Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario
Abstract
:Simple Summary
Abstract
1. Introduction
2. Global Climate Changes: Evidence and Causes
3. Plant Responses to Climate Change
4. The Role of Circular Economy in Mitigating Climate Change
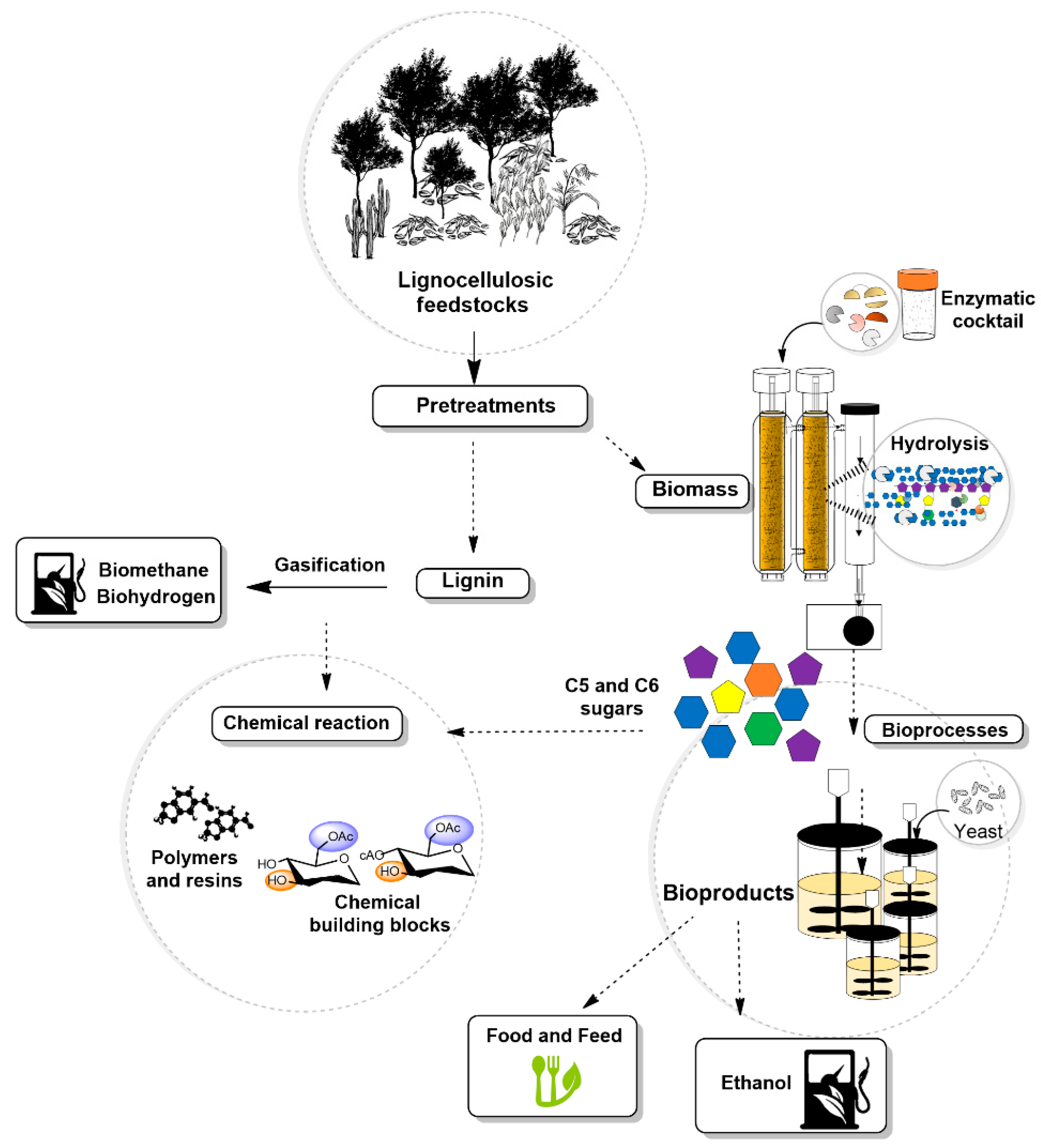
5. Potential Feedstocks for Bioenergy Purposes
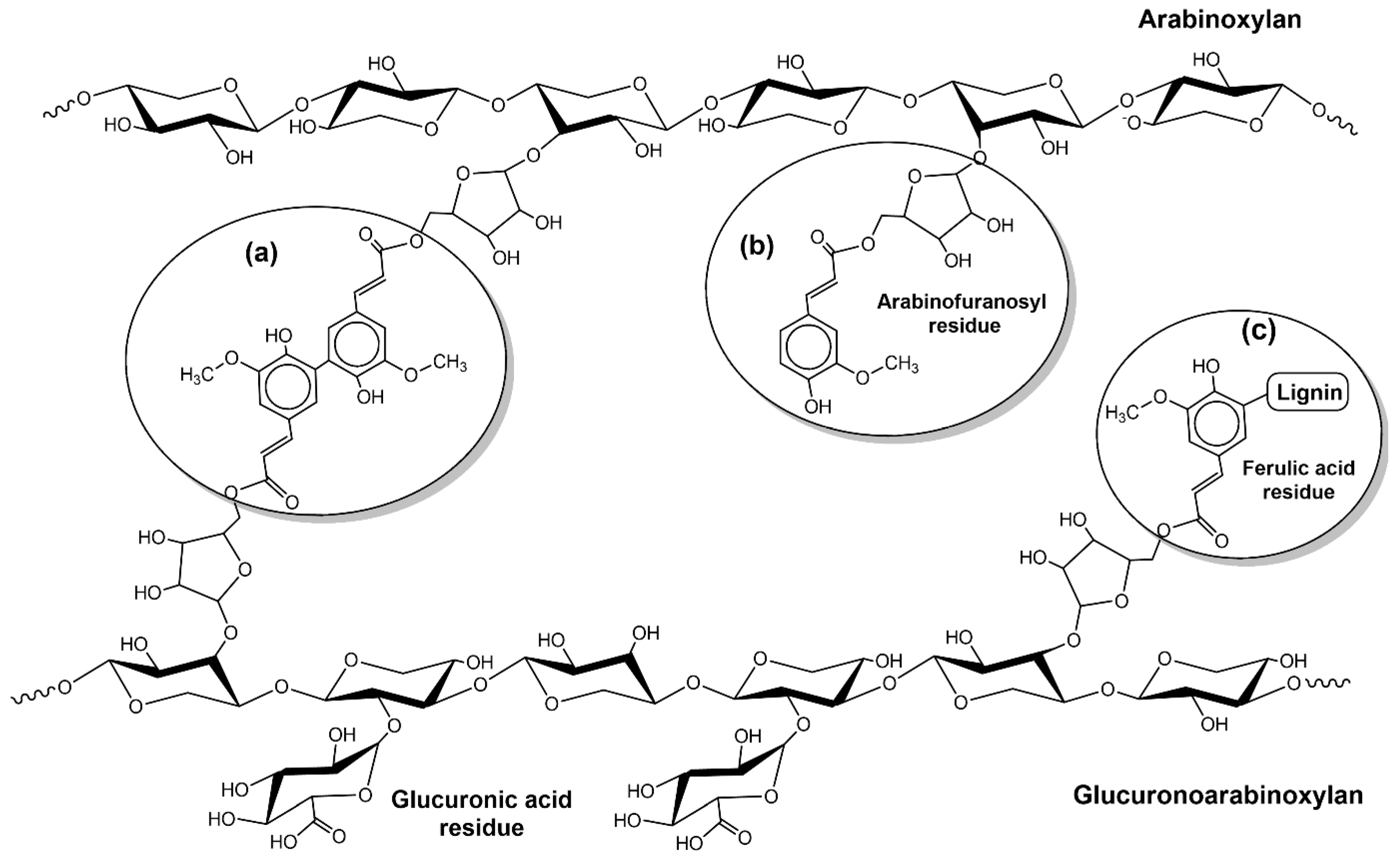
6. Chemical Composition of Lignocellulosic Biomass
7. Pretreatment
| Biomass | Pretreatment | Details | Conditions | Maximal Removal (%) | Glucose Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Miscanthus (Mx2779 and Mxg) | Steam explosion | 180 (9 bar), 200 (15 bar), 210 (20 bar), and 225 °C (25 bar) for 5, 10, or 15 min | 73.2% xylan (Mx2779) 78.9% xylan (Mxg) 200 °C, 15 bar, 10 min | 68% (Mx2779) 41% (Mxg) | [81] | |
| Microwave-assisted DES | ChCl:lactic acid (1:2) | 45 s, 800 W (152 °C) |
|
| [82] |
| Switchgrass | DES | ChCl:glycerol (1:2) with 20 wt% water additions | 120 °C for 1 h | 85.35% xylan 56.82 lignin | 89% | [83] |
| Elephant grass (leaf, stem, and whole plant) | Acid | H2SO4 | 5, 10, or 20% H2SO4 at 121 °C for 30 min | 85.02% hemicellulose from leaf (20% H2SO4) | 89.2% (leaf, 20% H2SO4) | [84] |
| High-pressure CO2/H2O | 180, 200, or 220 °C with a constant initial CO2 pressure of 50 bar |
|
(220 °C) | [85] | |
| Miscanthus | Biological | Bacteria (laccase) | 37 °C, 200 rpm, 96 h (with a mediator) | 59.5% lignin (Pseudomonas sp.) | 87% | [86] |
8. Enzymatic Deconstruction of Lignocellulosic Biomass
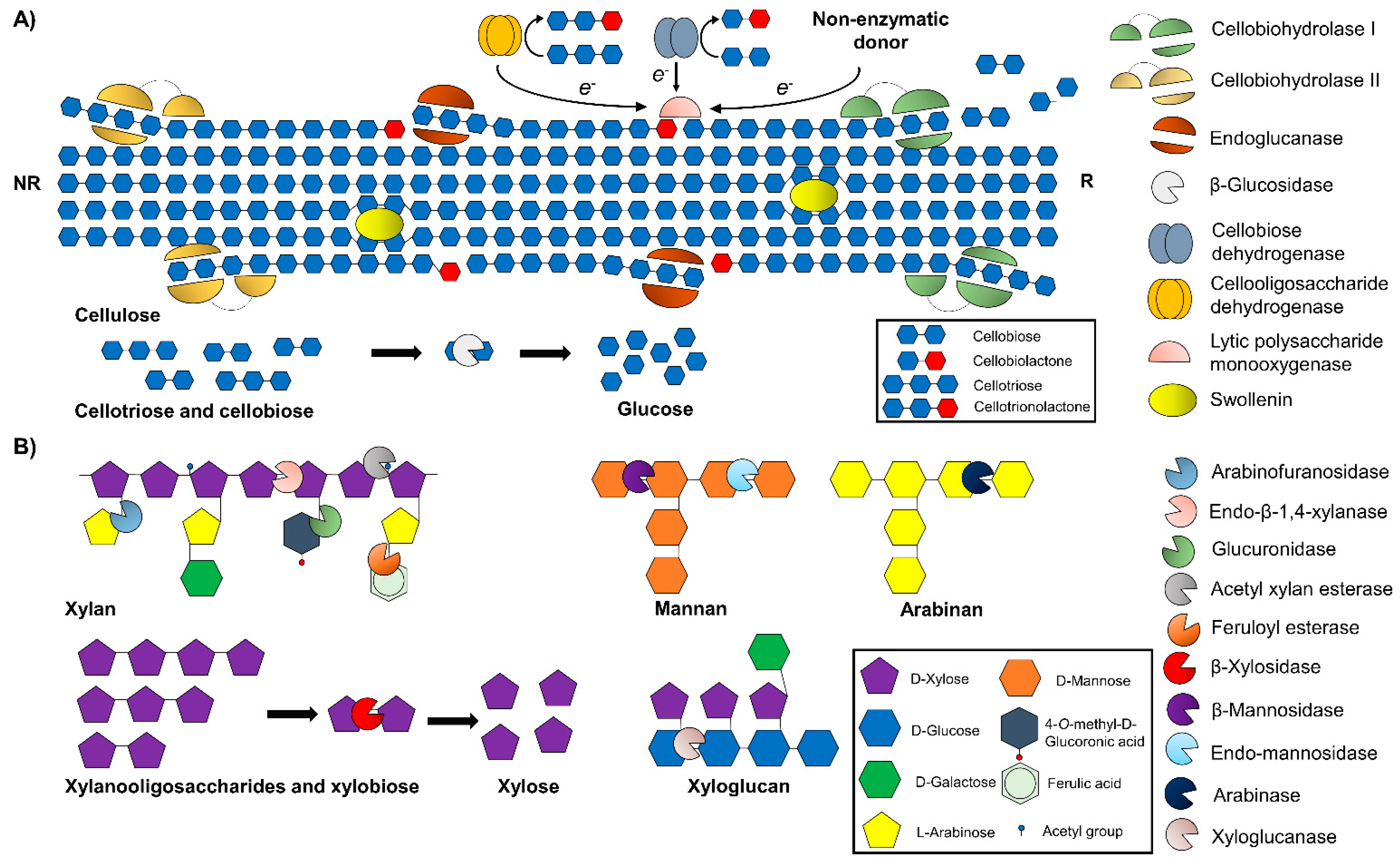
8.1. Core Cellulolytic Enzymes
8.2. Hemicellulolytic Enzymes
8.3. Accessory Enzymes
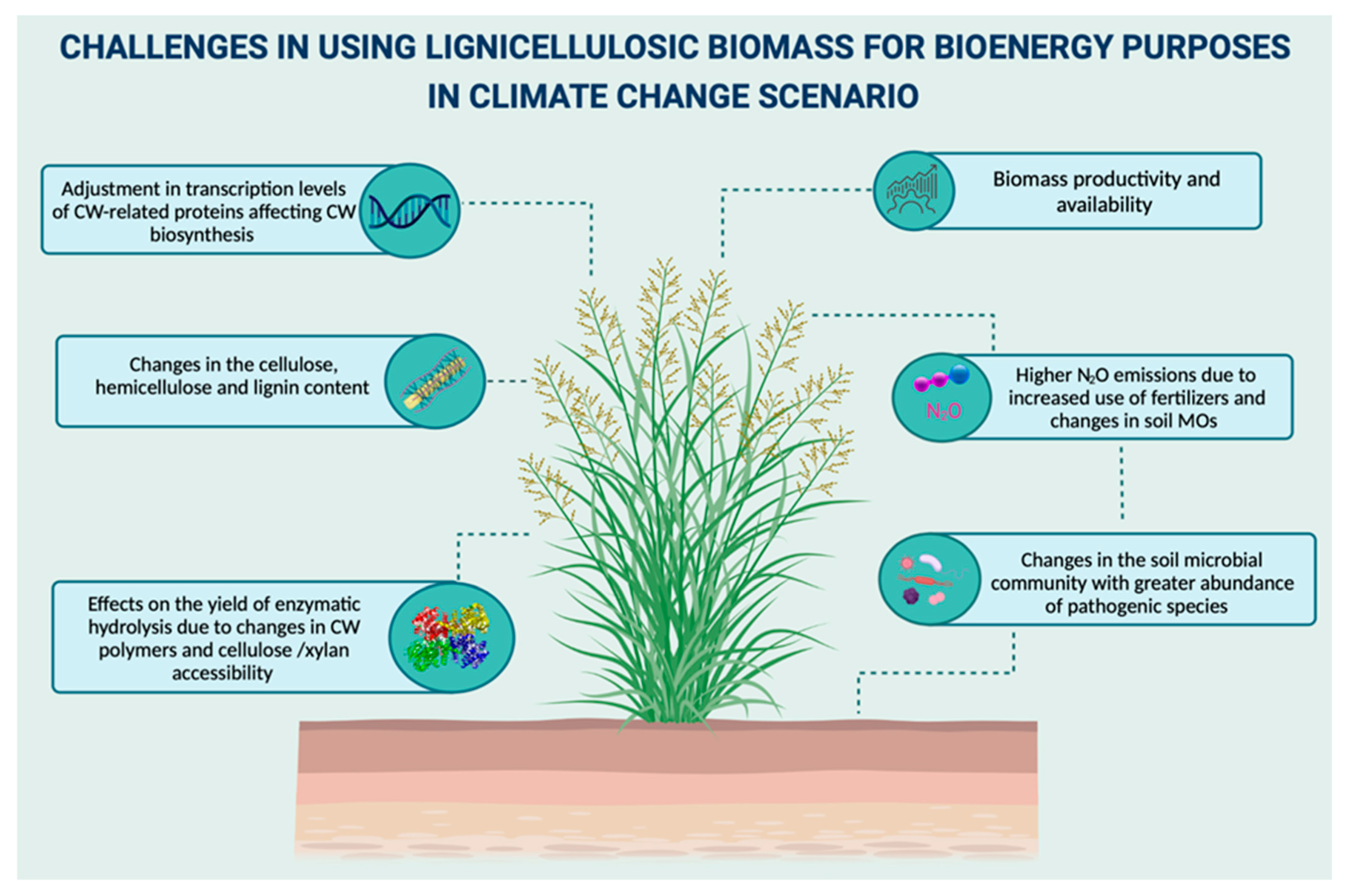
9. Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2020, 27, 27–49. [Google Scholar] [CrossRef]
- Gray, S.B.; Dermody, O.; Klein, S.P.; Locke, A.; McGrath, J.M.; Paul, R.E.; Rosenthal, D.M.; Vera, U.R.; Siebers, M.H.; Strellner, R.; et al. Intensifying drought eliminates the expected benefits of elevated carbon dioxide for soybean. Nat. Plants 2016, 2, 16132. [Google Scholar] [CrossRef]
- Slattery, R.A.; Ort, D.R. Carbon assimilation in crops at high temperatures. Plant Cell Environ. 2019, 42, 2750–2758. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Kumar, S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef]
- Amoah, J.; Kahar, P.; Ogino, C.; Kondo, A. Bioenergy and biorefinery: Feedstock, biotechnological conversion, and products. Biotechnol. J. 2019, 14, e1800494. [Google Scholar] [CrossRef] [PubMed]
- Da Matta, F.M.; Grandis, A.; Arenque, B.C.; Buckeridge, M.S. Impacts of climate changes on crop physiology and food quality. Food Res. Int. 2010, 43, 1814–1823. [Google Scholar] [CrossRef]
