Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. Sample Preparation for Scanning Electronic Microscopy
2.3. DNA Extraction, Molecular Marker Amplification, and Sequencing
2.4. Phylogenetic Analysis
3. Results and Discussion
3.1. Conserved Morphology and Sensilla Type of Antenna in Stink Bugs
3.2. Ultrastructural Variations of the Labia between Species with Different Feeding Habits
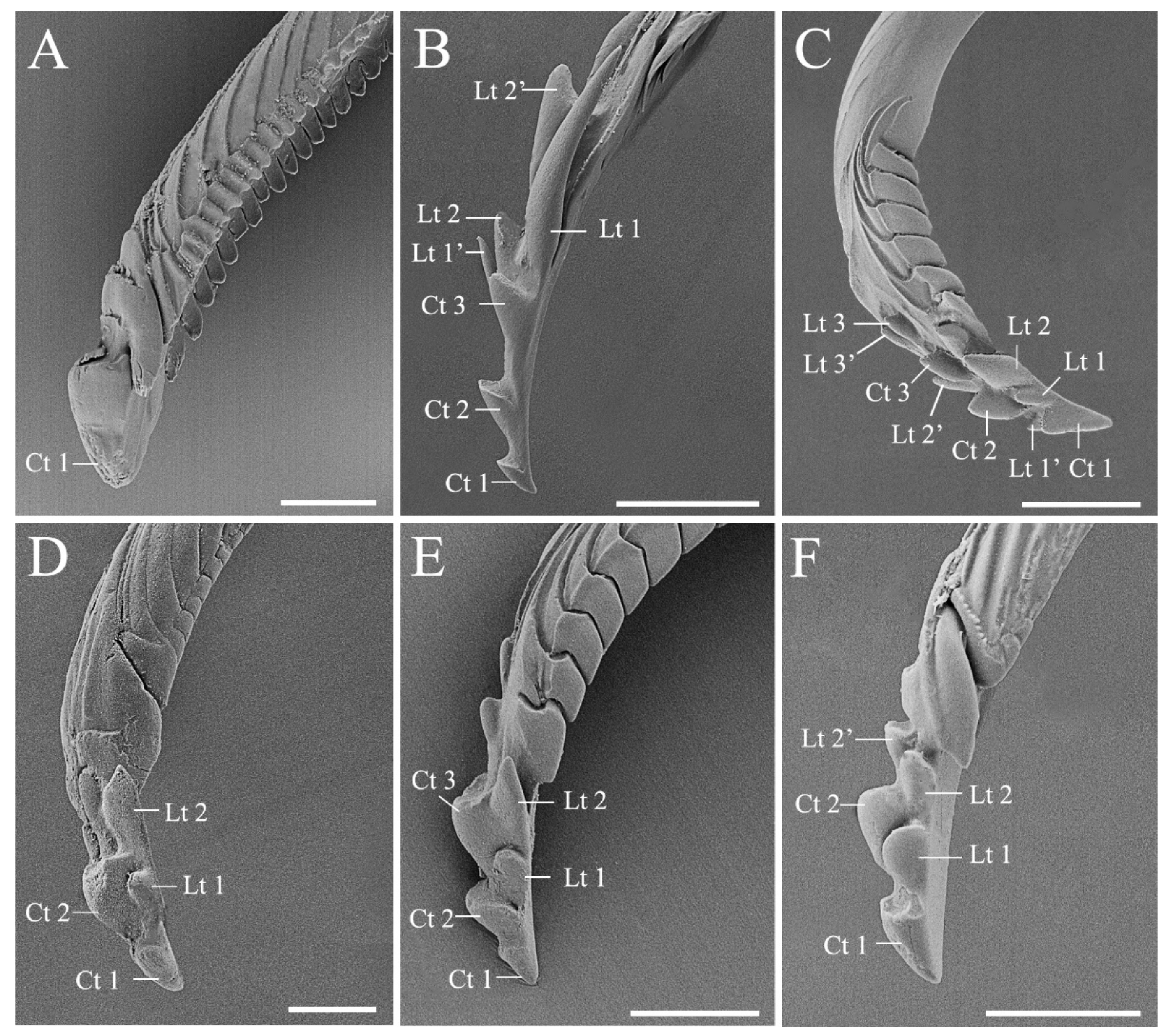
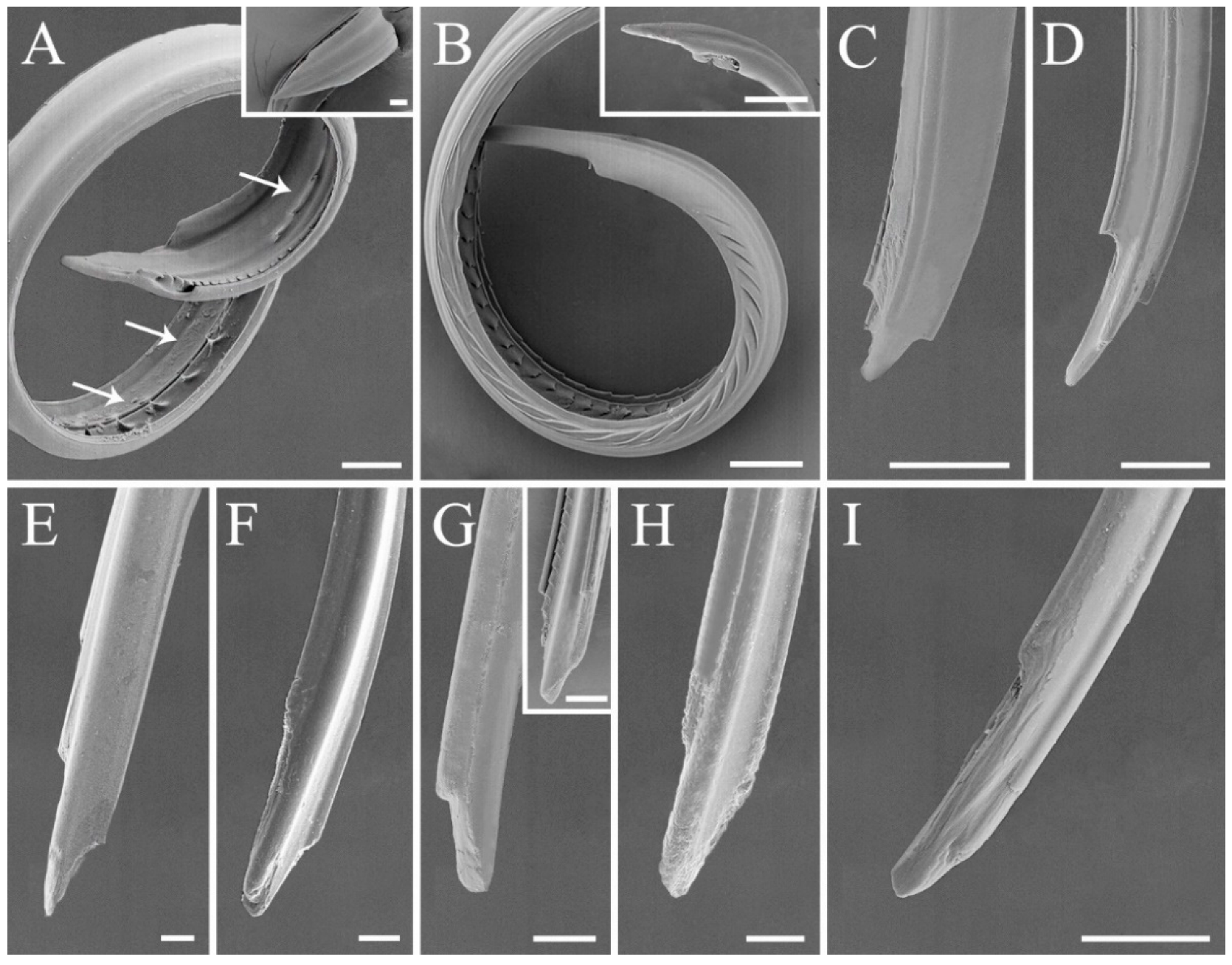
3.3. Modified Ultrastructure of Mandibles and Maxillae between Species with Different Feeding Habits
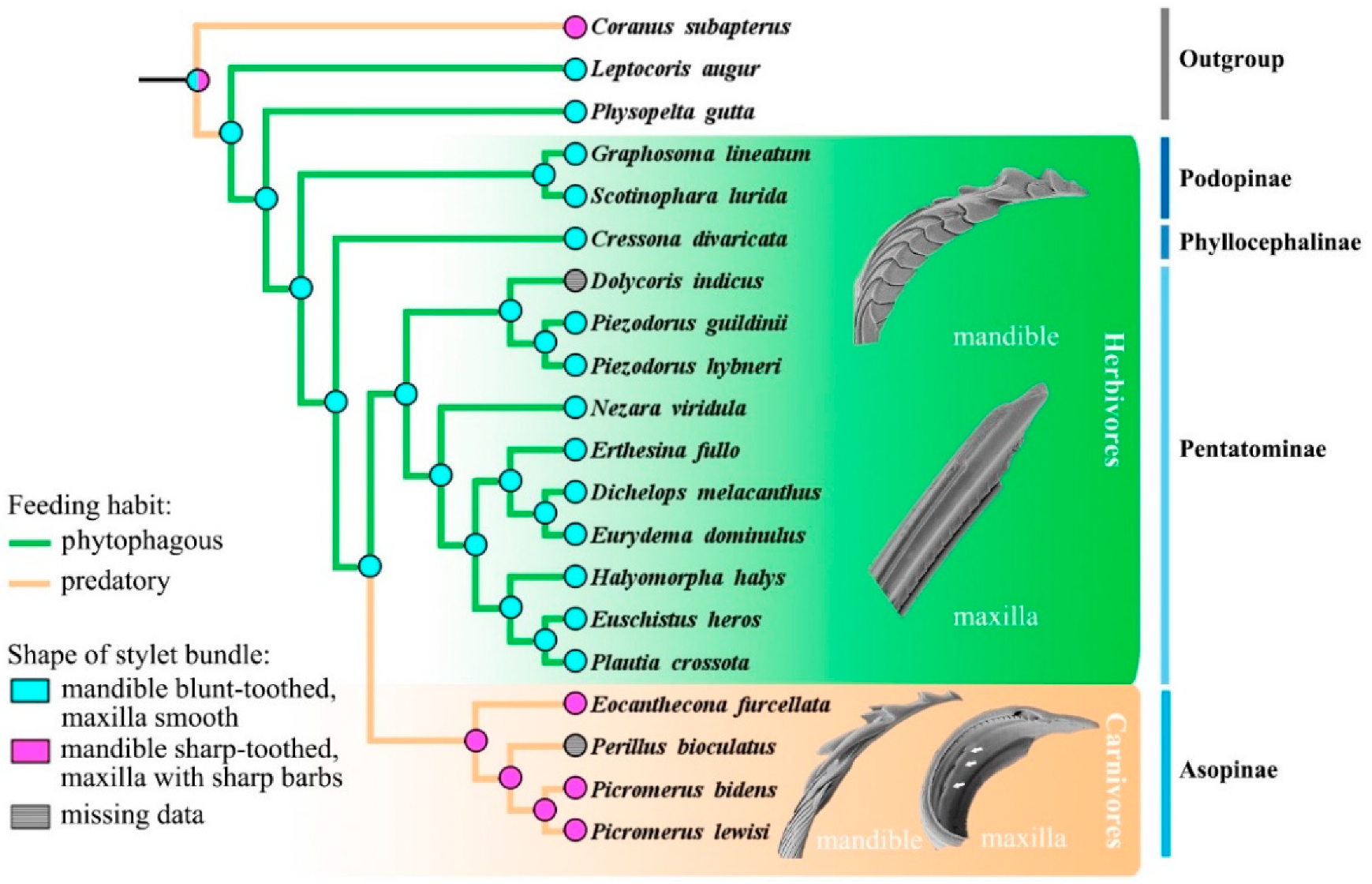
3.4. Ancestral States and Morphological Adaptations of Stink Bug Stylets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, T.J. Biodiversity of Heteroptera. In Insect Biodiversity: Science and Society; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2009; Volume 1, pp. 223–263. [Google Scholar]
- Schuh, R.T.; Weirauch, C. The True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History, 2nd ed.; Siri Scientific Press: Manchester, UK, 2020; pp. 1–769. [Google Scholar]
- Panizzi, A.R.; Grazia, J. True Bugs (Heteroptera) of the Neotropics; Springer: Dordrecht, The Netherlands, 2015; pp. 1–901. [Google Scholar]
- Cobben, R.H. Evolutionary Trends in Heteroptera. Part 2. Mouth Part Structures and Feeding Strategies. Mededlingen Landbouwhogeschool; H. Veeman & Zonen B.V.: Wageningen, The Netherlands, 1978; pp. 1–407. [Google Scholar]
- Cohen, A.C. Feeding adaptations of some predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990, 83, 1215–1223. [Google Scholar] [CrossRef]
- Krenn, H.W.; Aspöck, H. Form, function and evolution of the mouthparts of blood-feeding Arthropoda. Arthropod Struct. Dev. 2012, 41, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.; Zettel, H. A comparison of the external morphology and functions of labial tip sensilla. Eur. J. Entomol. 2014, 111, 275–297. [Google Scholar] [CrossRef]
- Swart, C.C.; Felgenhauer, B.E. Structure and function of the mouthparts and salivary gland complex of the giant waterbug, Belostoma lutarium (Stål) (Hemiptera: Belostomatidae). Ann. Entomol. Soc. Am. 2003, 96, 870–882. [Google Scholar] [CrossRef]
