Steam Explosion (STEX) of Citrus × Poncirus Hybrids with Exceptional Tolerance to Candidatus Liberibacter Asiaticus (CLas) as Useful Sources of Volatiles and Other Commercial Products
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Citrus Hybrid Fruit
2.2. Harvesting and Preparation of Citrus Hybrid Fruit
2.3. Continuous Pilot-Scale STEX of Citrus Hybrid Fruit
2.4. Peel Oil Content
2.5. Soluble and Compositional Sugar Content
2.6. Density and GC/MS Analysis of Condensed Volatiles
2.7. Acid Extraction of Pectin from Fresh Peel
2.8. Recovery of Pectic Hydrocolloids
2.9. Macromolecular Characterization of Pectic Hydrocolloids
2.10. Flavonoid Content
3. Results and Discussion
3.1. Soluble and Compositional Sugars
3.2. Peel Oil, Density, and Chemical Composition of Condensed Volatiles
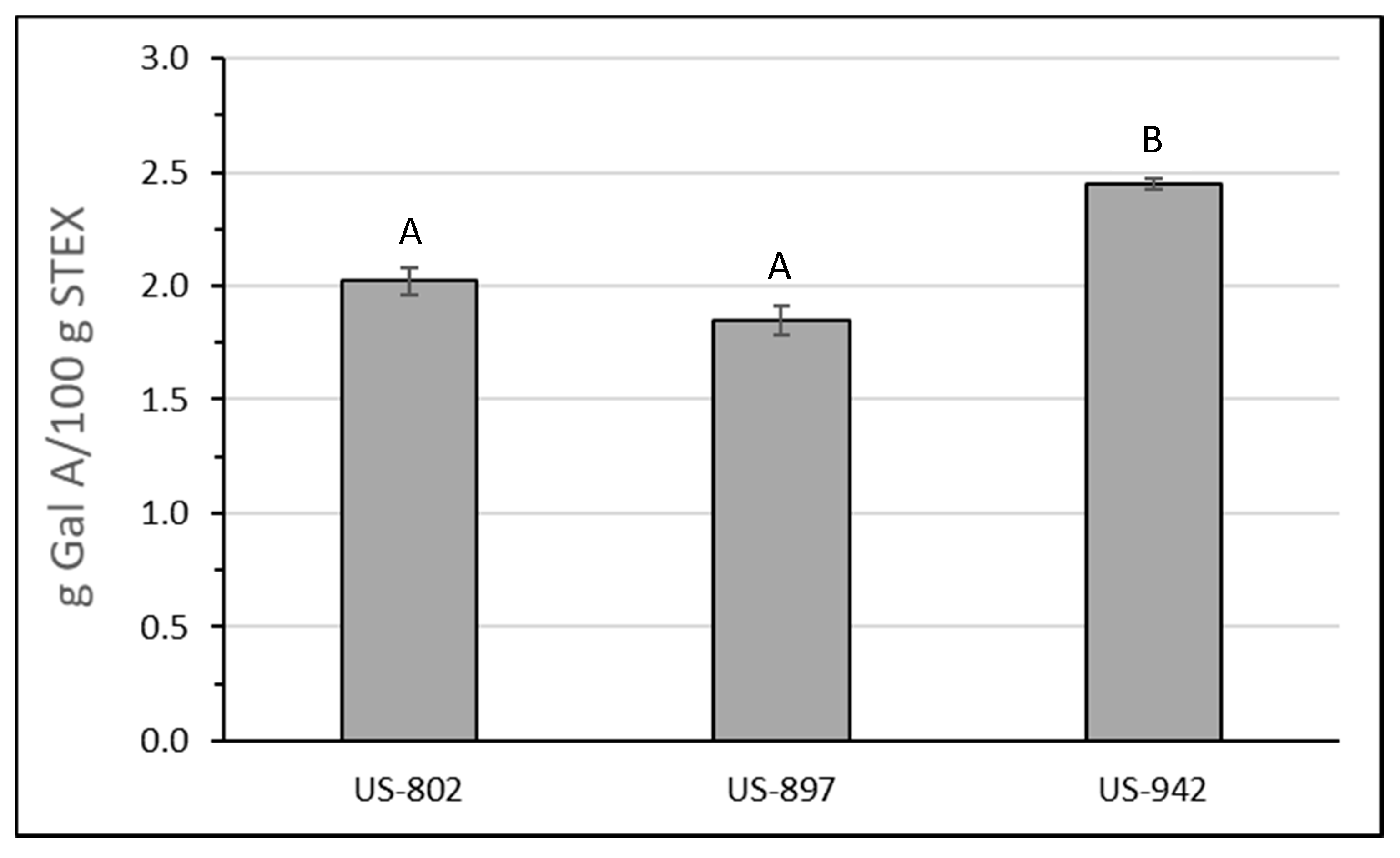
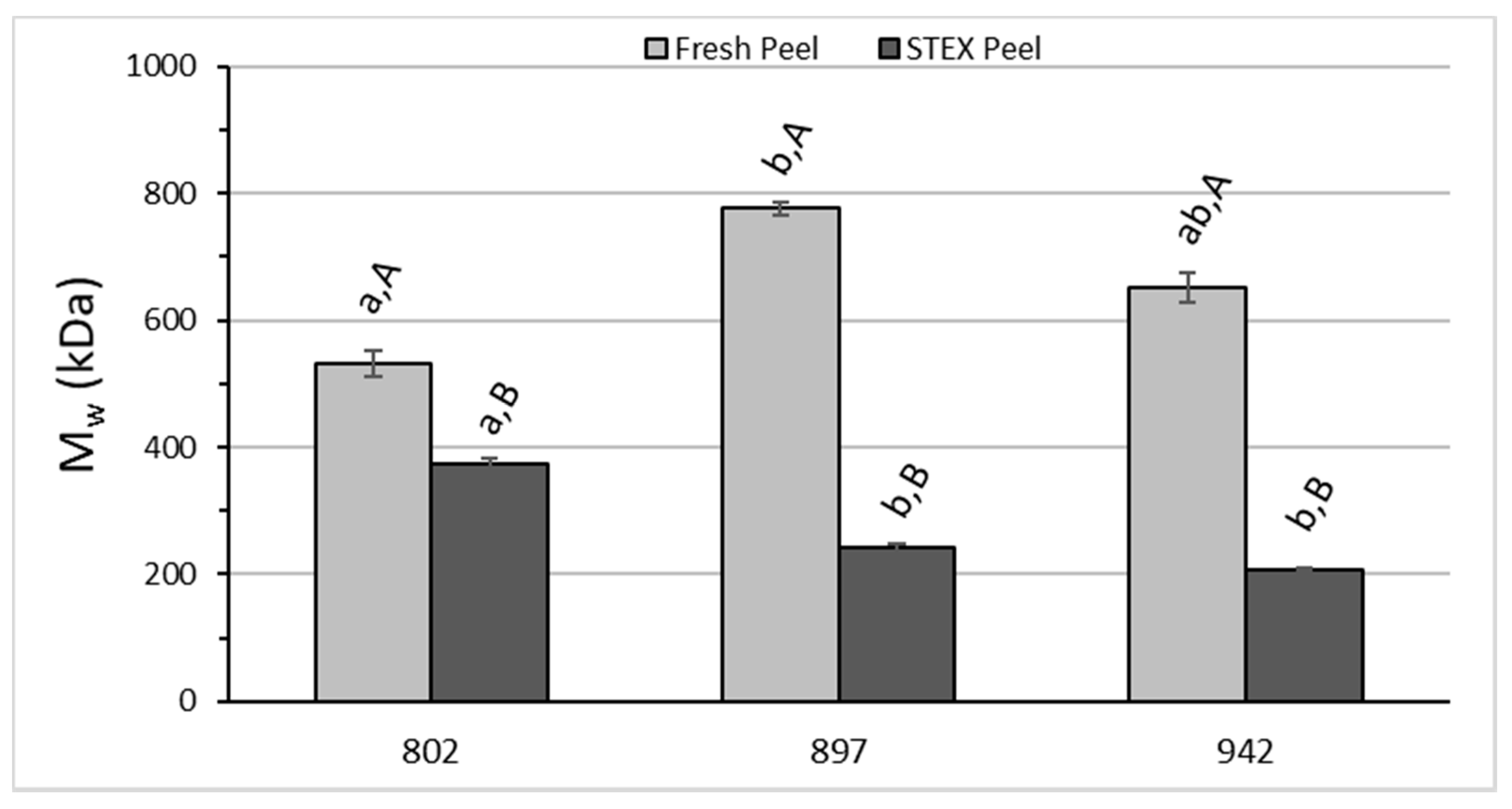
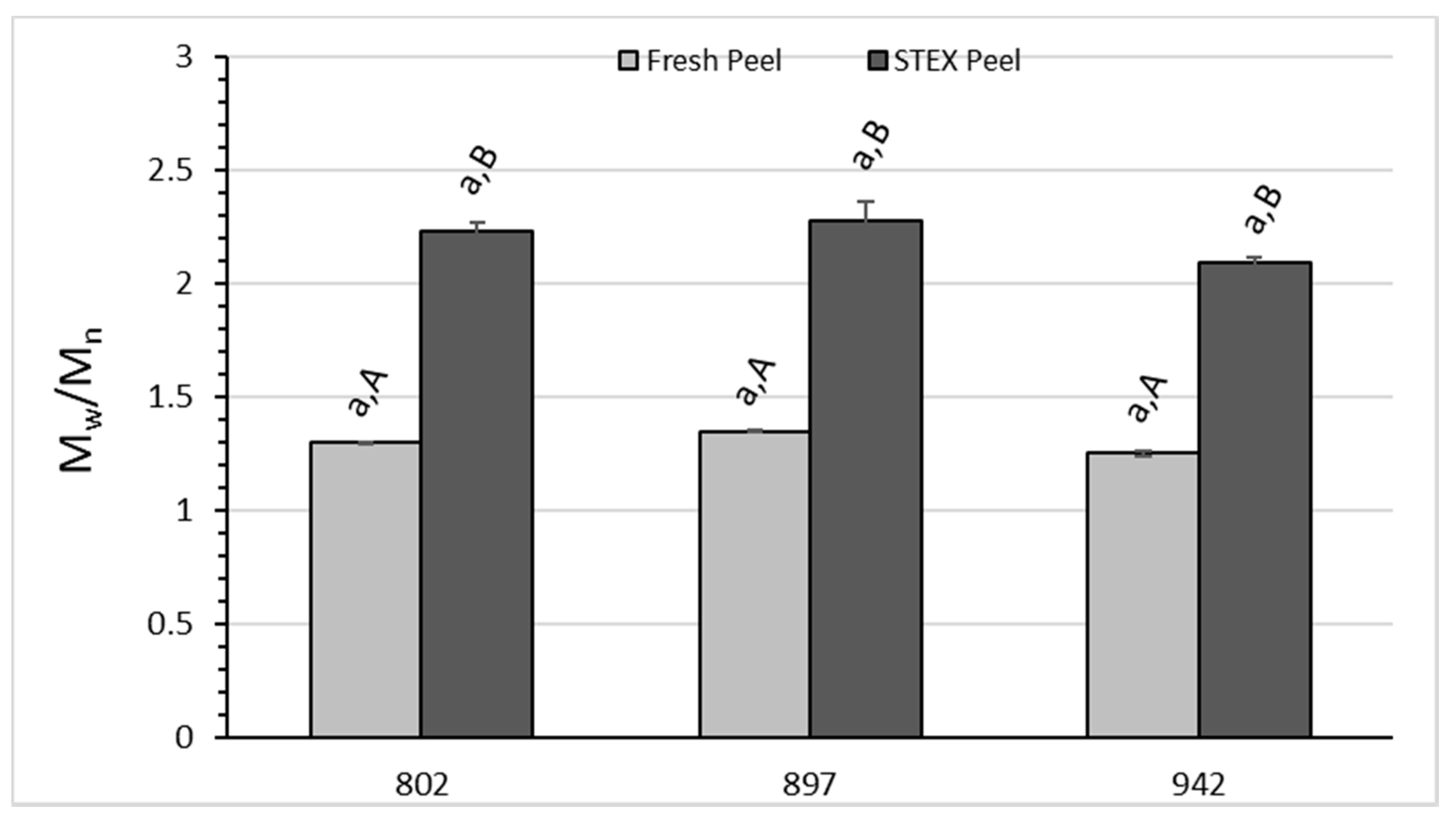
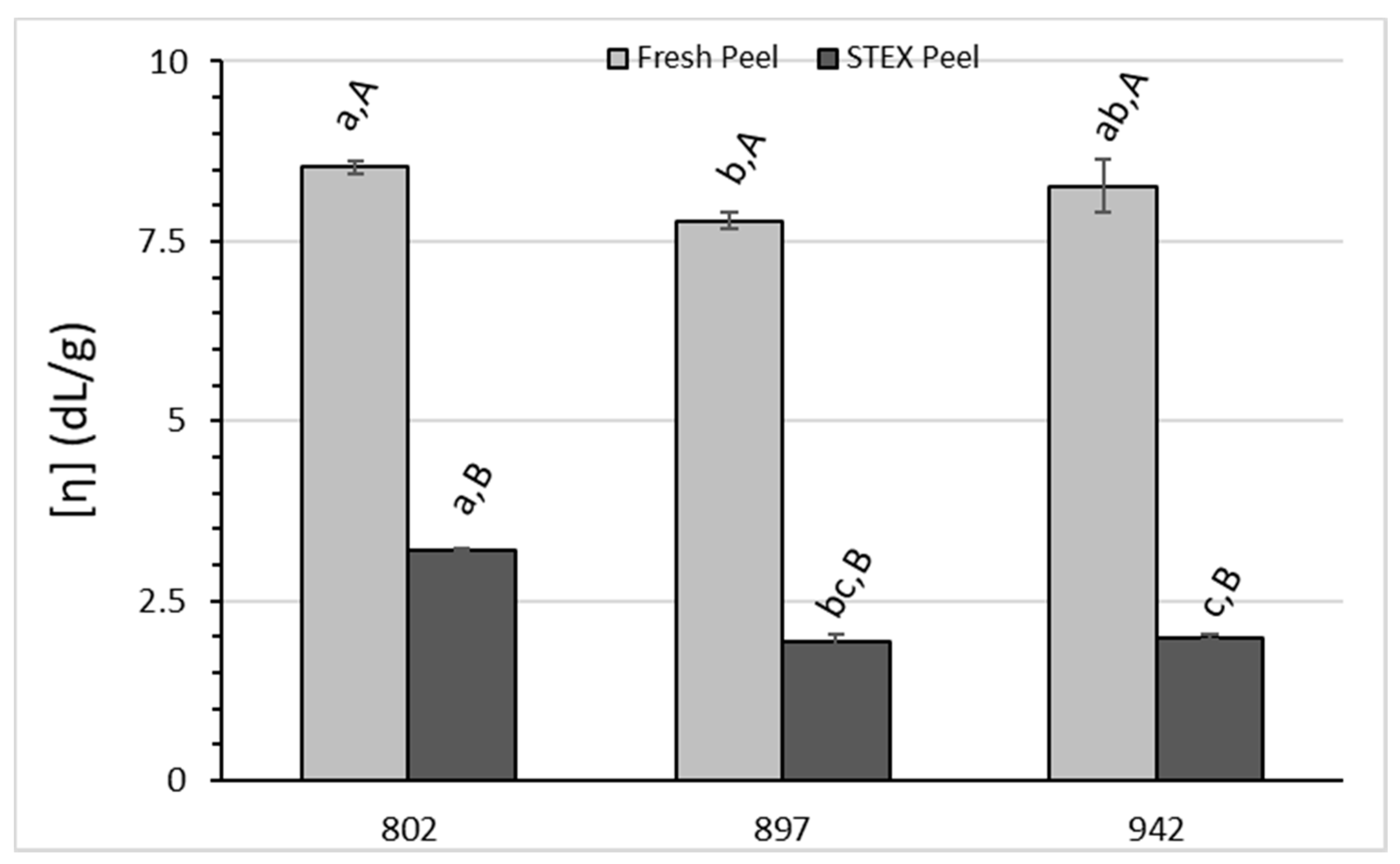
3.3. Recovery and Macromolecular Properties of Pectic Hydrocolloids from STEX
3.4. Flavonoids
3.4.1. Flavanones
3.4.2. PMFs
3.4.3. Coumarins
3.5. Potential Market Value
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United States Department of Agriculture. National Agricultural Statistics Service NASS, Florida Citrus Statistics 2019–2020. Available online: https://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/Citrus_Statistics/ (accessed on 20 July 2021).
- Singerman, A.; Bruani-Arouca, M.; Futch, S.H. The profitability of new citrus plantings in Florida in the era of Huanglongbing. Hort. Sci. 2018, 53, 1655–1663. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U.; Bowman, K.D. Tolerance of the trifoliate citrus hybrid US-897 (Citrus reticulata Blanco × Poncirus trifoliata L. Raf.) to huanglongbing. Hort. Sci. 2011, 46, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U.; Bowman, K.D. Tolerance of trifoliate citrus rootstock hybrids to Candidatus Liberibacter asiaticus. Sci. Hortic. 2012, 147, 71–80. [Google Scholar] [CrossRef]
- Albrecht, U.; Bowman, K.D. Reciprocal influences of rootstock and scion citrus cultivars challenged with Ca. Liberibacter asiaticus. Sci. Hortic. 2019, 254, 133–142. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Comparison of gene expression changes in susceptible, tolerant, and resistant hosts in response to infection with Citrus tristeza virus and huanglongbing. J. Cit. Pathol. 2015, 2. [Google Scholar] [CrossRef]
