | Thermobifida fusca | GH18 | 46.3 | 6.0–8.0/40–45 | Gaber et al. [54] |
| 4. | Saccharothrix yanglingensis Hhs.015 | - | 77.9 | 7.0/49 | Lu et al. [55] |
| 5. | Streptomyces albolongus ATCC 27414 | GH18 | 47 | 5.0/55 | Gao et al. [56] |
| 6. | Streptomyces chilikensis RC1830 | - | 10.5 | 7.0/60 | Ray et al. [57] |
| 7. | Streptomyces alfalfae | GH19 | 29 | 8.0/45 | Lv et al. [58] |
| Fungi | |||||
| 1. | Aspergillus terreus | - | 60 | 5.6/50 | Farag et al. [59] |
| 2. | Humicola grisea | - | 50 | 3.0/70 | Kumar et al. [60] |
| 3. | Myceliophthora thermophila C1 | - | 43 | 6.0/55 | Krolicka et al. [61] |
| 4. | Aspergillus griseoaurantiacus KX010988 | - | 130 | 4.5/40 | Shehata et al. [62] |
| 5. | Trichoderma harzianum GIM 3.442 | GH18 | 45 | 6.0/45 | Deng et al. [63] |
| Trade Name and Producing Firm | Producing Organism | Formulation Type (Solid, Liquid) | Activity (U/g) |
|---|---|---|---|
| Chitinase from Aspergillus niger (food grade)/Creative Enzymes® | Aspergillus niger | Light-brown powdered form | 200 * |
| Native Streptomyces griseus Chitinase/Creative Enzymes® | Streptomyces griseus | Lyophilized powder (essentially salt-free) | >200 * |
| Native Trichoderma viride Chitinase/Creative Enzymes® | Trichoderma viride | Lyophilized powder form | >600 * |
| Chitinase (Clostridium thermocellum)/Megazyme | Clostridium thermocellum | Solid | 300 ** |
| Chitinase from Streptomyces griseus/Merck (Sigma-Aldrich) | Streptomyces griseus | Solid | ≥200 *** |
| Chitinase from Streptomyces griseus/Merck (Sigma-Aldrich) | Streptomyces griseus | Solid | ≥1000 *** |
| Chitinase from Trichoderma viride/Merck (Sigma-Aldrich) | Trichoderma viride | Lyophilized powder form | ≥600 *** |
| Organism Name | Purification Technique | Molecular Weight (kDa) | Specific Activity (U/mg) | Optimum pH/Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Paenicibacillus barengoltzii | Ammonium sulfate precipitation (20–40%), ion-exchange chromatography | 67 | 12.4 | 3.5/60 | Fu, Yan, Wang, Yang and Jiang [66] |
| Aspergillus niveus | Ammonium sulfate precipitation, Sephadex G-100 gel filtration chromatography | 44 | 13.3 | 5.0/65 | Alves, de Oliveira Ornela, de Oliveira, Jorge and Guimarães [76] |
| Aspergillus griseoaurantiacus | Ammonium sulfate precipitation, DEAE-cellulose column chromatography, Sephacryl S-300 column chromatography | 130 | 93.75 | 4.5/40 | Shehata, Abd El Aty, Darwish, Abdel Wahab and Mostafa [62] |
| Bacillus altitudinis KA15 | Ammonium sulfate precipitation (30 and 60%), Sephacryl S-200 high-resolution size exclusion chromatography, high-performance ion-exchange chromatography (IEX) | 43 | 120,000 | 4.0/85 | Asmani et al. [95] |
| Myxococcus fulvus UM01 | Ni-NTA affinity chromatography | 26.99 | - | 7.0/35 | Shahbaz and Yu [64] |
| Paenibacillus sp. | Ammonium sulfate precipitation (60–80%), DEAE-IEC, and gel chromatography | 30 | 0.85 | 4.5/50 | Du, Duan, Miao, Zhai and Cao [51] |
| Shewanella inventionis HE3 | Ammonium sulfate precipitation (20–80%), gel filtration chromatography | 40 | 41,000 | 4.0/70 | Laribi-Habchi et al. [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications. Biology 2021, 10, 1319. https://doi.org/10.3390/biology10121319
Poria V, Rana A, Kumari A, Grewal J, Pranaw K, Singh S. Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications. Biology. 2021; 10(12):1319. https://doi.org/10.3390/biology10121319
Chicago/Turabian StylePoria, Vikram, Anuj Rana, Arti Kumari, Jasneet Grewal, Kumar Pranaw, and Surender Singh. 2021. "Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications" Biology 10, no. 12: 1319. https://doi.org/10.3390/biology10121319
APA StylePoria, V., Rana, A., Kumari, A., Grewal, J., Pranaw, K., & Singh, S. (2021). Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications. Biology, 10(12), 1319. https://doi.org/10.3390/biology10121319










