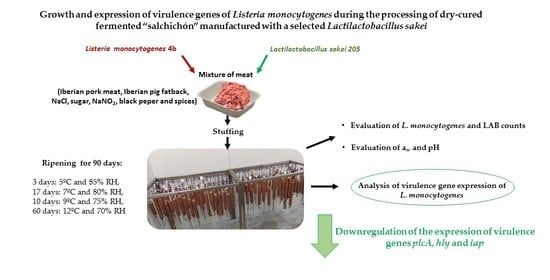
Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
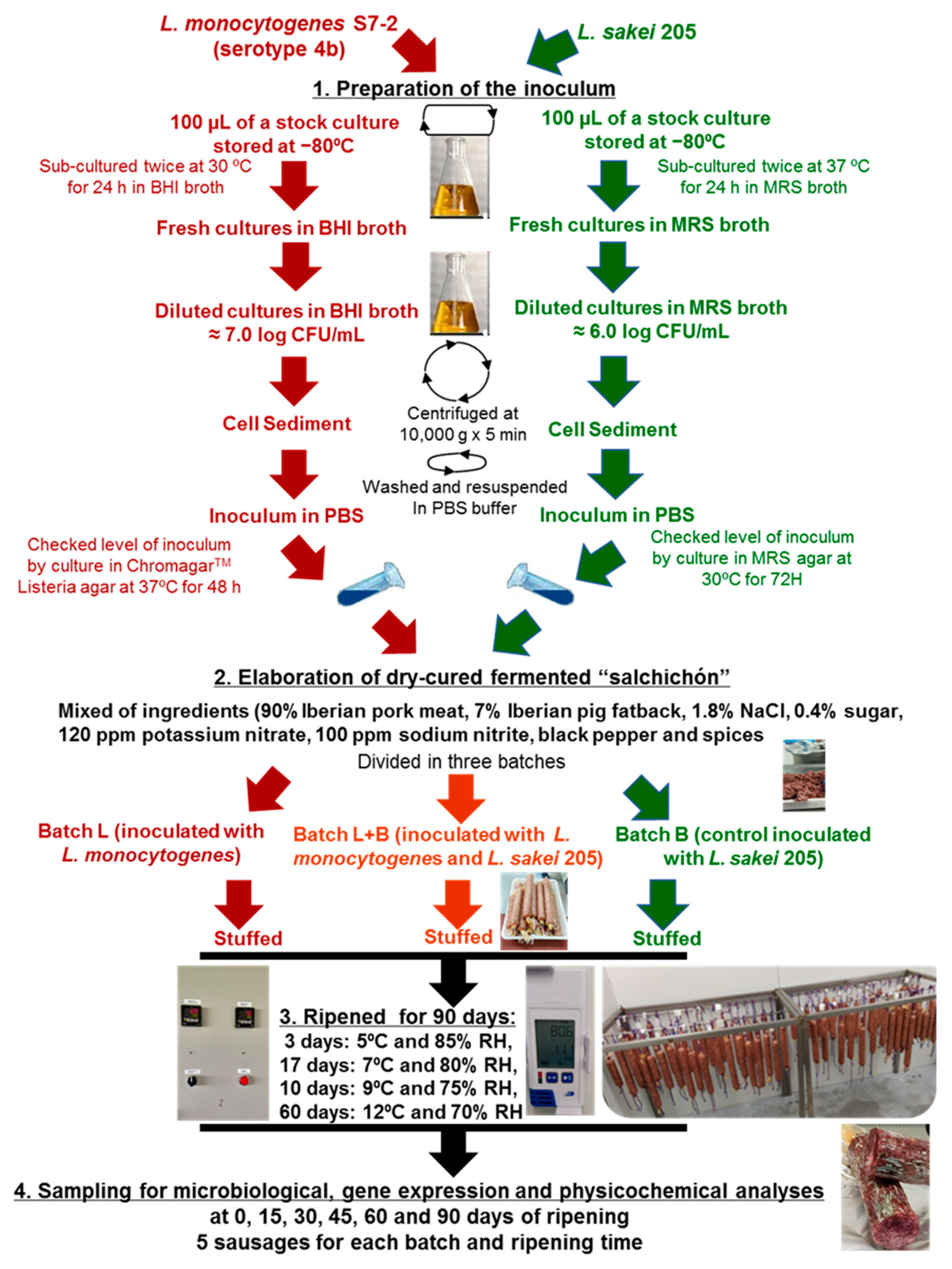
2.1. Bacterial and Culture Conditions
2.2. Elaboration of Dry-Cured Fermented “Salchichón”
2.3. Microbiological Analysis
2.4. RNA Extraction and Gene Expression Assay
2.5. Physicochemical Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Evolution of Water Activity and pH during Ripening of “Salchichón”
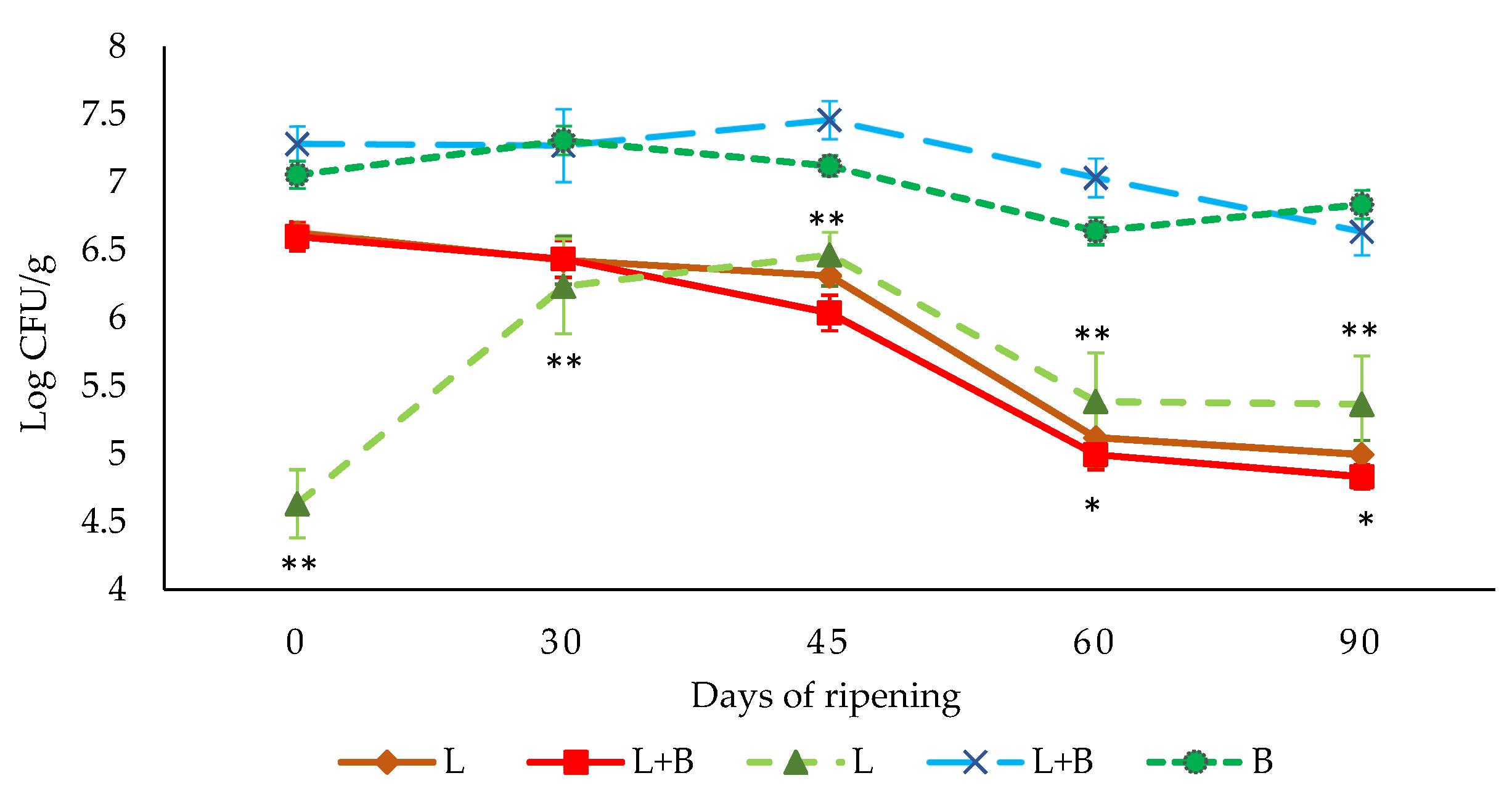
3.2. Evolution of Lactic-Acid Bacteria and L. monocytogenes Counts throughout the Ripening Process of “Salchichón”
3.3. Effect of Processing and Presence of L. sakei on the Absolute Transcription Levels of L. monocytogenes Virulence Genes in Dry-Cured Fermented Sausages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filipello, V.; Gallina, S.; Amato, E.; Losio, M.N.; Pontello, M.; Decastelli, L.; Lomonaco, S. Diversity and persistence of Listeria monocytogenes within the Gorgonzola PDO production chain and comparison with clinical isolates from the same area. Int. J. Food Microbiol. 2017, 245, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kathariou, S. Listeria monocytogenes virulence and pathogenicity, a food safety perspective. J. Food Prot. 2002, 65, 1811–1829. [Google Scholar] [CrossRef] [PubMed]