- Bowman, K.D.; Albrecht, U. Rootstock influences on health and growth following Candidatus Liberibacter Asiaticus infection in young sweet orange trees. Agronomy 2020, 10, 1907. [Google Scholar] [CrossRef]
- Deterre, S.C.; McCollum, G.; Leclair, C.; Manthey, J.A.; Bai, J.; Baldwin, E.A.; Raithore, S.; Stover, E.; Plotto, A. Effect of Poncirus trifoliata on the chemical composition of fruits in pedigrees of Citrus scion hybrids. Sci. Hortic. 2021, 277, 109816. [Google Scholar] [CrossRef]
- Panwar, D.; Panesar, P.S.; Chopra, H.K. Recent trends on the valorization strategies for the management of citrus by-products. Food Rev. Int. 2021, 37, 91–120. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern extraction and purification techniques for obtaining high purity food-grade bioactive compounds and value-added co-products from citrus wastes. Foods 2019, 8, 523. [Google Scholar] [CrossRef] [Green Version]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent progress in the utilization of industrial waste and by-products of citrus fruits: A review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Negro, V.; Mancini, G.; Ruggeri, B.; Fino, D. Citrus waste as feedstock for bio-based products recovery: Review on limonene case study and energy valorization. Bioresour. Technol. 2016, 214, 806–815. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Widmer, W.W.; Grohmann, K. Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem. 2007, 42, 1614–1619. [Google Scholar] [CrossRef]
- Zhou, W.; Widmer, W.; Grohmann, K. Developments in ethanol production from citrus peel waste. Proc. Fla. State Hortic. Soc. 2008, 121, 307–310. [Google Scholar]
- Widmer, W.W.; Narciso, J.A.; Grohmann, K.; Wilkins, M.R. Simultaneous saccharification and fermentation of orange processing waste to ethanol using Kluyveromyces marxianus. Biol. Eng. Trans. 2009, 2, 17–29. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment effects on orange processing waste for making ethanol by simultaneous saccharification and fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef]
