Cutaneous Melanocytic Tumor with CRTC1::TRIM11 Fusion: Review of the Literature of a Potentially Novel Entity
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Clinical Features
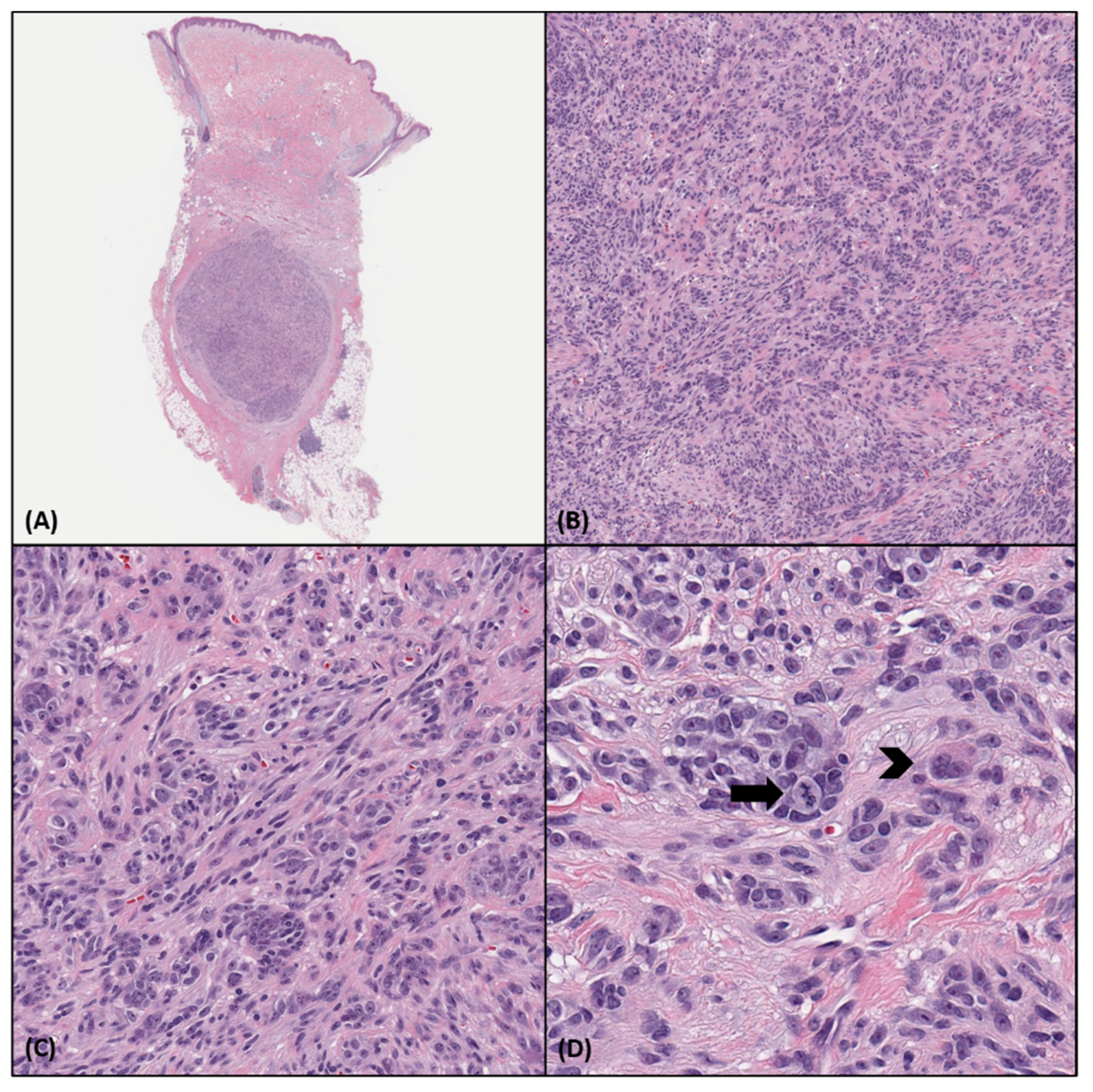
4. Histopathologic and Immunohistochemical Features
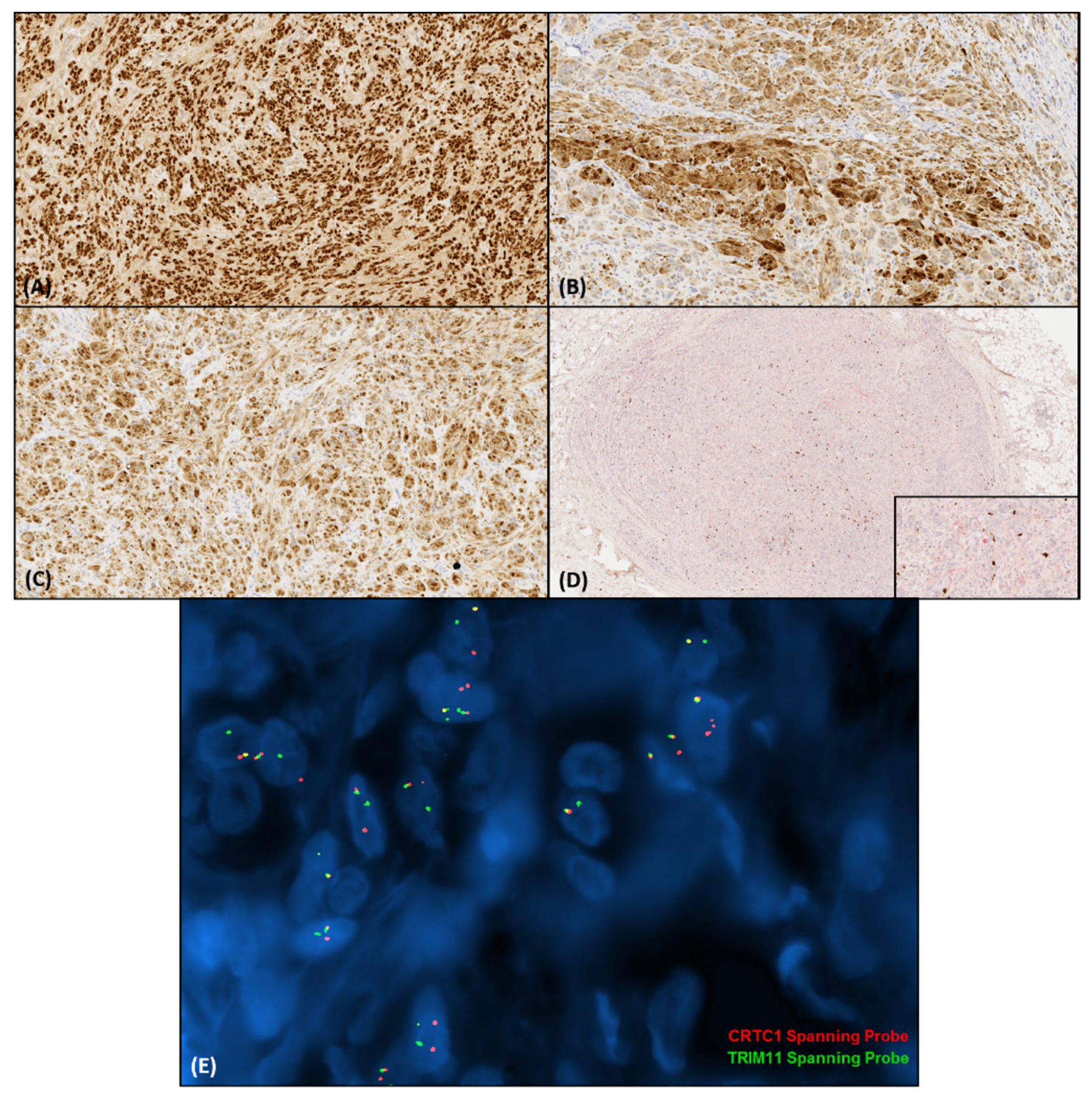
5. Molecular Diagnostics
6. Molecular Pathogenesis
7. Differential Diagnosis
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cellier, L.; Perron, E.; Pissaloux, D.; Karanian, M.; Haddad, V.; Alberti, L.; de la Fouchardière, A. Cutaneous melanocytoma with CRTC1-TRIM11 fusion: Report of 5 cases resembling clear cell sarcoma. Am. J. Surg. Pathol. 2018, 42, 382–391. [Google Scholar] [CrossRef]
- Bontoux, C.; Baroudjian, B.; Le Maignan, C.; Vercellino, L.; Farges, C.; Guillemot, D.; Pierron, G.; Lebbé, C.; Battistella, M. CRTC1-TRIM11 fusion in a case of metastatic clear cell sarcoma: Are CRTC1-TRIM11 fusion-bearing tumors melanocytomas or clear cell sarcomas? Am. J. Surg. Pathol. 2019, 43, 861–863. [Google Scholar] [CrossRef]
- Kashima, J.; Motoi, T.; Nishimaki, M.; Hayashi, Y.; Ogawa, M.; Kato, I.; Yamada, R.; Tonooka, A.; Horiguchi, S.I.; Funata, N.; et al. A case report of cutaneous melanocytoma with CRTC1-TRIM11 fusion: Is CMCT distinct from clear cell sarcoma of soft tissue? Pathol. Int. 2019, 69, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Wang, L.; Billings, S.D.; Pissaloux, D.; Tirode, F.; Berry, R.; de La Fouchardiere, A. CRTC1-TRIM11 fusion defined melanocytic tumors: A series of four cases. J. Cutan. Pathol. 2019, 46, 810–818. [Google Scholar] [CrossRef]
- Parra, O.; Bridge, J.A.; Busam, K.J.; Shalin, S.C.; Linos, K. Dermal melanocytic tumor with CRTC1-TRIM11 fusion: Report of two additional cases with review of the literature of an emerging entity. J. Cutan. Pathol. 2021, 48, 915–924. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, I.D. t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol. 2009, 45, 2–9. [Google Scholar] [CrossRef]