- Alía, A.; Andrade, M.J.; Rodríguez, A.; Martín, I.; Pérez-Baltar, A.; Medina, M.; Córdoba, J.J. Prevalence and characterization of Listeria monocytogenes in deboning and slicing areas of Spanish dry-cured ham processing. LWT-Food Sci. Technol. 2020, 128, 109498. [Google Scholar] [CrossRef]
- Samelis, J.; Bedie, G.K.; Sofos, J.N.; Belk, K.E.; Scanga, J.A.; Smith, G.C. Control of Listeria monocytogenes with combined antimicrobials after postprocess contamination and extended storage of frankfurters at 4 °C in vacuum packages. J. Food Prot. 2002, 65, 299–307. [Google Scholar] [CrossRef]
- EURL Lm Technical Guidance Document on Challenge Test and Durability Studies for Assessing Shelf-Life of Ready-to Eat Foods Related to Listeria monocytogenes. 2021. Available online: https://ec.europa.eu/food/system/files/2021-07/biosafety_fh_mc_tech-guide-doc_listeria-in-rte-foods_en_0.pdf (accessed on 28 July 2021).
- Mataragas, M.; Rovetto, F.; Bellio, A.; Alessandria, V.; Rantsiou, K.; Decastelli, L.; Cocolin, L. Differential gene expression profiling of Listeria monocytogenes in cacciatore and felino salami to reveal potential stress resistance biomarkers. Food Microbiol. 2015, 46, 408–417. [Google Scholar] [CrossRef]
- Janßen, D.; Eisenbach, L.; Ehrmann, M.A.; Vogel, R.F. Assertiveness of Lactobacillus sakei and Lactobacillus curvatus in a fermented sausage model. Int. J. Food Microbiol. 2018, 285, 188–197. [Google Scholar] [CrossRef]