- Grohman, K.; Cameron, R.; Kim, Y.; Widmer, W.; Luzio, G. Extraction and recovery of pectic fragments from citrus processing waste for co-production with ethanol. J. Chem. Technol. Biotechnol. 2013, 88, 395–407. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Cooper, K. Steam explosion and fermentation of sugar beets from Southern Florida and the Midwestern United States. Biocatal. Agric. Biotechnol. 2017, 11, 26–33. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Manthey, J.A.; Ferguson, K.L. Bench scale batch steam explosion of Florida red and white grapefruit juice processing residues. Future Foods 2021, 3, 100020. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Manthey, J.A.; Bai, J.; Ferguson, K.L. Analysis and potential value of compounds extracted from star ruby, rio red, and ruby red grapefruit, and grapefruit juice processing residues via steam explosion. Front. Nutr. 2021, 8, 691663. [Google Scholar] [CrossRef]
- Dorado, C.; Cameron, R.G.; Manthey, J.A. Study of static steam explosion of Citrus sinensis juice processing waste for the isolation of sugars, pectic hydrocolloids, flavonoids, and peel oil. Food Bioprocess Technol. 2019, 12, 1293–1303. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Hotchkiss, A.T.; Manthey, J.A. Release and recovery of pectic hydrocolloids and phenolics from culled citrus fruits. Food Hydrocoll. 2017, 72, 52–61. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Hotchkiss, A.T.; Manthey, J.A. Recovery of pectic hydrocolloids and phenolics from huanglongbing related dropped citrus fruit. J. Sci. Food Agric. 2017, 97, 4467–4475. [Google Scholar] [CrossRef]
- Cameron, R.G.; Chau, H.K.; Manthey, J.A. Continuous process for enhanced release and recovery of pectic hydrocolloids and phenolics from citrus biomass. J. Chem. Technol. Biotechnol. 2016, 91, 2597–2606. [Google Scholar] [CrossRef]
- Boman, B.J.; Obreza, T.A.; Morgan, K.T. Environmental Issues and Best Management Practices. In Nutrition of Florida Citrus Trees, 3rd ed.; Morgan, K.T., Kadyampakeni, D.M., Eds.; Electronic Data Information Source of University of Florida, No. 2; Institute of Food and Agricultural Sciences Extension: Gainesville, FL, USA, 2020; Volume 2020, pp. SL464–SS677. [Google Scholar] [CrossRef]
- Obreza, T.A.; Morgan, K.T.; Albrigo, L.G.; Boman, B.J.; Kadyampakeni, D.; Vashisth, T.; Zekri, M.; Graham, J.; Johnson, E. Recommended Fertilizer Rates and Timing. In Nutrition of Florida Citrus Trees, 3rd ed.; Morgan, K.T., Kadyampakeni, D.M., Eds.; Electronic Data Information Source of University of Florida, No. 2; Institute of Food and Agricultural Sciences Extension: Gainesville, FL, USA, 2020; Volume 2020, pp. SL462–SS675. [Google Scholar] [CrossRef]