- Schumacher, Y.; Aparicio, T.; Ourabah, S.; Baraille, F.; Martin, A.; Wind, P.; Dentin, R.; Postic, C.; Guilmeau, S. Dysregulated CRTC1 activity is a novel component of PGE2 signaling that contributes to colon cancer growth. Oncogene 2016, 35, 2602–2614. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Oliveira, M.E.; Wagner, V.P.; Araújo, A.L.D.; Martins, M.D.; Santos-Silva, A.R.; Bingle, L.; Vargas, P.A. Prognostic value of CRTC1-MAML2 translocation in salivary mucoepidermoid carcinoma: Systematic review and meta-analysis. J. Oral. Pathol. Med. 2020, 49, 386–394. [Google Scholar] [CrossRef]
- Bean, G.R.; Krings, G.; Otis, C.N.; Solomon, D.A.; García, J.J.; van Zante, A.; Camelo-Piragua, S.; van Ziffle, J.; Chen, Y.Y. CRTC1-MAML2fusion in mucoepidermoid carcinoma of the breast. Histopathology 2019, 74, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Kai, K.; Tanikawa, K.; Hiraki, M.; Mizukami, N.; Aishima, S.; Nakano, T.; Yamamoto, H. Primary mucoepidermoid carcinoma of the liver with CRTC1-MAML2 fusion: A case report. Diagn Pathol. 2019, 14, 84. [Google Scholar] [CrossRef] [PubMed]
- Winnes, M.; Mölne, L.; Suurküla, M.; Andrén, Y.; Persson, F.; Enlund, F.; Stenman, G. Frequent fusion of the CRTC1 and MAML2 genes in clear cell variants of cutaneous hidradenomas. Genes Chromosomes Cancer. 2007, 46, 559–563. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.-L.; Chen, Z.; Griffin, J.D.; Wu, L. Gene expression profiling analysis of CRTC1-MAML2 fusion oncogene-induced transcriptional program in human mucoepidermoid carcinoma cells. BMC Cancer 2015, 15, 803. [Google Scholar] [CrossRef]
- Alholle, A.; Karanian, M.; Brini, A.T.; Morris, M.R.; Kannappan, V.; Niada, S.; Niblett, A.; Ranchère-Vince, D.; Pissaloux, D.; Delfour, C.; et al. Genetic analyses of undifferentiated small round cell sarcoma identifies a novel sarcoma subtype with a recurrent CRTC1-SS18 gene fusion. J. Pathol. 2018, 245, 186–196. [Google Scholar] [CrossRef]
- Kao, Y.C.; Sung, Y.S.; Argani, P.; Swanson, D.; Alaggio, R.; Tap, W.; Wexler, L.; Dickson, B.C.; Antonescu, C.R. NTRK3 overexpression in undifferentiated sarcomas with YWHAE and BCOR genetic alterations. Mod. Pathol. 2020, 33, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, W.; Shi, H.; Lu, S.; Wang, K.; Sun, C.; He, J.; Jin, W.; Lv, X.; Zou, H.; et al. TRIM11 overexpression promotes proliferation, migration and invasion of lung cancer cells. J. Exp. Clin. Cancer Res. 2016, 35, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, L.; Qian, X.; Ge, Y.; Xu, G. High expression of TRIM11 correlates with poor prognosis in patients with hepatocellular carcinoma. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, R.; Chen, H.; Chen, W.; Wu, K.; Lv, J. Expression of Tripartite Motif-Containing Proteactiin 11 (TRIM11) is associated with the progression of human prostate cancer and is downregulated by microRNA-5193. Med. Sci. Mon. 2019, 25, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhong, J.; Li, S.W.; Li, J.Z.; Zhou, M.; Chen, Y.; Sang, Y.; Liu, L. TRIM11, a direct target of miR-24-3p, promotes cell proliferation and inhibits apoptosis in colon cancer. Oncotarget 2016, 7, 86755–86765. [Google Scholar] [CrossRef]
- Luo, N.; Wang, Z. TRIM11 stimulates the proliferation of gastric cancer through targeting CPEB3/EGFR axis. J. BUON 2020, 25, 2097–2104. [Google Scholar]
- Hou, Y.; Ding, M.; Wang, C.; Yang, X.; Ye, T.; Yu, H. TRIM11 promotes lymphomas by activating the β-catenin signaling and Axin1 ubiquitination degradation. Exp. Cell Res. 2020, 387, 111750. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Luo, Y.; Tian, Z.; Liao, X.; Cui, Q.; Yang, Q.; Wu, G. TRIM11 promotes breast cancer cell proliferation by stabilizing estrogen receptor α. Neoplasia 2020, 22, 343–351. [Google Scholar] [CrossRef]
- Di, K.; Linskey, M.E.; Bota, D.A. TRIM11 is overexpressed in high-grade gliomas and promotes proliferation, invasion, migration and glial tumor growth. Oncogene 2013, 32, 5038–5047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Ma, J. Proliferation and invasion of ovarian cancer cells are suppressed by knockdown of TRIM11. Oncol. Lett. 2017, 14, 2125–2130. [Google Scholar] [CrossRef]
- Huang, J.; Tang, L.; Zhao, Y.; Ding, W. TRIM11 promotes tumor angiogenesis via activation of STAT3/VEGFA signaling in lung adenocarcinoma. Am. J. Cancer Res. 2019, 9, 2019–2027. [Google Scholar] [PubMed]
- Hantschke, M.; Mentzel, T.; Rütten, A.; Palmedo, G.; Calonje, E.; Lazar, A.J.; Kutzner, H. Cutaneous clear cell sarcoma: A clinicopathologic, immunohistochemical, and molecular analysis of 12 cases emphasizing its distinction from dermal melanoma. Am. J. Surg. Pathol. 2010, 34, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.; Fitzhugh, V.A.; Groisberg, R.; Aviv, H.A.; Maghari, A. A rare case of primary dermal clear cell sarcoma with focal epidermotropism: An entity difficult to distinguish from melanoma. J. Cutan. Pathol. 