- Martín, B.; Jofré, A.; Garriga, M.; Pla, M.; Aymerich, T. Rapid quantitative detection of Lactobacillus sakei in meat and fermented sausages by real-time PCR. Appl. Environ. Microbiol. 2006, 72, 6040–6048. [Google Scholar] [CrossRef] [Green Version]
- Barmpalia-Davis, I.M.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Survival of Listeria monocytogenes in a simulated dynamic gastrointestinal model during storage of inoculated Bologna and Salami slices in vacuum packages. J. Food Prot. 2008, 71, 2014–2023. [Google Scholar] [CrossRef]
- Doménech, E.; Jimenez-Belenguer, A.; Amoros, J.A.; Ferrus, M.A.; Escriche, I. Prevalence and antimicrobial resistance of Listeria monocytogenes and Salmonella strains isolated in ready-to-eat foods in Eastern Spain. Food Control. 2015, 47, 120–125. [Google Scholar] [CrossRef]
- Ferreira, V.; Barbosa, J.; Silva, J.; Felício, M.T.; Mena, C.; Hogg, T.; Gibbs, P.; Teixeira, P. Characterisation of alheiras, traditional sausages produced in the North of Portugal, with respect to their microbiological safety. Food Control. 2007, 18, 436–440. [Google Scholar] [CrossRef]
- Meloni, D. Presence of Listeria monocytogenes in mediterranean-style dry fermented sausages. Foods 2015, 4, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Foodborne Ilness Outbreak Database Siena Foods Salame. Available online: http://www.outbreakdatabase.com/details/assi-market-pickles-2010/?vehicle=cucumber (accessed on 26 June 2021).