- Kimball, D. Citrus Processing Quality Control and Technology, 1st ed.; AVI/Van Nostrand Reinhold: New York, NY, USA, 1991; pp. 74–80. [Google Scholar] [CrossRef]
- Kertesz, Z.I. The Pectic Substances; Interscience Publishers, Inc.: New York, NY, USA, 1951. [Google Scholar]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef]
- Doner, L.W.; Irwin, P.L. Assay of reducing end-groups in oligosaccharide homologues with 2,2′-bicinchoninate. Anal. Biochem. 1992, 202, 50–53. [Google Scholar] [CrossRef]
- Kenealy, W.R.; Jeffries, T. Enzyme Processes for Pulp and Paper: A Review of Recent Developments. In Wood Deterioration and Preservation; Goodell, B., Nicholas, D.D., Schultz, T.P., Eds.; American Chemical Society: Washington, DC, USA, 2003; Volume 845, pp. 210–239. [Google Scholar]
- Anthon, G.E.; Barrett, D.M. Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. application to the assay of pectin methylesterase. J. Agric. Food Chem. 2004, 52, 3749–3753. [Google Scholar] [CrossRef]
- Quesenberry, M.S.; Lee, Y.C. A rapid formaldehyde assay using purpald reagent: Application under periodation conditions. Anal. Biochem. 1996, 234, 50–55. [Google Scholar] [CrossRef]
- Zurek, G.; Karst, U. Microplate photometric determination of aldehydes in disinfectant solutions. Anal. Chim. Acta 1997, 351, 247–257. [Google Scholar] [CrossRef]
- Kaya, M.; Sousa, A.G.; Crépeau, M.-J.; Sørensen, S.O.; Ralet, M.-C. Characterization of citrus pectin samples extracted under different conditions: Influence of acid type and pH of extraction. Ann. Bot. 2014, 114, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile compounds in citrus essential oils: A comprehensive review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Kesterson, J.W.; Braddock, R.J. Total peel oil content of the major florida citrus cultivars. J. Food Sci. 1975, 40, 931–933. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Bettarini, F.; Borgonovi, G.; Fiorani, T.; Gagliardi, I.; Caprioli, V.; Massardo, P.; Ogoche, J.I.J.; Hassanali, A.; Nyandat, E.; Chapya, A. Antiparasitic compounds from east african plants: Isolation and biological activity of anonaine, matricarianol, canthin-6-one and caryophyllene oxide. Int. J. Trop. Insect Sci. 1993, 14, 93–99. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Ultra International B.V. Essential Oils Market Report Autumn. 2019. Available online: http://ultranl.com/market/market-report-autumn_2019/ (accessed on 5 December 2019).
