Krüppel-homologue 1 Mediates Hormonally Regulated Dominance Rank in a Social Bee
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bees
2.2. Behavioral Observations
2.3. Assessing Ovarian Activity and Wax Deposition
2.4. Reverse-Transcription Real-Time Quantitative PCR (qPCR)
2.5. Synthesis and Delivery of dsRNA
2.5.1. Perfluorocarbon Nanoparticles
2.5.2. Assessing PFC Nanoparticles Toxicity
2.5.3. Determining the Efficiency of dsRNA Loading onto the PFCnp
2.5.4. Determining the Time of PFCnp Injection
2.6. dsRNA Mediated Kr-h1 Knock-Down
2.6.1. RNA Interference (RNAi) with Naked dsRNA
2.6.2. Down-Regulating Kr-h1 Expression Using dsRNA Loaded on PFCnp
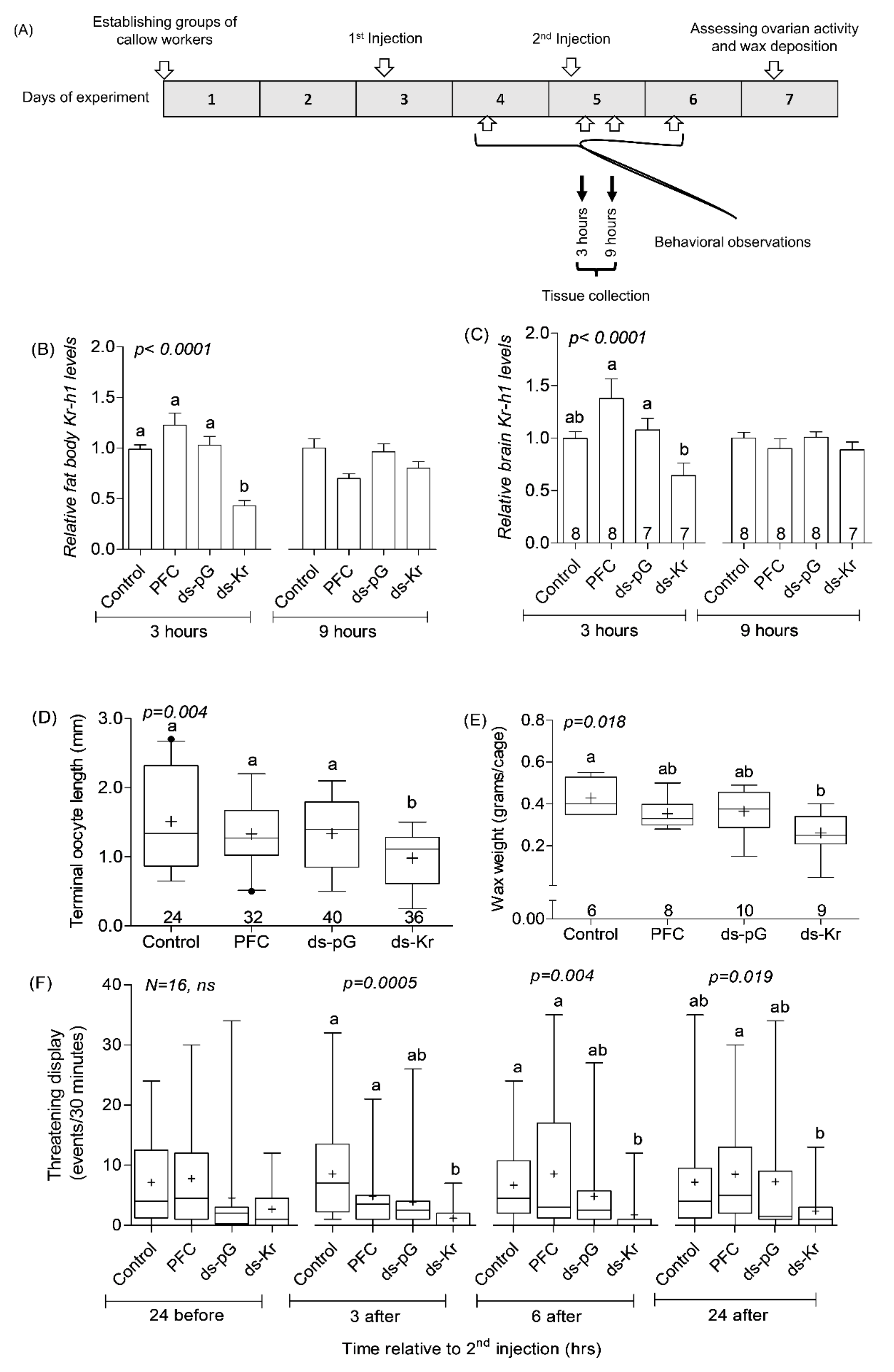
2.7. Experiment 1. The Influence of Kr-h1 on JH Regulated Physiology and Behavior in Groups of Similarly Treated Queenless Workers
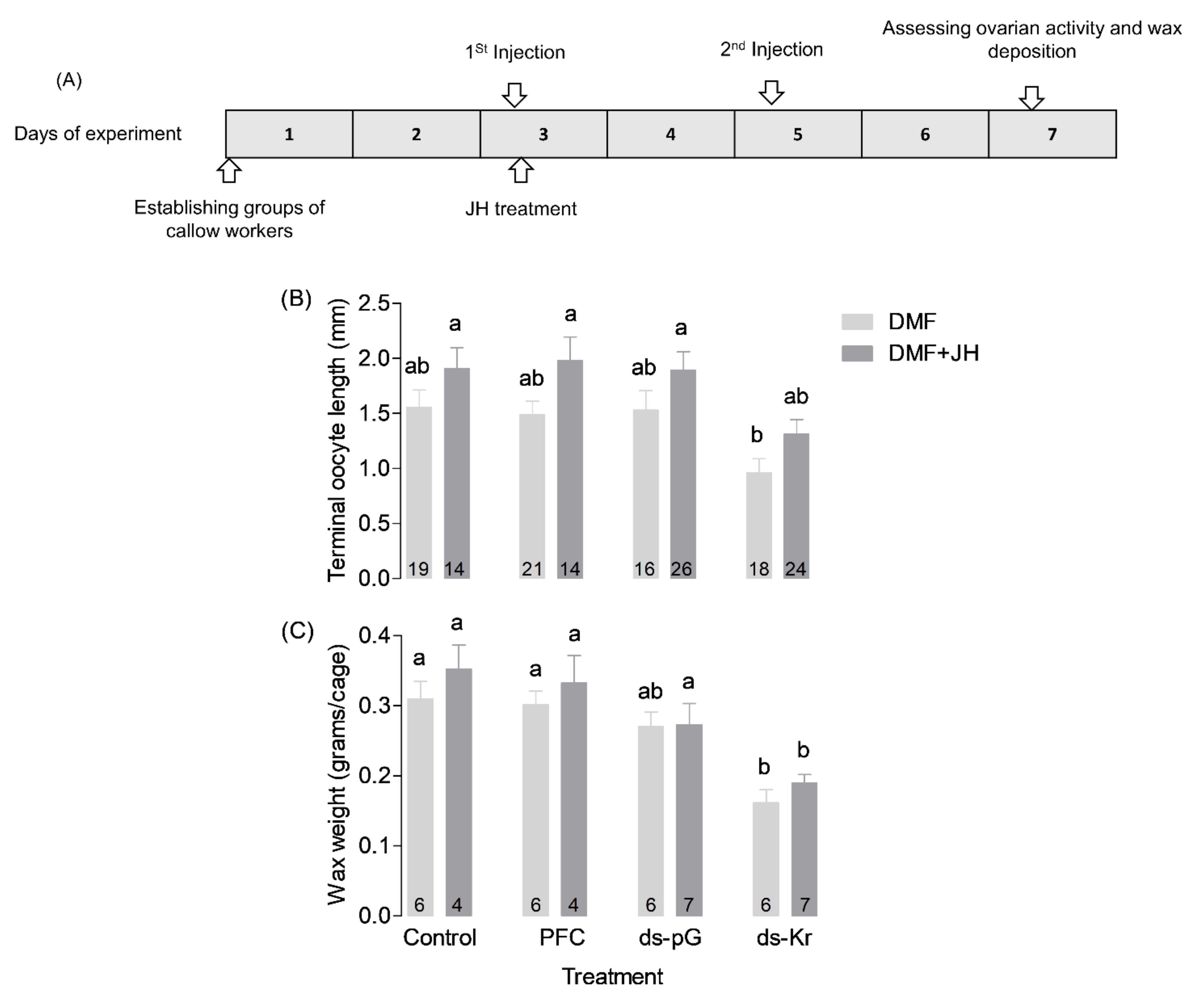
2.8. Experiment 2. The Influence of JH-III on Bees Treated with Kr-h1 dsRNA
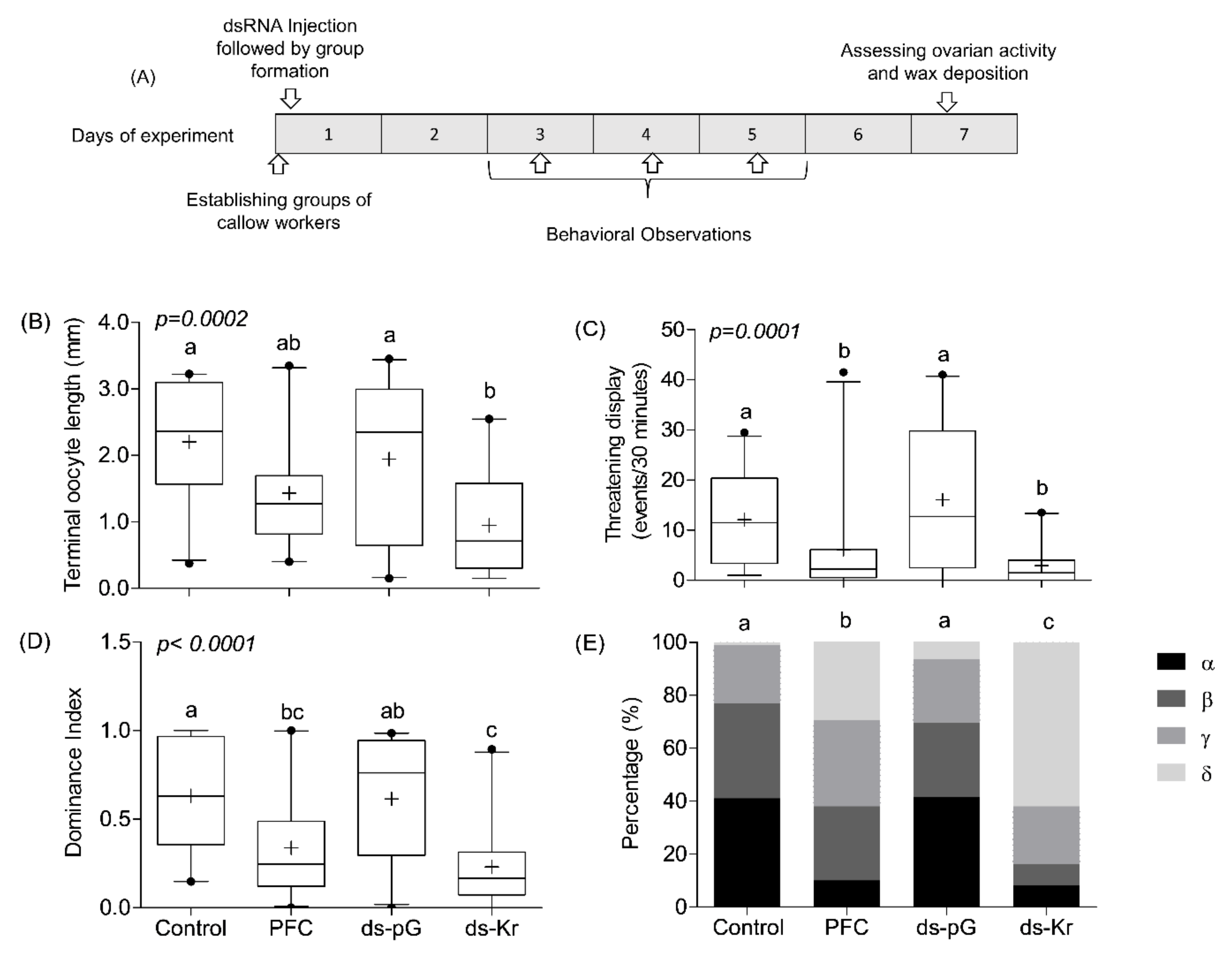
2.9. Experiment 3. The Influence of Kr-h1 on JH Regulated Physiology and Behavior in Groups of Queenless Workers, Each Subjected to a Different Treatment
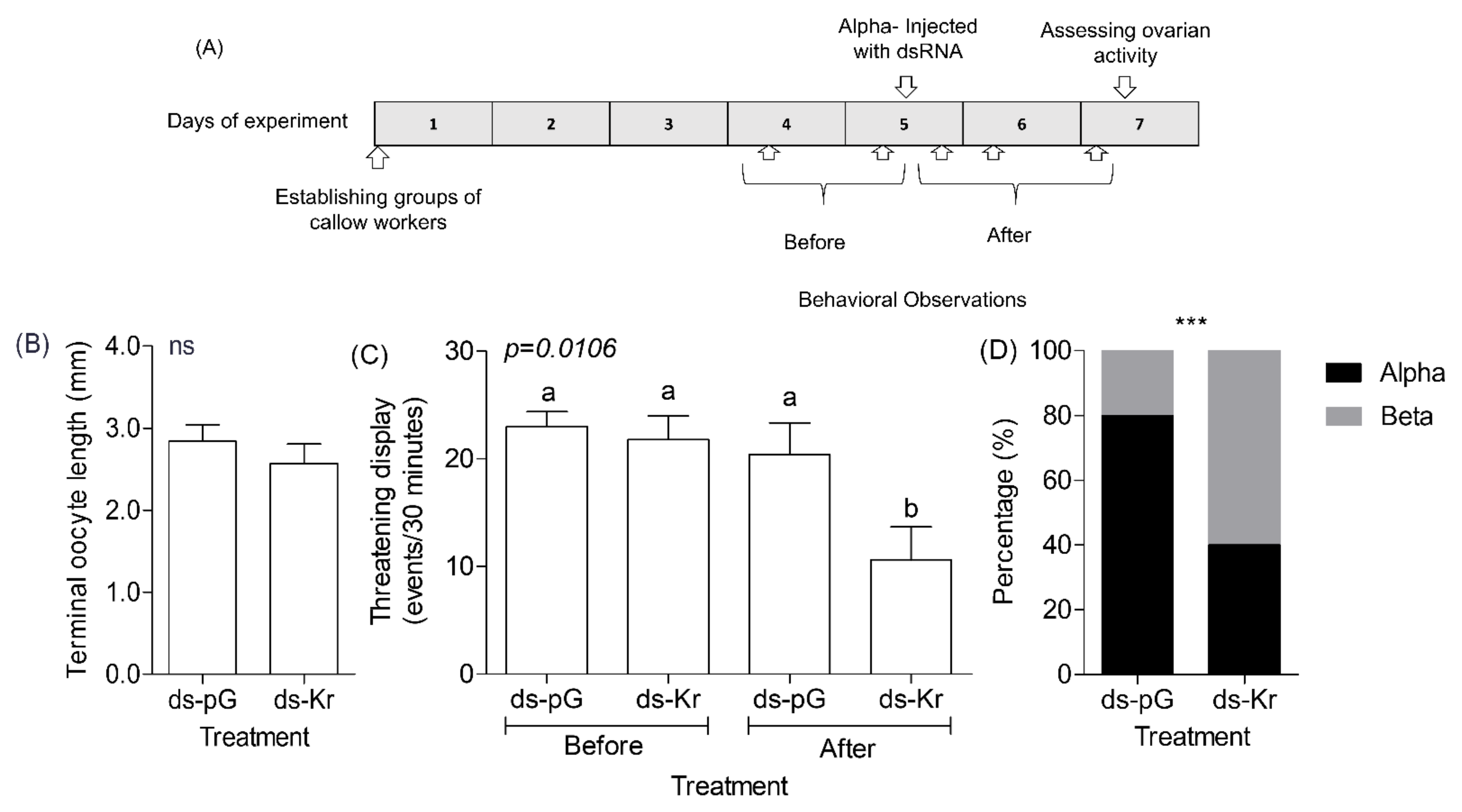
2.10. Experiment 4. The Influence of Kr-h1 on Aggression and Dominance in Groups of Queenless Workers That Have Already Established Dominance Hierarchy
2.11. Statistical Analyses
3. Results
3.1. Experiment 1. The Influence of Kr-h1 on JH Regulated Physiology and Behavior in Groups of Similarly Treated Queenless Workers
3.2. Experiment 2. The Influence of JH on Bees Treated with Kr-h1 dsRNA
3.3. Experiment 3. The Influence of Kr-h1 on JH Regulated Physiology and Behavior in Groups of Queenless Workers, Each Subjected to a Different Treatment
3.4. Experiment 4. The Influence of Kr-h1 on Aggression and Dominance in Groups of Queenless Workers That Have Already Established a Dominance Hierarchy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fero, K.; Simon, J.L.; Jourdie, V.; Moore, P.A. Consequences of social dominance on crayfish resource use. Behaviour 2007, 144, 61–82. [Google Scholar] [CrossRef]
- Georgiev, A.V.; Klimczuk, A.C.E.; Traficonte, D.M.; Maestripieri, D. When violence pays: A cost-benefit analysis of aggressive behavior in animals and humans. Evol. Psychol. 2013, 11, 678–699. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, G.A.; Cheng, J.T. Power, status, and hierarchy: Current trends and future challenges. Curr. Opin. Psychol. 2020, 33, iv–xiii. [Google Scholar] [CrossRef] [PubMed]
- Cowlishaw, G.; Dunbar, R.I.M. Dominance rank and mating success in male primates. Anim. Behav. 1991, 41, 1045–1056. [Google Scholar] [CrossRef]
- Gesquiere, L.R.; Learn, N.H.; Simao, M.C.M.; Onyango, P.O.; Alberts, S.C.; Altmann, J. Life at the top: Rank and stress in wild male baboons. Science 2011, 333, 357–360. [Google Scholar] [CrossRef]
- Archie, E.A.; Altmann, J.; Alberts, S.C. Social status predicts wound healing in wild baboons. Proc. Natl. Acad. Sci. USA 2012, 109, 9017–9022. [Google Scholar] [CrossRef]
- Snyder-Mackler, N.; Sanz, J.; Kohn, J.N.; Voyles, T.; Pique-Regi, R.; Wilson, M.E.; Barreiro, L.B.; Tung, J. Social status alters chromatin accessibility and the gene regulatory response to glucocorticoid stimulation in rhesus macaques. Proc. Natl. Acad. Sci. USA 2019, 116, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A. Social stress, immune functions and disease in rodents. Front. Neuroendocrinol. 2007, 28, 28–49. [Google Scholar] [CrossRef]
- Van der Kooij, M.A.; Sandi, C. The genetics of social hierarchies. Curr. Opin. Behav. Sci. 2015, 2, 52–57. [Google Scholar] [CrossRef]
- Barrette, C. Dominance cannot be inherited. Trends Ecol. Evol. 1987, 2, 251. [Google Scholar] [CrossRef]
- Fonberg, E. Dominance and aggression. Int. J. Neurosci. 1988, 41, 201–213. [Google Scholar] [CrossRef]
- Biro, P.A.; Stamps, J.A. Do consistent individual differences in metabolic rate promote consistent individual differences in behavior? Trends Ecol. Evol. 2010, 25, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.F.; McGregor, P.K.; Latruffe, C. Know thine enemy: Fighting fish gather information from observing conspecific interactions. Proc. R. Soc. B Biol. Sci. 1998, 265, 1045–1049. [Google Scholar] [CrossRef]
- Chase, I.D.; Bartolomeo, C.; Dugatkin, L.A. Aggressive interactions and inter-contest interval: How long do winners keep winning? Anim. Behav. 1994, 48, 393–400. [Google Scholar] [CrossRef]