- Depieri, R.A.; Panizzi, A.R. Rostrum length, mandible serration, and food and salivary canals areas of selected species of stink bugs (Heteroptera, Pentatomidae). Rev. Bras. Entomol. 2010, 54, 584–587. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Brożek, J.; Dai, W. Morphological disparity of the mouthparts in polyphagous species of Largidae (Heteroptera: Pentatomomorpha: Pyrrhocoroidea) reveals feeding specialization. Insects 2020, 11, 145. [Google Scholar] [CrossRef]
- Silva, C.C.A.; de Capdeville, G.; Moraes, M.C.B.; Falcão, R.; Solino, L.F.; Laumann, R.A.; Silva, J.P.; Borges, M. Morphology, distribution and abundance of antennal sensilla in three stink bug species (Hemiptera: Pentatomidae). Micron 2010, 41, 289–300. [Google Scholar] [CrossRef]
- Ahmad, A.; Parveen, S.; Brożek, J.; Dey, D. Antennal sensilla of phytophagous and predatory pentatomids (Hemiptera: Pentatomidae): A comparative study of four genera. Zool. Anz. 2016, 261, 48–55. [Google Scholar] [CrossRef]
- Li, H.; Leavengood, J.M.; Chapman, E.G.; Burkhardt, D.; Song, F.; Jiang, P.; Liu, J.; Zhou, X.; Cai, W. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171223. [Google Scholar] [CrossRef]
- Weirauch, C.; Schuh, R.T.; Cassis, G.; Wheeler, W.C. Revisiting habitat and lifestyle transitions in Heteroptera (Insecta: Hemiptera): Insights from a combined morphological and molecular phylogeny. Cladistics 2019, 35, 67–105. [Google Scholar] [CrossRef]
- Weirauch, C.; Schuh, R.T. Systematics and evolution of heteroptera: 25 years of progress. Annu. Rev. Entomol. 2011, 56, 487–510. [Google Scholar] [CrossRef]
- Roca-Cusachs, M.; Schwertner, C.F.; Kim, J.; Eger, J.; Grazia, J.; Jung, S. Opening Pandora’s box: Molecular phylogeny of the stink bugs (Hemiptera: Heteroptera: Pentatomidae) reveals great incongruences in the current classification. Syst. Entomol. 2021. [Google Scholar] [CrossRef]
- Panizzi, A.R.; McPherson, J.E.; James, D.G.; Javahery, M.; McPherson, M.R. Stink bugs (Pentatomidae). In Heteroptera of Economic Importance; Schaefer, W.C., Panizzi, A.R., Eds.; CRC Press: Boca Raton, FL, USA, 2000; pp. 421–474. [Google Scholar]
- Stål, C. Öfversigt af Kongl. Vetenskaps-Akademiens Forhandlingar; P. A. Norstedt & Söner: Stockholm, Sweden, 1855; Volume 12, p. 182. [Google Scholar]
- Todd, J.W. Ecology and behavior of Nezara viridula. Annu. Rev. Entomol. 1989, 34, 273–292. [Google Scholar] [CrossRef]