- Ringbloom, U. (Ed.) The Orange Book, 1st ed.; Tetra Pak Processing Systems AB: Lund, Sweden, 2004. [Google Scholar]
- Xu, B.M.; Sims, C.A.; Etxeberria, E.; Goodrich Schenider, R.M. Physicochemical and sensory properties of cold pressed oils from florida hamlin and valencia oranges affected by huanglongbing. J. Food Sci. 2017, 82, 2158–2166. [Google Scholar] [CrossRef]
- Mahato, N.; Sharma, K.; Koteswararao, R.; Sinha, M.; Baral, E.; Cho, M.H. Citrus essential oils: Extraction, authentication and application in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 611–625. [Google Scholar] [CrossRef]
- Mitiku, S.; Sawamura, M.; Itoh, T.; Ukeda, H. Volatile components of peel cold-pressed oils of two cultivars of sweet orange (Citrus sinensis (L.) Osbeck) from Ethiopia. Flavour Fragr. J. 2000, 15, 240–244. [Google Scholar] [CrossRef]
- Braddock, R.J. Handbook of Citrus By-Products and Processing Technology; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Kiefl, J.; Kohlenberg, B.; Hartmann, A.; Obst, K.; Paetz, S.; Krammer, G.; Trautzsch, S. Investigation on key molecules of Huanglongbing (HLB)-induced orange juice off-flavor. J. Agric. Food Chem. 2018, 66, 2370–2377. [Google Scholar] [CrossRef]
- Rolin, C.; Chrestensen, L.B.; Hansen, K.M.; Staunstrup, J.; Sorensen, S. Tailoring Pectin with Specific Shape, Composition and Esterification Pattern. In Gums and Stabilisers for the Food Industry 15, 1st ed.; Williams, P.A., Phillips, G.O., Eds.; Royal Society of Chemistry: Cambridge, UK, 2010; Volume 15, pp. 13–25. [Google Scholar] [CrossRef]
- Fishman, M.L.; Chau, H.K.; Hoagland, P.D.; Hotchkiss, A.T. Microwave-assisted extraction of lime pectin. Food Hydrocoll. 2006, 20, 1170–1177. [Google Scholar] [CrossRef]
- The Food and Agriculture Organization of the United Nations. Joint FAO/WHO Expert Committee on Food Additives Monographs 4 (681h JECFA); FAO: Rome, Italy, 2007. [Google Scholar]
- Ferguson, K.L.; da Cruz, M.A.; Ferrarezi, R.S.; Dorado, C.; Bai, J.; Cameron, R.G. Impact of Huanglongbing (HLB) on grapefruit pectin yield and quality during grapefruit maturation. Food Hydrocoll. 2021, 113, 106553. [Google Scholar] [CrossRef]
- Koh, J.; Morales-Contreras, B.E.; Guerra-Rosas, M.I.; Osorio-Hernández, E.; Culver, C.A.; Morales-Castro, J.; Wicker, L. Huanglongbing disease and quality of pectin and fruit juice extracted from Valencia oranges. LWT 2020, 131, 109692. [Google Scholar] [CrossRef]
- Ngouémazong, D.E.; Kabuye, G.; Fraeye, I.; Cardinaels, R.; van Loey, A.; Moldenaers, P.; Hendrickx, M. Effect of debranching on the rheological properties of Ca2+–pectin gels. Food Hydrocoll. 2012, 26, 44–53. [Google Scholar] [CrossRef]
- Cameron, R.G.; Luzio, G.A.; Goodner, K.; Williams, M.A.K. Demethylation of a model homogalacturonan with a salt-independent pectin methylesterase from citrus: I. Effect of pH on demethylated block size, block number and enzyme mode of action. Carbohydr. Polym. 2008, 71, 287–299. [Google Scholar] [CrossRef]
- Willats, W.G.; Orfila, C.; Limberg, G.; Buchholt, H.C.; van Alebeek, G.J.; Voragen, A.G.; Marcus, S.E.; Christensen, T.M.I.E.; Mikkelsen, J.D.; Murray, B.S.; et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion. J. Biol. Chem. 2001, 276, 19404–19413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapomarda, A.; de Acutis, A.; Chiesa, I.; Fortunato, G.M.; Montemurro, F.; de Maria, C.; Belmonte, M.M.; Gottardi, R.; Vozzi, G. Pectin-GPTMS based biomaterial: Toward a sustainable Bioprinting of 3D scaffolds for Tissue Engineering application. Biomacromolecules 2019, 21, 319–327. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servais, A.B.; Valenzuela, C.D.; Kienzle, A.; Ysasi, A.B.; Wagner, W.L.; Tsuda, A.; Ackermann, M.; Mentzer, S.J. Functional mechanics of a pectin-based pleural sealant after lung injury. Tissue Eng. Part A 2018, 24, 695–702. [Google Scholar] [CrossRef]
- White, R.J.; Budarin, V.L.; Clark, J.H. Pectin-derived porous materials. Chem. Eur. J. 2010, 16, 1326–1335. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Gentili, B. Phenolic glycosides of grapefruit: A relation between bitterness and structure. Arch. Biochem. Biophys. 1961, 92, 191–192. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Gentili, B. Flavonoids of citrus–VI. The structure of neohesperidose. Tetrahedron 1963, 19, 773–782. [Google Scholar] [CrossRef]
- Albach, R.F.; Redman, G.H. Composition and inheritance of flavanones in citrus fruit. Phytochemistry 1969, 8, 127–143. [Google Scholar] [CrossRef]
- Horowitz, R.M.; Gentili, B. Taste and structure of phenolic glycosides. J. Agric. Food Chem. 1969, 17, 696–700. [Google Scholar] [CrossRef]
- Rajkumar, S.; Jebanesan, A. Bioactivity of flavonoid compounds from Poncirus trifoliata L. (Family: Rutaceae) against the dengue vector, Aedes aegypti L. (Diptera: Culicidae). Parasitol. Res. 2008, 104, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Lee, S.-H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidin and poncirin isolated from the fruits of Poncirus trifoliata on potential gastric disease. Phytother. Res. 2009, 23, 1748–1753. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Hu, S.; Ma, P.; Che, D.; Liu, R.; Zhang, Y.; Wang, J.; Li, C.; Ding, Y.; Fu, J.; et al. Neohesperidin suppresses IgE-mediated anaphylactic reactions and mast cell activation via Lyn-PLC-Ca2+ pathway. Phytother. Res. 2019, 33, 2034–2043. [Google Scholar] [CrossRef]
- Lu, J.F.; Zhu, M.Q.; Zhang, H.; Liu, H.; Xia, B.; Wang, Y.L.; Shi, X.; Peng, L.; Wu, J.W. Neohesperidin attenuates obesity by altering the composition of the gut microbiota in high-fat diet-fed mice. FASEB J. 2020, 34, 12053–12071. [Google Scholar] [CrossRef]