- Feder, Y.; Nesher, E.; Ogran, A.; Kreinin, A.; Malatynska, E.; Yadid, G.; Pinhasov, A. Selective breeding for dominant and submissive behavior in Sabra mice. J. Affect. Disord. 2010, 126, 214–222. [Google Scholar] [CrossRef]
- Masur, J.; Benedito, M.A.C. Genetic selection of winner and loser rats in a competitive situation. Nature 1974, 249, 284. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Haynes, K.F.; Preziosi, R.F.; Moore, P.J. The evolution of interacting phenotypes: Genetics and evolution of social dominance. Am. Nat. 2002, 160, S186–S197. [Google Scholar] [CrossRef]
- Wang, F.; Kessels, H.W.; Hu, H. The mouse that roared: Neural mechanisms of social hierarchy. Trends Neurosci. 2014, 37, 674–682. [Google Scholar] [CrossRef]
- Maruska, K.P.; Zhang, A.; Neboori, A.; Fernald, R.D. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 2013, 25, 145–157. [Google Scholar] [CrossRef]
- Lema, S.C.; Sanders, K.E.; Walti, K.A. Arginine Vasotocin, Isotocin and nonapeptide receptor gene expression link to social status and aggression in sex-dependent patterns. J. Neuroendocrinol. 2015, 27, 142–157. [Google Scholar] [CrossRef]
- Timmer, M.; Cordero, M.I.; Sevelinges, Y.; Sandi, C. Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology 2011, 36, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, V.M.; Becker, R.O.; de Azevedo, M.S.; Morris, M.; Rigatto, K.; Almeida, S.; Lucion, A.B.; Giovenardi, M. Oxytocin modulates social interaction but is not essential for sexual behavior in male mice. Behav. Brain Res. 2013, 244, 130–136. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Dike, O.E.; Stevenson, E.L.; Storck, K.; Young, W.S. Social dominance in male vasopressin 1b receptor knockout mice. Horm. Behav. 2010, 58, 257–263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiao, J.Y. Neural basis of social status hierarchy across species. Curr. Opin. Neurobiol. 2010, 20, 803–809. [Google Scholar] [CrossRef]
- Holmes, A.; Murphy, D.L.; Crawley, J.N. Abnormal behavioral phenotypes of serotonin transporter knockout mice: Parallels with human anxiety and depression. Biol. Psychiatry 2003, 54, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Fox, M.A.; Gallagher, P.S.; Murphy, D.L. Hypolocomotion, anxiety and serotonin syndrome-like behavior contribute to the complex phenotype of serotonin transporter knockout mice. Genes Brain Behav. 2007, 6, 389–400. [Google Scholar] [CrossRef]
- Williamson, C.M.; Lee, W.; Curley, J.P. Temporal dynamics of social hierarchy formation and maintenance in male mice. Anim. Behav. 2016, 115, 259–272. [Google Scholar] [CrossRef]
- So, N.; Franks, B.; Lim, S.; Curley, J.P. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS ONE 2015, 10, e0134509. [Google Scholar] [CrossRef]
- Francis, R.C. On the relationship between aggression and social dominance. Ethology 1988, 78, 223–237. [Google Scholar] [CrossRef]
- Fernald, R.D. Cognitive skills needed for social hierarchies. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 229–236. [Google Scholar] [CrossRef]
- Langley, E.J.G.; Van Horik, J.O.; Whiteside, M.A.; Madden, J.R. Group social rank is associated with performance on a spatial learning task. R. Soc. Open Sci. 2018, 5, 171475. [Google Scholar] [CrossRef]
- Boogert, N.J.; Reader, S.M.; Laland, K.N. The relation between social rank, neophobia and individual learning in starlings. Anim. Behav. 2006, 72, 1229–1239. [Google Scholar] [CrossRef]
- Massen, J.J.M.; Pašukonis, A.; Schmidt, J.; Bugnyar, T. Ravens notice dominance reversals among conspecifics within and outside their social group. Nat. Commun. 2014, 5, 3679. [Google Scholar] [CrossRef] [PubMed]
- Vrontou, E.; Nilsen, S.P.; Demir, E.; Kravitz, E.A.; Dickson, B.J. Fruitless regulates aggression and dominance in drosophila. Nat. Neurosci. 2006, 9, 1469–1471. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.B.; Kravitz, E.A. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2007, 104, 19577–19582. [Google Scholar] [CrossRef]
- Pardi, L. Dominance order in Polistes wasps. Physiol. Zool. 1948, 21, 1–13. [Google Scholar] [CrossRef]
- Wilson, E.O. The Insect Societies; Harvard University Press: Cambridge, MA, USA, 1971; Volume 1, ISBN 0674454952. [Google Scholar]
- Free, J.B. The behaviour of egg-laying workers of bumblebee colonies. Br. J. Anim. Behav. 1955, 3, 147–153. [Google Scholar] [CrossRef]
