Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
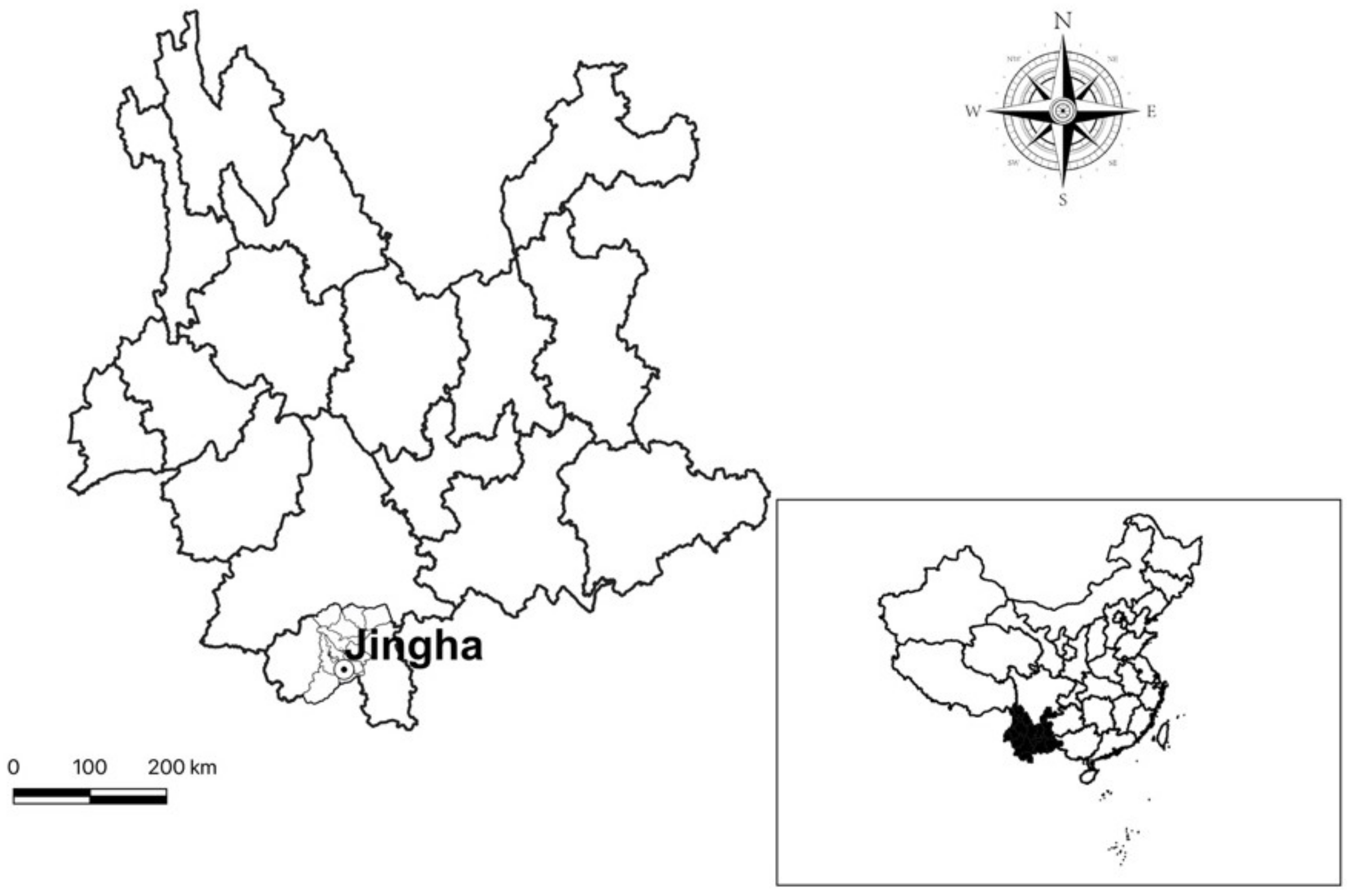
2.1. Field Investigation
2.2. Collection and Identification of Gamasid Mites and Their Hosts
2.3. Statistical Analysis
3. Results
3.1. Collection of Gamasid Mites and Their Hosts
3.2. Community Structure and Overall Infestations of Gamasid Mites on Rattus andamanensis
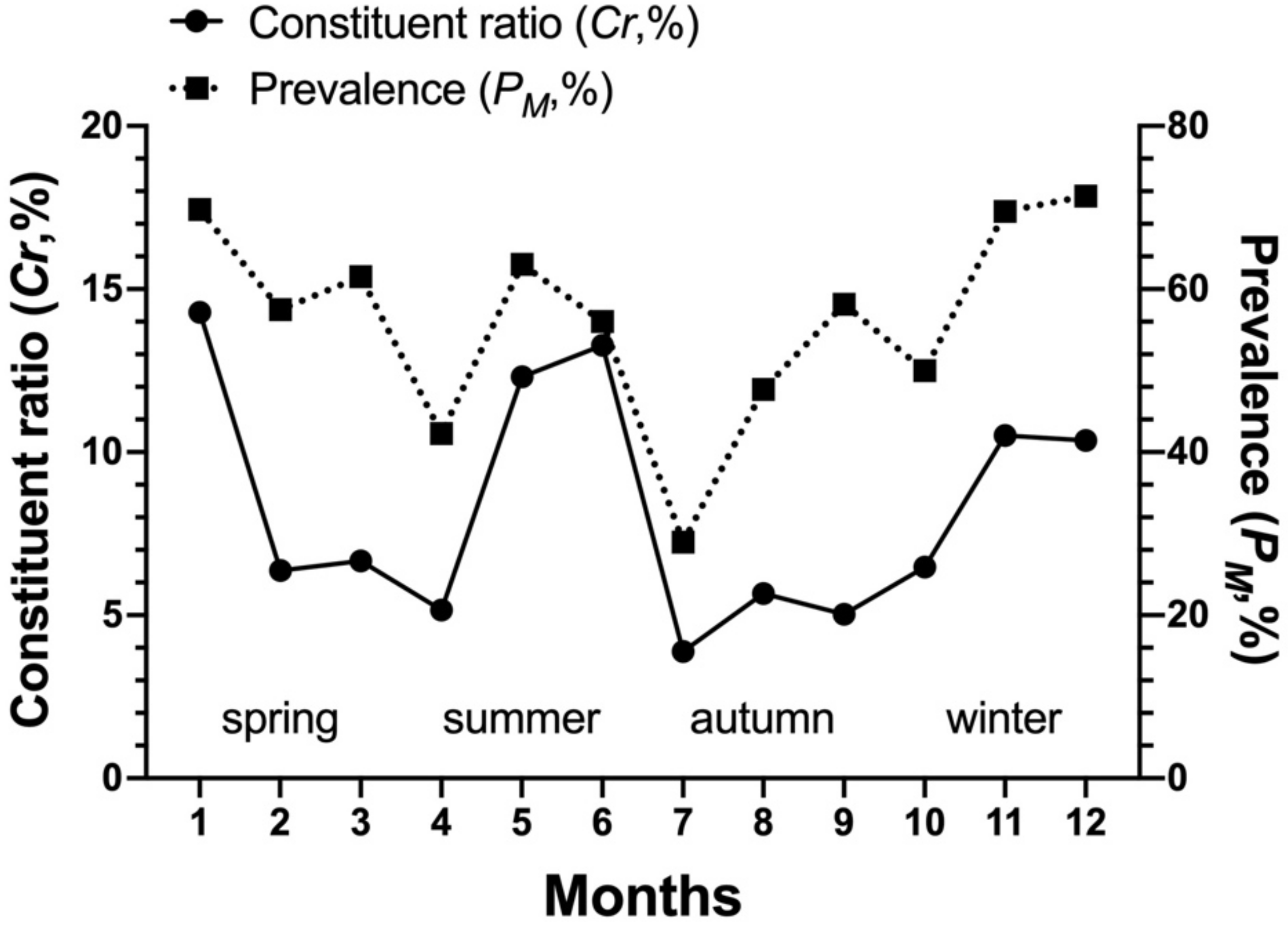
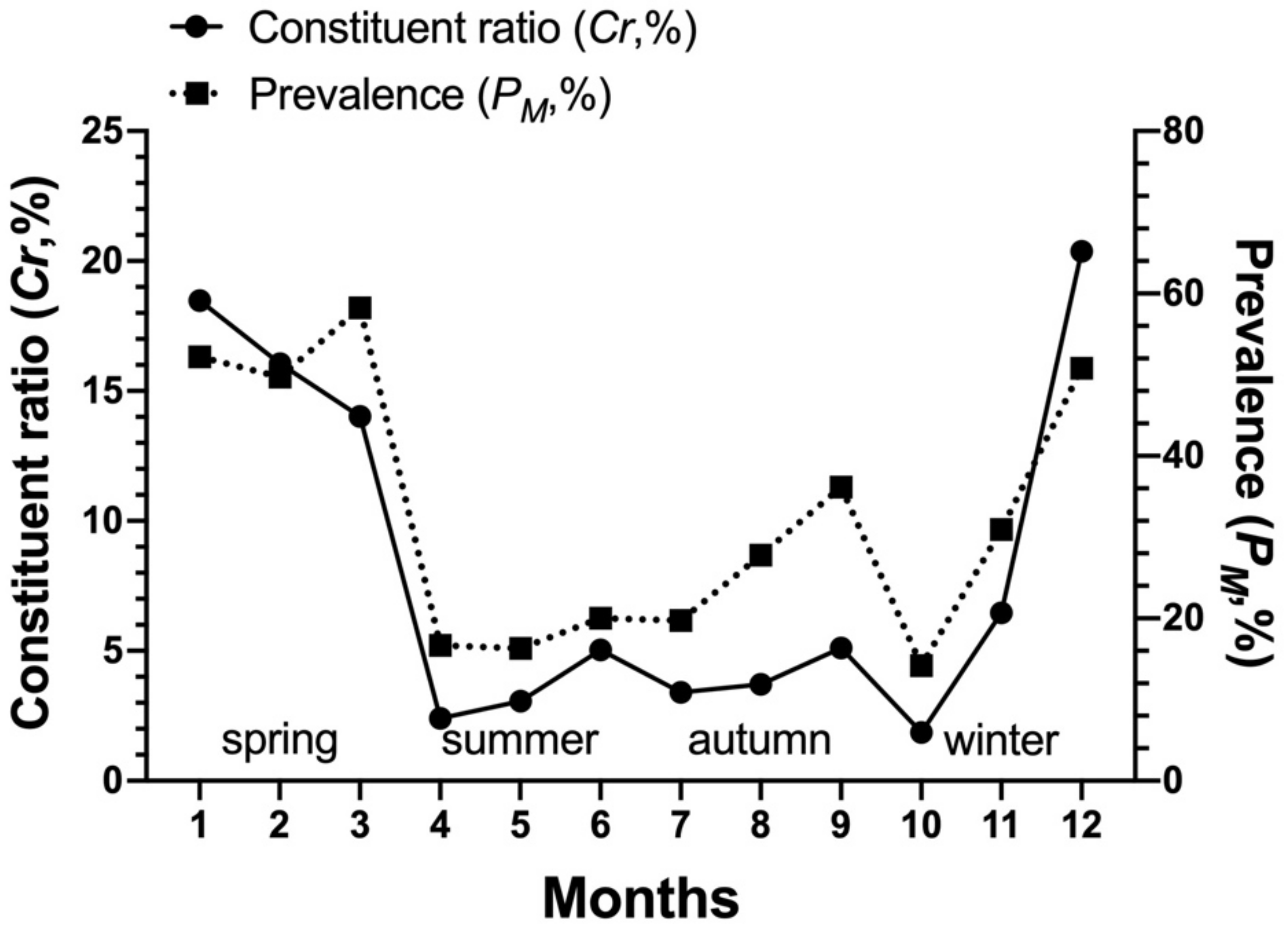
3.3. Dominant Species of Gamasid Mites and Their Seasonal Fluctuations
3.4. Relationship between the Seasonal Fluctuation of Dominant Gamasid Mites and the Fluctuation of Climatic Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Varma, M. Ticks and Mites (Acari). In Medical Insects and Arachnids; Lane, R.P., Crosskey, R.W., Eds.; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Huang, L.Q.; Guo, X.G.; Speakman, J.R.; Dong, W.G. Analysis of Gamasid Mites (Acari: Mesostigmata) Associated with the Asian House Rat, Rattus Tanezumi (Rodentia: Muridae) in Yunnan Province, Southwest China. Parasitol. Res. 2013, 112, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Dhooria, M.S. Fundamentals of Applied Acarology; Springer: Singapore, 2016. [Google Scholar]
- Gulvik, M. Mites (Acari) as Indicators of Soil Biodiversity and Land Use Monitoring: A Review. Pol. J. Ecol. 2007, 55, 415. [Google Scholar]