- Lai, C.-S.; Wu, J.-C.; Ho, C.-T.; Pan, M.-H. Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. BioFactors 2015, 41, 301–313. [Google Scholar] [CrossRef]
- Tung, Y.-C.; Chou, Y.-C.; Hung, W.-L.; Cheng, A.-C.; Yu, R.-C.; Ho, C.-T.; Pan, M.-H. Polymethoxyflavones: Chemistry and molecular mechanisms for cancer prevention and treatment. Curr. Pharmacol. Rep. 2019, 5, 98–113. [Google Scholar] [CrossRef]
- Zhao, G.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.-H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Owis, A.I. Chapter 15—Citrus polymethoxyflavones: Biofunctional molecules of therapeutic interest. Stud. Nat. Prod. Chem. 2018, 59, 509–530. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Yu, K.-Y.; Guo, L.; Bai-Zhong, C.; Li, P.; Liu, E.-H. Polymethoxyflavones in peel of Citrus reticulata ‘Chachi’ and their biological activities. Food Chem. 2017, 234, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-P.; Yan, G.; Xie, Y.-T.; Lin, T.-C.; Zhang, W.; Li, J.; Wu, Y.J.; Zhou, J.-Z.; Wu, Y.-H. Bioactive prenylated coumarins as potential anti-inflammatory and anti-HIV agents from Clausena lenis. Bioorg. Chem. 2020, 97, 103699. [Google Scholar] [CrossRef]
- Liang, H.; Shi, Y.; Zeng, K.; Zhao, M.; Tu, P.; Jiang, Y. Coumarin derivatives from the leaves and twigs of Murraya exotica L. and their anti-inflammatory activities. Phytochemistry 2020, 177, 112416. [Google Scholar] [CrossRef]
- Zhang, Z.-R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid.-Based Complement. Altern. Med. 2015, 919616. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; Guo, D.-S.; Lu, M.-H.; Yue, J.-Y.; Liu, Y.; Shang, C.-M.; An, D.-R.; Zhao, M.M. Inhibitory effect of osthole from Cnidium monnieri on Tobacco mosaic virus (tmv) infection in Nicotiana glutinosa. Molecules 2019, 25, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, M.S.; Lee, B.H.; Kim, J.-K.; Ahn, E.K.; Ko, H.-J.; Cho, R.-Y.; Lee, S.-J.; Bae, G.-U.; Kim, Y.K.; et al. Marmesin-mediated suppression of VEGF/VEGFR and integrin β1 expression: Its implication in non-small cell lung cancer cell responses and tumor angiogenesis. Oncol. Rep. 2017, 37, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Znati, M.; Ben Jannet, H.; Cazaux, S.; Souchard, J.P.; Harzallah Skhiri, F.; Bouajila, J. Antioxidant, 5-lipoxygenase inhibitory and cytotoxic activities of compounds isolated from the Ferula lutea flowers. Molecules 2014, 19, 16959–16975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Yu, A.L.; Wang, Z.L.; Chen, K.; Zheng, W.; Zhou, J.J.; Xie, Q.; Yan, H.-B.; Ren, P.; Huang, X. Chaihu-Shugan-San and absorbed meranzin hydrate induce anti-atherosclerosis and behavioral improvements in high-fat diet ApoE−/− mice via anti-inflammatory and BDNF-TrkB pathway. Biomed. Pharmacother. 2019, 115, 108893. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. (Eds.) Top Value Added Chemicals from Biomass Volume I-Results of Screening for Potential Candidates from Sugars and Synthesis Gas; United States Department of Energy, Office of Energy, Efficiency And Renewable Energy: Springfield, VA, USA, 2004. [CrossRef] [Green Version]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisted. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M. Bio-Based Chemicals: Value Added Products from Biorefineries, International Energy Agency, Bioenergy Task 42; Biorefinery: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Straathof, A.J.J.; Bampouli, A. Potential of commodity chemicals to become bio-based according to maximum yields and petrochemical prices. Biofuels Bioprod. Biorefin. 2017, 11, 798–810. [Google Scholar] [CrossRef] [Green Version]
- Pacific Pectin. Pacific Pectin Products. 2021. Available online: https://pacificpectin.com/products/ (accessed on 27 April 2021).
- Lara-Espinoza, C.; Carvajal-Millán, E.; Balandrán-Quintana, R.; López-Franco, Y.; Rascón-Chu, A. Pectin and pectin-based composite materials: Beyond food texture. Molecules 2018, 23, 942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wholesale Organic Grapefruit Seed Extract 98% Naringin from Coolflying. Available online: https://www.alibaba.com/product-detail/Wholesale-Organic-Grapefruit-Seed-Extract-98_1600083983518.html?spm=a2700.7724857.normal_offer.d_title.46009e8aiYQH6Y (accessed on 12 August 2021).
- Natural Sweetener Citrus Aurantium Extract/Bitter Orange Extract Powder Neohesperidin. Available online: https://www.alibaba.com/product-detail/Neohesperidin-Natural-Sweetener-Citrus-Aurantium-Extract_1600261028323.html?spm=a2700.7724857.normal_offer.d_title.542f7545aX1SlK&s=p> (accessed on 12 August 2021).
- High Purity Poncirin CAS NO 14941-083. Available online: https://m.alibaba.com/amp/product/1600276176419.html (accessed on 12 August 2021).
- Global Flavonones Market-2027; Fior Markets: Pune, India, 2021.
- Singerman, A.; Burani-Arouca, M.; Hutch, S.H. The Economics of Planting New Citrus Groves in Florida in the Era of HLB. Electronic Data Information Source of University of Florida; Institute of Food and Agricultural Sciences Extension: Gainesville, FL, USA, 2018; p. FE1050. Available online: https://edis.ifas.ufl.edu/publication/FE1050 (accessed on 20 October 2021).
- Singerman, A. The real cost of HLB in Florida. Citrus Industry Magazine, 30 July 2019; 10–13. Available online: https://crec.ifas.ufl.edu/media/crecifasufledu/economics/cost_manuscript_20190801.pdf(accessed on 20 October 2021).