- Zwarts, L.; Versteven, M.; Callaerts, P. Genetics and neurobiology of aggression in drosophila. Fly 2012, 6, 35–48. [Google Scholar] [CrossRef]
- Williamson, C.M.; Lee, W.; DeCasien, A.R.; Lanham, A.; Romeo, R.D.; Curley, J.P. Social hierarchy position in female mice is associated with plasma corticosterone levels and hypothalamic gene expression. Sci. Rep. 2019, 9, 7324. [Google Scholar] [CrossRef]
- Grieb, Z.A.; Ross, A.P.; McCann, K.E.; Lee, S.; Welch, M.; Gomez, M.G.; Norvelle, A.; Michopoulos, V.; Huhman, K.L.; Albers, H.E. Sex-dependent effects of social status on the regulation of arginine-vasopressin (AVP) V1a, oxytocin (OT), and serotonin (5-HT) 1A receptor binding and aggression in Syrian hamsters (Mesocricetus auratus). Horm. Behav. 2021, 127, 104878. [Google Scholar] [CrossRef]
- Toth, A.L.; Tooker, J.F.; Radhakrishnan, S.; Minard, R.; Henshaw, M.T.; Grozinger, C.M. Shared genes related to aggression, rather than chemical communication, are associated with reproductive dominance in paper wasps (Polistes metricus). BMC Genomics 2014, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Brown, M.J.F.; Toth, A.L. Candidate genes for cooperation and aggression in the social wasp Polistes dominula. J. Comp. Physiol. A 2018, 204, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, F.; Riba-Grognuz, O.; Wurm, Y.; Keller, L.; Shoemaker, D.W.; Grozinger, C.M. Sociogenomics of cooperation and conflict during colony founding in the fire ant Solenopsis invicta. PLoS Genet. 2013, 9, 1003633. [Google Scholar] [CrossRef]
- Steffen, M.A.; Rehan, S.M. Genetic signatures of dominance hierarchies reveal conserved cis-regulatory and brain gene expression underlying aggression in a facultatively social bee. Genes Brain Behav. 2020, 19, e12597. [Google Scholar] [CrossRef]
- Withee, J.R.; Rehan, S.M. Social aggression, experience, and brain gene expression in a subsocial bee. Integr. Comp. Biol. 2017, 57, 640–648. [Google Scholar] [CrossRef]
- Shpigler, H.Y.; Herb, B.; Drnevich, J.; Band, M.; Robinson, G.E.; Bloch, G. Juvenile hormone regulates brain-reproduction tradeoff in bumble bees but not in honey bees. Horm. Behav. 2020, 126, 104844. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Watanabe, Y.; Tin, M.M.Y.; Tsuji, K.; Mikheyev, A.S. Social dominance alters nutrition-related gene expression immediately: Transcriptomic evidence from a monomorphic queenless ant. Mol. Ecol. 2017, 26, 2922–2938. [Google Scholar] [CrossRef]
- Wingfield, J.C.; Hegner, R.E.; Dufty, A.M.; Ball, G.F. The “Challenge Hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am. Nat. 1990, 136, 829–846. [Google Scholar] [CrossRef]
- Terburg, D.; Van Honk, J. Approach-avoidance versus dominance-submissiveness: A multilevel neural framework on how testosterone promotes social status. Emot. Rev. 2013, 5, 296–302. [Google Scholar] [CrossRef]
- Adams, M.E. Juvenile hormones. In Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009; ISBN 9780123741448. [Google Scholar] [CrossRef]
- Goodman, W.G.; Cusson, M. The juvenile hormones. In Insect Endocrinology; Gilbert, L.I., Ed.; Academic Press: Cambridge, MA, USA, 2012; pp. 310–365. ISBN 9780123847492. [Google Scholar] [CrossRef]
- Jindra, M.; Palli, S.R.; Riddiford, L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013, 58, 181–204. [Google Scholar] [CrossRef]
- Jindra, M.; Bellés, X.; Shinoda, T. Molecular basis of juvenile hormone signaling. Curr. Opin. Insect Sci. 2015, 11, 39–46. [Google Scholar] [CrossRef]
- Pandey, A.; Bloch, G. Juvenile hormone and ecdysteroids as major regulators of brain and behavior in bees. Curr. Opin. Insect Sci. 2015, 12, 26–37. [Google Scholar] [CrossRef]
- Riddiford, L.M. Juvenile hormone action: A 2007 perspective. J. Insect Physiol. 2008, 54, 895–901. [Google Scholar] [CrossRef]
- Truman, J.W.; Riddiford, L.M. Endocrine insights into the evolution of metamorphosis in insects. Annu. Rev. Entomol. 2002, 47, 467–500. [Google Scholar] [CrossRef]
- De Loof, A.; Baggerman, G.; Breuer, M.; Claeys, U.; Cerstiaens, A.; Clynen, E.; Janssen, T.; Schoofs, L.; Broeck, J. Vanden Gonadotropins in insects: An overview. Arch. Insect Biochem. Physiol. 2001, 47, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Riddiford, L.M. How does juvenile hormone control insect metamorphosis and reproduction? Gen. Comp. Endocrinol. 2012, 179, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Delisle, J.; Cusson, M. Juvenile hormone biosynthesis, oocyte growth and vitellogenin accumulation in Choristoneura fumiferana and C. rosaceana: A comparative study. J. Insect Physiol. 1999, 45, 515–523. [Google Scholar] [CrossRef]