- Polizeli, M.L.T.M.; Somera, A.F.; de Lucas, R.C.; Nozawa, M.S.F.; Michelin, M. Enzymes Involved in the Biodegradation of Sugarcane Biomass: Challenges and Perspectives. In Advances of Basic Science for Second Generation Bioethanol from Sugarcane; Buckeridge, M.S., de Souza, A.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 55–79. [Google Scholar]
- Monteiro, L.M.O.; Vici, A.C.; Messias, J.M.; Heinen, P.R.; Pinheiro, V.E.; Rechia, C.G.V.; Buckeridge, M.S.; Polizeli, M.L.T.M. Increased Malbranchea pulchella β-glucosidase production and its application in agroindustrial residue hydrolysis: A research based on experimental designs. Biotechnol. Rep. 2021, 30, e00618. [Google Scholar] [CrossRef]
- Contato, A.G.; de Oliveira, T.B.; Aranha, G.M.; Freitas, E.N.; Vici, A.; Nogueira, K.M.V.; de Lucas, R.C.; Scarcella, A.; Buckeridge, M.; Silva, R.N.; et al. Prospection of fungal lignocellulolytic enzymes produced from jatoba (Hymenaea courbaril) and tamarind (Tamarindus indica) seeds: Scaling for bioreactor and saccharification profile of sugarcane bagasse. Microorganisms 2021, 9, 533. [Google Scholar] [CrossRef]
- de Lucas, R.C.; de Oliveira, T.B.; Lima, M.S.; Pasin, T.M.; Scarcella, A.S.A.; Ribeiro, L.F.C.; Carvalho, C.; Damasio, A.R..L.; Buckeridge, M.S.; Prade, R.A.; et al. The profile secretion of Aspergillus clavatus: Different pre-treatments of sugarcane bagasse distinctly induces holocellulases for the lignocellulosic biomass conversion into sugar. Renew. Energy 2020, 165, 748–757. [Google Scholar] [CrossRef]
- De Lucas, R.C.; De Oliveira, T.B.; Lima, M.S.; Pasin, T.; Scarcella, A.S.A.; Prade, R.A.; Segato, F.; Polizeli, M.L.T.M. Effect of enzymatic pretreatment of sugarcane bagasse with recombinant hemicellulases and esterase prior to the application of the cellobiohydrolase CBH I Megazyme®. Biomass Convers. Biorefinery 2020, 1–9. [Google Scholar] [CrossRef]
- Silva, J.C.R.; Salgado, J.C.S.; Vici, A.C.; Ward, R.J.; Polizeli, M.L.T.M.; Guimarães, L.H.S.; Furriel, R.P.M.; Jorge, J.A. A novel Trichoderma reesei mutant RP698 with enhanced cellulase production. Braz. J. Microbiol. 2020, 51, 537–545. [Google Scholar] [CrossRef]
- Scarcella, A.S.A.; Somera, A.F.; Nunes, C.D.C.C.; Gomes, E.; Vici, A.C.; Buckeridge, M.S.; Polizeli, M.L.T.M. Matrix discriminant analysis evidenced surface-lithium as an important factor to increase the hydrolytic saccharification of sugarcane bagasse. Molecules 2019, 24, 3614. [Google Scholar] [CrossRef] [Green Version]
- Scarcella, A.S.A.; Pasin, T.M.; de Oliveira, T.B.; de Lucas, R.C.; Ferreira-Nozawa, M.S.; Freitas, E.N.; Vici, A.C.; Buckeridge, M.S.; Michelin, M.; Polizeli, M.L.T.M. Saccharification of different sugarcane bagasse varieties by enzymatic cocktails produced by Mycothermus thermophilus and Trichoderma reesei RP698 cultures in agro-industrial residues. Energy 2021, 226, 120360. [Google Scholar] [CrossRef]