- Koch, R.L.; Pezzini, D.T.; Michel, A.P.; Hunt, T.E. Identification, biology, impacts, and management of stink bugs (Hemiptera: Heteroptera: Pentatomidae) of soybean and corn in the midwestern United States. J. Integr. Pest Manag. 2017, 8, 11. [Google Scholar] [CrossRef]
- Powell, G. The biology and control of an emerging shield bug pest, Pentatoma rufipes (L.) (Hemiptera: Pentatomidae). Agric. For. Entomol. 2020, 22, 298–308. [Google Scholar] [CrossRef]
- Leskey, T.C.; Nielsen, A.L. Impact of the invasive brown marmorated stink bug in north America and Europe: History, biology, ecology, and management. Annu. Rev. Entomol. 2018, 63, 599–618. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.; Tirello, P.; Scaccini, D.; Toews, M.D.; Duso, C.; Pozzebon, A. Characterizing damage potential of the brown marmorated stink bug in cherry orchards in Italy. Entomol. Gen. 2019, 39, 271–283. [Google Scholar] [CrossRef]
- Wolff, J.F. Icones Cimicum Descriptionibus Illustratae; Palm: Erlangen, Germany, 1811; Volume 5, p. 182. [Google Scholar]
- Roca-Cusachs, M.; Kim, J.; Park, J.; Jung, S. Taxonomic review of the predatory stink bugs of the Korean Peninsula (Heteroptera: Pentatomidae: Asopinae), with a key to the Korean species and a discussion of their usefulness as biological control agents. J. Asia Pac. Entomol. 2020, 23, 113–123. [Google Scholar] [CrossRef]
- Schaefer, C.W.; Panizzi, A.R. Heteroptera of Economic Importance; CRC Press: Boca Raton, FL, USA, 2000; pp. 3–828. [Google Scholar]
- Rani, P.U.; Madhavendra, S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stål) and Nezara viridula L. (Hemiptera). Int. J. Insect Morphol. Embryol. 1995, 24, 119–132. [Google Scholar] [CrossRef]
- Brézot, P.; Tauban, D.; Renou, M. Sense organs on the antennal flagellum of the green stink bug, Nezara viridula (L.) (Heteroptera: Pentatomidae): Sensillum types and numerical growth during the post-embryonic development. Int. J. Insect Morphol. Embryol. 1996, 25, 427–441. [Google Scholar] [CrossRef]
- Parveen, S.; Ahmad, A.; Broek, J.; Ramamurthy, V.V. Morphological diversity of the labial sensilla of phytophagous and predatory Pentatomidae (Hemiptera: Heteroptera), with reference to their possible functions. Zootaxa 2015, 4039, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brożek, J.; Dai, W. Comparative morphology of the mouthparts in three predatory stink bugs (Heteroptera: Asopinae) reveals feeding specialization of stylets and sensilla. Insects 2020, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.Y.; Zou, H.G. Records of heteropterous insects on bamboo from Yunnan. Zool. Res. 1982, 3, 113–120. [Google Scholar]