- Dong, S.-Z.; Ye, G.-Y.; Guo, J.Y.; Hu, C. Roles of ecdysteroid and juvenile hormone in vitellogenesis in an endoparasitic wasp, Pteromalus puparum (Hymenoptera: Pteromalidae). Gen. Comp. Endocrinol. 2009, 160, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Klowden, M.J. Endocrine aspects of mosquito reproduction. Arch. Insect Biochem. Physiol. 1997, 35, 491–512. [Google Scholar] [CrossRef]
- Panaitof, S.C.; Scott, M.P. Effect of juvenile hormone on vitellogenin gene expression in the fat body of burying beetles, Nicrophorus orbicollis. Arch. Insect Biochem. Physiol. 2006, 63, 82–91. [Google Scholar] [CrossRef]
- Bloch, G.; Wheeler, D.E.; Robinson, G.E. Endocrine influences on the organization of insect societies. In Hormones, Brain and Behavior; Pfaff, D.W., Arnold, A.P., Rubin, R.T., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 195–235. ISBN 9780125321044. [Google Scholar] [CrossRef]
- Robinson, G.E.; Vargo, E.L. Juvenile hormone in adult eusocial hymenoptera: Gonadotropin and behavioral pacemaker. Arch. Insect Biochem. Physiol. 1997, 35, 559–583. [Google Scholar] [CrossRef]
- Bloch, G.; Borst, D.W.; Huang, Z.-Y.; Robinson, G.E.; Cnaani, J.; Hefetz, A. Juvenile hormone titers, juvenile hormone biosynthesis, ovarian development and social environment in Bombus terrestris. J. Insect Physiol. 2000, 46, 47–57. [Google Scholar] [CrossRef]
- Röseler, P.F. Juvenile hormone control of oogenesis in bumblebee workers, Bombus terrestris. J. Insect Physiol. 1977, 23, 985–992. [Google Scholar] [CrossRef]
- Amsalem, E.; Teal, P.; Grozinger, C.M.; Hefetz, A. Precocene-I inhibits juvenile hormone biosynthesis, ovarian activation, aggression and alters sterility signal production in bumble bee (Bombus terrestris) workers. J. Exp. Biol. 2014, 217, 3178–3185. [Google Scholar] [CrossRef]
- Pandey, A.; Motro, U.; Bloch, G. Juvenile hormone interacts with multiple factors to modulate aggression and dominance in groups of orphan bumble bee (Bombus terrestris) workers. Horm. Behav. 2020, 117, 104602. [Google Scholar] [CrossRef] [PubMed]
- Shpigler, H.; Amsalem, E.; Huang, Z.Y.; Cohen, M.; Siegel, A.J.; Hefetz, A.; Bloch, G. Gonadotropic and physiological functions of juvenile hormone in bumblebee (Bombus terrestris) workers. PLoS ONE 2014, 9, e100650. [Google Scholar] [CrossRef] [PubMed]
- Shpigler, H.Y.; Magory Cohen, T.; Ben-Shimol, E.; Ben-Betzalel, R.; Levin, E. Juvenile hormone functions as a metabolic rate accelerator in bumble bees (Bombus terrestris). Horm. Behav. 2021, 136, 105073. [Google Scholar] [CrossRef]
- Van Doorn, A. Factors influencing dominance behaviour in queenless bumblebee workers (Bombus terrestris). Physiol. Entomol. 1989, 14, 211–221. [Google Scholar] [CrossRef]
- Larrere, M.; Couillaud, F. Role of juvenile hormone biosynthesis in dominance status and reproduction of the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol. 1993, 33, 335–338. [Google Scholar] [CrossRef]
- Pandey, A.; Motro, U.; Bloch, G. Juvenile hormone affects the development and strength of circadian rhythms in young bumble bee (Bombus terrestris) workers. Neurobiol. Sleep Circadian Rhythms 2020, 9, 100056. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Liu, Y.; An, Y.; Fang, H.; Michaud, J.P.; Zhang, H.; Li, Y.; Zhang, Q.; Li, Z. Molecular characterization of primary juvenile hormone responders Methoprene-Tolerant (Met) and Krüppel Homolog 1 (Kr-h1) in Grapholita molesta (Lepidoptera: Tortricidae) with clarification of their roles in metamorphosis and reproduction. J. Econ. Entomol. 2019, 112, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; He, H.; Qu, X.; Cai, Y.; Ding, W.; Qiu, L.; Li, Y. RNA interference-mediated knockdown of the transcription factor Krüppel homologue 1 suppresses vitellogenesis in Chilo suppressalis. Insect Mol. Biol. 2020, 29, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yang, R.-L.; Wang, W.-P.; Zhou, Q.-H.; Chen, E.-H.; Yuan, G.-R.; Wang, J.-J.; Dou, W. Involvement of Met and Kr-h1 in JH-mediated reproduction of female Bactrocera dorsalis (Hendel). Front. Physiol. 2018, 9, 482. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Yuan, S.Y.; Nanda, S.; Wang, W.X.; Lai, F.X.; Fu, Q.; Wan, P.J. The roles of E93 and Kr-h1 in metamorphosis of Nilaparvata lugens. Front. Physiol. 2018, 9, 1677. [Google Scholar] [CrossRef] [PubMed]