- Michelin, M.; Ruiz, H.A.; Polizeli, M.L.T.M.; Teixeira, J. Multi-step approach to add value to corncob: Production of biomass-degrading enzymes, lignin and fermentable sugars. Bioresour. Technol. 2018, 247, 582–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, V.E.; Horváth, I.S.; Lundin, M.; Polizeli, M.L.T.M. Screening and cocktail optimization using experimental mixture design: Enzymatic saccharification as a biological pretreatment strategy. Biofuels Bioprod. Biorefining 2021, 15, 1447–1460. [Google Scholar] [CrossRef]
- Pasin, T.M.; Scarcella, A.S.A.; de Oliveira, T.B.; Lucas, R.C.; Cereia, M.; Betini, J.H.; Polizeli, M.L.T.M. Paper industry wastes as carbon sources for aspergillus species Cultivation and production of an enzymatic cocktail for biotechnological applications. Ind. Biotechnol. 2020, 16, 56–60. [Google Scholar] [CrossRef]
- Pasin, T.; Salgado, J.C.S.; Scarcella, A.S.A.; De Oliveira, T.B.; De Lucas, R.C.; Cereia, M.; Rosa, J.C.; Ward, R.J.; Buckeridge, M.S.; Polizeli, M.L.T.M. A halotolerant endo-1,4-β-xylanase from Aspergillus clavatus with potential application for agroindustrial residues saccharification. Appl. Biochem. Biotechnol. 2020, 191, 1111–1126. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, A.; Connors, S.L.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.I.; et al. IPCC, Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Perkins-Kirkpatrick, S.E.; Lewis, S.C. Increasing trends in regional heatwaves. Nat. Commun. 2020, 11, 3357. [Google Scholar] [CrossRef]
- Coumou, D.; Rahmstorf, S. A decade of weather extremes. Nat. Clim. Chang. 2012, 2, 491–496. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef] [Green Version]
- Hubbe, A.; Hubbe, M. Current climate change and the future of life on the planet. Front. Young Minds 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Feeley, K.J.; Bravo-Avila, C.; Fadrique, B.; Perez, T.M.; Zuleta, D. Climate-driven changes in the composition of New World plant communities. Nat. Clim. Chang. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Steffen, W.; Richardson, K.; Rockström, J.; Cornell, S.E.; Fetzer, I.; Bennett, E.M.; Biggs, R.; Carpenter, S.R.; De Vries, W.; De Wit, C.A.; et al. Planetary boundaries: Guiding human development on a changing planet. Science 2015, 347, 1259855. [Google Scholar] [CrossRef] [Green Version]
- Gatti, L.V.; Basso, L.S.; Miller, J.B.; Gloor, M.; Domingues, L.G.; Cassol, H.L.G.; Tejada, G.; Aragão, L.E.O.C.; Nobre, C.; Peters, W.; et al. Amazonia as a carbon source linked to deforestation and climate change. Nat. Cell Biol. 2021, 595, 388–393. [Google Scholar] [CrossRef]
