An Andrographolide from Helichrysum caespitium (DC.) Sond. Ex Harv., (Asteraceae) and Its Antimicrobial, Antiquorum Sensing, and Antibiofilm Potentials
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Collection and Identification
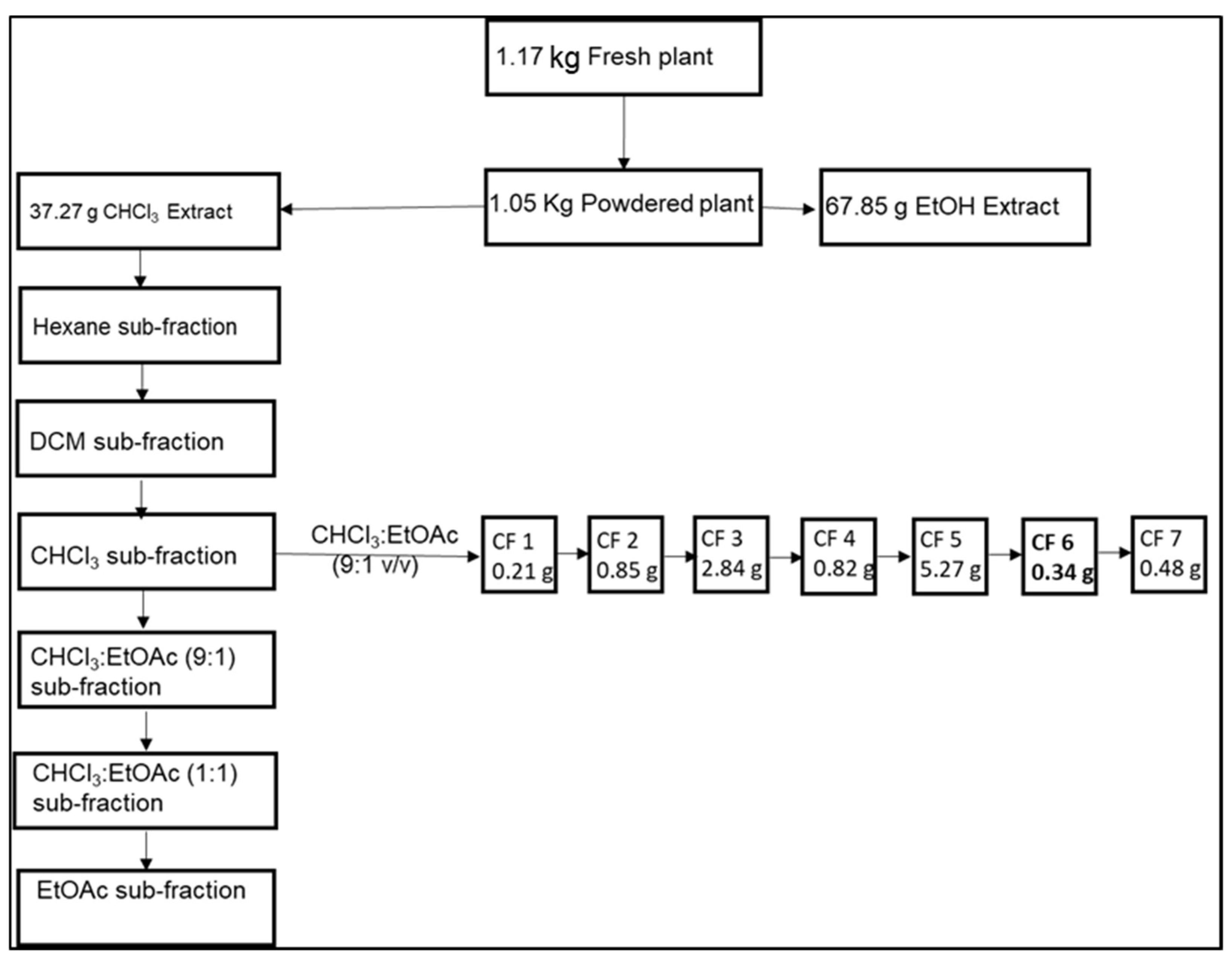
2.2. Helichrysum Caespititium Extraction
2.3. Isolation from the Chloroform Extract
2.4. Column Chromatography
2.5. UPLC-MS and NMR Instrumentation Used for Structural Elucidation
2.6. Test Pathogens
2.7. Minimum Inhibitory Concentration
2.8. Anti-Quorum Sensing Activity of CF6
2.9. AntiBiofilm Assays
2.9.1. Cell Attachment and Biofilm Development
2.9.2. Biofilm Development
3. Results
3.1. Evaluation of the Purity of the Isolated Compound by TLC and UPLC-MS Analysis
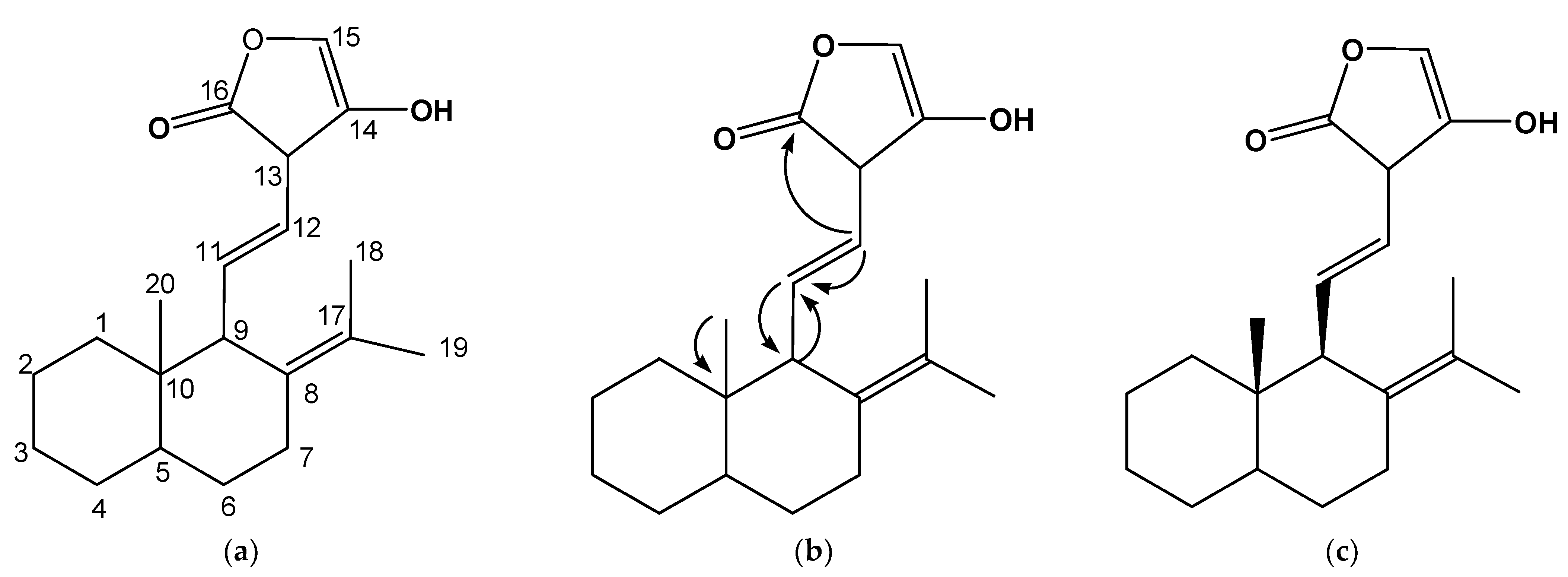
3.2. Elucidation of the Structure of CF6 from Spectrometric and Chromatographic Data
3.3. Evaluation of the Biological Activity Potentials of 10-Methyl-8-(propan-17-ylidene)naphthalen-9-yl)-11-vinyl-14-hydroxyfuran-16-one
3.3.1. In Vitro Antibacterial Activities
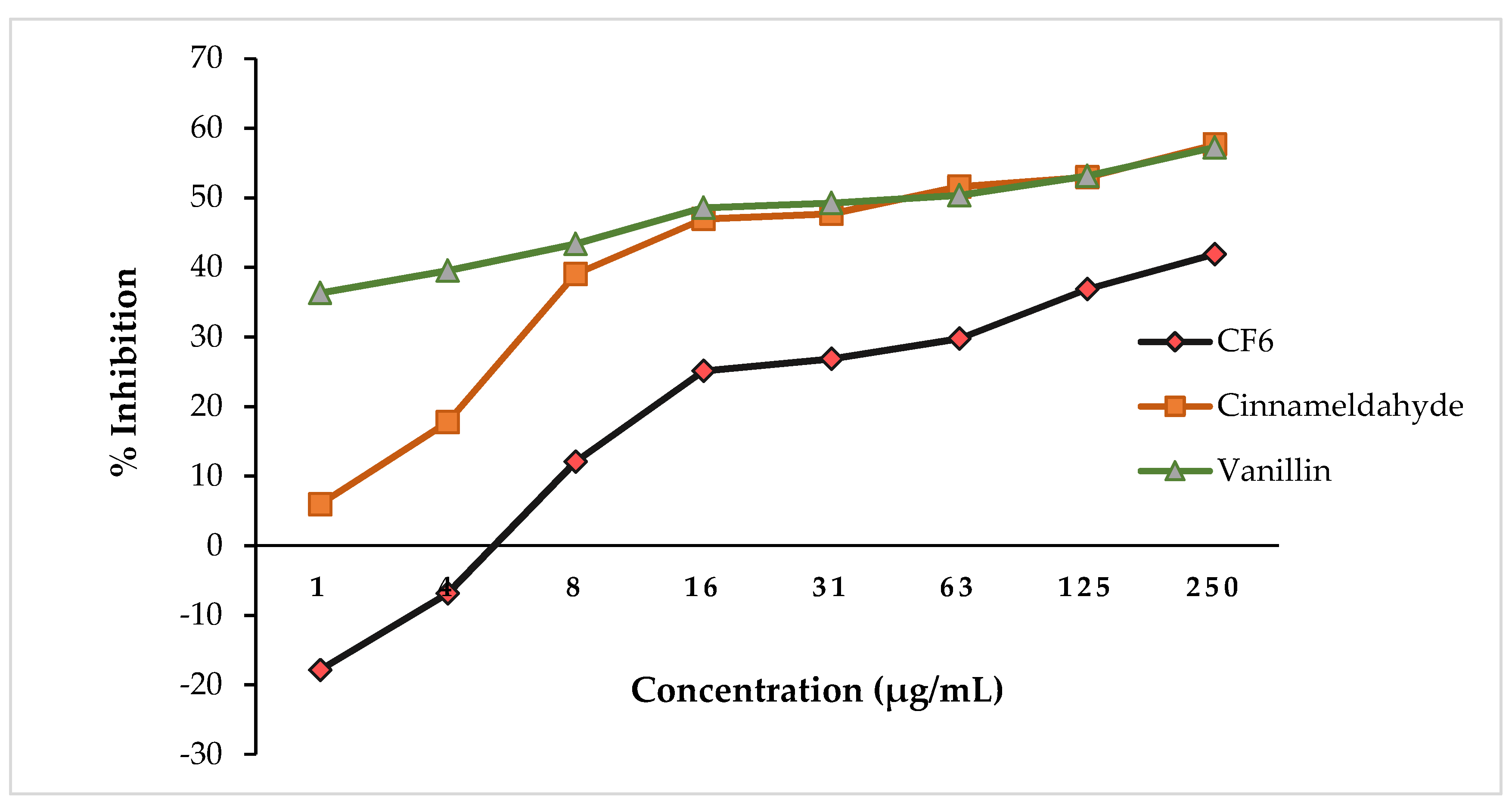
3.3.2. In Vitro Antiquorum Sensing Activities of CF6