- Holtof, M.; Van Lommel, J.; Gijbels, M.; Dekempeneer, E.; Nicolai, B.; Broeck, J.V.; Marchal, E. Crucial role of juvenile hormone receptor components Methoprene-Tolerant and Taiman in sexual maturation of adult male desert locusts. Biomolecules 2021, 11, 244. [Google Scholar] [CrossRef]
- Zhu, Z.; Tong, C.; Qiu, B.; Yang, H.; Xu, J.; Zheng, S.; Song, Q.; Feng, Q.; Deng, H. 20E-mediated regulation of BmKr-h1 by BmKRP promotes oocyte maturation. BMC Biol. 2021, 19, 39. [Google Scholar] [CrossRef]
- Guo, W.; Song, J.; Yang, P.; Chen, X.; Chen, D.; Ren, D.; Kang, L.; Wang, X. Juvenile hormone suppresses aggregation behavior through influencing antennal gene expression in locusts. PLoS Genet. 2020, 16, e1008762. [Google Scholar] [CrossRef]
- Fussnecker, B.; Grozinger, C. Dissecting the role of Kr-h1 brain gene expression in foraging behavior in honey bees (Apis mellifera). Insect Mol. Biol. 2008, 17, 515–522. [Google Scholar] [CrossRef]
- Shpigler, H.; Patch, H.M.; Cohen, M.; Fan, Y.; Grozinger, C.M.; Bloch, G. The transcription factor Krüppel homolog 1 is linked to hormone mediated social organization in bees. BMC Evol. Biol. 2010, 10, 120. [Google Scholar] [CrossRef]
- Grozinger, C.M.; Sharabash, N.M.; Whitfield, C.W.; Robinson, G.E. Pheromone-mediated gene expression in the honey bee brain. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. 2), 14519–14525. [Google Scholar] [CrossRef]
- Minakuchi, C.; Zhou, X.; Riddiford, L.M. Krüppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech. Dev. 2008, 125, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wu, Z.; Wang, Z.; Deng, S.; Zhou, S. Krüppel-homolog 1 mediates juvenile hormone action to promote vitellogenesis and oocyte maturation in the migratory locust. Insect Biochem. Mol. Biol. 2014, 52, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Konopova, B.; Jindra, M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2007, 104, 10488–10493. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Albishi, N.M.; Palli, S.R. Identification of juvenile hormone-induced posttranslational modifications of Methoprene tolerant and Krüppel homolog 1 in the yellow fever mosquito, Aedes aegypti. J. Proteom. 2021, 242, 104257. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wu, Q.-W.; Tian, Z.; Zhu, L.; King-Jones, K.; Zhu, F.; Wang, X.; Liu, W. Krüppel homolog 1 regulates photoperiodic reproductive plasticity in the cabbage beetle Colaphellus bowringi. Insect Biochem. Mol. Biol. 2021, 134, 103582. [Google Scholar] [CrossRef] [PubMed]
- Kayukawa, T.; Minakuchi, C.; Namiki, T.; Togawa, T.; Yoshiyama, M.; Kamimura, M.; Mita, K.; Imanishi, S.; Kiuchi, M.; Ishikawa, Y.; et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. USA 2012, 109, 11729–11734. [Google Scholar] [CrossRef]
- Zhang, T.; Song, W.; Li, Z.; Qian, W.; Wei, L.; Yang, Y.; Wang, W.; Zhou, X.; Meng, M.; Peng, J.; et al. Krüppel homolog 1 represses insect ecdysone biosynthesis by directly inhibiting the transcription of steroidogenic enzymes. Proc. Natl. Acad. Sci. USA 2018, 115, 3960–3965. [Google Scholar] [CrossRef]
- Zhu, Z.-D.; Hu, Q.-H.; Tong, C.-M.; Yang, H.-G.; Zheng, S.-C.; Feng, Q.-L.; Deng, H.-M. Transcriptomic analysis reveals the regulation network of BmKrüppel homolog 1 in the oocyte development of Bombyx mori. Insect Sci. 2021, 28, 47–62. [Google Scholar] [CrossRef]
- Ling, L.; Raikhel, A. Cross-talk of insulin-like peptides, juvenile hormone, and 20-hydroxyecdysone in regulation of metabolism in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2021, 118, e2023470118. [Google Scholar] [CrossRef]
- Hu, K.; Tian, P.; Yang, L.; Tang, Y.; Qiu, L.; He, H.; Ding, W.; Li, Y. Molecular characterization of the Krüppel-homolog 1 and its role in ovarian development in Sogatella furcifera (Hemiptera: Delphacidae). Mol. Biol. Rep. 2020, 47, 1099–1106. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, Y.; Lin, X. Role of Broad-Complex (Br) and Krüppel homolog 1 (Kr-h1) in the ovary development of Nilaparvata lugens. Front. Physiol. 2017, 8, 1013. [Google Scholar] [CrossRef]
- Lin, X.; Yao, Y.; Wang, B. Methoprene-tolerant (Met) and Krüpple-homologue 1 (Kr-h1) are required for ovariole development and egg maturation in the brown plant hopper. Sci. Rep. 2015, 5, 18064. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, L.; Liu, C.; Chen, L.; Xiao, H.-J.; Liang, G.-M. Dissecting the role of Krüppel homolog 1 in the metamorphosis and female reproduction of the cotton bollworm, Helicoverpa armigera. Insect Mol. Biol. 2018, 27, 492–504. [Google Scholar] [CrossRef]