2020, 47, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka, M.; Ishida, T.; Kuo, T.T.; Matsuyama, A.; Imamura, T.; Nishida, K.; Kuroda, H.; Inayama, Y.; Oshiro, H.; Kobayashi, H.; et al. Clear cell sarcoma of soft tissue: A clinicopathologic, immunohistochemical, and molecular analysis of 33 cases. Am. J. Surg. Pathol. 2008, 32, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Wakai, S.; Ryo, E.; Miyata, K.; Miyazawa, M.; Yoshida, K.; Motoi, T.; Ogawa, C.; Iwata, S.; Kobayashi, E.; et al. Expanding the phenotypic spectrum of mesenchymal tumors harboring the EWSR1-CREM fusion. Am. J. Surg. Pathol. 2019, 43, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Cabral, E.S.; Kartha, R.V.; Swetter, S.M. Primary dermal melanoma: Distinct immunohistochemical findings and clinical outcome compared with nodular and metastatic melanoma. Arch. Dermatol. 2008, 144, 49–56. [Google Scholar] [CrossRef]
- Sidiropoulos, M.; Obregon, R.; Cooper, C.; Sholl, L.M.; Guitart, J.; Gerami, P. Primary dermal melanoma: A unique subtype of melanoma to be distinguished from cutaneous metastatic melanoma. J. Am. Acad. Dermatol. 2014, 71, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.K.; Wang, H.; Kim, J.; Chen, J.K.; Sun, L.; Zhang, Y.; Swetter, S.M. Mutational profile of primary dermal melanoma: A case series. J. Am. Acad. Dermatol. 2016, 75, 1263–1265.e5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Namiki, T.; Yanagawa, S.; Izumo, T.; Ishikawa, M.; Tachibana, M.; Kawakami, Y.; Yokozeki, H.; Nishioka, K.; Kaneko, Y. Genomic alterations in primary cutaneous melanomas detected by metaphase comparative genomic hybridization with laser capture or manual microdissection: 6p gains may predict poor outcome. Cancer Genet. Cytogenet. 2005, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Ecker, P.M.; Johnson, D.L.; Harvell, J.D. Primary dermal melanoma: A distinct subtype of melanoma. Arch Dermatol. 2004, 140, 99–103. [Google Scholar] [CrossRef]
