Simple Summary
Scientific knowledge should transcend the barriers between the laboratory and the field to act in the service of humanity. Considering the enormous potential that soil offers for organic carbon (SOC) sequestration for the mitigation of greenhouse gas (GHG) emissions, and considering the recognized ecological importance of biological soil crusts (biocrusts) to be applied in the soil–plant continuum, we propose three perspectives to apply biocrusts to sustainable agriculture.
Abstract
The major priority of research in the present day is to conserve the environment by reducing GHG emissions. A proposed solution by an expert panel from 195 countries meeting at COP 21 was to increase global SOC stocks by 0.4% year−1 to compensate for GHG emissions, the ‘4 per 1000′ agreement. In this context, the application of biocrusts is a promising framework with which to increase SOC and other soil functions in the soil–plant continuum. Despite the importance of biocrusts, their application to agriculture is limited due to: (1) competition with native microbiota, (2) difficulties in applying them on a large scale, (3) a lack of studies based on carbon (C) balance and suitable for model parameterization, and (4) a lack of studies evaluating the contribution of biocrust weathering to increase C sequestration. Considering these four challenges, we propose three perspectives for biocrust application: (1) natural microbiome engineering by a host plant, using biocrusts; (2) quantifying the contribution of biocrusts to C sequestration in soils; and (3) enhanced biocrust weathering to improve C sequestration. Thus, we focus this opinion article on new challenges by using the specialized microbiome of biocrusts to be applied in a new environment to counteract the negative effects of climate change.
1. Introduction
Among the main immediate challenges of humanity, the reduction in atmospheric CO2 concentrations and the emissions of other greenhouse gases (GHGs) is a major priority to counteract the negative effects of climate change [1,2]. Because soils can store two to three times more carbon (C) than the atmosphere, soil organic carbon (SOC) sequestration is a possible and efficient solution to diminish GHG emissions to the atmosphere [2]. In grasslands and agriculture, 47% of total potential mitigation arises from SOC protection and sequestration, while 20% involves other GHGs involved with improved soil management practices [3,4].
The enormous potential of soils to sequester C motivated the governments of 195 countries in 2015, convened in Paris for the 21st Conference of the Parties (COP 21), to launch a new global climate change agreement, the ‘4 per 1000′, with the main objective to increase global SOC stocks by 4 per 1000 (or 0.4%) per year as a compensation for global GHG emissions from anthropogenic sources (https://www.4p1000.org/, access on 1 August 2021). Despite the high aim of 4% per year being almost impossible, the development of strategies that increase C accumulation in soil should be a priority of governments, scientific communities, and modern agriculture [5].
In agriculture, many approaches have been focused on soil C regeneration through increased residue returns and biomass production (cover crops), and on decreasing C losses via reduced disturbance (no-till farming). These agronomic approaches, however, do not always produce net C gains since soil C accumulation is not a linear function of inputs [6]. One of the determinant keys of the C balance is the microbial mineralization of SOC, which results in CO2 losses [7]. Thus, the efficiency of soil microbes to process C is gaining interest as an important management strategy to increase SOC sequestration [6]. In this context, the interactions within biological soil crusts (biocrusts) are currently attracting scientific attention because of their ability to increase the fixation of nitrogen (N) and C, phosphorus (P) availability, and reduce nutrient leaching. Biocrusts are therefore considered as a key ecological microbial community in the continuity of soil biogeochemical cycles [8,9,10]. In order to obtain a closed cycle, all the different sources, cycling processes, and sinks need to be assessed by means of suitable methods, some of which will require a new approach.
1.1. Biocrust, a Photosynthetic Cell Factory for C Sequestration in Agriculture
Biocrusts cover about 12% of the Earth’s landmasses, thereby providing ecosystem services, affecting biogeochemical fluxes on a global scale, and strongly influencing soil–plant relationships; thus, facilitating edaphic engineering effects [11,12]. Biocrusts are found on almost all soil types, but are more commonly found in arid and cold regions, where plant coverage is low and more widely spaced. Across the globe, biocrusts can be found on all continents, including Antarctica [13,14].
Biocrusts are formed by an association of soil mineral particles and micro-organisms, composed by free-living, lichenized, and mycorrhizal fungi, chemoheterotrophic bacteria, cyanobacteria, diazotrophic bacteria, archaea, eukaryotic algae, and bryophytes. Biocrusts can aggregate soil particles through excreting exopolysaccharides (EPSs), glycoproteins, and forming filament networks [15]. However, the species composition and physical appearance of biocrusts depend on the climate, soil properties, and disturbance conditions. For example, biocrusts are more dominated by green algae on more acidic and less salty soils. In contrast, cyanobacteria are more favored on alkaline soils [16]. Independent of climate, the abundance of lichens and mosses in biocrusts generally increases with increasing clay and silt content and decreasing sand (Table 1). Moist habitats generally support more lichens and mosses [17]. Due to their abilities, biocrusts are one of the major players of global C and N sequestration in soils. On the other hand, biocrusts also increase soil stability against erosion by forming aggregates, increasing porosity and water retention, leading to better seed germination [8,9,16,18]. Thus, an improved understanding of the structures, composition, and functions of biocrust microbiomes and their geological implication (geomicrobiology) allows changes in soil ecosystem structures to be forecast for long-term restoration due their essential roles related to physiological or chemical properties (Figure 1).

