Environmental DNA (eDNA) Metabarcoding in the Fish Market and Nearby Seafood Restaurants in Taiwan Reveals the Underestimation of Fish Species Diversity in Seafood
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
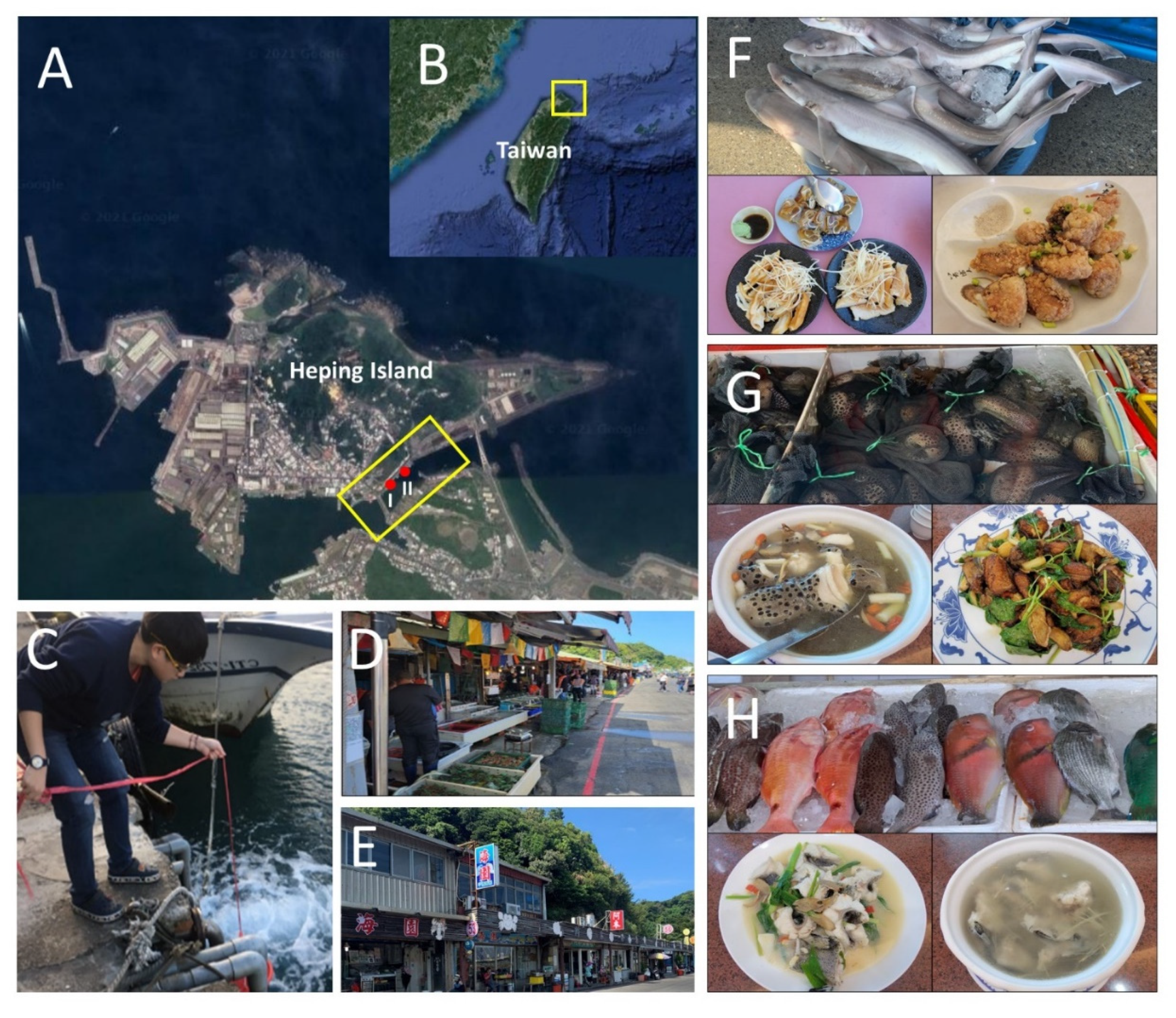
2.1. Sample Collection
2.2. DNA Extraction
2.3. DNA Library Preparation
2.4. Data Process
2.5. Taxonomic Assignment
2.6. Statistical Analysis
3. Results
3.1. Identification and Classification of Fish Species from eDNA Samplings
3.2. Seafood and Its Species Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asche, F.; Bellemare, M.F.; Roheim, C.; Smith, M.D.; Tveteras, S. Fair Enough? Food Security and the International Trade of Seafood. World Dev. 2015, 67, 151–160. [Google Scholar] [CrossRef]
- The State of World Fisheries and Aquaculture 2020. Available online: http://www.fao.org/state-of-fisheries-aquaculture/en/ (accessed on 1 October 2021).