- Minakuchi, C.; Namiki, T.; Shinoda, T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009, 325, 341–350. [Google Scholar] [CrossRef]
- Amsalem, E.; Galbraith, D.A.; Cnaani, J.; Teal, P.E.A.; Grozinger, C.M. Conservation and modification of genetic and physiological toolkits underpinning diapause in bumble bee queens. Mol. Ecol. 2015, 24, 5596–5615. [Google Scholar] [CrossRef] [PubMed]
- Jedlička, P.; Ernst, U.R.; Votavová, A.; Hanus, R.; Valterová, I. Gene expression dynamics in major endocrine regulatory pathways along the transition from solitary to social life in a bumblebee, Bombus terrestris. Front. Physiol. 2016, 7, 574. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.; Borst, D.W.; Huang, Z.-Y.; Robinson, G.E.; Hefetz, A. Effects of social conditions on juvenile hormone mediated reproductive development in Bombus terrestris workers. Physiol. Entomol. 1996, 21, 257–267. [Google Scholar] [CrossRef]
- Duchateau, M.J. Agonistic behaviours in colonies of the bumblebee Bombus terrestris. J. Ethol. 1989, 7, 141–151. [Google Scholar] [CrossRef]
- Geva, S.; Hartfelder, K.; Bloch, G. Reproductive division of labor, dominance, and ecdysteroid levels in hemolymph and ovary of the bumble bee Bombus terrestris. J. Insect Physiol. 2005, 51, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, A.; Heringa, J. The ontogeny of a dominance hierarchy in colonies of the bumblebee Bombus terrestris (Hymenoptera, Apidae). Insectes Sociaux 1986, 33, 3–25. [Google Scholar] [CrossRef]
- Duchateau, M.J.; Velthuis, H.H.W. Ovarian development and egg laying in workers of Bombus terrestris. Entomol. Exp. Appl. 1989, 51, 199–213. [Google Scholar] [CrossRef]
- Hagai, T.; Cohen, M.; Bloch, G. Genes encoding putative Takeout/juvenile hormone binding proteins in the honeybee (Apis mellifera) and modulation by age and juvenile hormone of the takeout-like gene GB19811. Insect Biochem. Mol. Biol. 2007, 37, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Winter, P.M.; Yu, X.; Fuhrhop, R.W.; Scott, M.J.; Hockett, F.; Robertson, J.D.; Gaffney, P.J.; Lanza, G.M.; Wickline, S.A. Quantitative “magnetic resonance immunohistochemistry” with ligand-targeted 19F nanoparticles. Magn. Reson. Med. 2004, 52, 1255–1262. [Google Scholar] [CrossRef]
- Chen, J.; Pan, H.; Lanza, G.M.; Wickline, S.A. Perfluorocarbon nanoparticles for physiological and molecular imaging and therapy. Adv. Chronic Kidney Dis. 2013, 20, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Caruthers, S.; Tran, T.; Marsh, J.; Wallace, K.; Cyrus, T.; Partlow, K.; Scott, M.; Lijowski, M.; Neubauer, A.; et al. Perfluorocarbon nanoparticles for molecular imaging and targeted therapeutics. Proc. IEEE 2008, 96, 397–415. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High drug-loading nanomedicines: Progress, current status, and prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Perez, J.M. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. Am. Chem. Soc. Nano 2010, 4, 5321–5331. [Google Scholar] [CrossRef]
- Yerushalmi, S.; Bodenhaimer, S.; Bloch, G. Developmentally determined attenuation in circadian rhythms links chronobiology to social organization in bees. J. Exp. Biol. 2006, 209, 1044–1051. [Google Scholar] [CrossRef]
- Jandt, J.M.; Tibbetts, E.A.; Toth, A.L. Polistes paper wasps: A model genus for the study of social dominance hierarchies. Insectes Sociaux 2013, 61, 11–27. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Peng, Y.; Pu, J.; Fu, W.; Wang, J.; Han, Z. Variation in RNAi efficacy among insect species is attributable to dsRNA degradation in vivo. Insect Biochem. Mol. Biol. 2016, 77, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.W.; Yu, Z.; Biondi, M.; Song, H.; Silver, K.; Zhang, J.; Zhu, K.Y. Stability of double-stranded RNA in gut contents and hemolymph of Ostrinia nubilalis larvae. Pestic. Biochem. Physiol. 2020, 169, 104672. [Google Scholar] [CrossRef]
- Wang, K.; Peng, Y.; Chen, J.; Peng, Y.; Wang, X.; Shen, Z.; Han, Z. Comparison of efficacy of RNAi mediated by various nanoparticles in the rice striped stem borer (Chilo suppressalis). Pestic. Biochem. Physiol. 2020, 165, 104467. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Braukmann, F.; Jordan, D.; Miska, E.A. A genetic pathway encoding double-stranded RNA transporters and interactors regulates growth and plasticity in Caenorhabditis elegans. bioRxiv 2019, 694414. [Google Scholar] [CrossRef]