Table 1.
Preference of biocrust-composing microbial groups to climatic and edaphic conditions (nd = not determined).
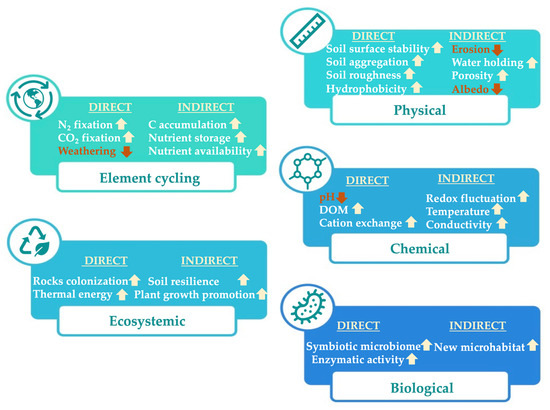
Figure 1.
Effects of biocrusts on soil properties and element cycling. The direct and indirect effects are summarized according to their main mechanisms based on physical, chemical, biological, ecosystemic, and element cycling. The arrows before each point show an increase (↑) or decrease (↓) in the presence of biocrusts.
1.2. Biocrust, a Photosynthetic Cell Factory for C Sequestration in Agriculture
Baumann, Jung, Samolov, Lehnert, Büdel, Karsten, Bendix, Achilles, Schermer and Matus [16] evaluated the richness of green algae and cyanobacteria of biocrusts in four climate zones: arid, semi-arid, Mediterranean, and humid temperate. According to the morphological identification of the enrichment cultures, a total of twenty-four taxa of green algae and eighteen of cyanobacteria, regardless of climatic conditions, were found. Each biocrust was comprised of twelve to fifteen phototrophic species that used sunlight as an energy source to assimilate CO2, directly affecting the C cycle by C fixation [26]. Cyanobacteria are the oldest oxygenic photosynthetic organisms, producing O2 during photosynthesis as they fix CO2 dissolved in the water, thus having one of the most important metabolisms to have evolved on Earth [27,28]. The adaptation ability of cyanobacteria has allowed them to live in various conditions, including marine, freshwater, and terrestrial environments [29,30]. Cyanobacteria have been applied in medicine, cosmetic manufacturing, bioremediation, biofuel, and agriculture [29,31,32,33]. In fact, some filamentous cyanobacteria have evolved specialized cells to fix atmospheric N, known as heterocysts [34]. However, in recent years, more attention has been paid to their C sequestration potential [10,35]. For example, Kheirfam, Sadeghi and Darki [10] studied a factorial combination of bacteria and cyanobacteria from biocrusts and nutrients added to the field-collected soil. The authors showed that the inoculation of biocrusts had the potential to remove 0.85–1.07 g CO2 m−2 from the atmosphere.
Such scientific interest in the understanding of biocrusts’ contribution to global climate change advances the prediction, scaling, restoration, and C sequestration options that are at the forefront of contemporary biocrust science. In the present opinion manuscript, we discuss the structural, benefic, and evolutionary mechanisms to overcome environmental limitations by biocrusts. In addition, we suggest a new and revolutionary challenge for the new generation of bioinoculants based in biocrusts, such as host natural microbial engineering, where plants recruit their microbiome to increase C sequestration: new challenges in a new environment.
2. Limitations of the Broad-Scale Application of Biocrusts in Agriculture
Despite the demonstrated importance of biocrust-residing microorganisms to improve C sequestration, field applications are limited because (Figure 2):
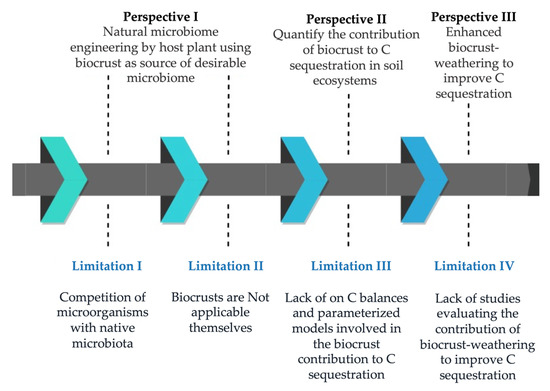
Figure 2.
Proposal perspectives to solve practical limitations to the use of biocrusts for increased carbon sequestration.
(1) Competition of micro-organisms (desirable strains from biocrusts) with native microbiota in a new environment;
(2) Biocrusts are not applicable themselves;
(3) A lack of parameterized C balance models simulating the contribution of biocrusts to the C sequestration processes;
(4) A lack of studies evaluating the contribution of biocrust weathering to improve C sequestration.
3. Perspectives to Solve Practical Limitations of Biocrust Use for Carbon Sequestration
Most research on biocrust inoculation has been conducted at a laboratory or on a plot scale, which is a necessary starting point; however, we need methods that can scale up the application, innovative methodologies, and treat ecological- and management-relevant areas. For these purposes, we propose three perspectives to implement effective biocrust application and monitoring to understand the main functions and services of the ecosystem (Figure 3).