- Martín, I.; Rodríguez, A.; Sánchez-Montero, L.; Padilla, P.; Córdoba, J.J. Effect of the dry-cured fermented sausage “salchichón” processing with a selected Lactobacillus sakei in Listeria monocytogenes and microbial population. Foods 2021, 10, 856. [Google Scholar] [CrossRef]
- Commission, E. Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuff. Off. J. Eur. Communities 2007, 322, 12–29. [Google Scholar]
- Rolhion, N.; Cossart, P. How the study of Listeria monocytogenes has led to new concepts in biology. Futur. Microbiol. Futur. Med. 2017, 12, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Alía, A.; Rodríguez, A.; Andrade, M.J.; Gómez, F.; Córdoba, J.J. Combined effect of temperature, water activity and salt content on the growth and gene expression of Listeria monocytogenes in a dry-cured ham model system. Meat Sci. 2019, 155, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Hadjilouka, A.; Molfeta, C.; Panagiotopoulou, O.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Expression of Listeria monocytogenes key virulence genes during growth in liquid medium, on rocket and melon at 4, 10 and 30 °C. Food Microbiol. 2016, 55, 7–15. [Google Scholar] [CrossRef]
- Alía, A.; Córdoba, J.J.; Rodríguez, A.; García, C.; Andrade, M.J. Evaluation of the efficacy of Debaryomyces hansenii as protective culture for controlling Listeria monocytogenes in sliced dry-cured ham. LWT 2020, 119, 108886. [Google Scholar] [CrossRef]
- Olesen, I.; Vogensen, F.; Jespersn, L. Gene Transcription and virulence potential of Listeria monocytogenes strains after exposure to acidic and NaCl stress. Foodborne Pathog. Dis. 2009, 6, 669–680. [Google Scholar] [CrossRef]
- Olesen, I.; Thorsen, L.; Jespersen, L. Relative transcription of Listeria monocytogenes virulence genes in liver pâtés with varying NaCl content. Int. J. Food Microbiol. 2010, 141, S60–S68. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.R.; James, K.E.; Callahan, M.C.; Wiedmann, M.; Boor, K.J. Exposure to salt and organic acids increases the ability of Listeria monocytogenes to invade Caco-2 cells but decreases its ability to survive gastric stress. Appl. Environ. Microbiol. 2006, 72, 5384–5395. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Burall, L.; Mammel, M.K.; Datta, A.R. Global transcriptomic response of Listeria monocytogenes during growth on cantaloupe slices. Food Microbiol. 2019, 77, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Wang, C.; Tsai, H.J.; Chou, C.H. Growth of Listeria monocytogenes on a RTE-meat matrix enhances cell invasiveness to mouse J774A.1 macropahges. Int. J. Food Microbiol. 2010, 144, 199–201. [Google Scholar] [CrossRef]
- Upadhyay, A.; Johny, A.K.; Amalaradjou, M.A.R.; Baskaran, S.A.; Kim, K.S.; Venkitanarayanan, K. Plant-derived antimicrobials reduce Listeria monocytogenes virulence factors in vitro, and down-regulate expression of virulence genes. Int. J. Food Microbiol. 2012, 157, 88–94. [Google Scholar] [CrossRef]
- Delgado, J.; Peromingo, B.; Rodríguez, A.; Rodríguez, M. Biocontrol of Penicillium griseofulvum to reduce cyclopiazonic acid contamination in dry-fermented sausages. Int. J. Food Microbiol. 2019, 293, 1–6. [Google Scholar] [CrossRef]
- Mataragas, M.; Alessandria, V.; Rantsiou, K.; Cocolin, L. Evaluation of the Listeria monocytogenes inactivation during post-process storage of fermented sausages: A basis for the development of a decision support tool. Food Control. 2015, 50, 568–573. [Google Scholar] [CrossRef]
- Cano-García, L.; Belloch, C.; Flores, M. Impact of Debaryomyces hansenii strains inoculation on the quality of slow dry-cured fermented sausages. Meat Sci. 2014, 96, 1469–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín, A.; Córdoba, J.J.; Aranda, E.; Córdoba, M.G.; Asensio, M.A. Contribution of a selected fungal population to the volatile compounds on dry-cured ham. Int. J. Food Microbiol. 2006, 110, 8–18. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.-C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef]