3.3.3. Antibiofilm Activities of CF6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiavari-Frederico, M.O.; Barbosa, L.N.; Carvalho Dos Santos, I.; Ratti da Silva, G.; Fernandes de Castro, A.; de Campos Bortolucci, W.; Barboza, L.N.; Campos, C.F.A.A.; Gonçalves, J.E.; Menetrier, J.V.; et al. Antimicrobial activity of Asteraceae species against bacterial pathogens isolated from postmenopausal women. PLoS ONE 2020, 15, e0227023. [Google Scholar] [CrossRef]
- Freitas, P.R.; de Araújo, A.C.J.; Barbosa, C.R.D.S.; Muniz, D.F.; Rocha, J.E.; Neto, J.B.D.A.; da Silva, M.M.C.; Pereira, R.L.S.; da Silva, L.E.; Amaral, W.D.; et al. Characterization and antibacterial activity of the essential oil obtained from the leaves of Baccharis coridifolia DC against multiresistant strains. Microb. Pathog. 2020, 145, 104223. [Google Scholar] [CrossRef]
- García-Risco, M.R.; Mouhid, L.; Salas-Pérez, L.; López-Padilla, A.; Santoyo, S.; Jaime, L.; Ramírez de Molina, A.; Reglero, G.; Fornari, T. Biological Activities of Asteraceae (Achillea millefolium and Calendula officinalis) and Lamiaceae (Melissa officinalis and Origanum majorana) Plant Extracts. Plant Foods Hum. Nutr. 2017, 72, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Naeim, H.; El-Hawiet, A.; Abdel Rahman, R.A.; Hussein, A.; El Demellawy, M.A.; Embaby, A.M. Antibacterial activity of Centaurea pumilio L. root and aerial part extracts against some multidrug resistant bacteria. Complement. Med. Ther. 2020, 20, 79. [Google Scholar] [CrossRef] [Green Version]