- Habermann, E.; de Oliveira, E.A.D.; Delvecchio, G.; Belisário, R.; Barreto, R.F.; Viciedo, D.O.; Rossingnoli, N.O.; Costa, K.A.D.P.; Prado, R.D.M.; Gonzalez-Meler, M.; et al. How does leaf physiological acclimation impact forage production and quality of a warmed managed pasture of Stylosanthes capitata under different conditions of soil water availability? Sci. Total Environ. 2021, 759, 143505. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A. The response of photosynthesis and stomatal conductance to rising [CO2]: Mechanisms and environmental interactions. Plant Cell Environ. 2007, 30, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Streck, N.A. Climate change and agroecosystems: The effect of elevated atmospheric CO2 and temperature on crop growth, development, and yield. Ciência Rural 2005, 35, 730–740. [Google Scholar] [CrossRef] [Green Version]
- Habermann, E.; Martin, J.A.B.S.; Contin, D.R.; Bossan, V.P.; Barboza, A.; Braga, M.R.; Groppo, M.; Martinez, C.A. Increasing atmospheric CO2 and canopy temperature induces anatomical and physiological changes in leaves of the C4 forage species Panicum maximum. PLoS ONE 2019, 14, e0212506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Souza, A.; Gaspar, M.; Da Silva, E.A.; Ulian, E.C.; Waclawovsky, A.J.; Nishiyama, M.Y.N., Jr.; Dos Santos, R.V.; Teixeira, M.M.; Souza, G.; Buckeridge, M. Elevated CO2 increases photosynthesis, biomass and productivity, and modifies gene expression in sugarcane. Plant Cell Environ. 2008, 31, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Wedow, J.M.; Yendrek, C.R.; Mello, T.R.; Creste, S.; Martinez, C.; Ainsworth, E.A. Metabolite and transcript profiling of Guinea grass (Panicum maximum Jacq) response to elevated [CO2] and temperature. Metabolomics 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habermann, E.; De Oliveira, E.A.D.; Contin, D.R.; Martin, J.A.B.S.; Curtarelli, L.; Gonzalez-Meler, M.; Martinez, C.A. Stomatal Development and conductance of a tropical forage legume are regulated by elevated [CO2] under moderate warming. Front. Plant Sci. 2019, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- AbdElgawad, H.; Vignolo, E.R.F.; de Vos, D.; Asard, H. Elevated CO2 mitigates drought and temperature-induced oxidative stress differently in grasses and legumes. Plant Sci. 2015, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Sage, R.F.; Way, D.A.; Kubien, D.S. Rubisco, Rubisco activase, and global climate change, J. Exp. Bot. 2008, 59, 1581–1595. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. J. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Carvalho, J.M.; Barreto, R.F.; Prado, R.D.M.; Habermann, E.; Branco, R.; Martinez, C. Elevated CO2 and warming change the nutrient status and use efficiency of Panicum maximum Jacq. PLoS ONE 2020, 15, e0223937. [Google Scholar] [CrossRef] [Green Version]