- Lezcano, C.; Shoushtari, A.N.; Ariyan, C.; Hollmann, T.J.; Busam, K.J. Primary and metastatic melanoma with NTRK fusions. Am. J. Surg. Pathol. 2018, 42, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, M.T.; Reuben, A.; Billings, S.D.; Prieto, V.G.; Curry, J.L. Toward a molecular-genetic classification of spitzoid neoplasms. Clin. Lab. Med. 2017, 37, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Quan, V.L.; Panah, E.; Zhang, B.; Shi, K.; Mohan, L.S.; Gerami, P. The role of gene fusions in melanocytic neoplasms. J. Cutan. Pathol. 2019, 46, 878–887. [Google Scholar] [CrossRef]
- Yeh, I.; Busam, K.J.; McCalmont, T.H.; LeBoit, P.E.; Pissaloux, D.; Alberti, L.; de la Fouchardière, A.; Bastian, B.C. Filigree-like rete ridges, lobulated nests, rosette-like structures, and exaggerated naturation characterize Spitz Tumors with NTRK1 fusion. Am. J. Surg. Pathol. 2019, 43, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.M.; Haugh, A.M.; Lee, C.Y.; Zhang, B.; Bubley, J.A.; Merkel, E.A.; Verzì, A.E.; Gerami, P. A Comparison of morphologic and molecular features of BRAF, ALK, and NTRK1 fusion spitzoid neoplasms. Am. J. Surg. Pathol. 2017, 41, 491–498. [Google Scholar] [CrossRef]
- De la Fouchardiere, A.; Pissaloux, D.; Tirode, F.; Karanian, M.; Fletcher, C.D.M.; Hanna, J. Clear Cell Tumor with melanocytic differentiation and ACTIN-MITF translocation: Report of 7 Cases of a novel entity. Am. J. Surg. Pathol. 2020, 45, 962–968. [Google Scholar] [CrossRef]
- De la Fouchardiere, A.; Pissaloux, D.; Tirode, F.; Hanna, J. Clear cell tumor with melanocytic differentiation and MITF-CREM translocation: A novel entity similar to clear cell sarcoma [published online ahead of print, 2021 Jan 18]. Virchows Arch. 2021, 479, 1–6. [Google Scholar] [CrossRef]
- Jo, V.Y. Myoepithelial Tumors: An Update. Surg. Pathol. Clin. 2015, 8, 445–466. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.; Gardner, J.M.; Edgar, M.; Weiss, S.W. Epithelioid schwannomas. Am. J. Surg. Pathol. 2016, 40, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Fletcher, C.D.M. Epithelioid malignant peripheral nerve sheath tumor. Am. J. Surg. Pathol. 2015, 39, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Deyrup, A.T.; Althof, P.; Zhou, M.; Morgan, M.; Solomon, A.R.; Bridge, J.A.; Weiss, S.W. Paraganglioma-like dermal melanocytic tumor: A unique entity distinct from cellular blue nevus, clear cell sarcoma, and cutaneous melanoma. Am. J. Surg. Pathol. 2004, 28, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