| Cultivar | Fruit Length (mm) 1 | Fruit Diameter (mm) 1 | Fruit Weight (g) 1 | Fruit Juice (% wt, wet) | Fruit Seed (% wt, wet) | Fruit Peel (% wt, wet) | Fruit per Tree (kg) 1 | Fruit per Hectare (kg) 1 |
|---|---|---|---|---|---|---|---|---|
| US-802 | 88 ± 6 | 83 ± 7 | 217.1 ± 8.4 | 39.6 | 7.5 | 52.9 | 67.8 ± 3.8 | 36,500 ± 2100 |
| US-897 | 41 ± 2 | 35 ± 3 | 29.8 ± 1.3 | 36.9 | 9.7 | 53.4 | 35.0 ± 6.1 | 18,900 ± 3300 |
| US-942 | 41 ± 4 | 39 ± 3 | 38.7 ± 2.7 | 39.9 | 8.8 | 51.3 | 54.0 ± 8.0 | 29,100 ± 4300 |
| Items | Total Mass Run (kg) | Average Temperature (°C) | Total Run Time (min) | % dw before STEX 1 (wt %, Dry) | % dw after STEX 1 (wt %, Dry) |
|---|---|---|---|---|---|
| US-802 | 133 | 135 | 92 | 16.36 ± 0.30 | 13.72 ± 0.20 |
| US-897 | 82 | 134 | 27 | 16.25 ± 0.36 | 12.37 ± 0.05 |
| US-942 | 79 | 135 | 34 | 20.69 ± 0.36 | 15.35 ± 0.04 |
| Items | SOLUBLE | |||||
|---|---|---|---|---|---|---|
| Fresh | STEX | |||||
| Variety | Glucose | Fructose | Sucrose | Glucose | Fructose | Sucrose |
| US-802 | 9.60 ± 0.13 | 8.49 ± 0.12 | 6.22 ± 0.18 | 11.86 ± 0.76 (0.04) | 10.15 ± 0.71 (0.07) | 2.29 ±0.17 (0.00) |
| US-897 | 10.06 ± 0.39 | 9.49 ± 0.37 | 6.31 ± 0.27 | 12.23 ± 0.26 (0.02) | 11.08 ± 0.37 (0.04) | 0.00 ±0.00 (0.00) |
| US-942 | 9.76 ± 0.50 | 9.09 ± 0.57 | 5.43 ± 0.34 | 12.62 ± 0.71 (0.01) | 11.42 ± 0.66 (0.02) | 0.00 ±0.00 (0.00) |
| Hamlin 1 | 12.21 | 13.85 | 7.37 | |||
| Valencia 1 | 9.58 | 8.86 | 7.86 | |||
| Marsh 2 | 13.69 | 12.71 | 9.81 | |||
| Ruby, Star, Flame 2 | 9.85 | 10.08 | 13.94 | |||
| COMPOSITIONAL | ||||||
| Fresh | STEX | |||||
| Variety | Glucose | Fructose | Glucose | Fructose | ||
| US-802 | 22.41 ± 0.99 | 11.37 ± 0.25 | 20.98 ± 1.42 (0.37) | 10.95 ± 0.09 (0.09) | ||
| US-897 | 22.39 ± 0.89 | 13.49 ± 0.32 | 25.11 ± 1.11 (0.00) | 16.58 ± 0.11 (0.00) | ||
| US-942 | 16.57 ± 1.54 | 10.26 ± 0.96 | 20.22 ± 0.89 (0.07) | 13.35 ± 0.09 (0.03) | ||
| Hamlin 1 | 24.05 | 16.18 | ||||
| Valencia 1 | 20.94 | 12.98 | ||||
| Marsh 2 | 27.25 | 18.89 | ||||
| Ruby, Star, Flame 2 | 24.74 | 15.66 | ||||
| Items | Fresh (% w/w, Wet) | Fresh (% w/w, Dry) | STEX (% w/w, Dry) | Volatiles Released (%) |
|---|---|---|---|---|
| US-802 | 0.13 ± 0.00 | 0.79 ± 0.00 | 0.37 ± 0.01 (0.00) | 53 |
| US-897 | 0.48 ± 0.02 | 2.95 ± 0.13 | 0.51 ± 0.00 (0.00) | 83 |
| US-942 | 0.69 ± 0.04 | 3.32 ± 0.17 | 0.49 ± 0.04 (0.00) | 85 |
| Valencia [38] | 0.7 | - | - | |
| Hamlin [38] | 0.4 | - | - | |
| Ruby Red [38] | 0.3 | - | - | |
| Marsh [38] | 0.3 | - | - |
| Reference | [21] | Deng et al. 2020 | [44] | ||||
|---|---|---|---|---|---|---|---|
| Treatment | Steam Explosion | Steam Explosion | Molecular Distillation | Cold Pressed | |||
| De-Seeded Fruit | Brown Extracted Peel | Essential Oil | JBT Processed Fruit | ||||
| Variety | US-802 | US-942 | US-897 | Ruby Red | Marsh | Late AS Hamlin | Late AS Valencia |
| Density (g/mL) | 0.9263 ± 0.0003 | 0.8436 ± 0.0004 | 0.8452 ± 0.0003 | 0.8477 ± 0.0002 | nr | nr | nr |
| Compound | % based on total ion current | ||||||
| α-Pinene | 1.01 | 0.82 | 0.75 | 0.36 | 0.76 | 0.50 | 0.48 |
| β-Pinene | 0.00 | 3.30 | 2.59 | 0.00 a | 0.05 | nr | nr |
| β-Myrcene | 4.63 | 3.39 | 3.81 | 1.66 | 2.16 | 1.38 | 1.21 |
| Octanal | 0.00 | 0.01 | 0.02 | 0.07 | 0.36 | 0.67 | 0.97 |
| o-Cymene | 1.66 | 0.20 | 0.19 | 0.06 a | nr | nr | nr |
| D-Limonene | 60.07 | 78.92 | 79.69 | 87.94 | 93.33 b | 96.6 b | 95.9 b |
| Linalool | 0.20 | 0.41 | 0.36 | 0.16 | 0.12 | 0.04 | 0.23 |
| Nonanal | 0.00 | 0.00 | 0.00 | 0.07 | 0.05 | 0.01 | 0.04 |
| Decanal | 0.00 | 0.02 | 0.02 | 0.07 | 0.19 | 0.09 | 0.29 |
| Carvone | 1.80 | 0.14 | 0.25 | 1.60 | 0.41 | trace | 0.01 |
| Geranyl acetate | 0.09 | 0.00 | 0.03 | 0.12 | nr | nr | nr |
| α-Copaene | 0.10 | 0.08 | 0.07 | 0.32 | 0.13 | trace | trace |
| ß-Elemene | 1.13 | 0.30 | 0.33 | 0.28 | nr | nr | nr |