- Scopoli, J.A. Entomologia Carniolica Exhibens Insecta Carnioliae Indigena et Distributa in Ordines, Genera, Species, Varietatis. Methodo Linnaeana; Trattner: Vindobonae, Austria, 1763; p. 124. [Google Scholar]
- Dallas, W.S. List of the Specimens of Hemipterous Insects in the Collection of the British Museum. Part I; Trustees of the British Museum: London, UK, 1851; pp. 1–368. [Google Scholar]
- Burmeister, H. Rhyngota seu Hemiptera. In: Meyen, F.J.F Beiträge zur Zoologie, gesammelt auf einer Reise um die Erde, und W. Erichson’s und H. Burmeister’s Beschreibungen und Abbildungen der von Herrn Meyen auf dieser Reise gesammelten Insekten. Nov. Acta Acad. Caesareae Leopold.-Carol. Nat. Curiosorum 1834, 16, 285–308. [Google Scholar]
- Schneider, D. Insect antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Simon, C.; Buckley, T.R.; Frati, F.; Stewart, J.B.; Beckenbach, A.T. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 545–579. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Cao, L.; Shi, A.; Yang, H.; Cai, W. The complete mitochondrial genome of the damsel bug Alloeorhynchus bakeri (Hemiptera: Nabidae). Int. J. Biol. Sci. 2011, 8, 93–107. [Google Scholar] [CrossRef]
- Kuraku, S.; Zmasek, C.M.; Nishimura, O.; Katoh, K. aLeaves facilitates on-demand exploration of metazoan gene family trees on MAFFT sequence alignment server with enhanced interactivity. Nucleic Acids Res. 2013, 41, W22–W28. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2018, 20, 1160–1166. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Rider, D.A.; Schwertner, C.F.; Vilímová, J.; Rédei, D.; Kment, P.; Thomas, D.B. Higher systematics of the Pentatomoidea. In Invasive Stink Bugs and Related Species (Pentatomoidea); McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 25–202. [Google Scholar]
- Liu, Y.; Li, H.; Song, F.; Zhao, Y.; Wilson, J.J.; Cai, W. Higher-level phylogeny and evolutionary history of Pentatomomorpha (Hemiptera: Heteroptera) inferred from mitochondrial genome sequences. Syst. Entomol. 2019, 44, 810–819. [Google Scholar] [CrossRef]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.51. 2018. Available online: http://www.mesquiteproject.org (accessed on 1 December 2018).