- Leakey, A.; Ainsworth, E.A.; Bernacchi, C.; Rogers, A.; Long, S.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Leakey, A.D.; Uribelarrea, M.; Ainsworth, E.A.; Naidu, S.L.; Rogers, A.; Ort, D.R.; Long, S. Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of Co2 concentration in the absence of drought. Plant Physiol. 2006, 140, 779–790. [Google Scholar] [CrossRef] [Green Version]
- Leakey, A.D.B.; Bernacchi, C.J.; Dohleman, F.G.; Ort, D.R.; Long, S.P. Will photosynthesis of maize (Zea mays ) in the US Corn Belt increase in future [CO2 ] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob. Chang. Biol. 2004, 10, 951–962. [Google Scholar] [CrossRef]
- Fay, P.A.; Polley, H.; Jin, V.L.; Aspinwall, M. Productivity of well-watered Panicum virgatum does not increase with CO2 enrichment. J. Plant Ecol. 2012, 5, 366–375. [Google Scholar] [CrossRef] [Green Version]
- Vera, U.R.; Siebers, M.; Gray, S.B.; Drag, D.W.; Rosenthal, D.M.; Kimball, B.A.; Ort, D.R.; Bernacchi, C.J. Global warming can negate the expected Co2 stimulation in photosynthesis and productivity for soybean grown in the midwestern United States. Plant Physiol. 2013, 162, 410–423. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, E.D.; Bramley, H.; Siddique, K.; Henty, S.; Berger, J.; Palta, J.A. Can elevated CO2 combined with high temperature ameliorate the effect of terminal drought in wheat? Funct. Plant Biol. 2013, 40, 160–171. [Google Scholar] [CrossRef]
- De Oliveira, E.A.D.; Siddique, K.; Bramley, H.; Stefanova, K.; Palta, J.A. Response of wheat restricted-tillering and vigorous growth traits to variables of climate change. Glob. Chang. Biol. 2014, 21, 857–873. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doman, L.E.; Vipin, A.; Singer, L.E.; Zaretskaya, V.; Jones, A.; Huetteman, T.; Bowman, M.; Slater-Thompson, N.; Hojjati, B.; Peterson, D.; et al. International Energy Outlook 2016; U.S. Energy Information Administration: Washington, DC, USA, 2016.
- De Bhowmick, G.; Sarmah, A.K.; Sen, R. Lignocellulosic biorefinery as a model for sustainable development of biofuels and value added products. Bioresour. Technol. 2018, 247, 1144–1154. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for biorefineries: A review of common methods for efficient utilisation of lignocellulosic materials. Biotechnol. Biofuels 2019, 12, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bio-Based Chemicals. Value Added Products from Biorefineries. Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Biobased-Chemicals-value-added-products-from-biorefineries.pdf (accessed on 3 November 2021).
- Fitzpatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef] [PubMed]
- Wagemann, K.; Tippkötter, N. A short introduction. In Biorefineries; Springer: Berlin/Heidelberg, Germany, 2018; Volume 166, pp. 1–11. [Google Scholar]
- Biofuel International, “Next Generation Enzymes”. Available online: https://www.dyadic.com/wp-content/uploads/2014/11/Next-Generation-Enzymes.pdf (accessed on 4 November 2021).