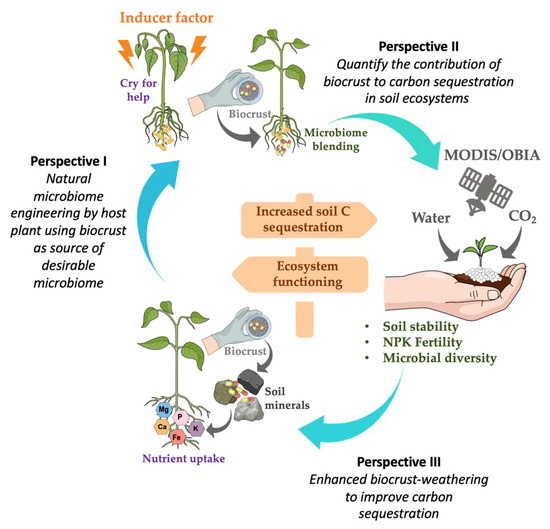
Figure 3.
Proposal perspectives: biocrust applications in agriculture and in a new environment. The link between soil processes, microbiome, ecosystem services, weathering, and soil monitoring perspectives. The arrows indicate the relationship between soil–plant processes and biocrusts. The yellow arrow indicates the impact of the soil perspectives on regulating and provisioning ecosystem functioning and C sequestration.
3.1. Perspective I. Natural Microbiome Engineering by a Host Plant, Using a Biocrust as Source of Desirable Micro-Organisms
To decrease the first and second limitations, we suggest natural microbiome engineering by a host plant, using a biocrust as a source of a desirable microbiome. Host-mediated microbiota engineering (HMME) is a novel biological strategy that utilizes the intrinsic ability of plants to recruit and select their associated microbiome in their rhizobiome through cyclic differentiation and propagation [36,37]. HMME is a promising approach for improving host performance by engineering microbial communities for beneficial effects on growth, stress tolerance, and plant health [38,39]. This strategy enables the selection of a particular microbiome by visualizing changes in the host phenotype after several generations of growing the plant in the same place [40]. We recently report that the natural selection of microbiota over multiple generations can be induced through the existence of three factors: (1) a plant model, (2) an abiotic/biotic stressor or inducer factor, and (3) a desirable microbiome [41]. Thus, when a host plant is subjected to some type of abiotic/biotic stress or inducer factor, the plant can modify the composition of their exudates, resulting in the reassembly of the associated microbiomes, which in turn is reflected in modifications of the plant phenotype [42,43,44], entailing an adaptation of the host plant [42,45]. These ‘selected’ micro-organisms would have an advantage over external microbiota, and the reassembled microbiome can optimize the plant’s response against stress or an inducer factor [46,47]. Bakker, et al. [48] suggested the concept of ‘cry for help’, wherein the stage of an outbreak of biotic or abiotic disease plants recruit protective microbiota, mainly by the exudation of photo-synthetically fixed C in the rhizobiome and favoring endosphere colonization [49,50]. Thus, plants are able to recruit a specialized microbiota when subjected to an adverse condition.
We propose the use of HMME using a (i) model plant, which could be a representative grassland species, (ii) subjected to elevated CO2 (eCO2) as an inducer factor, due to eCO2 influencing the richness, composition, and structure of soil microbial community to C sequestration [51], (iii) grown in soil mixed with a biocrust (desirable microbiome). After some growth cycles under these eCO2 conditions, plants (themselves) could select those micro-organisms that give them a comparative advantage in C sequestration (induced by eCO2). These micro-organisms can be horizontally transferred among cycles and vertically to descending generations by seeds. From this, is possible to obtain bioinoculants by culturable micro-organisms or whole microbiota by using rhizobiome extracts (Figure 3). For example, Jochum and collaborators induced drought tolerance after six rounds of HMME selection, and core microbiota functionality was transferable in a subsequent assay [36]. Another study induced earlier or later flowering times in Arabidopsis thaliana plants using HMME selection [37]. Recently, HMME was also applied in Nasonia; the insects were exposed over successive generations to subtoxic levels of atrazine, and changes were observed in the structure and function of the gut microbiome that conveyed that microbiome-mediated atrazine resistance was inherited and increased over successive generations of Nasonia vitripennis [44]. This innovative biotechnology allowed for the endophytic bioinoculant (inside the roots) naturally selected by the host plant to be obtained, which avoids competition with native microbiota (limitation one) and is applicable under field conditions because it could be directly applied to seeds (limitation two).
3.2. Perspective II. Quantify the Contribution of Biocrusts to Carbon Sequestration in Soils
The monitoring of C fluxes in terrestrial ecosystems to accurately quantify and predict C balance is, globally, one of the highest research priorities [52]. These predictions are essential for identifying and quantifying sinks and sources of greenhouse gas (GHG, e.g., CO2) emissions (limitation three). Research about biocrusts related to the C cycle has been carried out mainly under controlled conditions with temperate forest soils, agricultural ecosystems, and drylands. However, field-scale studies are scarce [19]. Quantifying the C stocks and fluxes in these biomes and determining the processes that regulate them are crucial for a basic understanding of the C cycling of these ecosystems that cover 45% of the Earth’s land area [53]. It is estimated that increasing soil C reserves in these low-productive ecosystems could significantly reduce atmospheric CO2 levels [54], considering that soil C uptake estimates are 3.5 to 5.2 Gt year−1 [55].