- Steinbrecht, R.A. Structure and function of insect olfactory sensilla. In Olfaction in Mosquito–Host Interactions; Bock, G., Cardew, G., Hildebrand, J., Eds.; Wiley: Chichester, UK, 1996; pp. 158–177. [Google Scholar]
- Grabe, V.; Sachse, S. Fundamental principles of the olfactory code. Biosystems 2018, 164, 94–101. [Google Scholar] [CrossRef]
- Hallem, E.A.; Dahanukar, A.; Carlson, J.R. Insect odor and taste receptors. Annu. Rev. Entomol. 2006, 51, 113–135. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, W. How does the intricate mouthpart apparatus coordinate for feeding in the hemimetabolous insect pest Erthesina fullo? Insects 2020, 11, 503. [Google Scholar] [CrossRef] [PubMed]
- Usha Rani, P.; Wakamura, S. Host acceptance behaviour of a predatory pentatomid, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae) towards larvae of Spodoptera litura (Lepidoptera: Noctuidae). Int. J. Trop. Insect Sci. 1993, 14, 141–147. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Dai, W. Fine morphology of the mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) reflects adaptation for phytophagous habits. Insects 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brożek, J.; Dai, W. Sensory armature and stylets of the mouthparts of Stephanitis nashi (Hemiptera: Cimicomorpha: Tingidae), their morphology and function. Micron 2020, 132, 102840. [Google Scholar] [CrossRef]
- Baker, G.T.; Xiong, C.; Ma, P.W.K. Labial tip sensilla of Blissus leucopterus leucopterus (Hemiptera: Blissidae): Ultrastructure and behavior. Insect Sci. 2008, 15, 271–275. [Google Scholar] [CrossRef]