- Kurbakov, K.A.; Konorov, E.A.; Minaev, M.Y.; Kuznetsova, O.A. Multiplex real-time PCR with HRM for detection of Lactobacillus sakei and Lactobacillus curvatus in Food Samples. Food Technol. Biotechnol. 2019, 57, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Quijada, N.M.; De Filippis, F.; Sanz, J.J.; García-Fernández, M.D.C.; Rodríguez-Lázaro, D.; Ercolini, D.; Hernández, M. Different Lactobacillus populations dominate in “Chorizo de León” manufacturing performed in different production plants. Food Microbiol. 2018, 70, 94–102. [Google Scholar] [CrossRef]
- Sidira, M.; Mitropoulou, G.; Galanis, A.; Kanellaki, M.; Kourkoutas, Y. Effect of Sugar Content on Quality Characteristics and Shelf-Life of Probiotic Dry-Fermented Sausages Produced by Free or Immobilized Lactobacillus casei ATCC 393. Foods 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobrino, O.J.; Rodríguez, J.M.; Moreira, W.L.; Fernández, M.F.; Sanz, B.; Hernández, P.E. Antibacterial activity of Lactobacillus strains isolated from dry fermented sausages. Int. J. Food Microbiol. 1993, 75, 344–349. [Google Scholar] [CrossRef]
- Lucke, F.K. Utilization of microbes to process and preserve meat. Meat Sci. 2000, 56, 105–115. [Google Scholar] [CrossRef]
- Chaillou, S.; Champomier-Vergès, M.C.; Cornet, M.; Crutz-Le Coq, A.M.; Dudez, A.M.; Martin, V.; Beaufils, S.; Darbon-Rongère, E.; Bossy, R.; Loux, V. The complete genome sequence of the meat-borne lactic acid bacterium Lactobacillus sakei 23K. Nat. Biotechnol. 2005, 23, 1527–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bošković, M.; Tadić, V.; Dordević, J.; Glišić, M.; Lakićević, B.; Dimitrijević, M.; Ž Baltić, M. Effect of starter cultures on survival of Listeria monocytogenes in Čajna sausage. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012074. [Google Scholar] [CrossRef] [Green Version]
- Prpich, N.Z.P.; Garro, O.A.; Romero, M.; Judis, M.A.; Cayré, M.E.; Castro, M.P. Evaluation of an autochthonous starter culture on the production of a traditional dry fermented sausage from Chaco (Argentina) at a small-scale facility. Meat Sci. 2016, 115, 41–44. [Google Scholar] [CrossRef]
- Prpich, N.Z.P.; Castro, M.P.; Cayré, M.E.; Garro, O.A.; Vignolo, G.M. Indigenous starter cultures to improve quality of artisanal dry fermented sausages from chaco (Argentina). Int. J. Food Sci. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mataragas, M.; Greppi, A.; Rantsiou, K.; Cocolin, L. Gene Transcription patterns of pH and salt stressed Listeria monocytogenes cells in simulated gastric and pancreatic conditions. J. Food Prot. 2014, 77, 254–261. [Google Scholar] [CrossRef]
- Beaufort, A.; Cornu, M.; Bergis, H.; Lardeux, A.L.; Lombard, B. EURL Lm Technical Guidance Document for Conducing Shelf-life Studies on Listeria monocytogenes in Ready to Eat Foods. Available online: https://www.fsai.ie/uploadedFiles/EURL%20Lm_Technical%20Guidance%20Document%20Lm%20shelf-life%20studies_V3_2014-06-06%20(2).pdf (accessed on 6 November 2021).
- Alía, A.; Andrade, M.J.; Córdoba, J.J.; Martín, I.; Rodríguez, A. Development of a multiplex real-time PCR to differentiate the four major Listeria monocytogenes serotypes in isolates from meat processing plants. Food Microbiol. 2020, 87, 856. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.B.; Whitney, D. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 2, 347–370. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; Córdoba, M.G. Effect of autochthonous starter cultures in the production of “salchichón”, a traditional Iberian dry-fermented sausage, with different ripening processes. LWT-Food Sci. Technol. 2011, 44, 1562–1571. [Google Scholar] [CrossRef]
- El Adab, S.; Essid, I.; Hassouna, M. Microbiological, biochemical and textural characteristics of a tunisian dry fermented poultry meat sausage inoculated with selected starter cultures. J. Food Saf. 2015, 35, 75–85. [Google Scholar] [CrossRef]