| Author | Case | Age | Sex | Location | SOX-10 | MITF | S100 | MelanA | HMB-45 | TRIM11 | NTRK1 | CRTC1:: TRIM11 Fusion (RNA Sequencing) | TRIM11 FISH | NTRK1 FISH | CGH | Recurrence | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellier et al. [1] | 1 | 28 | F | Leg | + | + | + | + a | + d | + | + | + | + | − | +7 | No | 36 |
| 2 | 82 | M | Lumbar | + | + | + | + a | + a | + | + | + | + | − | − | No | 6 | |
| 3 | 25 | F | Elbow | + a | + | + | + | − | NP | + | + | + | − | NP | No | 14 | |
| 4 | 28 | F | Thigh | + | + | + | + | + d | + | + | + | + | NP | +7 | No | 72 | |
| 5 | 64 | M | Neck | + | + | + | − | + d | + | + | + | + | NP | NP | No | 3 | |
| Bontoux et al. [2] | 6 | 31 | F | Arm | + | NP | + | + | + a | NP | NP | + | NP | NP | NP | Yes e | 156 |
| Kashima et al. [3] | 7 | 77 | M | Thigh | + | + | + | + b | + a | + | NP | + | CISH + | NP | NP | No | 12 |
| Ko et al. [4] | 8 | 32 | M | Ear lobe | + | NP | + a | − | − | NP | NP | NP | + | NP | NP | No | 16 |
| 9 | 59 | F | Face | + | NP | − | − | − | NP | NP | NP | + | NP | NP | No | 12 | |
| 10 | 11 | F | Lower leg | + | NP | − | + a | + a | NP | NP | + | + | NP | NP | No | 10 | |
| 11 | 49 | M | Leg | + | NP | + b | − | − | NP | NP | + | + | NP | NP | No | 10 | |
| Parra et al. [5] | 12 | 65 | F | Back | + | + | + | + c | + c | NP | + | + | − | − | − | No | 48 |
| 13 | 33 | F | Bicep | + | NP | + | + b | NP | NP | NP | + | + | NP | NP | No | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra, O.; Linos, K. Cutaneous Melanocytic Tumor with CRTC1::TRIM11 Fusion: Review of the Literature of a Potentially Novel Entity. Biology 2021, 10, 1286. https://doi.org/10.3390/biology10121286
Parra O, Linos K. Cutaneous Melanocytic Tumor with CRTC1::TRIM11 Fusion: Review of the Literature of a Potentially Novel Entity. Biology. 2021; 10(12):1286. https://doi.org/10.3390/biology10121286
Chicago/Turabian StyleParra, Ourania, and Konstantinos Linos. 2021. "Cutaneous Melanocytic Tumor with CRTC1::TRIM11 Fusion: Review of the Literature of a Potentially Novel Entity" Biology 10, no. 12: 1286. https://doi.org/10.3390/biology10121286
APA StyleParra, O., & Linos, K. (2021). Cutaneous Melanocytic Tumor with CRTC1::TRIM11 Fusion: Review of the Literature of a Potentially Novel Entity. Biology, 10(12), 1286. https://doi.org/10.3390/biology10121286