- Guo, X.G.; Qian, T.J.; Guo, L.J.; Wang, J.; Dong, W.G.; Zhang, L.; Ma, Z.M.; Li, W. Species Diversity and Community Structure of Sucking Lice in Yunnan, China. Insect Sci. 2004, 11, 61–70. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, X.G.; Fan, R.; Zhao, C.F.; Mao, K.Y.; Zhang, Z.W.; Zhao, Y. Ecological Analysis of Gamasid Mites on the Body Surface of Norway Rats (Rattus Norvegicus) in Yunnan Province, Southwest China. Biologia 2020, 75, 1325–1336. [Google Scholar] [CrossRef]
- Yin, P.W.; Guo, X.G.; Jin, D.C.; Fan, R.; Zhao, C.F.; Zhang, Z.W.; Huang, X.B.; Mao, K.Y. Distribution and Host Selection of Tropical Rat Mite, Ornithonyssus bacoti, in Yunnan Province of Southwest China. Animals 2021, 11, 110. [Google Scholar] [CrossRef]
- Xiang, R.; Guo, X.G.; Zhao, C.F.; Fan, R.; Mao, K.Y.; Zhang, Z.W.; Huang, X.B. Infestation and Distribution of Gamasid Mites on Himalayan Field Rat (Rattus Nitidus) in Yunnan Province of Southwest China. Biologia 2021, 76, 1763–1773. [Google Scholar] [CrossRef]
- Korallo, N.P.; Vinarski, M.V.; Krasnov, B.R.; Shenbrot, G.I.; Mouillot, D.; Poulin, R. Are There General Rules Governing Parasite Diversity? Small Mammalian Hosts and Gamasid Mite Assemblages. Divers. Distrib. 2007, 13, 353–360. [Google Scholar] [CrossRef]