- Oppedisano, F.; Muscoli, C.; Musolino, V.; Carresi, C.; Macrì, R.; Giancotta, C.; Bosco, F.; Maiuolo, J.; Scarano, F.; Paone, S.; et al. The Protective Effect of Cynara cardunculus Extract in Diet-Induced NAFLD: Involvement of OCTN1 and OCTN2 Transporter Subfamily. Nutrients 2020, 12, 1435. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha. India Parasite 2018, 25, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolnik, A.; Olas, B. The Plants of the Asteraceae Family as Agents in the Protection of Human Health. Int. J. Mol. Sci. 2021, 22, 3009. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Mnayer, D.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Coutinho, H.D.M.; Salehi, B.; Martorell, M.; Del Mar Contreras, M.; Soltani-Nejad, A.; et al. Echinacea plants as antioxidant and antibacterial agents: From traditional medicine to biotechnological applications. Phytother. Res. 2018, 32, 1653–1663. [Google Scholar] [CrossRef]
- Lourens, A.C.U.; Viljoen, A.M.; Heerden, F.R. South African Helichrysum species: A review of the traditional uses, biological activity and phytochemistry. J. Ethnopharmacol. 2008, 119, 630–652. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.K.; Marnewick, J.L.; Rautenbach, F.; Iwuoha, E.I.; Hussein, A.A. Acylphloroglucinol derivatives from the South African Helichrysum niveum and their biological activities. Molecules 2015, 20, 17309–17324. [Google Scholar] [CrossRef] [Green Version]
- Dekker, T.G.; Fourie, T.G.; Snyckers, F.O.; van der Schyf, C.J. Studies of South African medicinal plants. Part 2. Caespitin, a new phloroglucinol derivative with antimicrobial properties from Helichrysum caespititium. S. Afr. J. Chem. 1983, 36, 114–116. Available online: https://journals.co.za/doi/pdf/10.10520/AJA03794350_1036 (accessed on 13 August 2021).
- Mathekga, A.D.M.; Meyer, J.J.M.; Horn, M.M.; Drewes, S.E. An acylated phloroglucinol with antimicrobial properties from Helichrysum caespitium. Phytochemistry 2000, 53, 93–96. [Google Scholar] [CrossRef]
- Mamabolo, M.P.; Muganza, F.M.; Olivier, M.T. Free radical scavenging and antibacterial activities of Helichrysum caespititium (DC) Harv. extracts. Biol. Med. Aligarh 2017, 9, 1–6. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on Antimicrobial Resistance; World Health Organization (WHO): Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 13 August 2021).