The C budgets of these forest and agriculture ecosystems can be increased with the application of a biocrust. However, we must also consider how microbial compositions affect the responses of the C flux to abiotic conditions [56,57], since each taxon may have a different response and could form different interactions among them (limitation one). Quantifying the magnitude of the contribution of each species or functional group to the ecosystem C balance will improve estimates of C budgets in these ecosystems. A biocrust can contribute differently to C storage: (a) aggregating soil particles through the secretion of exopolysaccharides, forming networks of filaments that increase the stability of the soil against erosion and other degradation factors [58,59]; (b) increasing porosity [60]; (c) retaining water and/or infiltrating it [61,62]; (d) increasing soil fertility by accumulating nutrients [27,59]; and (e) helping in the establishment of other organisms such as mosses, lichens, cyanobacterias, micro-fungi, and plants, increasing the storage potential of C [63]. These functions performed by a biocrust are relevant since it is one of the predominant soil covers, covering up to 70% of the surface in some areas (e.g., dryland), and it is the primary source of soil organic carbon (SOC) in many of them. It is estimated that biocrusts represent ~15% of global terrestrial C stock and ~40–85% of N fixation worldwide [64,65]. Therefore, quantifying C fluxes and nutrients through the assembly of biocrust communities under field conditions would significantly enhance our understanding and prediction, through mathematical models, of how specific pressures derived from global change could alter the structure of the biocrust community and, accordingly, guide our efforts towards enhancing C sequestration and nutrient availability in soils [66,67].
With this goal, we suggest that it is important to consider: (i) in situ monitoring of CO2 and other GHG fluxes with the microbiota derived from an inoculated biocrust from perspective I in the soil and to relate these fluxes with environmental factors and physico-chemical properties that control GHG emissions; (ii) a second significant contribution to knowledge would be to map the distribution of biocrust types (in successional stages) in the basins of various ecosystems using ground-based remote sensing techniques. As low- and moderate-resolution satellite imagery (e.g., Landsat and Moderate Resolution Imaging Spectroradiometer (MODIS)) or object-based image analysis (OBIA) approaches are often used for mapping with very-high-resolution imagery when the pixel resolution is inadequate.; (iii) all contributions (balance) on C fluxes (both biocrusts and micro-organisms) under natural field conditions to include them in model-based soil C monitoring systems; and (iv) possible associations between specific groups of micro-organisms below the biocrust, enzymatic activities involved in the C, N, and P turnover, and soil physico-chemical variables (e.g., TOC, TN, P, pH, and carbonates). Improving the production of reliable maps of biocrust cover further depends on the availability of imaging systems which provide not only adequate spatial and spectral resolution but are also capable of collecting images sufficiently frequently.
3.3. Perspective III. Enhanced Biocrust Weathering to Improve Carbon Sequestration
Enhanced mineral weathering is a C sequestration process that could remove more than 2 billion tons of CO2 each year. Silicate minerals exposed to the weathering surface can sequester atmospheric CO2 and transform it into HCO3-, thereby reducing the intensity of atmospheric greenhouse effects. However, it is a process that usually takes thousands of years. Even so, rock weathering is an important component to consider for geological carbon sinks. Carbon sinks derived from carbonate weathering and silicate weathering are the two primary mechanisms underlying rock weathering carbon sinks [68]. Unfortunately, the time this process takes is too long to compensate for CO2 flux from human activities. This limitation can be compensated through an inoculated biocrust from perspectives I and II to increase weathering rates. Because when the biocrust comes into contact with the rock, it triggers a chemical process known as the Urey reaction. This reaction removes CO2 from the atmosphere and combines it with water and calcium or magnesium silicates, leaving the CO2 trapped in these carbonates in the soil. C capture’s accelerated chemical weathering process could remove more than 2 billion tons of CO2 each year [65]. This is because approximately 95% of the Earth’s crust is made of silicate minerals, which are silicon and oxygen compounds.