- Peng, P.Y.; Guo, X.G.; Ren, T.G.; Song, W.Y.; Dong, W.G.; Fan, R. Species Diversity of Ectoparasitic Chigger Mites (Acari: Prostigmata) on Small Mammals in Yunnan Province, China. Parasitol. Res. 2016, 115, 3605–3618. [Google Scholar] [CrossRef] [PubMed]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Walter, D.E.; Proctor, H.C. Mites: Ecology, Evolution & Behaviour. Life at a Microscale, 2nd ed.; Springer Science+Business Media: Dordrecht, The Netherlands; New York, NY, USA, 2013. [Google Scholar]
- Musser, G.; Carleton, M.; Wilson, D.; Reeder, D. Mammal Species of the World: A Taxonomic and Geographic Reference; Johns Hopkins University Press: London, UK; Baltimore, MD, USA, 2005. [Google Scholar]
- Smith, A.T.; Xie, Y.; Hoffmann, R.S.; Lunde, D.; MacKinnon, J.; Wilson, D.E.; Wozencraft, W.C.; Gemma, F. A Guide to the Mammals of China; Princeton University Press: Princeton, NJ, USA, 2008; p. 179. [Google Scholar]
- Sun, K.; Qiao, Y.; Qin, B. A Green Pearl of the Southwestern Frontier of China-the Topography, Climate, Plant Species and Collection of Xishuangbanna. Biol Teach. 2000, 25, 37–38. [Google Scholar]
- Yu, Y.; Meng, G.Y.; Zhang, C.L. Characteristics of Climate Change in Xishuangbanna Area in the Past 45 years. Meteorol. Sci. 2008, 36, 410–413. [Google Scholar]
- Weather Prediction. Available online: https://tianqi.911cha.com/jinghong (accessed on 30 September 2021).