- Thunberg, C.P. Dissertatio Entomologica Novas Insectorum Species, Sistens, Cujus Partem Secundum; Edman: Upsaliae, Sweden, 1783; p. 42. [Google Scholar]
- Brożek, J.; Chłond, D. Morphology, arrangement and classification of sensilla on the apical segment of labium in Peiratinae (Hemiptera: Heteroptera: Reduviidae). Zootaxa 2010, 2476, 39. [Google Scholar] [CrossRef]
- Panizzi, A.R. Wild hosts of pentatomids: Ecological significance and role in their pest status on crops. Annu. Rev. Entomol. 1997, 42, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Scaccini, D.; Pozzebon, A. Invasive brown marmorated stink bug (Hemiptera: Pentatomidae) facilitates feeding of European wasps and ants (Hymenoptera: Vespidae, Formicidae) on plant exudates. Eur. J. Entomol. 2021, 118, 24–30. [Google Scholar] [CrossRef]
- Martinson, H.M.; Raupp, M.J.; Shrewsbury, P.M. Invasive stink bug wounds trees, liberates sugars, and facilitates native Hymenoptera. Ann. Entomol. Soc. Am. 2013, 106, 47–52. [Google Scholar] [CrossRef]
- Miles, P.W. The saliva of Hemiptera. Adv. Insect Physiol. 1972, 9, 183–255. [Google Scholar] [CrossRef]
- Backus, E.A. Sensory systems and behaviours which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Wang, W.; Brożek, J.; Dai, W. Unique fine morphology of mouthparts in Haematoloecha nigrorufa (Stål) (Hemiptera: Reduviidae) adapted to millipede feeding. Insects 2020, 11, 386. [Google Scholar] [CrossRef]