- Thilsted, S.H.; Thorne-Lyman, A.; Webb, P.; Bogard, J.R.; Subasinghe, R.; Phillips, M.J.; Allison, E.H. Sustaining Healthy Diets: The Role of Capture Fisheries and Aquaculture for Improving Nutrition in the Post-2015 Era. Food Policy 2016, 61, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Kroetz, K.; Luque, G.M.; Gephart, J.A.; Jardine, S.L.; Lee, P.; Moore, K.C.; Cole, C.; Steinkruger, A.; Donlan, C.J. Consequences of Seafood Mislabeling for Marine Populations and Fisheries Management. Proc. Natl. Acad. Sci. USA 2020, 117, 30318–30323. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, D.-M.; Baillie, C.; Mariani, S. Generic Names and Mislabeling Conceal High Species Diversity in Global Fisheries Markets. Conserv. Lett. 2018, 11, e12573. [Google Scholar] [CrossRef]
- Fernandes, T.J.R.; Amaral, J.S.; Mafra, I. DNA Barcode Markers Applied to Seafood Authentication: An Updated Review. Crit. Rev. Food Sci. Nutr. 2020, 1–32. [Google Scholar] [CrossRef]
- Barendse, J.; Roel, A.; Longo, C.; Andriessen, L.; Webster, L.M.I.; Ogden, R.; Neat, F. DNA Barcoding Validates Species Labelling of Certified Seafood. Curr. Biol. 2019, 29, R198–R199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, D.C.; Palhares, R.M.; Drummond, M.G.; Frigo, T.B. DNA Barcoding Identification of Commercialized Seafood in South Brazil: A Governmental Regulatory Forensic Program. Food Control 2015, 50, 784–788. [Google Scholar] [CrossRef]
- Chin Chin, T.; Adibah, A.B.; Danial Hariz, Z.A.; Siti Azizah, M.N. Detection of Mislabelled Seafood Products in Malaysia by DNA Barcoding: Improving Transparency in Food Market. Food Control 2016, 64, 247–256. [Google Scholar] [CrossRef]
- Liu, S.-Y.V.; Chan, C.-L.C.; Lin, O.; Hu, C.-S.; Chen, C.A. DNA Barcoding of Shark Meats Identify Species Composition and CITES-Listed Species from the Markets in Taiwan. PLoS ONE 2013, 8, e79373. [Google Scholar] [CrossRef] [Green Version]
- Shehata, H.R.; Naaum, A.M.; Garduño, R.A.; Hanner, R. DNA Barcoding as a Regulatory Tool for Seafood Authentication in Canada. Food Control 2018, 92, 147–153. [Google Scholar] [CrossRef]
- Armani, A.; Guardone, L.; La Castellana, R.; Gianfaldoni, D.; Guidi, A.; Castigliego, L. DNA Barcoding Reveals Commercial and Health Issues in Ethnic Seafood Sold on the Italian Market. Food Control 2015, 55, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Clare, E.L.; Economou, C.K.; Faulkes, C.G.; Gilbert, J.D.; Bennett, F.; Drinkwater, R.; Littlefair, J.E. EDNAir: Proof of Concept That Animal DNA Can Be Collected from Air Sampling. PeerJ 2021, 9, e11030. [Google Scholar] [CrossRef] [PubMed]
- Ficetola, G.F.; Miaud, C.; Pompanon, F.; Taberlet, P. Species Detection Using Environmental DNA from Water Samples. Biol. Lett. 2008, 4, 423–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentini, A.; Taberlet, P.; Miaud, C.; Civade, R.; Herder, J.; Thomsen, P.F.; Bellemain, E.; Besnard, A.; Coissac, E.; Boyer, F. Next-generation Monitoring of Aquatic Biodiversity Using Environmental DNA Metabarcoding. Mol. Ecol. 2016, 25, 929–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deiner, K.; Bik, H.M.; Mächler, E.; Seymour, M.; Lacoursière-Roussel, A.; Altermatt, F.; Creer, S.; Bista, I.; Lodge, D.M.; De Vere, N. Environmental DNA Metabarcoding: Transforming How We Survey Animal and Plant Communities. Mol. Ecol. 2017, 26, 5872–5895. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ezpeleta, N.; Zinger, L.; Kinziger, A.; Bik, H.M.; Bonin, A.; Coissac, E.; Emerson, B.C.; Lopes, C.M.; Pelletier, T.A.; Taberlet, P.; et al. Biodiversity Monitoring Using Environmental DNA. Mol. Ecol. Resour. 2021, 21, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Evans, N.T.; Renshaw, M.A.; Jerde, C.L.; Olds, B.P.; Shogren, A.J.; Deiner, K.; Lodge, D.M.; Lamberti, G.A.; Pfrender, M.E. Estimating Fish Alpha-and Beta-Diversity along a Small Stream with Environmental DNA Metabarcoding. Metabarcoding Metagenomics 2018, 2, e24262. [Google Scholar] [CrossRef]
- Jeunen, G.-J.; Knapp, M.; Spencer, H.G.; Lamare, M.D.; Taylor, H.R.; Stat, M.; Bunce, M.; Gemmell, N.J. Environmental DNA (EDNA) Metabarcoding Reveals Strong Discrimination among Diverse Marine Habitats Connected by Water Movement. Mol. Ecol. Resour. 2019, 19, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Stoeckle, M.Y.; Soboleva, L.; Charlop-Powers, Z. Aquatic Environmental DNA Detects Seasonal Fish Abundance and Habitat Preference in an Urban Estuary. PLoS ONE 2017, 12, e0175186. [Google Scholar] [CrossRef]