- Zheng, Z.M.; Jiang, Z.K.; Chen, A.G. Glires (Nie Chi Dong Wu Xue), 2nd ed.; Shanghai Jiaotong University Press: Shanghai, China, 2012; p. 62. (In Chinese) [Google Scholar]
- Wilson, D.E.; Lacher, T.E.; Mittermeier, R.A. Handbook of the Mammals of the World, Volume 7: Rodents II; Lynx Ediciones: Barcelona, Spain, 2017. [Google Scholar]
- Nowak, R.M.; Walker, E.P. Walker’s Mammals of the World; JHU Press: Baltimore, MD, USA, 1999; Volume 1. [Google Scholar]
- Huang, W.J.; Chen, Y.X.; Wen, Y.X. Glires of China (Zhong Guo Nie Chi Lei); Fudan University Press: Shanghai, China, 1995. (In Chinese) [Google Scholar]
- Deng, G.F.; Teng, K.F. Economic Insect Fauna of China Fasc. 40 Acari: Dermanyssoidese; Science Press: Beijing, China, 1993. (In Chinese) [Google Scholar]
- Pan, Z.W.; Deng, G.F. Economic Insect Fauna of China. Fasc 17, Acari: Gamasina; Science Press: Beijing, China, 1980. (In Chinese) [Google Scholar]
- Evans, G.O.; Till, W. Mesostigmatic Mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes): An Introduction to Their External morphology and Classification. Trans. Zool. Soc. Lond. 1979, 35, 139–262. [Google Scholar] [CrossRef]
- Hyatt, K.H.; Embersom, R.M. A Review of the Macrochelidae (Acari: Mesostigmata) of the British Isles. Bull. Br. Mus. Nat. History Zool. 1988, 54, 63–125. [Google Scholar]
- Radovsky, F.J. The Macronyssidae and Laelapidae (Acarina: Mesostigmata) Parasitic on Bats; University of California Publications in Entomology: Berkeley, CA, USA; Los Angeles, CA, USA, 1967. [Google Scholar]
- Peng, P.Y.; Guo, X.G.; Jin, D.C.; Dong, W.G.; Qian, T.J.; Qin, F.; Yang, Z.H.; Fan, R. Landscapes with Different Biodiversity Influence Distribution of Small Mammals and Their Ectoparasitic Chigger Mites: A Comparative Study from Southwest China. PLoS ONE 2018, 13, e0189987. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Zhan, Y.Z.; Guo, X.G.; Speakman, J.R.; Zuo, X.H.; Wu, D.; Wang, Q.H.; Yang, Z.H. Abundances and Host Relationships of Chigger Mites in Yunnan Province, China. Med. Vet. Entomol. 2013, 27, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.G.; Qian, T.J.; Meng, X.Y.; Dong, W.G.; Shi, W.X.; Wu, D. Preliminary Analysis of Chigger Communities Associated with House Rats (Rattus flavipectus) from Six Counties in Yunnan, China. Syst. Appl. Acarol. 2006, 11, 13–21. [Google Scholar] [CrossRef]
- Hinton, M.A. The Zoology of Rats and Mice, with Special Reference to the Question of the Future Control of the Rat Population. J. R. Sanit. Inst. 1919, 40, 44–52. [Google Scholar] [CrossRef]
- Ellerman, J. Fauna of India, including Pakistan, Burma and Ceylon. Mammalia. Vol. 3, Rodentia; Zoological Survey of India: Calcutta, India, 1961. [Google Scholar]
- Wang, Y.X. A Complete Checklist of Mammal Species and Subspecies in China: A Taxonomic and Geographic Reference; China Forestry Publishing House: Beijing, China, 2003; pp. 41–46. [Google Scholar]
- Musser, G.G.; Newcomb, C. Malaysian Murids and the Giant Rat of Sumatra. Bull. Am. Mus. Nat. Hist. 1983, 174, 327–598. [Google Scholar]
- Musser, G.G.; Heaney, L.R. Philippine Rattus: A New Species from the Sulu Archipelago; American Museum of Natural History: New York, NY, USA, 1985. [Google Scholar]
- Musser, G.; Carleton, M.; Wilson, D.; Reeder, D. Mammal Species of the World. In Family Muridae; Smithsonian Institution Press: Washington, DC, USA, 1993. [Google Scholar]
- Corbet, G.B.; Hill, J.E. The Mammals of the Indomalayan Region: A Systematic Review; Oxford University Press: Oxford, UK, 1992; Volume 488. [Google Scholar]
- Ellerman, J.R.; Morrison-Scott, T.C.S. Checklist of Palaearctic and Indian Mammals, 2nd ed.; Alden Press: Oxford, UK, 1966; pp. 1758–1946. [Google Scholar]
- Allen, G.M. Rats (Genus Rattus) from the Asiatic Expeditions; By Order of the Trustees of American Museum of Natural History: New York, NY, USA, 1926. [Google Scholar]