- Aleson-Carbonell, L.; Fernández-López, J.; Pérez-Alvarez, J.A.; Kuri, V. Functional and sensory effects of fibre-rich ingredients on breakfast fresh sausages manufacture. Food Sci. Technol. Int. 2005, 11, 89–97. [Google Scholar] [CrossRef]
- Pérez-Álvarez, J.A.; Sayas-Barberá, M.E.; Fernández-López, J.; Aranda-Catalá, V. Physicochemical characteristics of Spanish-type dry-cured sausage. Food Res. Int. 1999, 32, 599–607. [Google Scholar] [CrossRef]
- Benkerroum, N.; Daoudi, A.; Hamraoui, T.; Ghalfi, H.; Thiry, C.; Duroy, M.; Evrart, P.; Roblain, D.; Thonart, P. Lyophilized preparations of bacteriocinogenic Lactobacillus curvatus and Lactococcus lactis subsp. lactis as potential protective adjuncts to control Listeria monocytogenes in dry-fermented sausages. J. Appl. Microbiol. 2005, 98, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Nolan, D.A.; Chamblin, D.C.; Troller, J.A. Minimal water activity levels for growth and survival of Listeria monocytogenes and Listeria innocua. Int. J. Food Microbiol. 1992, 16, 323–335. [Google Scholar] [CrossRef]
- Vermeulen, A.; Gysemans, K.P.M.; Bernaerts, K.; Geeraerd, A.H.; Van Impe, J.F.; Debevere, J.; Devlieghere, F. Influence of pH, water activity and acetic acid concentration on Listeria monocytogenes at 7 °C: Data collection for the development of a growth/no growth model. Int. J. Food Microbiol. 2007, 114, 332–341. [Google Scholar] [CrossRef]
- Bowman, J.P.; Bittencourt, C.R.; Ross, T. Differential gene expression of Listeria monocytogenes during high hydrostatic pressure processing. Microbiology 2008, 154, 462–475. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Olesen, I.; Andersen, T.; Fang, W.; Jespersen, L. Survival of Listeria monocytogenes in simulated gastrointestinal system and transcriptional profiling of stress- and adhesion-related genes. Foodborne Pathog. Dis. 2010, 7, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, M.; Henriksson, A. The effect of processed meat and meat starter cultures on gastrointestinal colonization and virulence of Listeria monocytogenes in mice. Int. J. Food Microbiol. 2003, 84, 255–261. [Google Scholar] [CrossRef]
- Ye, K.; Zhang, X.; Huang, Y.; Liu, J.; Liu, M.; Zhou, G. Bacteriocinogenic Enterococcus faecium inhibits the virulence property of Listeria monocytogenes. Food Sci. Technol. 2018, 89, 87–92. [Google Scholar] [CrossRef]

| Genes | Batches | Days of Ripening | ||||
|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 60 | 90 | ||
| hly | L | 3.01 ± 0.372 1 | 3.07 ± 0.099 1 | 3.26 ± 0.216 1 | 1.89 ± 0.226 2,b | 1.82 ± 0.370 2 |
| L + B | 3.28 ± 0.132 1,2 | 2.96 ± 0.180 2 | 3.42 ± 0.208 1 | 2.48 ± 0.272 3,a | 1.49 ± 0.316 4 | |
| plcA | L | 4.17 ± 0.286 1 | 4.32 ± 0.282 1,a | 4.41 ± 0.410 1 | 2.47 ± 0.236 2 | 3.06 ± 0.505 2 |
| L + B | 4.01 ± 0.265 1 | 3.74 ± 0.440 1,b | 4.35 ± 0.271 1 | 2.91 ± 0.494 2 | 2.68 ± 0.483 2 | |
| iap | L | 2.72 ± 0.181 1 | 2.69 ± 0.067 1 | 2.82 ± 0.283 1 | 1.65 ± 0.368 2 | 1.96 ± 0.249 2 |
| L + B | 2.92 ± 0.165 1 | 2.70 ± 0.076 1 | 3.16 ± 0.378 1 | 1.66 ± 0.433 2 | 1.53 ± 0.491 2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, I.; Alía, A.; Rodríguez, A.; Gómez, F.; Córdoba, J.J. Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei. Biology 2021, 10, 1258. https://doi.org/10.3390/biology10121258
Martín I, Alía A, Rodríguez A, Gómez F, Córdoba JJ. Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei. Biology. 2021; 10(12):1258. https://doi.org/10.3390/biology10121258
Chicago/Turabian StyleMartín, Irene, Alberto Alía, Alicia Rodríguez, Francisco Gómez, and Juan J. Córdoba. 2021. "Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei" Biology 10, no. 12: 1258. https://doi.org/10.3390/biology10121258
APA StyleMartín, I., Alía, A., Rodríguez, A., Gómez, F., & Córdoba, J. J. (2021). Growth and Expression of Virulence Genes of Listeria monocytogenes during the Processing of Dry-Cured Fermented “Salchichón” Manufactured with a Selected Lactilactobacillus sakei. Biology, 10(12), 1258. https://doi.org/10.3390/biology10121258