Exemplarily, the chemical reaction can be followed through the dissolution of anorthite (Equation (1)). The dissolution of primary silicates leads to secondary precipitates, releasing cations, and transforming CO2 into HCO3:
Ca2Al2Si2O8 + 2CO2 + 3H2O → Al2Si2O5(OH)4 + Ca2+ + 2HCO3
If supersaturation concerning individual carbonate phases is reached, solid carbonates might form (Equation (2)):
Ca2+ + 2HCO3− → CaCO3 + CO2 + H2O
Carbonate formation is an important mechanism for the in situ fixation of CO2 through carbon capture and storage [69]. However, enhanced weathering aims to convert CO2 into alkalinity, as the formation of carbonates will reduce the process’s efficiency (Equation (2)). The maximum CO2 amount drawn from the atmosphere through silicate dissolution is a function of the cation flux (mostly Ca2+, Mg2+, K+, and Na+); which is charge-balanced by HCO3− formation. In addition, CO2 fixation through the dissolution is based on Al conservation through the formation of secondary minerals (Equation (1)). However, the dissolution of the aluminosilicate can precede the formation of the secondary phase [70] (Equation (3)), and far from equilibrium conditions can be sustained during basalt weathering, for instance, through the complexation of Al3+ with organic acids [71]:
Ca2Al2Si2O8 + 8CO2 + 4H2O → 2Al3+ +Ca2+ + 8HCO3− + 2SiO2(aq)
It is essential to consider that the investigations of the patterns, mechanisms, and rates of weathering with biocrusts are in their infancy (limitation four). What we know comes largely from geomicrobial interactions studied in similar settings, that are endolytically within rocks. Because of its importance in global carbon cycling, many scientists have carried out research on silicate rock weathering carbon sink and made progress toward understanding the related mechanisms. In this sense, Chen, et al. [72] analyzed the effects of biocrust incorporation on the soil, showing that it enriched the soil with biogenic elements (C, N, and P) and generated the leaching of many metals and metalloids from the mineral phase. This effect on the rock is achieved through the reactivity of the biocrust metabolic products (e.g., extracellular polymeric substances) excreted by the micro-organisms on the mineral surface, acidification or decrease in the redox potential due to the permanent coating of minerals by exopolymeric substances, and the secretion of specific metallic ligands and other organic complexes [73,74]. Moreover, in the rock fragments (because of weathering), more Mg, Fe, and Ca silicates are exposed, increasing porosity and permeability [75,76]. Additionally, the reactive mineral fraction (Fe and Mn) forming associations with the organic matter has been suggested as another C sequestration strategy to mitigate climate change [77,78,79]. The combination and intensity of these mechanisms in biocrusts likely vary, but microbial stabilization is determining in all of them, and abiotic factors have not been examined in much detail. In this sense, carrying out studies about the relationships between natural chemical weathering rates and controlling parameters (e.g., temperature, precipitation, and pH) can help to clarify the discrepancy observed between field and laboratory data [80,81] and could allow the up-scaling of local measurements to a global scale (perspective II), contributing to the refinement of global CO2 consumption estimations. Future research can help to elucidate the generality of biocrust responses to the suite of global changes with which they are faced and increase our understanding of the mechanisms that drive this change. Studying the parameters controlling weathering rates associated with biocrust in natural settings can improve the feedback between climate and weathering and its role in the short-term carbon cycle and climate change [82,83,84].
The responses of biocrusts to climate change appear to be particularly strong and, while different biocrust organisms will respond differently to changing climatic conditions, the data suggest that increasing temperatures and altered precipitation patterns, as well as strong interactions between the two, are significantly modifying the structure, function, and resilience of biocrust communities. Thus, depending on how microbial community profiles change, there may be more implications for mineralization and organic C storage [65]. Unfortunately, studies on the relationship between biocrusts and weathering are few and appear only to account for current research. Thus, we propose an intensive study of the relationship between the effects of micro-organisms derived from biocrusts (perspective I) on mineral weathering, soil formation, and continuous C sequestration (perspective II) to validate the advantage and attributes of biocrusts. Consequently, we postulate that the exhaustive study of the composition and dynamics of biocrust and their interactions with the physico-chemical properties of soils under the new prevailing conditions as a result of the atmospheric increase in GHGs has enormous potential to be used as a biotechnological tool to increase the sequestration of C in soils.
4. Conclusions
Investigations of biocrust weathering patterns, mechanisms, and rates are in their infancy. Most research on biocrust inoculation has been conducted at a laboratory, which is a necessary starting point. However, we need to scale-up the research using common protocols in relevant areas to land managers, and bridge the gap between science and practice. The next frontiers for biocrusts need to better document how biological (e.g., species composition/function and organism condition) and physical factors (e.g., activity rates and times as determined by climatic factors, soils) influence C fixation and loss. Across all scales, we need to understand and observe biocrust photosynthesis and respiration, and what portion of C losses is due to other sources, such as bacteria, fungi, and soil carbonates by indirect inoculation, where host plants recruit specific microbiomes in the rhizobiome to induce C sequestration. Future research can help elucidate the generality of biocrust responses to the suite of global changes they face and increase our understanding of the mechanisms that drive this change. For this, we proposed three perspectives to solve practical limitations to using biocrusts to increase carbon sequestration: utilizing the natural microbiome of a host plant, quantifying the contribution of biocrusts to carbon sequestration in soil ecosystems, and enhanced biocrust weathering to increase the level of nutrients released and carbon sequestration, addressing the spatial relationships between biocrusts and ecological processes and quantifying their contribution to ecosystem functionality at local to landscape scales.