- Gao, Z.H.; Huang, T.H.; Jiang, B.G.; Jia, N.; Liu, Z.X.; Shao, Z.T.; Jiang, R.R.; Liu, H.B.; Wei, R.; Li, Y.Q. Wide Distribution and Genetic Diversity of Babesia microti in Small Mammals from Yunnan Province, Southwestern China. PLoS Negl. Trop. Dis. 2017, 11, e0005898. [Google Scholar] [CrossRef]
- Institute of Zoology, Chinese Academy of Sciences. Rattus Rattus Sladeni. China Animal Species Catalog Database; Institute of Zoology, Chinese Academy of Sciences: Beijing, China, 2009. [Google Scholar]
- Han, C.X.; Li, J.G.; Yang, X.J.; Zhang, H.L.; Wang, M.C.; Yang, Q.E. Agricultural and Forestry Rodents and Scientific Management in China (Zhong Guo Nong Lin Nie Chi Dong Wu Yu Ke Xue Guan Li); Northwest A&F University Press: Yangling, China, 2005; p. 323. (In Chinese) [Google Scholar]
- Wang, Y.; Wang, J.M.; Qi, R.Z.; Li, C.; Cao, Z.N.; Zhang, X.L. Molecular Identification of an Intercepted. Ratt. Ratt. Sladeni. Chin. J. Front. Health Quar. 2019, 1, 40–42. [Google Scholar]
- Lv, Y.; Guo, X.; Jin, D.; Song, W.; Peng, P.; Lin, H.; Fan, R.; Zhao, C.; Zhang, Z.; Mao, K.; et al. Infestation and Seasonal Fluctuation of Chigger Mites on the Southeast Asian House Rat (Rattus Brunneusculus) in Southern Yunnan Province, China. Int. J. Parasitol. Parasites Wildl. 2021, 14, 141–149. [Google Scholar] [CrossRef]
- Meng, X.X.; Li, H.F.; Zhang, Z.H. Investigation on Population Distribution and Seasonal Extinction of Gamasid Mites in Dezhou. Chin. J. Vector Biol. Control 2005, 16, 390–391. (In Chinese) [Google Scholar]
- Zhou, S.H.; Li, S.Y.; Chen, W.J.; Yuan, G.l.; Wang, L.L. Investigation on Ectoparasitc Mites on Rodent in Sanduao Island of Fujian Province. Chin. J. Vector Biol. Control 2008, 19, 546–549. (In Chinese) [Google Scholar]
- Liu, G.P.; Ren, Q.M.; Xing, A.H.; Wang, F.; Liu, J.Q. Investigation on Rattus norvegicus and Its Ectoparasites from Frontiers of Northeast China. Chin. J. Hyg. Insectic. 2010, 16, 196–198. (In Chinese) [Google Scholar]
- Jameson, E.W., Jr. The Genus Laelaps (Acarina: Laelapidae) in Taiwan. J. Med. Entomol. 1965, 2, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Paramasvaran, S.; Sani, R.A.; Hassan, L.; Krishnasamy, M.; Jeffery, J.; Oothuman, P.; Salleh, I.; Lim, K.H.; Sumarni, M.G.; Santhana, R.L. Ectoparasite Fauna of Rodents and Shrews from four Habitats in Kuala Lumpur and the States of Selangor and Negeri Sembilan, Malaysia and its Public Health Significance. Trop. Biomed. 2009, 26, 303–311. [Google Scholar] [PubMed]
- Mariana, A.; Zuraidawati, Z.; Ho, T.; Kulaimi, B.M.; Saleh, I.; Shukor, M.; Shahrul-Anuar, M. Ticks (Ixodidae) and Other Ectoparasites in Ulu Muda Forest Reserve, Kedah, Malaysia. Southeast Asian J. Trop. Med. Public Health 2008, 39, 496. [Google Scholar] [PubMed]
- Lasim, A.M.; Asyikha, R.; Ali, R.; Ishak, S.N.J.S. A Preliminary Survey of Ectoparasites of Small Mammals in Pangkor Island, Perak, Malaysia. Serangga. 2018, 23, 73–82. [Google Scholar]
- Madinah, A.; Mariana, A.; Fatimah, A.; Abdullah, M.J.T.B. A Preliminary Field Survey of Ectoparasites of Rodents in Urban Park, Sarawak, Malaysian Borneo. Trop. Biomed. 2013, 30, 1–5. [Google Scholar]
- Ng, Y.; Hamdan, N.; Tuen, A.; Mohd-Azlan, J.; Chong, Y. Co-Infections of Ectoparasite Species in Synanthropic Rodents of Western SARAWAK, Malaysian Borneo. Trop. Biomed. 2017, 34, 1–9. [Google Scholar]
- Wiratsudakul, A. Occurrence of Ectoparasites on Rodents in Sukhothai Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1324–1330. [Google Scholar]
- Kia, E.; Moghddas-Sani, H.; Hassanpoor, H.; Vatandoost, H.; Zahabiun, F.; Akhavan, A.; Hanafi-Bojd, A.; Telmadarraiy, Z. Ectoparasites of Rodents Captured in Bandar Abbas, Southern Iran. Iran. J. Arthropod-Borne Dis. 2009, 3, 44. [Google Scholar] [PubMed]