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic biomass: Hurdles and challenges in its valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gent, S.; Twedt, M.; Gerometta, C.; Almberg, E. Introduction to Feedstocks. In Theoretical and Applied Aspects of Biomass Torrefaction; Butterworth-Heinemann: Burlington, MA, USA, 2017; pp. 17–39. [Google Scholar]
- Brown, T.; Wright, M.; Román-Leshkov, Y.; Brown, R. Techno-economic assessment (TEA) of advanced biochemical and thermochemical biorefineries. In Advances in Biorefineries; Woodhead Publishing: Sawston, UK, 2014; pp. 34–66. [Google Scholar]
- Lima, A.M.; Gomez, L.D.; Steele-King, C.G.; Simister, R.; Bernardinelli, O.D.; Carvalho, A.M.; Rezende, A.C.; Labate, A.C.; Deazevedo, E.R.; McQueen-Mason, S.J.; et al. Evaluating the composition and processing potential of novel sources of Brazilian biomass for sustainable biorenewables production. Biotechnol. Biofuels 2014, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Anderson, E.K.; Voigt, T.B.; Kim, S.; Lee, D. Determining effects of sodicity and salinity on switchgrass and prairie cordgrass germination and plant growth. Ind. Crop. Prod. 2015, 64, 79–87. [Google Scholar] [CrossRef]
- Sosa, L.L.; Jozami, E.; Oakley, L.J.; Montero, G.A.; Ferreras, L.A.; Venturi, G.; Feldman, S.R. Using C4 perennial range land grasses for bioenergy. Biomass Bioenergy 2019, 128, 105299. [Google Scholar] [CrossRef]
- Brandt, A.; Gräsvik, J.; Hallett, J.P.; Welton, T. Deconstruction of lignocellulosic biomass with ionic liquids. Green Chem. 2013, 15, 550–583. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chundawat, S.P.; Beckham, G.T.; Himmel, M.E.; Dale, B.E. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.; Finger-Teixeira, A.; Mota, T.; Salvador, V.H.; Moreira-Vilar, F.C.; Molinari, H.B.C.; Mitchell, R.; Marchiosi, R.; Ferrarese-Filho, O.; Dos Santos, W.D. Ferulic acid: A key component in grass lignocellulose recalcitrance to hydrolysis. Plant Biotechnol. J. 2014, 13, 1224–1232. [Google Scholar] [CrossRef]
- Hong, J.; Ye, A.X.; Zhang, Y.-H.P. quantitative determination of cellulose accessibility to cellulase based on adsorption of a nonhydrolytic fusion protein containing CBM and GFP with its applications. Langmuir 2007, 23, 12535–12540. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, E.C. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2011, 2, 421–449. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass–An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Adsul, M.; Sandhu, S.K.; Singhania, R.R.; Gupta, R.; Puri, S.K.; Mathur, A. Designing a cellulolytic enzyme cocktail for the efficient and economical conversion of lignocellulosic biomass to biofuels. Enzym. Microb. Technol. 2020, 133, 109442. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Raghavi, S.; Sindhu, R.; Binod, P.; Gnansounou, E.; Pandey, A. Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour. Technol. 2016, 199, 202–210. [Google Scholar] [CrossRef]
- Lavoie, J.-M.; Capek-Menard, E.; Gauvin, H.; Chornet, E. Production of pulp from Salix viminalis energy crops using the FIRSST process. Bioresour. Technol. 2010, 101, 4940–4946. [Google Scholar] [CrossRef]
- Kong, W.; Fu, X.; Wang, L.; Alhujaily, A.; Zhang, J.; Ma, F.; Zhang, X.; Yu, H. A novel and efficient fungal delignification strategy based on versatile peroxidase for lignocellulose bioconversion. Biotechnol. Biofuels 2017, 10, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, H.; Chang, Y.; Lee, D.-J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef]
- Li, M.-F.; Yang, S.; Sun, R.-C. Recent advances in alcohol and organic acid fractionation of lignocellulosic biomass. Bioresour. Technol. 2016, 200, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Bai, F.; Zhao, X. Overproduction of cellulase by Trichoderma reesei RUT C30 through batch-feeding of synthesized low-cost sugar mixture. Bioresour. Technol. 2016, 216, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, R.; Winters, A.; Bryant, D.N.; Bosch, M.; Clifton-Brown, J.; Leak, D.; Gallagher, J. Pilot-scale production of xylo-oligosaccharides and fermentable sugars from Miscanthus using steam explosion pretreatment. Bioresour. Technol. 2020, 296, 122285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wan, C. Ultrafast fractionation of lignocellulosic biomass by microwave-assisted deep eutectic solvent pretreatment. Bioresour. Technol. 2018, 250, 532–537. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep eutectic solvent pretreatment enabling full utilization of switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef]
- Santos, C.C.; de Souza, W.; ’Anna, C.S.; Brienzo, M. Elephant grass leaves have lower recalcitrance to acid pretreatment than stems, with higher potential for ethanol production. Ind. Crop. Prod. 2018, 111, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Toscan, A.; Morais, A.; Paixão, S.M.; Alves, S.P.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. High-pressure carbon dioxide/water pre-treatment of sugarcane bagasse and elephant grass: Assessment of the effect of biomass composition on process efficiency. Bioresour. Technol. 2017, 224, 639–647. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chen, X.; Shao, Q.; Qin, W. Pretreatment of Miscanthus with biomass-degrading bacteria for increasing delignification and enzymatic hydrolysability. Microb. Biotechnol. 2019, 12, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Marques, S.; Alves, L.; Roseiro, J.; Gírio, F. Conversion of recycled paper sludge to ethanol by SHF and SSF using Pichia stipitis. Biomass Bioenergy 2008, 32, 400–406. [Google Scholar] [CrossRef]
- Soleimani, S.; Ranaei-Siadat, S.-O. Preparation and optimization of cellulase cocktail to improve the bioethanol process. Biofuels 2017, 8, 291–296. [Google Scholar] [CrossRef]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Kumar, A. Biomass breakdown A review on pretreatment instrumentations and methods. Front. Biosci. 2018, 10, 155–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polizeli, M.L.T.M.; Vici, A.; Scarcella, A.; Cereia, M.; Pereira, M. Enzyme System from Aspergillus in Current Industrial Uses and Future Applications in the Production of Second-Generation Ethanol; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 127–140. [Google Scholar]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Heinen, P.R.; Bauermeister, A.; Ribeiro, L.; Messias, J.; Almeida, P.; Moraes, L.A.; Vargas-Rechia, C.; de Oliveira, A.; Ward, R.; Filho, E.; et al. GH11 xylanase from Aspergillus tamarii Kita: Purification by one-step chromatography and xylooligosaccharides hydrolysis monitored in real-time by mass spectrometry. Int. J. Biol. Macromol. 2018, 108, 291–299. [Google Scholar] [CrossRef]