- Baloyi, I.T.; Adeosun, I.J.; Yusuf, A.A.; Cosa, S. In silico and in vitro screening of antipathogenic properties of Melianthus comosus (Vahl) against Pseudomonas aeruginosa. Antibiotics 2021, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Mamabolo, M.P.; Muganza, F.M.; Olivier, M.T.; Olaokun, O.O.; Nemutavhanani, L.D. Evaluation of antigonorrhea activity and cytotoxicity of Helichrysum caespititium (DC) Harv. whole plant extracts. Biol. Med. Aligarh 2017, 9, 422. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosa, S.; Rakoma, J.R.; Yusuf, A.A.; Tshikalange, T.E. Calpurnia aurea (Aiton) Benth extracts reduce quorum sensing controlled virulence factors in Pseudomonas aeruginosa. Molecules 2020, 25, 2283. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Gomes, A.C.; Jesus, M.L.; Rodilla, J.L.M.L. Diterpene Lactones with Labdane, Halimane and ClerodaneFrameworks. Nat. Prod. Commun. 2011, 6, 497–504. [Google Scholar]
- Fabry, W.; Okemo, P.O.; Ansorg, R. Antibacterial activity of east African medicinal plants. J. Ethnopharmacol. 1998, 60, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, S. Anti-staphylococcal plant natural products. Nat. Prod. Rep. 2004, 21, 263–277. [Google Scholar] [CrossRef]
- Sineenard Songsri, S.; Nuntawong, N. Cytotoxic Labdane Diterpenes from Hedychium ellipticum Buch.-Ham. ex Sm. Molecules 2016, 21, 749. [Google Scholar] [CrossRef]
- Kenmogne, M.; Prost, E.; Jacquier, M.J.; Frederich, M.; Sondengam, L.B.; Zeches, M.; Waffo-Tegue, P. Five labdane diterpenoids from the seeds of Aframomum zambesiacum. Phytochemistry 2006, 67, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Campoli-Richards, D.M.; Monk, J.P.; Price, A.; Benfield, P.; Todd, P.A.; Ward, A. Ciprofloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35, 373–447. [Google Scholar] [CrossRef]
- Maroyi, A. Helichrysum caespitutium (DC.) Harv.: Review of its medicinal uses, phytochemistry and biological activities. J. Appl. Pharm. Sci. 2019, 9, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Van der Schyf, C.J.; Dekker, T.G.; Fourie, T.G.; Synckers, F.O. Synthesis and antimicrobial activity of a series of Caespitin derivatives. Antimicrob. Agents Chemother 1986, 30, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutluk, I.; Aslan, M.; Orhan, I.E.; Ozcelik, B. Antibacterial, antifungal and antiviral bioactivities of selected Helichrysum species. S. Afr. J. Bot. 2018, 119, 252–257. [Google Scholar] [CrossRef]
- Ahmad, W.A.; Yusof, N.Z.; Nordin, N.; Zakaria, Z.A.; Rezali, M.F. Production and characterization of violacein by locally isolated Chromobacterium violaceum in agricultural wastes. Appl. Biochem. Biotechnol. 2012, 167, 1220–1234. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
| Position | CF6 | |||
|---|---|---|---|---|
| Assignment | δH | Multiplicity (J/Hz) | δC | |