Author Contributions
Conceptualization, P.D. and C.M., investigation, P.D., M.d.l.L.M., F.M., P.J.B., I.J., Y.K. and C.M.; data curation, P.D., M.d.l.L.M., F.M., P.J.B. and I.J.; writing—original draft preparation, P.D., C.M., P.J.B. and I.J.; writing—review and editing, P.D., C.M. and Y.K.; supervision, M.d.l.L.M., F.M. and Y.K.; project administration, P.D. and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FONDECYT regular project 1201196, Iniciation No. 11200377, INACH regular RT_06-17, Iniciation No. 11180521 and Postdoctoral Fellowship No. 3200758. Y.K. thanks for the support of the Program of Attraction of Advanced Human Capital from Abroad, Short Stays Modality (MEC), International Cooperation Program (CONICYT); RUDN University Strategic Academic Leadership Program and the Government Program of Competitive Growth of Kazan Federal University, and the RUDN University Strategic Academic Leadership Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Scientific and Technological Bioresource.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Minasny, B.; Malone, B.P.; McBratney, A.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Cardenas, M.G.; Kaonga, M.; Koutika, L.-S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 2019, 49, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Ortiz, A.M.D.; Outhwaite, C.L.; Dalin, C.; Newbold, T. A review of the interactions between biodiversity, agriculture, climate change, and international trade: Research and policy priorities. One Earth 2021, 4, 88–101. [Google Scholar] [CrossRef]
- Fu, X.; Zhou, Y.; Yang, F.; Ma, L.; Long, H.; Zhong, Y.; Ni, P. A Review of Key Technologies and Trends in the Development of Integrated Heating and Power Systems in Agriculture. Entropy 2021, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Wallenstein, M.; Schipanksi, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Bowling, D.R.; Grote, E.E.; Belnap, J. Rain pulse response of soil CO2 exchange by biological soil crusts and grasslands of the semiarid Colorado Plateau, United States. J. Geophys. Res. Space Phys. 2011, 116, G03028. [Google Scholar] [CrossRef] [Green Version]
- Kheirfam, H. Increasing soil potential for carbon sequestration using microbes from biological soil crusts. J. Arid. Environ. 2019, 172, 104022. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Darki, B.Z. Soil conservation in an abandoned agricultural rain-fed land through inoculation of cyanobacteria. CATENA 2019, 187, 104341. [Google Scholar] [CrossRef]
- Maier, S.; Tamm, A.; Wu, D.; Caesar, J.; Grube, M.; Weber, B. Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J. 2018, 12, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Szyja, M.; Menezes, A.G.D.S.; Oliveira, F.D.A.; Leal, I.; Tabarelli, M.; Büdel, B.; Wirth, R. Neglected but Potent Dry Forest Players: Ecological Role and Ecosystem Service Provision of Biological Soil Crusts in the Human-Modified Caatinga. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Belnap, J.; Büdel, B.; Lange, O.L. Biological Soil Crusts: Characteristics and Distribution. In Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin/Heidelberg, Germany, 2001; pp. 3–30. [Google Scholar] [CrossRef]
- Felde, V.J.M.N.L.; Chamizo, S.; Felix-Henningsen, P.; Drahorad, S.L. What stabilizes biological soil crusts in the Negev Desert? Plant Soil 2018, 429, 9–18. [Google Scholar] [CrossRef]
- Warren, S.D.; Clair, L.L.S.; Stark, L.R.; Lewis, L.; Pombubpa, N.; Kurbessoian, T.; Stajich, J.E.; Aanderud, Z.T. Reproduction and Dispersal of Biological Soil Crust Organisms. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Baumann, K.; Jung, P.; Samolov, E.; Lehnert, L.W.; Büdel, B.; Karsten, U.; Bendix, J.; Achilles, S.; Schermer, M.; Matus, F.; et al. Biological soil crusts along a climatic gradient in Chile: Richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol. Biochem. 2018, 127, 286–300. [Google Scholar] [CrossRef]
- Chock, T.; Antoninka, A.J.; Faist, A.M.; Bowker, M.A.; Belnap, J.; Barger, N.N. Responses of biological soil crusts to rehabilitation strategies. J. Arid. Environ. 2018, 163, 77–85. [Google Scholar] [CrossRef]
- Adessi, A.; de Carvalho, R.C.; De Philippis, R.; Branquinho, C.; da Silva, J.M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Baumann, K.; Glaser, K.; Mutz, J.-E.; Karsten, U.; MacLennan, A.; Hu, Y.; Michalik, D.; Kruse, J.; Eckhardt, K.-U.; Schall, P.; et al. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Chilton, A.M.; Neilan, B.A.; Eldridge, D.J. Biocrust morphology is linked to marked differences in microbial community composition. Plant Soil 2017, 429, 65–75. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Bahr, J.; Robinson, D.M.; Belnap, J.; Campbell, T.; Gill, R.A.; McMILLIAN, B.; Clair, S.S. The Burning of Biocrusts Facilitates the Emergence of a Bare Soil Community of Poorly-Connected Chemoheterotrophic Bacteria With Depressed Ecosystem Services. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Gallardo, A.; Covelo, F.; Prado-Comesaña, A.; Ochoa, V.; Maestre, F.T. Differences in thallus chemistry are related to species-specific effects of biocrust-forming lichens on soil nutrients and microbial communities. Funct. Ecol. 2015, 29, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Concostrina-Zubiri, L.; Matos, P.; Giordani, P.; Branquinho, C. Biocrust tissue traits as potential indicators of global change in the Mediterranean. Plant Soil 2017, 429, 159–174. [Google Scholar] [CrossRef]