- Carapito, R.; Imberty, A.; Jeltsch, J.-M.; Byrns, S.C.; Tam, P.-H.; Lowary, T.; Varrot, A.; Phalip, V. molecular basis of arabinobio-hydrolase activity in phytopathogenic fungi. J. Biol. Chem. 2009, 284, 12285–12296. [Google Scholar] [CrossRef] [Green Version]
- Saha, B.C. α-l-Arabinofuranosidases: Biochemistry, molecular biology and application in biotechnology. Biotechnol. Adv. 2000, 18, 403–423. [Google Scholar] [CrossRef]
- Nasseri, S.A.; Betschart, L.; Opaleva, D.; Rahfeld, P.; Withers, S.G. A Mechanism-Based Approach to Screening Metagenomic Libraries for Discovery of Unconventional Glycosidases. Angew. Chem. 2018, 130, 11529–11534. [Google Scholar] [CrossRef]
- Pasin, T.M.; de Almeida, P.Z.; Scarcella, A.S.D.A.; Infante, J.D.C.; Polizeli, M.L.D.T.M. Bioconversion of agro-industrial residues to second-generation bioethanol. In Biorefinery of Alternative Resources: Targeting Green Fuels and Platform Chemicals; Springer: Berlin/Heidelberg, Germany, 2020; pp. 23–47. [Google Scholar]
- Haddad Momeni, M.; Fredslund, F.; Bissaro, B.; Raji, O.; Vuong, T.V.; Meier, S.; Nielsen, T.S.; Lombard, V.; Guigliarelli, B.; Biaso, F.; et al. Discovery of fungal oligosaccharide-oxidising flavo-enzymes with previously unknown substrates, redox-activity profiles and interplay with LPMOs. Nat. Commun. 2021, 12, 2132. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Chylenski, P.; Bissaro, B.; Eijsink, V.G.H.; Horn, S.J. The impact of hydrogen peroxide supply on LPMO activity and overall saccharification efficiency of a commercial cellulase cocktail. Biotechnol. Biofuels 2018, 11, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arantes, V.; Pribowo, A.; Gourlay, K.; Saddler, J.N. Substrate factors that influence the synergistic interaction of AA9 and cellulases during the enzymatic hydrolysis of biomass. Energy Environ. Sci. 2014, 7, 2308–2315. [Google Scholar] [CrossRef]
- Biely, P.; Mastihubová, M.; Tenkanen, M.; Eyzaguirre, J.; Li, X.-L.; Vršanská, M. Action of xylan deacetylating enzymes on monoacetyl derivatives of 4-nitrophenyl glycosides of β-D-xylopyranose and α-l-arabinofuranose. J. Biotechnol. 2011, 151, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.-M.; Tao, H.; Liu, Y.-Y.; Wang, Y.-D.; Zhang, J.-R.; Tang, A.-X. A novel non-hydrolytic protein from Pseudomonas oryzihabitans enhances the enzymatic hydrolysis of cellulose. J. Biotechnol. 2013, 168, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.M.; Elshahed, M.S.; Youssef, N.H. Defined enzyme cocktail from the anaerobic fungus Orpinomyces sp. strain C1A effectively releases sugars from pretreated corn stover and switchgrass. Sci. Rep. 2016, 6, 29217. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Yu, X.; Sun, Y.; Wang, G.; Chen, H.; Chen, G. Evaluation of screened lignin-degrading fungi for the biological pretreatment of corn stover. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliva-Taravilla, A.; Tomás-Pejó, E.; Demuez, M.; González-Fernández, C.; Ballesteros, M. Inhibition of cellulose enzymatic hydrolysis by laccase-derived compounds from phenols. Biotechnol. Prog. 2015, 31, 700–706. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.-G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Challinor, A.; Watson, J.; Lobell, D.; Howden, S.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Ferreira, T.H.S.; Tsunada, M.S.; Bassi, D.; Araujo, P.; Mattiello, L.; Guidelli, G.V.; Righetto, G.L.; Gonçalves, V.R.; Lakshmanan, P.; Menossi, M. Sugarcane water stress tolerance mechanisms and its implications on developing biotechnology solutions. Front. Plant Sci. 2017, 8, 1077. [Google Scholar] [CrossRef]
- Anwar, K.; Joshi, R.; Dhankher, O.; Singla-Pareek, S.; Pareek, A. Elucidating the response of crop plants towards individual, combined and sequentially occurring abiotic stresses. Int. J. Mol. Sci. 2021, 22, 6119. [Google Scholar] [CrossRef]
- Prasad, V.B.R.; Govindaraj, M.; Djanaguiraman, M.; Djalovic, I.; Shailani, A.; Rawat, N.; Singla-Pareek, S.L.; Pareek, A.; Prasad, P.V.V. Drought and high temperature stress in sorghum: Physiological, genetic, and molecular insights and breeding approaches. Int. J. Mol. Sci. 2021, 22, 9826. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Sicher, R.C.; Bunce, J.A. The Impact of Enhanced Atmospheric Co2 concentrations on the responses of maize and soybean to elevated growth temperatures. In Combined Stresses in Plants; Springer: Berlin/Heidelberg, Germany, 2014; pp. 27–48. [Google Scholar]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morpho-physiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Habermann, E.; De Oliveira, E.A.D.; Contin, D.R.; DelVecchio, G.; Viciedo, D.O.; De Moraes, M.A.; Prado, R.D.M.; Costa, K.; Braga, M.R.; Martinez, C.A. Warming and water deficit impact leaf photosynthesis and decrease forage quality and digestibility of a C4 tropical grass. Physiol. Plant. 2019, 165, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.H.B.D.A.; De Camargo-Bortolin, L.H.G.; Castro, É.; Martínez, C.A. Leaf dynamics of Panicum maximum under future climatic changes. PLoS ONE 2016, 11, e0149620. [Google Scholar] [CrossRef]
- Dias de Oliveira, E.A.; Manchon, F.T.; Ricketts, M.P.; Bianconi, M.; Martinez, C.A.; Gonzalez-Meler, M. Plant diurnal cycle drives the variation in soil respiration in a C4-dominated tropical managed grassland exposed to high CO2 and warming. Plant Soil 2020, 456, 391–404. [Google Scholar] [CrossRef]
- Looby, C.I.; Treseder, K.K. Shifts in soil fungi and extracellular enzyme activity with simulated climate change in a tropical montane cloud forest. Soil Biol. Biochem. 2018, 117, 87–96. [Google Scholar] [CrossRef]
- Schmidt, P.-A.; Schmitt, I.; Otte, J.; Bandow, C.; Römbke, J.; Bálint, M.; Rolshausen, G. Season-long experimental drought alters fungal community composition but not diversity in a grassland soil. Microb. Ecol. 2017, 75, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Solly, E.F.; Lindahl, B.D.; Dawes, M.A.; Peter, M.; Souza, R.C.; Rixen, C.; Hagedorn, F. Experimental soil warming shifts the fungal community composition at the alpine treeline. New Phytol. 2017, 215, 766–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, T.B.; De Lucas, R.C.; Scarcella, A.S.A.; Contato, A.G.; Pasin, T.; Martinez, C.; Polizeli, M.L.T.M. Fungal communities differentially respond to warming and drought in tropical grassland soil. Mol. Ecol. 2020, 29, 1550–1559. [Google Scholar] [CrossRef]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Matić, S.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emergence of leaf spot disease on leafy vegetable and ornamental crops caused by Paramyrothecium and Albifimbria Species. Phytopathology 2019, 109, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, T.B.; De Lucas, R.C.; Scarcella, A.S.A.; Pasin, T.; Martinez, C.; Polizeli, M.L.T.M. Perspectives on exploring denitrifying fungi as a model to evaluate nitrous oxide production and reduce emissions from agricultural soils. J. Agric. Food Chem. 2019, 67, 12153–12154. [Google Scholar] [CrossRef] [PubMed]