- Gholipoury, M.; Rezai, H.R.; Namroodi, S.; Khazaeli, F.A. Zoonotic and Non-Zoonotic Parasites of Wild Rodents in Turkman Sahra, Northeastern Iran. Iran. J. Parasitol. 2016, 11, 350. [Google Scholar] [PubMed]
- Chuluun, B.; Mariana, A.; Ho, T.; Mohd Kulaimi, B. A Preliminary Survey of Ectoparasites of Small Mammals in Kuala Selangor Nature Park. Trop. Biomed. 2005, 22, 243–247. [Google Scholar] [PubMed]
- Alonso, R.; Ruiz, M.; Lovera, R.; De Oca, D.M.; Cavia, R.; Sánchez, J. Norway Rat (Rattus Norvegicus) Ectoparasites in Livestock Production Systems from Central Argentina: Influencing Factors on Parasitism. Acta Trop. 2020, 203, 105299. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.H.; Abdelgadir, M.; AlRashidi, M. Ectoparasites Burden of House Mouse (Mus Musculus Linnaeus, 1758) from Hai’l Region, Kingdom of Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Lee, P.L.; Wang, H.C. Molecular Detection of Rickettsia Species and Host Associations of Laelaps Mites (Acari: LAELAPIDAE) in Taiwan. Exp. Appl. Acarol. 2020, 81, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Palmeirim, M.; Bordes, F.; Chaisiri, K.; Siribat, P.; Ribas, A.; Morand, S. Helminth Parasite Species Richness in Rodents from Southeast Asia: Role of Host Species and Habitat. Parasitol. Res. 2014, 113, 3713–3726. [Google Scholar] [CrossRef] [PubMed]
- Karnchanabanthoeng, A.; Morand, S.; Jittapalapong, S.; Carcy, B. Babesia Occurrence in Rodents in Relation to Landscapes of Mainland Southeast Asia. Vector-Borne Zoonotic Dis. 2018, 18, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Pages, M.; Latinne, A.; Johan, M. Inter-and Intraspecific Genetic Biodiversity in South East Asian Rodents: New Insights for Their Conservation. Biodivers. Hotspots 2011, 363–382. [Google Scholar]
- Pakdeenarong, N.; Siribat, P.; Chaisiri, K.; Douangboupha, B.; Ribas, A.; Chaval, Y.; Herbreteau, V.; Morand, S. Helminth Communities in Murid Rodents from Southern and Northern Localities in Lao PDR: The Role of Habitat and Season. J. Helminthol. 2014, 88, 302–309. [Google Scholar] [CrossRef]
- Jittapalapong, S.; Herbreteau, V.; Hugot, J.P.; Arreesrisom, P.; Karnchanabanthoeng, A.; Rerkamnuaychoke, W.; Morand, S. Relationship of parasites and pathogens diversity to rodents in Thailand. Kasetsart Journal of Natural Science. 2009, 43, 106–117. [Google Scholar]
- Liu, S.Y.; He, K.; Chen, S.D.; Jin, W.; Murphy, R.W.; Tang, M.K.; Liao, R.; Li, F.J. How Many Species of Apodemus and Rattus Occur in China? A Survey Based on Mitochondrial Cyt b and Morphological Analyses. Zool. Res. 2018, 39, 309. [Google Scholar] [PubMed]
- Aplin, K.P.; Suzuki, H.; Chinen, A.A.; Chesser, R.T.; Ten Have, J.; Donnellan, S.C.; Austin, J.; Frost, A.; Gonzalez, J.P.; Herbreteau, V. Multiple Geographic Origins of Commensalism and Complex Dispersal History of Black Rats. PLoS ONE 2011, 6, e26357. [Google Scholar] [CrossRef] [PubMed]
- Chakma, N.; Sarker, N.J.; Belmain, S.; Sarker, S.U.; Aplin, K.; Sarker, S.K. New Records of Rodent Species in Bangladesh: Taxonomic Studies from Rodent Outbreak Areas in the Chittagong Hill Tracts. Bangladesh J. Zool. 2018, 46, 217–230. [Google Scholar] [CrossRef][Green Version]
- Ristiyanto, R.; Mulyono, A.; Agustina, M.; Yuliadi, B.; Muhidin, M. Indeks Keragaman Ektoparasit Pada Tikus Rumah Rattus Tanezumi Temminck, 1844 Dan Tikus Polinesia R. Exulans (Peal, 1848) Di Daerah Enzootik Pes Lereng Gunung Merapi, Jawa Tengah. Vektora J. Vektor Dan Reserv. Penyakit 2009, 1, 73–83. [Google Scholar]
- Linardi, P.M.; Botelha, J.R.; Cunha, H.C. Ectoparasites of Rodents of the Urban Region of Belo Horizonte, MG: II. Variations of the Infestation in Rattus norvegiens norvegecus; Memorias do Instituto Oswaldo Cruz: Rio de Janeiro, Brazil, 1985; pp. 227–232. [Google Scholar]