- Lemos, W.P.; Zanuncio, J.C.; Serrão, J.E. Attack behavior of Podisus rostralis (Heteroptera: Pentatomidade) adults on caterpillars of Bombyx mori (Lepidoptera: Bombycidae). Braz. Arch. Biol. Technol. 2005, 48, 975–981. [Google Scholar] [CrossRef]
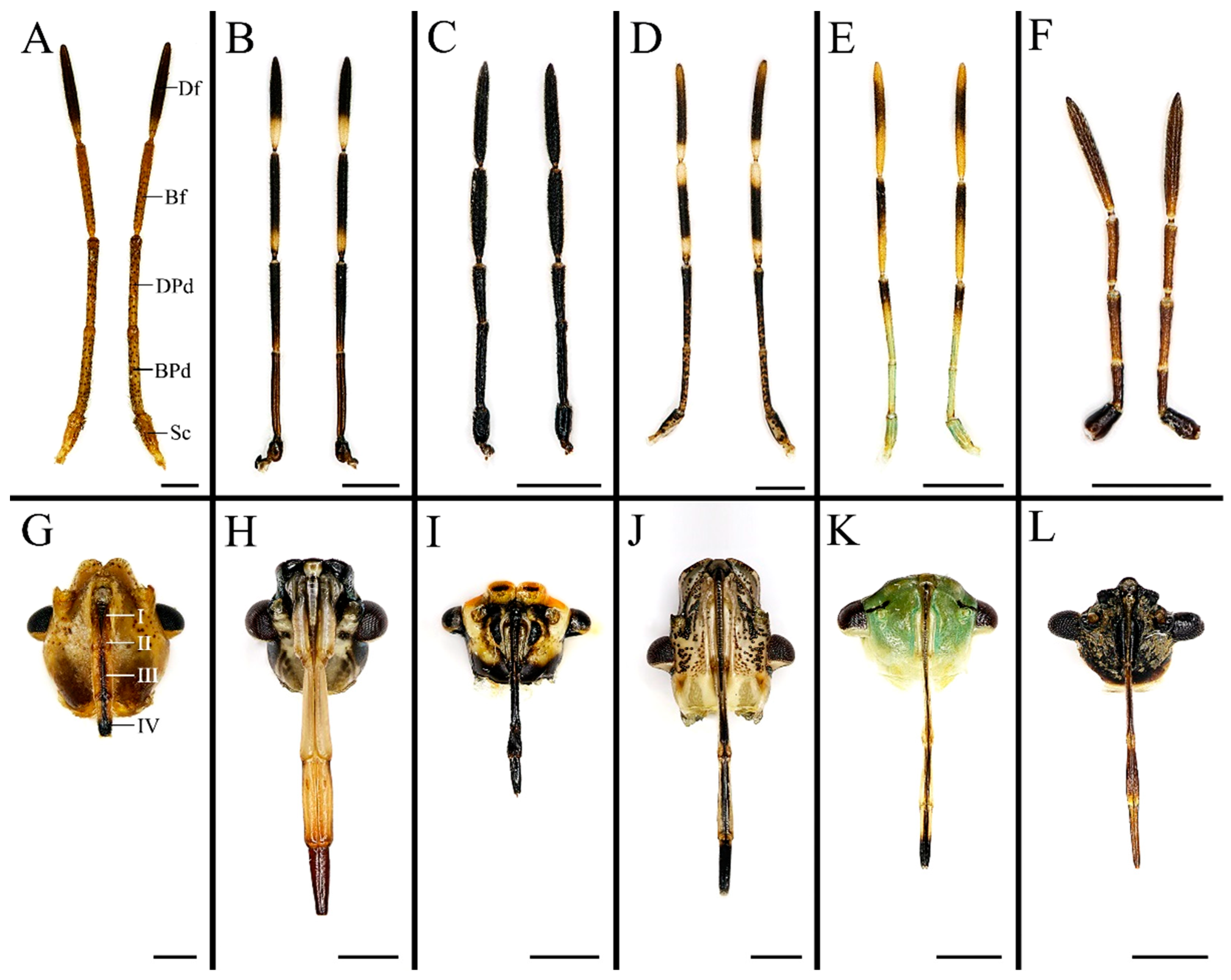

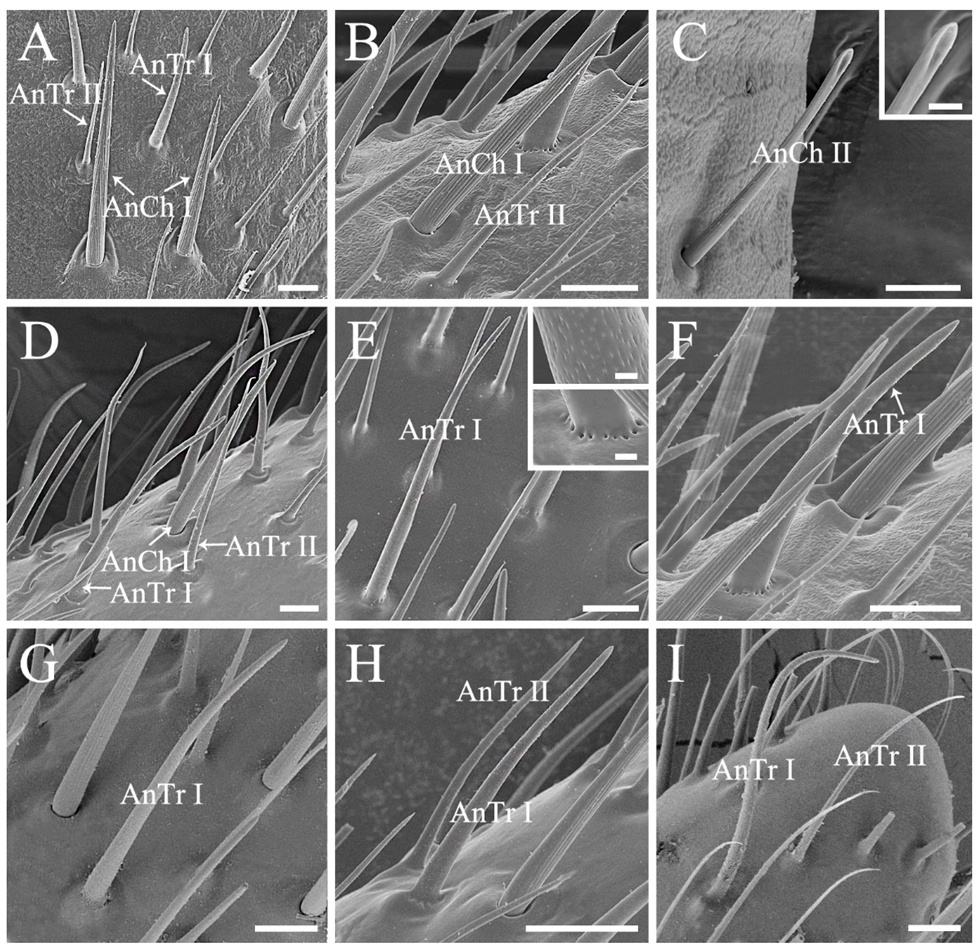
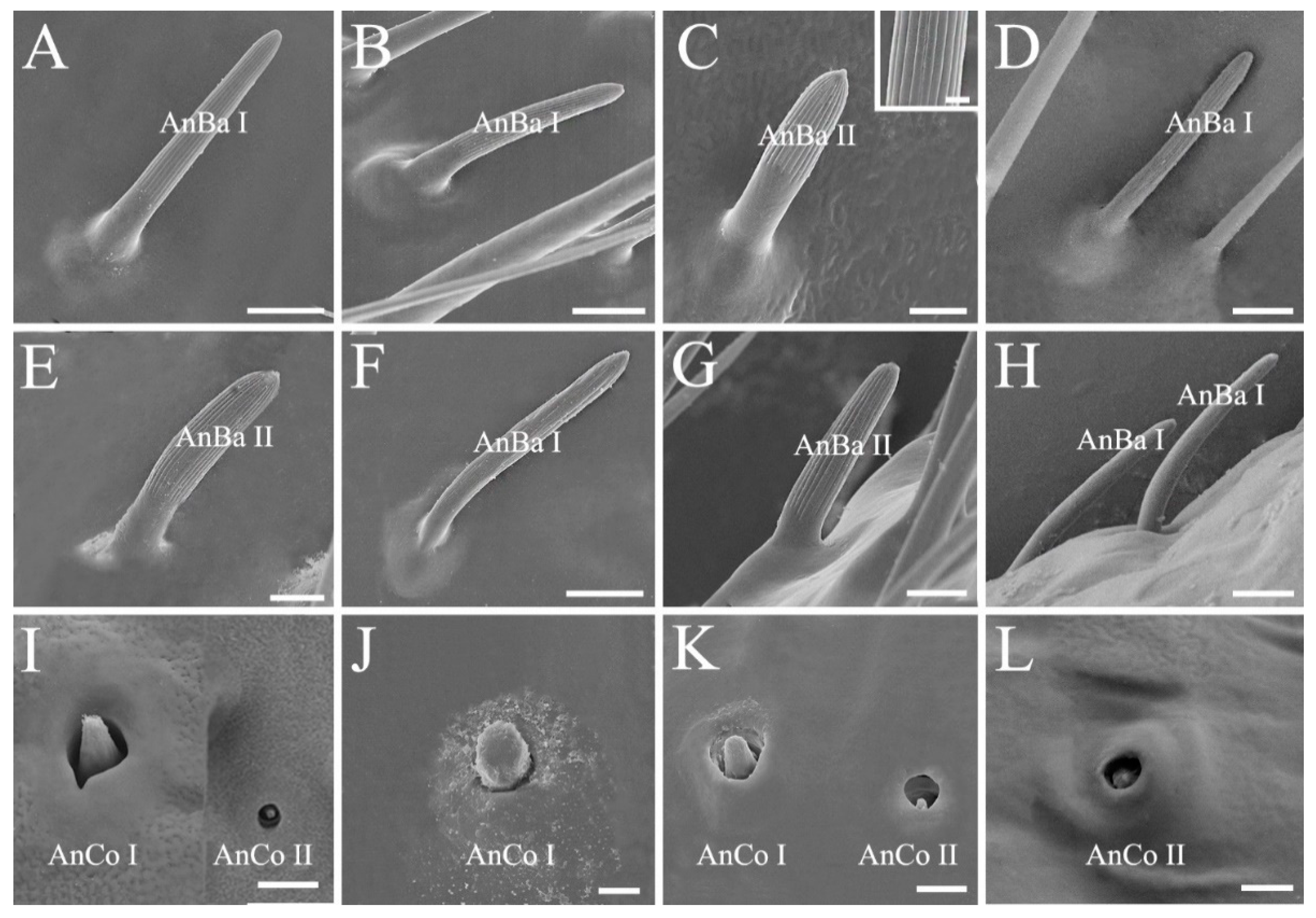
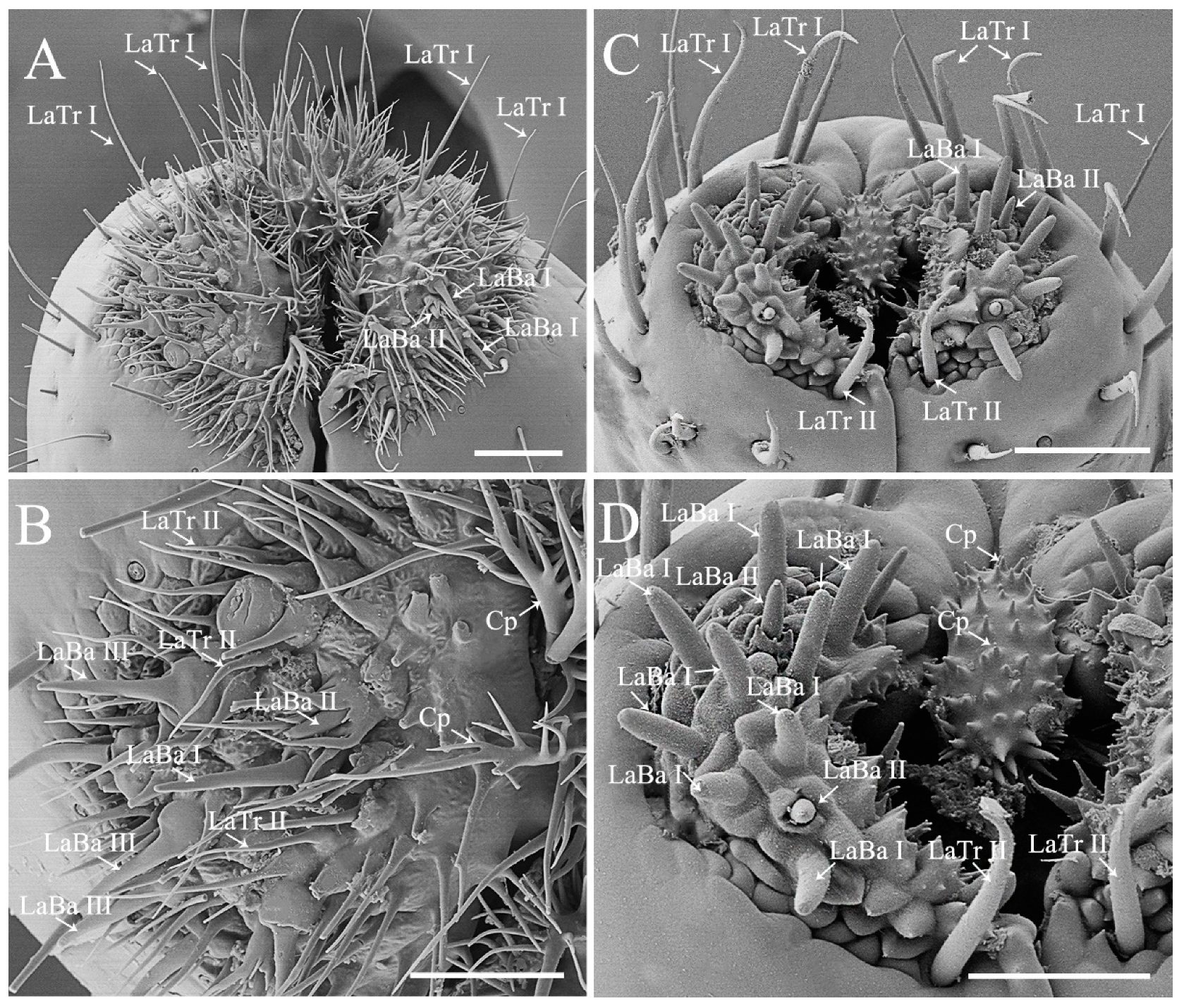

| Morphological Characters | Similarity | Disparity |
|---|---|---|
| Antennal morphology | Five segments, including a proximal scape, a subdivided pedicel forming two segments, a basiflagellomere, and a distiflagellomere | N.A. |
| Antennal sensilla type and arrangement | Mainly four types, antennal sensilla chaetica, sensilla trichodea, sensilla basiconica, and sensilla coeloconica | A higher density of sensilla basiconica is observed on distiflagellomere in predatory Eocanthecona furcellata than in phytophagous species |
| Labial morphology | Tubular; straight; and segmented into four, namely labial segment I to IV from base to distal end | Slender in phytophagous species while stout in predators |
| Labial sensilla complex and arrangement | Mainly two types, labial sensilla trichodea and sensilla basiconica | Labial sensillum basiconica is the main type in phytophagous species (except for Erthesina fullo), while both sensillum trichodea and basiconica are the two main types in predators. |