| (E)-Caryophyllene | 6.89 | 2.18 | 3.13 | 0.89 | 0.20 c | 0.01 d | 0.02 d |
| α-Humulene | 1.11 | 0.28 | 0.36 | 0.15 a | 0.03 e | trace | trace |
| δ-Cadinene | 0.18 | 0.30 | 0.17 | 0.27 | 0.04 | trace | nd |
| Caryophyllene oxide | 7.66 | 0.26 | 0.36 | 0.43 a | 0.04 | trace | 0.01 |
| Total | 86.53 | 90.62 | 92.11 | 94.45 | 97.87 | 99.30 | 99.16 |
| Oxygenated | 9.75 | 0.84 | 1.04 | 2.52 | 1.17 | 0.81 | 1.55 |
| Sample | DM (Mean ± SE) | Percent GalA (Mean ± SE) | GalA/Rha (Mean ± SE) | DBr (Mean ± SE) | |
|---|---|---|---|---|---|
| STEX | US-802 | 60.34 ± 5.04 a,A | 69.46 ± 1.49 a,A | 21.93 ± 0.64 a,A | 8.25 ± 0.14 a,A |
| US-897 | 63.21 ± 4.58 a,A | 61.02 ± 1.07 a,A | 16.33 ± 0.38 ab,A | 9.26 ± 0.10 ab,A | |
| US-942 | 70.48 ± 2.64 a,A | 62.03 ± 0.55 a,A | 9.07 ± 0.11 c,A | 4.55 ± 0.01 c,A | |
| Fresh | US-802 | 53.86 ± 2.29 abc,A | 65.32 ± 1.79 ab,A | 31.25 ± 1.64 a,B | 12.58 ± 0.60 a,B |
| US-897 | 45.23 ± 4.07 b,B | 70.17 ± 0.51 a,B | 25.82 ± 0.23 a.B | 9.49 ± 0.11 a,B | |
| US-942 | 52.15 ± 3.53 c,B | 67.03 ± 1.85 b,B | 28.15 ± 1.33 a,B | 11.82 ± 0.40 a,B | |
| Items | Hesperidin | Nobiletin | Tangeretin | Naringin | Neohesperidin | Poncirin |
|---|---|---|---|---|---|---|
| ppm or ug/g Dry Weight | ||||||
| Hamlin Fresh [22] | 5625 ± 788 | 1068 ± 124 | 79 ± 16 | nr | nr | nr |
| Hamlin STEX (170 °C 8 min) [22] | 64,611 ± 3764 | 1137 ± 29 | 116 ± 19 | nr | nr | nr |
| Valencia Fresh [22] | 6372 ± 4901 | 994 ± 69 | 87 ± 17 | nr | nr | nr |
| Valencia STEX (170 °C 8 min) [22] | 39,712 ± 6836 | 786 ± 91 | 113 ± 18 | nr | nr | nr |
| US-802 Fresh | 0 | 0 | 0 | 15,014 ± 127 | 0 | 3974 ± 7 |
| US-802 STEX | 0 | 0 | 0 | 17,513 ± 212 (0.42) | 0 | 3675 ± 149 (0.21) |
| US-897 Fresh | 1273 ± 225 | 171 ± 4 | 118 ± 4 | 2905 ± 53 | 11,177 ± 1278 | 21,823 ± 174 |
| US-897 STEX | 1296 ± 135 (0.77) | 177 ± 2 (0.11) | 110 ± 1 (0.16) | 2633 ± 145 (0.30) | 10,911 ± 113 (0.83) | 22,172 ± 216 (0.05) |
| US-942 Fresh | 1427 ± 3 | 474 ± 12 | 417 ± 21 | 2757 ± 83 | 14,569 ± 21 | 5686 ± 12 |
| US-942 STEX | 1258 ± 22 (0.07) | 355 ± 25 (0.05) | 268 ± 4 (0.07) | 2158 ± 352 (0.20) | 11,026 ± 116 (0.02) | 4072 ± 46 (0.02) |
| Items | Glucose | Peel Oil | Pectic Hydrocolloids | Flavonoid | Specific Flavonoid |
|---|---|---|---|---|---|
| Dry Weight kg/Hectare | Compound Name | ||||
| US-802 | 323 | 11 | 55 | 48 | Naringin |
| US-897 | 158 | 27 | 24 | 29 | Poncirin |
| US-942 | 304 | 58 | 59 | 27 | Neohesperidin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorado, C.; Bowman, K.D.; Cameron, R.G.; Manthey, J.A.; Bai, J.; Ferguson, K.L. Steam Explosion (STEX) of Citrus × Poncirus Hybrids with Exceptional Tolerance to Candidatus Liberibacter Asiaticus (CLas) as Useful Sources of Volatiles and Other Commercial Products. Biology 2021, 10, 1285. https://doi.org/10.3390/biology10121285
Dorado C, Bowman KD, Cameron RG, Manthey JA, Bai J, Ferguson KL. Steam Explosion (STEX) of Citrus × Poncirus Hybrids with Exceptional Tolerance to Candidatus Liberibacter Asiaticus (CLas) as Useful Sources of Volatiles and Other Commercial Products. Biology. 2021; 10(12):1285. https://doi.org/10.3390/biology10121285
Chicago/Turabian StyleDorado, Christina, Kim D. Bowman, Randall G. Cameron, John A. Manthey, Jinhe Bai, and Kyle L. Ferguson. 2021. "Steam Explosion (STEX) of Citrus × Poncirus Hybrids with Exceptional Tolerance to Candidatus Liberibacter Asiaticus (CLas) as Useful Sources of Volatiles and Other Commercial Products" Biology 10, no. 12: 1285. https://doi.org/10.3390/biology10121285
APA StyleDorado, C., Bowman, K. D., Cameron, R. G., Manthey, J. A., Bai, J., & Ferguson, K. L. (2021). Steam Explosion (STEX) of Citrus × Poncirus Hybrids with Exceptional Tolerance to Candidatus Liberibacter Asiaticus (CLas) as Useful Sources of Volatiles and Other Commercial Products. Biology, 10(12), 1285. https://doi.org/10.3390/biology10121285