- Viciedo, D.O.; Prado, R.D.M.; Martinez, C.; Habermann, E.; Piccolo, M.D.C. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.M.; Mota, T.R.; Salatta, F.V.; Sinzker, R.C.; Končitíková, R.; Kopečný, D.; Simister, R.; Silva, M.; Goeminne, G.; Morreel, K.; et al. Cell wall remodeling under salt stress: Insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020, 43, 2172–2191. [Google Scholar] [CrossRef] [PubMed]
- Warming, G. Plant cell walls tackling climate change: Biotechnological strategies to improve crop adaptations and photosynthesis in response to global warming. Plants 2020, 9, 212. [Google Scholar] [CrossRef] [Green Version]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; McFarlane, H.E.; Persson, S. The impact of abiotic factors on cellulose synthesis. J. Exp. Bot. 2015, 67, 543–552. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.N.; Khatri, V.; Wu, J.; Takada, M.; de Scarcella, A.S.; Martinez, C.A.; Saddler, J.N.; Polizeli, M.L.T.M. Structural and compositional changes induced by hydrothermal and organosolv pretreatments impacts enzymatic hydrolysis of a tropical forage grass grown under future climate conditions. Ind. Crop. Prod. 2021, 171, 113937. [Google Scholar] [CrossRef]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Santos, T.; Vieira, L.G.E.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.B.; Petkowicz, C. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.). Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.N.; Khatri, V.; Contin, D.; Oliveira, T.; Contato, A.G.; Peralta, R.M.; dos Santos, W.D.; Martínez, C.A.; Saddler, J.; Polizeli, M.L.T.M. Climate change affects cell wall structure and hydrolytic performance of a tropical forage grass as an energy crop. Biofuels Bioprod. Biorefining 2021. [Google Scholar] [CrossRef]
- Rakszegi, M.; Lovegrove, A.; Balla, K.; Láng, L.; Bedő, Z.; Veisz, O.; Shewry, P.R. Effect of heat and drought stress on the structure and composition of arabinoxylan and β-glucan in wheat grain. Carbohydr. Polym. 2014, 102, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, R.D.; Rancour, D.M.; Marita, J.M. Grass cell walls: A story of cross-linking. Front. Plant Sci. 2017, 7, 2056. [Google Scholar] [CrossRef] [Green Version]
- Freitas, E.N.; Alnoch, R.C.; Contato, A.G.; Nogueira, K.M.V.; Crevelin, E.J.; de Moraes, L.A.B.; Silva, R.N.; Martínez, C.A.; Polizeli, M.L.T.M. Enzymatic pretreatment with laccases from Lentinus sajor-caju induces structural modification in lignin and enhances the digestibility of tropical forage grass (Panicum maximum) grown under future climate conditions. Int. J. Mol. Sci. 2021, 22, 9445. [Google Scholar] [CrossRef]
- Lee, M.A.; Davis, A.P.; Chagunda, M.G.G.; Manning, P. Forage quality declines with rising temperatures, with implications for livestock production and methane emissions. Biogeosciences 2017, 14, 1403–1417. [Google Scholar] [CrossRef] [Green Version]
- Moura-Sobczak, J.; Souza, U.; Mazzafera, P. Drought stress and changes in the lignin content and composition in Eucalyptus. BMC Proc. 2011, 5, P103. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Lee, B.-H.; Dellinger, M.; Cui, X.; Zhang, C.; Wu, S.; Nothnagel, E.A.; Zhu, J.-K. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 2010, 63, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Le Gall, H.; Philippe, F.; Domon, J.-M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell wall metabolism in response to abiotic stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.; Chowdhury, J.; Shirley, N.; Little, A. The plant cell wall: A complex and dynamic structure as revealed by the responses of genes under stress conditions. Front. Plant Sci. 2016, 7, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persia, D.; Cai, G.; Del Casino, C.; Faleri, C.; Willemse, M.T.; Cresti, M. Sucrose synthase is associated with the cell wall of tobacco pollen tubes. Plant Physiol. 2008, 147, 1603–1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Yañez, Á.; Beltrán, D.; Campano-Romero, C.; Molinett, S.; Herrera, R.; Moya-León, M.A.; Morales-Quintana, L. Glycosylation is important for FcXTH1 activity as judged by its structural and biochemical characterization. Plant Physiol. Biochem. 2017, 119, 200–210. [Google Scholar] [CrossRef]
- Campbell, P.; Braam, J. In vitro activities of four xyloglucan endotransglycosylases from Arabidopsis. Plant J. 1999, 18, 371–382. [Google Scholar] [CrossRef]
- Guillaumie, S.; Goffner, D.; Barbier, O.; Martinant, J.-P.; Pichon, M.; Barrière, Y. Expression of cell wall related genes in basal and ear internodes of silking brown-midrib-3, caffeic acid O-methyltransferase (COMT) down-regulated, and normal maize plants. BMC Plant Biol. 2008, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chantreau, M.; Sibout, R.; Hawkins, S. Plant cell wall lignification and monolignol metabolism. Front. Plant Sci. 2013, 4, 220. [Google Scholar] [CrossRef] [Green Version]
- Cass, C.L.; Peraldi, A.; Dowd, P.F.; Mottiar, Y.; Santoro, N.; Karlen, S.D.; Bukhman, Y.V.; Foster, C.E.; Thrower, N.; Bruno, L.C.; et al. Effects of phenylalanine ammonia lyase(pal) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses inBrachypodium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahinnia, F.; Carrillo, N.; Hajirezaei, M.-R. Engineering climate-change-resilient crops: New tools and approaches. Int. J. Mol. Sci. 2021, 22, 7877. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, E.N.d.; Salgado, J.C.S.; Alnoch, R.C.; Contato, A.G.; Habermann, E.; Michelin, M.; Martínez, C.A.; Polizeli, M.d.L.T.M. Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario. Biology 2021, 10, 1277. https://doi.org/10.3390/biology10121277
Freitas ENd, Salgado JCS, Alnoch RC, Contato AG, Habermann E, Michelin M, Martínez CA, Polizeli MdLTM. Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario. Biology. 2021; 10(12):1277. https://doi.org/10.3390/biology10121277
Chicago/Turabian StyleFreitas, Emanuelle Neiverth de, José Carlos Santos Salgado, Robson Carlos Alnoch, Alex Graça Contato, Eduardo Habermann, Michele Michelin, Carlos Alberto Martínez, and Maria de Lourdes T. M. Polizeli. 2021. "Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario" Biology 10, no. 12: 1277. https://doi.org/10.3390/biology10121277
APA StyleFreitas, E. N. d., Salgado, J. C. S., Alnoch, R. C., Contato, A. G., Habermann, E., Michelin, M., Martínez, C. A., & Polizeli, M. d. L. T. M. (2021). Challenges of Biomass Utilization for Bioenergy in a Climate Change Scenario. Biology, 10(12), 1277. https://doi.org/10.3390/biology10121277