- Azad, A. Mites of Public Health Importance and Their Control. Vector Control Series Training and Information Guide: XIII; WHO/VBC: Geneva, Switzerland, 1986. [Google Scholar]
- Men, X.Y.; Guo, X.G.; Dong, W.G.; Niu, A.Q.; Qian, T.J.; Wu, D. Ectoparasites of Chevrier’s Field Mouse, Apodemus chevrieri, in a Focus of Plague in Southwest China. Med Vet. Entomol. 2007, 21, 297–300. [Google Scholar] [CrossRef]





| Taxa | N | Taxa | N | Taxa | N | Taxa | N |
|---|---|---|---|---|---|---|---|
| Family Lealapidae | 13,530 | L. jettmari Vitzthum, 1930 | 1 | Genus Cosmolaelaps | 6 | Subfamily Haemogamasinae | 11 |
| Subfamily Lealapinae | 13,410 | L. extremi Zachvatkin, 1948 | 3 | C. yerulyuae Ma, 1995 | 6 | Genus Eulaelaps | 9 |
| Genus Laelaps | 13,337 | Genus Androlaelaps | 4 | Subfamily Hypoaspidinae | 109 | E. jilinensis Wen, 1976 | 2 |
| L. nuttalli Hirst, 1915 | 9557 | A. singularis Wang et Li, 1965 | 4 | Genus Hypoaspis | 109 | E. pratentis Zhou, 1981 | 1 |
| L. liui Wang et Li, 1965 | 104 | Genus Tricholaelaps | 15 | H. concinna Teng, 1982 | 21 | E. substabularis Yang et Gu, 1986 | 1 |
| L. guizhouensis Gu et Wang, 1981 | 34 | T. myonysognathus Grochovskaya et Nguen-Xuan-hoe, 1961 | 15 | H. aculeifer Canestrini, 1884 | 16 | E. stabularis Koch, 1836 | 5 |
| L. echidninus Berlese, 1887 | 2797 | Genus Dipolaelaps | 26 | H. chelaris Teng, Zhang et Cui, 1992 | 16 | Genus Haemogamasus | 2 |
| L. turkestanicus Lange, 1955 | 216 | D. jiangkouensis Gu, 1985 | 25 | H. ovatus Ma, Ning et Wei, 2003 | 8 | H. monticola Wang et Li, 1965 | 1 |
| L. traubi Domrow, 1962 | 219 | D. chimmarogalis Gu, 1983 | 1 | H. lubrica Voigts et Oudemans, 1904 | 6 | H. nidi Michael, 1892 | 1 |
| L. fukienensis Wang, 1963 | 110 | Genus Haemolaelaps | 22 | H. pavlovskii Bregetova, 1956 | 35 | ||
| L. algericus Hirst, 1925 | 84 | H. casalis Berlese, 1887 | 4 | H. kirinensis Chang, Cheng et Yin, 1963 | 3 | Family Macronyssidae | 1 |
| L. cheni Li, 1965 | 116 | H. orientalis Teng et Pan, 1964 | 13 | H. leeae Tseng, 1977 | 2 | Genus Ornithonyssus | 1 |
| L. jinghaensis sp.nov | 22 | H. cordatus Teng et Pan, 1964 | 3 | H. hrdyi Samsinak, 1961 | 1 | O. bacoti Hirst, 1913 | 1 |
| L. chin Wang et Li, 1965 | 29 | H. glasgowi Ewing, 1925 | 1 | H. linteyini Samsinak, 1964 | 1 | ||
| L. clethsionomydis Lange, 1955 | 45 | H. petauristae Gu et Wang, 1980 | 1 |
| Years and Months | Community Structure of Gamasid Mites | ||||
|---|---|---|---|---|---|
| Years | Months | S | H’ | E | D |
| 2017 | 1 | 16 | 1.098 | 0.396 | 0.396 |
| 2 | 14 | 1.053 | 0.399 | 0.428 | |
| 3 | 13 | 0.891 | 0.347 | 0.485 | |
| 2016 | 4 | 12 | 1.053 | 0.424 | 0.504 |
| 5 | 15 | 0.451 | 0.166 | 0.818 | |
| 6 | 8 | 0.751 | 0.361 | 0.665 | |
| 7 | 8 | 1.032 | 0.496 | 0.498 | |
| 8 | 10 | 0.901 | 0.391 | 0.564 | |
| 9 | 9 | 1.165 | 0.530 | 0.456 | |
| 10 | 6 | 0.340 | 0.190 | 0.839 | |
| 11 | 11 | 0.647 | 0.270 | 0.681 | |
| 12 | 12 | 0.770 | 0.310 | 0.518 | |
| Total | 41 | 1.013 | 0.273 | 0.542 | |
| Months | Host (R. andamanensis) | Mites | Host Infestation | ||||
|---|---|---|---|---|---|---|---|
| N | Cr (%) | No. of Mites | Cr (%) | PM | MA | MI | |
| (%) | |||||||
| 1 | 182 | 8.87 | 2165 | 16.00 | 80.77 | 11.90 | 14.73 |
| 2 | 167 | 8.13 | 1160 | 8.57 | 73.05 | 6.95 | 9.51 |
| 3 | 182 | 8.87 | 1074 | 7.94 | 83.52 | 5.90 | 7.07 |
| 4 | 168 | 8.18 | 720 | 5.32 | 51.79 | 4.29 | 8.28 |
| 5 | 184 | 8.96 | 1304 | 9.64 | 68.48 | 7.09 | 10.35 |
| 6 | 150 | 7.31 | 1570 | 11.60 | 63.33 | 10.47 | 16.53 |
| 7 | 152 | 7.40 | 549 | 4.06 | 40.13 | 3.61 | 9.00 |
| 8 | 151 | 7.36 | 738 | 5.45 | 59.60 | 4.89 | 8.20 |
| 9 | 141 | 6.87 | 748 | 5.53 | 69.50 | 5.30 | 7.63 |
| 10 | 190 | 9.25 | 678 | 5.01 | 54.21 | 3.57 | 6.58 |
| 11 | 197 | 9.60 | 1237 | 9.14 | 71.57 | 6.28 | 8.77 |
| 12 | 189 | 9.21 | 1588 | 11.74 | 77.25 | 8.40 | 10.88 |