- Maestre, F.T.; Escolar, C.; de Guevara, M.L.; Quero, J.L.; Lázaro, R.; Delgado-Baquerizo, M.; Ochoa, V.; Berdugo, M.; Gozalo, B.; Gallardo, A. Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob. Chang. Biol. 2013, 19, 3835–3847. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Jeffries, T.C.; Singh, B.K. Biocrust-forming mosses mitigate the impact of aridity on soil microbial communities in drylands: Observational evidence from three continents. New Phytol. 2018, 220, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, M.-H.; Ding, G.-D.; He, Y.; Liu, W.; Wang, C.-Y. Soil biocrusts reduce seed germination and contribute to the decline in Artemisia ordosica Krasch. shrub populations in the Mu Us Sandy Land of North China. Glob. Ecol. Conserv. 2021, 26, e01467. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Miralles, I.; Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 2012, 49, 96–105. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2019, 225, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Adessi, A.; Torzillo, G.; De Philippis, R. Exopolysaccharide Features Influence Growth Success in Biocrust-forming Cyanobacteria, Moving From Liquid Culture to Sand Microcosms. Front. Microbiol. 2020, 11, 568224. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Lu, X. Engineering cyanobacteria chassis cells toward more efficient photosynthesis. Curr. Opin. Biotechnol. 2019, 62, 1–6. [Google Scholar] [CrossRef]
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.R. Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8. [Google Scholar]
- Jochum, M.D.; McWilliams, K.L.; Pierson, E.A.; Jo, Y.-K. Host-mediated microbiome engineering (HMME) of drought tolerance in the wheat rhizosphere. PLoS ONE 2019, 14, e0225933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panke-Buisse, K.; Poole, A.; Goodrich, J.K.; Ley, R.; Kao-Kniffin, J. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2014, 9, 980–989. [Google Scholar] [CrossRef]
- Mueller, U.; Sachs, J. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, R.; Durán, P. Natural Holobiome Engineering by Using Native Extreme Microbiome to Counteract the Climate Change Effects. Front. Bioeng. Biotechnol. 2020, 8, 568. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Sadowsky, M.J.; Viscardi, S.; Barra, P.J.; Mora, M.D.L.L. Engineering Multigenerational Host-Modulated Microbiota against Soilborne Pathogens in Response to Global Climate Change. Biology 2021, 10, 865. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Viscardi, S.; Barra, P.J.; Carrión, V.J.; Mora, M.L.; Pozo, M.J. Microbial community composition in take-all suppressive soils. Front. Microbiol. 2018, 9, 2198. [Google Scholar] [CrossRef] [PubMed]
- Harkes, P.; van Steenbrugge, J.; Elsen, S.J.J.V.D.; Suleiman, A.K.A.; De Haan, J.J.; Holterman, M.H.M.; Helder, J. Shifts in the Active Rhizobiome Paralleling Low Meloidogyne chitwoodi Densities in Fields Under Prolonged Organic Soil Management. Front. Plant Sci. 2020, 10, 1697. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Zhu, K.; Hou, S.; Chen, L.; Mi, D.; Gui, Y.; Qi, Y.; Jiang, C.; Guo, J.-H. Plant Root Exudates Are Involved in Bacillus cereus AR156 Mediated Biocontrol Against Ralstonia solanacearum. Front. Microbiol. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Carrión, V.J.; Cordovez, V.; Tyc, O.; Etalo, D.W.; De Bruijn, I.; De Jager, V.C.L.; Medema, M.H.; Eberl, L.; Raaijmakers, J.M. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J. 2018, 12, 2307–2321. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O.; Ayangbenro, A. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2018, 103, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.; Pieterse, C.; de Jonge, R.; Berendsen, R. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Maheswari, M.; Desai, S.; Gopinath, K.; Venkateswarlu, B. Elevated CO2: Plant associated microorganisms and carbon sequestration. Appl. Soil Ecol. 2015, 95, 73–85. [Google Scholar] [CrossRef]
- Musche, M.; Adamescu, M.; Angelstam, P.; Bacher, S.; Bäck, J.; Buss, H.L.; Duffy, C.; Flaim, G.; Gaillardet, J.; Giannakis, G.V.; et al. Research questions to facilitate the future development of European long-term ecosystem research infrastructures: A horizon scanning exercise. J. Environ. Manag. 2019, 250, 109479. [Google Scholar] [CrossRef]
- Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Ahlström, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO 2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Chapin, F.S. Effects of Plant Traits on Ecosystem and Regional Processes: A Conceptual Framework for Predicting the Consequences of Global Change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavorel, S. Plant functional effects on ecosystem services. J. Ecol. 2012, 101, 4–8. [Google Scholar] [CrossRef]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Darki, B.Z. Quality improvement of an erosion-prone soil through microbial enrichment. Soil Tillage Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Miralles-Mellado, I.; Cantón, Y.; Solé-Benet, A. Two-dimensional porosity of crusted silty soils: Indicators of soil quality in semiarid rangelands? Soil Sci. Soc. Am. J. 2011, 75, 1330–1342. [Google Scholar] [CrossRef] [Green Version]