- Lichtenberg, S.S.; Tsyusko, O.V.; Palli, S.R.; Unrine, J.M. Uptake and bioactivity of Chitosan/double-stranded RNA polyplex nanoparticles in Caenorhabditis elegans. Environ. Sci. Technol. 2019, 53, 3832–3840. [Google Scholar] [CrossRef]
- Sadd, B.M.; Barribeau, S.M.; Bloch, G.; de Graaf, D.C.; Dearden, P.; Elsik, C.G.; Gadau, J.; Grimmelikhuijzen, C.J.P.; Hasselmann, M.; Lozier, J.D.; et al. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol. 2015, 16, 76. [Google Scholar] [CrossRef]
- Dekkers, T.J.; van Rentergem, J.A.A.; Meijer, B.; Popma, A.; Wagemaker, E.; Huizenga, H.M. A meta-analytical evaluation of the dual-hormone hypothesis: Does cortisol moderate the relationship between testosterone and status, dominance, risk taking, aggression, and psychopathy? Neurosci. Biobehav. Rev. 2019, 96, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Anestis, S.F. Testosterone in juvenile and adolescent male chimpanzees (Pan troglodytes): Effects of dominance rank, aggression, and behavioral style. Am. J. Phys. Anthropol. 2006, 130, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.H.; Lester, L.J.; Sroka, P.; Kessler, T.; Hearn, R. Juvenile hormone promotes dominance behavior and ovarian development in social wasps (Polistes annularis). Experientia 1975, 31, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Bloch, G.; Wheeler, D.E.; Robinson, G.E. Endocrine influences on the organization of insect societies. In Hormones, Brain and Behavior Online; Pfaff, D.W., Arnold, A.P., Rubin, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 1027–1070. ISBN 9780080887838. [Google Scholar] [CrossRef]
- Bortolotti, L.; Bogo, G.; Pošćić, F.; Duchateau, M.J. Effect of a topical treatment with juvenile hormone analogues on dominance, ovarian development and corpora allata size in Bombus terrestris workers. Bull. Insectology 2020, 73, 303–311. [Google Scholar]
- Tibbetts, E.A.; Huang, Z.Y. The challenge hypothesis in an insect: Juvenile hormone increases during reproductive conflict following queen loss in Polistes Wasps. Am. Nat. 2010, 176, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, E.A.; Izzo, A.S. Endocrine mediated phenotypic plasticity: Condition-dependent effects of juvenile hormone on dominance and fertility of wasp queens. Horm. Behav. 2009, 56, 527–531. [Google Scholar] [CrossRef]
- Moda, L.M.; Vieira, J.; Guimarães Freire, A.C.; Bonatti, V.; Bomtorin, A.D.; Barchuk, A.R.; Simões, Z.L.P. Nutritionally driven differential gene expression leads to heterochronic brain development in honeybee castes. PLoS ONE 2013, 8, e64815. [Google Scholar] [CrossRef]
- Roy, A.; Palli, S.R. Epigenetic modifications acetylation and deacetylation play important roles in juvenile hormone action. BMC Genomics 2018, 19, 934. [Google Scholar] [CrossRef]
- Gassias, E.; Maria, A.; Couzi, P.; Demondion, E.; Durand, N.; Bozzolan, F.; Aguilar, P.; Debernard, S. Involvement of Methoprene-tolerant and Krüppel homolog 1 in juvenile hormone-signaling regulating the maturation of male accessory glands in the moth Agrotis ipsilon. Insect Biochem. Mol. Biol. 2021, 132, 103566. [Google Scholar] [CrossRef]
- Li, X.; Chen, T.; Jiang, H.; Huang, J.; Huang, M.; Xu, R.; Xie, Q.; Zhu, H.; Su, S. Effects of methyl farnesoate on Krüppel homolog 1 (Kr-h1) during vitellogenesis in the Chinese mitten crab (Eriocheir sinensis). Anim. Reprod. Sci. 2021, 224, 106653. [Google Scholar] [CrossRef]
- Duportets, L.; Bozzolan, F.; Abrieux, A.; Maria, A.; Gadenne, C.; Debernard, S. The transcription factor Krüppel homolog 1 is linked to the juvenile hormone-dependent maturation of sexual behavior in the male moth, Agrotis ipsilon. Gen. Comp. Endocrinol. 2012, 176, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Shpigler, H.Y.; Siegel, A.J.; Huang, Z.Y.; Bloch, G. No effect of juvenile hormone on task performance in a bumblebee (Bombus terrestris) supports an evolutionary link between endocrine signaling and social complexity. Horm. Behav. 2016, 85, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Argue, K.J.; Yun, A.J.; Neckameyer, W.S. Early manipulation of juvenile hormone has sexually dimorphic effects on mature adult behavior in Drosophila melanogaster. Horm. Behav. 2013, 64, 589–597. [Google Scholar] [CrossRef][Green Version]