| Total | 2053 | 100.00 | 13,531 | 100.00 | 66.63 | 6.59 | 9.89 |
| Dominant Mite Species | Mites | Host Infestation | |||
|---|---|---|---|---|---|
| N | Cr (%) | PM (%) | MA | MI | |
| L. nuttalli | 9557 | 70.63 | 57.04 | 4.66 | 8.16 |
| L. echidninus | 2797 | 20.67 | 33.07 | 1.36 | 4.12 |
| Total | 12,699 | 91.30 | |||
| Months | Examined Hosts | Infested Hosts | No. of Mites | Cr (%) | PM (%) | MA | MI |
|---|---|---|---|---|---|---|---|
| 1 | 182 | 127 | 1366 | 14.29 | 69.78 | 7.51 | 10.76 |
| 2 | 167 | 96 | 609 | 6.37 | 57.49 | 3.65 | 6.34 |
| 3 | 182 | 112 | 637 | 6.67 | 61.54 | 3.50 | 5.69 |
| 4 | 168 | 71 | 493 | 5.16 | 42.26 | 2.93 | 6.94 |
| 5 | 184 | 116 | 1176 | 12.31 | 63.04 | 6.39 | 10.14 |
| 6 | 150 | 84 | 1269 | 13.28 | 56.00 | 8.46 | 15.11 |
| 7 | 152 | 44 | 372 | 3.89 | 28.95 | 2.45 | 8.45 |
| 8 | 151 | 72 | 541 | 5.66 | 47.68 | 3.58 | 7.51 |
| 9 | 141 | 82 | 481 | 5.03 | 58.16 | 3.41 | 5.87 |
| 10 | 190 | 95 | 619 | 6.48 | 50.00 | 3.26 | 6.52 |
| 11 | 197 | 137 | 1004 | 10.51 | 69.54 | 5.10 | 7.33 |
| 12 | 189 | 135 | 990 | 10.36 | 71.43 | 5.24 | 7.33 |
| Total | 2053 | 1171 | 9557 | 100.00 | 57.04 | 4.66 | 8.16 |
| Months | Examined Hosts | Infested Hosts | No. of Mites | Cr (%) | PM (%) | MA | MI |
|---|---|---|---|---|---|---|---|
| 1 | 182 | 95 | 517 | 18.48 | 52.20 | 2.84 | 5.44 |
| 2 | 167 | 83 | 449 | 16.05 | 49.70 | 2.69 | 5.41 |
| 3 | 182 | 106 | 392 | 14.02 | 58.24 | 2.15 | 3.70 |
| 4 | 168 | 28 | 67 | 2.40 | 16.67 | 0.40 | 2.39 |
| 5 | 184 | 30 | 86 | 3.07 | 16.30 | 0.47 | 2.87 |
| 6 | 150 | 30 | 141 | 5.04 | 20.00 | 0.94 | 4.70 |
| 7 | 152 | 30 | 95 | 3.40 | 19.74 | 0.63 | 3.17 |
| 8 | 151 | 42 | 104 | 3.72 | 27.81 | 0.69 | 2.48 |
| 9 | 141 | 51 | 143 | 5.11 | 36.17 | 1.01 | 2.80 |
| 10 | 190 | 27 | 52 | 1.86 | 14.21 | 0.27 | 1.93 |
| 11 | 197 | 61 | 181 | 6.47 | 30.96 | 0.92 | 2.97 |
| 12 | 189 | 96 | 570 | 20.38 | 50.79 | 3.02 | 5.94 |
| Total | 2053 | 679 | 2797 | 100.00 | 33.07 | 1.36 | 4.12 |
| Species of Gamasid Mites | Infestation Indices | Pearson Correlation, r (p) | ||
|---|---|---|---|---|
| Total Rainfall (mm) | Average Temperature (°C) | Average Humidity (%) | ||
| L. nuttalli | Cr | 0.0946 (0.77) | −0.2698 (0.3963) | 0.1023(0.7517) |
| PM | −0.5441 (0.0674) | −0.6922 (0.0126) * | −0.0211(0.948) | |
| MA | 0.035 (0.9212) | −0.3627 (0.2466) | 0.1761 (0.5841) | |
| MI | 0.3706 (0.2367) | 0.1761 (0.5841) | 0.2747 (0.3876) | |
| L. echidninus | Cr | −0.5874 (0.0488) * | −0.6373 (0.0258) * | −0.1408 (0.6624) |
| PM | −0.6224 (0.0348) * | −0.6444 (0.0237) * | −0.1408 (0.6624) | |
| MA | −0.5734 (0.0555) | −0.6549 (0.0208) * | −0.0845 (0.794) | |
| MI | −0.3279 (0.2981) | −0.3591 (0.2516) | −0.2113 (0.5098) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, P.-W.; Guo, X.-G.; Jin, D.-C.; Song, W.-Y.; Zhang, L.; Zhao, C.-F.; Fan, R.; Zhang, Z.-W.; Mao, K.-Y. Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China. Biology 2021, 10, 1297. https://doi.org/10.3390/biology10121297
Yin P-W, Guo X-G, Jin D-C, Song W-Y, Zhang L, Zhao C-F, Fan R, Zhang Z-W, Mao K-Y. Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China. Biology. 2021; 10(12):1297. https://doi.org/10.3390/biology10121297
Chicago/Turabian StyleYin, Peng-Wu, Xian-Guo Guo, Dao-Chao Jin, Wen-Yu Song, Lei Zhang, Cheng-Fu Zhao, Rong Fan, Zhi-Wei Zhang, and Ke-Yu Mao. 2021. "Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China" Biology 10, no. 12: 1297. https://doi.org/10.3390/biology10121297
APA StyleYin, P.-W., Guo, X.-G., Jin, D.-C., Song, W.-Y., Zhang, L., Zhao, C.-F., Fan, R., Zhang, Z.-W., & Mao, K.-Y. (2021). Infestation and Seasonal Fluctuation of Gamasid Mites (Parasitiformes: Gamasida) on Indochinese Forest Rat, Rattus andamanensis (Rodentia: Muridae) in Southern Yunnan of China. Biology, 10(12), 1297. https://doi.org/10.3390/biology10121297