| Labial cuticular projections | Present in phytophagous and predatory species | Short and slightly branched in phytophagous species, while long and multi-branched in predators |
| Mandibular stylet shape | With scale-like patterns, several central teeth, and lateral teeth; and the central teeth are flattened, rounded, and broad in phytophagous and predatory species | The lateral teeth are short, blunted, and symmetrically aligned in phytophagous species, while predators possess elongated and hook-like lateral teeth irregularly arranged on the mandible |
| Maxillary stylet shape | Left–right asymmetrical, with one stylet narrower than the other on the distal end | With short and sharp tipped barbs exclusively on the maxillae of predatory species |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Tian, L.; Li, H.; Cai, W. Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae). Biology 2021, 10, 1161. https://doi.org/10.3390/biology10111161
Li X, Tian L, Li H, Cai W. Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae). Biology. 2021; 10(11):1161. https://doi.org/10.3390/biology10111161
Chicago/Turabian StyleLi, Xinyu, Li Tian, Hu Li, and Wanzhi Cai. 2021. "Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae)" Biology 10, no. 11: 1161. https://doi.org/10.3390/biology10111161
APA StyleLi, X., Tian, L., Li, H., & Cai, W. (2021). Ultrastructural Variations of Antennae and Labia Are Associated with Feeding Habit Shifts in Stink Bugs (Heteroptera: Pentatomidae). Biology, 10(11), 1161. https://doi.org/10.3390/biology10111161