| 1 | CH2 | 2.11 | t(2H, J = 2.2 Hz) | 21.08 |
| 2 | CH2 | 3.43 | d(2H, J = 6.8 Hz) | 21.17 |
| 3 | CH2 | 1.79 | m(2H) | 19.37 |
| 4 | CH2 | 1.72 | m(2H) | 21.25 |
| 5 | CH | 3.92 | m(H) | 39.17 |
| 6 | CH2 | 1.24 | m(2H) | 29.72 |
| 7 | CH2 | 2.17 | t(2H, J = 2.1 Hz) | 31.90 |
| 8 | Cq | - | - | 105.78 |
| 9 | CH | 3.04 | t(H, J = 7.2 Hz) | 45.98 |
| 10 | Cq | - | - | 22.68 |
| 11 | C = C-H | 7.03 | dd(H, J = 10.2, 16.0) | 128.96 |
| 12 | C = C-H | 5.41 | dd(H, J = 8.0 Hz) | 129.86 |
| 13 | CH | 4.75 | m(1H) | 64.17 |
| 14 | OCq | - | - | 95.09 |
| 15 | OCH | 5.89 | s(H) | 95.20 |
| 16 | O = Cq | - | - | 160.78 |
| 17 | Cq | - | - | 104.75 |
| 18 | CH3 | 0.96 | s(3H) | 14.00 |
| 19 | CH3 | 0.96 | s(3H) | 14.11 |
| 20 | CH3 | 1.69 | s(3H)) | 18.24 |
| Compounds | S. pyogenes | S. aureus | E. coli | K. pneumoniae | P. aeruginosa | N. gonorrhoeae |
|---|---|---|---|---|---|---|
| CF6 | 125 | 125 | 250 | 250 | 250 | 60 |
| Controls (Ciprofloxacin = 1 µg/mL for all test pathogens) | ||||||
| Cell Attachment (%) | ||||||
| Compounds | S. pyogenes | S. aureus | E. coli | K. pneumoniae | P. aeruginosa | N. gonorrhoeae |
| CF6 | 80.70 ± 0.10 | 77.62 ± 0.07 | 65.89 ± 0.23 | 40.76 ± 0.17 | 42.03 ± 0.39 | 81.19 ± 0.11 |
| Ciprofloxacin | 84.33 ± 0.04 | 78.24 ± 0.02 | 76.80 ± 0.08 | 78.58 ± 0.19 | 79.79 ± 0.04 | 94.52 ± 0.01 |
| Biofilm Development (%) | ||||||
| Compounds | S. pyogenes | S. aureus | E. coli | K. pneumoniae | P. aeruginosa | N. gonorrhoeae |
| CF6 | 29.08 ± 0.01 | 35.55 ± 0.14 | −4.65 ± 0.19 | 25.64 ± 0.19 | 25.28 ± 0.35 | 32.57 ± 0.35 |
| Ciprofloxacin | 42.89 ± 0.04 | 42.36 ± 0.08 | 42.91 ± 0.03 | 43.62 ± 0.14 | 48.31 ± 0.07 | 40.72 ± 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bassey, K.; Mamabolo, P.; Cosa, S. An Andrographolide from Helichrysum caespitium (DC.) Sond. Ex Harv., (Asteraceae) and Its Antimicrobial, Antiquorum Sensing, and Antibiofilm Potentials. Biology 2021, 10, 1224. https://doi.org/10.3390/biology10121224
Bassey K, Mamabolo P, Cosa S. An Andrographolide from Helichrysum caespitium (DC.) Sond. Ex Harv., (Asteraceae) and Its Antimicrobial, Antiquorum Sensing, and Antibiofilm Potentials. Biology. 2021; 10(12):1224. https://doi.org/10.3390/biology10121224
Chicago/Turabian StyleBassey, Kokoette, Patience Mamabolo, and Sekelwa Cosa. 2021. "An Andrographolide from Helichrysum caespitium (DC.) Sond. Ex Harv., (Asteraceae) and Its Antimicrobial, Antiquorum Sensing, and Antibiofilm Potentials" Biology 10, no. 12: 1224. https://doi.org/10.3390/biology10121224
APA StyleBassey, K., Mamabolo, P., & Cosa, S. (2021). An Andrographolide from Helichrysum caespitium (DC.) Sond. Ex Harv., (Asteraceae) and Its Antimicrobial, Antiquorum Sensing, and Antibiofilm Potentials. Biology, 10(12), 1224. https://doi.org/10.3390/biology10121224