- Chamizo, S.; Cantón, Y.; Domingo, F.; Belnap, J. Evaporative losses from soils covered by physical and different types of biological soil crusts. Hydrol. Process. 2011, 27, 324–332. [Google Scholar] [CrossRef]
- Sadeghi, S.H.; Kheirfam, H.; Homaee, M.; Darki, B.Z.; Vafakhah, M. Improving runoff behavior resulting from direct inoculation of soil micro-organisms. Soil Tillage Res. 2017, 171, 35–41. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Oses, R.; Torres-Díaz, C.; Atala, C.; Zurita-Silva, A.; Ruiz-Lara, S. Root-endophytes improve the ecophysiological performance and production of an agricultural species under drought condition. AoB Plants 2016, 8, plw062. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, E.; Chamizo, S.; Roncero-Ramos, B.; Roman, R.; Canton, Y. Runoff from biocrust: A vital resource for vegetation performance on Mediterranean steppes. Ecohydrology 2018, 11, e1977. [Google Scholar] [CrossRef]
- Samolov, E.; Baumann, K.; Büdel, B.; Jung, P.; Leinweber, P.; Mikhailyuk, T.; Karsten, U.; Glaser, K. Biodiversity of Algae and Cyanobacteria in Biological Soil Crusts Collected Along a Climatic Gradient in Chile Using an Integrative Approach. Microorganisms 2020, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler-Robinson, E.; Nuanez, M.; Litvak, M.E. Biocrust contribution to ecosystem carbon fluxes varies along an elevational gradient. Ecosphere 2018, 9, e02315. [Google Scholar] [CrossRef]
- Heindel, R.C.; Governali, F.C.; Spickard, A.M.; Virginia, R.A. The Role of Biological Soil Crusts in Nitrogen Cycling and Soil Stabilization in Kangerlussuaq, West Greenland. Ecosystems 2018, 22, 243–256. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, X.; Zhao, C.; Tan, Q.; Luo, G.; Wang, J.; Li, Q.; Wu, L.; Chen, F.; Li, C.; et al. Global CO 2 Consumption by Silicate Rock Chemical Weathering: Its Past and Future. Earth’s Futur. 2021, 9, e2020EF001938. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Maher, K.; Steefel, C.I.; White, A.F.; Stonestrom, D. The role of reaction affinity and secondary minerals in regulating chemical weathering rates at the Santa Cruz Soil Chronosequence, California. Geochim. Cosmochim. Acta 2009, 73, 2804–2831. [Google Scholar] [CrossRef] [Green Version]
- Perez-Fodich, A.; Derry, L.A. Organic acids and high soil CO2 drive intense chemical weathering of Hawaiian basalts: Insights from reactive transport models. Geochim. Cosmochim. Acta 2019, 249, 173–198. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Li, Y.; Wei, W.; Zhang, J.; Wu, N. The variation of morphological features and mineralogical components of biological soil crusts in the Gurbantunggut Desert of Northwestern China. Environ. Earth Sci. 2008, 57, 1135–1143. [Google Scholar] [CrossRef]
- Suchet, P.A.; Probst, J. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: Application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993, 107, 205–210. [Google Scholar] [CrossRef]
- Finlay, R.D.; Mahmood, S.; Rosenstock, N.; Bolou-Bi, E.B.; Köhler, S.J.; Fahad, Z.; Rosling, A.; Wallander, H.; Belyazid, S.; Bishop, K. Biological weathering and its consequences at different spatial levels–from nanoscale to global scale. Biogeosciences 2020, 17, 1507–1533. [Google Scholar] [CrossRef] [Green Version]
- Beraldi-Campesi, H.; Hartnett, H.; Anbar, A.; Gordon, G.; Garcia-Pichel, F. Effect of biological soil crusts on soil elemental concentrations: Implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 2009, 7, 348–359. [Google Scholar] [CrossRef]
- Celle, H. Caractérisation des Précipitations sur le Pourtour de la Méditerranée Occidentale: Approche Isotopique et Chimique. Ph.D. Thesis, Université d’Avignon et des, Pays de Vaucluse, France, 2000. [Google Scholar]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: The cause of contrasting top–down controls on freshwater and marine phytoplankton. Oecologia 2005, 147, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Buss, H. Natural Weathering Rates of Silicate Minerals. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 115–155. [Google Scholar] [CrossRef]
- Gruber, C.; Zhu, C.; Georg, B.; Zakon, Y.; Ganor, J. Resolving the gap between laboratory and field rates of feldspar weathering. Geochim. Cosmochim. Acta 2014, 147, 90–106. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.; Eiriksdottir, E.S.; Kardjilov, M.I.; Gisladottir, G.; Sigfusson, B.; Snorrason, A.; Elefsen, S.; Hardardottir, J.; Torssander, P.; et al. Direct evidence of the feedback between climate and weathering. Earth Planet. Sci. Lett. 2009, 277, 213–222. [Google Scholar] [CrossRef]
- Beaulieu, E.; Godderis, Y.; Donnadieu, Y.; Labat, D.; Roelandt, C. High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nat. Clim. Chang. 2012, 2, 346–349. [Google Scholar] [CrossRef]
- Donnini, M.; Frondini, F.; Probst, J.-L.; Probst, A.; Cardellini, C.; Marchesini, I.; Guzzetti, F. Chemical weathering and consumption of atmospheric carbon dioxide in the Alpine region. Glob. Planet. Chang. 2016, 136, 65–81. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).