- Lee, S.S.; Ding, Y.; Karapetians, N.; Rivera-Perez, C.; Noriega, F.G.; Adams, M.E. Hormonal signaling cascade during an early-adult critical period required for courtship memory retention in drosophila. Curr. Biol. 2017, 27, 2798–2809.e3. [Google Scholar] [CrossRef]
- Mathiron, A.G.E.; Earley, R.L.; Goubault, M. Juvenile hormone manipulation affects female reproductive status and aggressiveness in a non-social parasitoid wasp. Gen. Comp. Endocrinol. 2019, 274, 80–86. [Google Scholar] [CrossRef]
- Montagna, T.S.; Raizer, J.; Antonialli-Junior, W.F. Effect of larval topical application of juvenile hormone on caste determination in the independent-founding eusocial wasp Mischocyttarus consimilis (Hymenoptera: Vespidae). Open J. Anim. Sci. 2015, 5, 174–184. [Google Scholar] [CrossRef][Green Version]
- Norman, V.C.; Hughes, W.O.H.H. Behavioural effects of juvenile hormone and their influence on division of labour in leaf-cutting ant societies. J. Exp. Biol. 2016, 219, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Sasagawa, H.; Sasaki, M.; Okada, I. Hormonal control of the division of labor in adult honeybees (Apis mellifera L.). I. Effect of methoprene on Corpora allata and hypopharyngeal gland, and its α-Glucosidase activity. Appl. Entomol. Zool. 1989, 24, 66–77. [Google Scholar] [CrossRef]
- Whitmore, D.; Whitmore, E.; Gilbert, L.I. Juvenile hormone induction of esterases: A mechanism for the regulation of juvenile hormone titer. Proc. Natl. Acad. Sci. USA 1972, 69, 1592–1595. [Google Scholar] [CrossRef]
- Borovsky, D.; Carlson, D.A.; Ujváry, I. In Vivo and In Vitro biosynthesis and metabolism of methyl farnesoate, juvenile hormone III, and juvenile hormone III acid in the mosquito Aedes aegypti. J. Med. Entomol. 1992, 29, 619–629. [Google Scholar] [CrossRef]
- Ajami, A.M.; Riddiford, L.M. Comparative metabolism of the Cecropia juvenile hormone. J. Insect Physiol. 1973, 19, 635–645. [Google Scholar] [CrossRef]
- Kramer, S.J.; Wieten, M.; de Kort, C.A.D. Metabolism of juvenile hormone in the colorado potato beetle, Leptinotarsa decemlineata. Insect Biochem. 1977, 7, 231–236. [Google Scholar] [CrossRef]
- Lanzrein, B. The activity and stability of injected juvenile hormones (JH I, JH II, and JH III) in last-instar larvae and adult females of the cockroach Nauphoeta cinerea. Gen. Comp. Endocrinol. 1979, 39, 69–78. [Google Scholar] [CrossRef]
- George, S.; Gaddelapati, S.C.; Palli, S.R. Histone deacetylase 1 suppresses Krüppel homolog 1 gene expression and influences juvenile hormone action in Tribolium castaneum. Proc. Natl. Acad. Sci. USA 2019, 116, 17759–17764. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jia, Q.-Q.; Li, S. Juvenile hormone signaling—A mini review. Insect Sci. 2019, 26, 600–606. [Google Scholar] [CrossRef]
- Mao, Y.; Li, Y.; Gao, H.; Lin, X. Krüppel homologue 1 interacts directly with Hairy and regulates ecdysis in the brown planthopper. Insect Mol. Biol. 2020, 29, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.T.; Roy, S.; Pei, G.; Dou, W.; Zou, Z.; Raikhel, A.S. Synergistic action of the transcription factors Krüppel homolog 1 and Hairy in juvenile hormone/Methoprene-tolerant-mediated gene-repression in the mosquito Aedes aegypti. PLoS Genet. 2019, 15, e1008443. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.S.K.; Law, S.T.S.; Li, C.; Qu, Z.; Bendena, W.G.; Tobe, S.S.; Hui, J.H.L. Diversity of insect sesquiterpenoid regulation. Front. Genet. 2020, 11, 1027. [Google Scholar] [CrossRef]
| Body Size Category | Marginal Wing Cell Length (mm) | Injection Volume (µL/Bee) | dsRNA Amount (µg/Bee) |
|---|---|---|---|
| Small | 2.6–2.8 | 10 | 1.0 |
| Medium | 2.9–3.0 | 15 | 1.5 |
| Large | 3.1–3.3 | 20 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandey, A.; Bloch, G. Krüppel-homologue 1 Mediates Hormonally Regulated Dominance Rank in a Social Bee. Biology 2021, 10, 1188. https://doi.org/10.3390/biology10111188
Pandey A, Bloch G. Krüppel-homologue 1 Mediates Hormonally Regulated Dominance Rank in a Social Bee. Biology. 2021; 10(11):1188. https://doi.org/10.3390/biology10111188
Chicago/Turabian StylePandey, Atul, and Guy Bloch. 2021. "Krüppel-homologue 1 Mediates Hormonally Regulated Dominance Rank in a Social Bee" Biology 10, no. 11: 1188. https://doi.org/10.3390/biology10111188
APA StylePandey, A., & Bloch, G. (2021). Krüppel-homologue 1 Mediates Hormonally Regulated Dominance Rank in a Social Bee. Biology, 10(11), 1188. https://doi.org/10.3390/biology10111188