- Tittensor, D.P.; Mora, C.; Jetz, W.; Lotze, H.K.; Ricard, D.; Berghe, E.V.; Worm, B. Global Patterns and Predictors of Marine Biodiversity across Taxa. Nature 2010, 466, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Shao, K.-T.; Lin, H.-Y.; Chiu, Y.-C.; Lee, M.-Y.; Liu, S.-H.; Lin, P.-L. DNA Barcodes of the Native Ray-Finned Fishes in Taiwan. Mol. Ecol. Resour. 2017, 17, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Su, N.-J.; Lee, H.-T.; Hsu, W.W.-Y.; Liao, C.-H. Application of Métier-Based Approaches for Spatial Planning and Management: A Case Study on a Mixed Trawl Fishery in Taiwan. J. Mar. Sci. Eng. 2021, 9, 480. [Google Scholar] [CrossRef]
- Wong, M.K.-S.; Nakao, M.; Hyodo, S. Field Application of an Improved Protocol for Environmental DNA Extraction, Purification, and Measurement Using Sterivex Filter. Sci. Rep. 2020, 10, 21531. [Google Scholar] [CrossRef] [PubMed]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a Set of Universal PCR Primers for Metabarcoding Environmental DNA from Fishes: Detection of More than 230 Subtropical Marine Species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef] [Green Version]
- Shokralla, S.; Porter, T.M.; Gibson, J.F.; Dobosz, R.; Janzen, D.H.; Hallwachs, W.; Golding, G.B.; Hajibabaei, M. Massively Parallel Multiplex DNA Sequencing for Specimen Identification Using an Illumina MiSeq Platform. Sci. Rep. 2015, 5, 9687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, S.; Hanner, R.; Steinke, D. Five Years of FISH-BOL: Brief Status Report. Mitochondrial DNA 2011, 22, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Zahn, R.J.; Silva, A.J.; Hellberg, R.S. Development of a DNA Mini-Barcoding Protocol Targeting COI for the Identification of Elasmobranch Species in Shark Cartilage Pills. Food Control 2020, 109, 106918. [Google Scholar] [CrossRef]
- Babraham Bioinformatics. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 15 April 2021).
- Bushnell, B.; Rood, J.; Singer, E. BBMerge–Accurate Paired Shotgun Read Merging via Overlap. PLoS ONE 2017, 12, e0185056. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M.; et al. MitoFish and MitoAnnotator: A Mitochondrial Genome Database of Fish with an Accurate and Automatic Annotation Pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef]
- Sato, Y.; Miya, M.; Fukunaga, T.; Sado, T.; Iwasaki, W. MitoFish and MiFish Pipeline: A Mitochondrial Genome Database of Fish with an Analysis Pipeline for Environmental DNA Metabarcoding. Mol. Biol. Evol. 2018, 35, 1553–1555. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.O.D.; Smouse, P.E. GENALEX 6: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Andruszkiewicz, E.A.; Starks, H.A.; Chavez, F.P.; Sassoubre, L.M.; Block, B.A.; Boehm, A.B. Biomonitoring of Marine Vertebrates in Monterey Bay Using EDNA Metabarcoding. PLoS ONE 2017, 12, e0176343. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Masuda, R.; Sato, Y.; Sado, T.; Araki, H.; Kondoh, M.; Minamoto, T.; Miya, M. Environmental DNA Metabarcoding Reveals Local Fish Communities in a Species-Rich Coastal Sea. Sci. Rep. 2017, 7, 40368. [Google Scholar] [CrossRef] [Green Version]
- Bylemans, J.; Gleeson, D.M.; Duncan, R.P.; Hardy, C.M.; Furlan, E.M. A Performance Evaluation of Targeted EDNA and EDNA Metabarcoding Analyses for Freshwater Fishes. Environ. DNA 2019, 1, 402–414. [Google Scholar] [CrossRef] [Green Version]
- Ushio, M.; Murakami, H.; Masuda, R.; Sado, T.; Miya, M.; Sakurai, S.; Yamanaka, H.; Minamoto, T.; Kondoh, M. Quantitative Monitoring of Multispecies Fish Environmental DNA Using High-Throughput Sequencing. Metabarcoding Metagenomics 2018, 2, e23297. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, J.; Yao, M. A Comprehensive and Comparative Evaluation of Primers for Metabarcoding EDNA from Fish. Methods Ecol. Evol. 2020, 11, 1609–1625. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoon, T.-H.; Lee, C.I.; Kang, C.-K.; Kim, H.-W. Metabarcoding Analysis of Ichthyoplankton in the East/Japan Sea Using the Novel Fish-Specific Universal Primer Set. Front. Mar. Sci. 2021, 8, 141. [Google Scholar] [CrossRef]
- Nester, G.M.; De Brauwer, M.; Koziol, A.; West, K.M.; DiBattista, J.D.; White, N.E.; Power, M.; Heydenrych, M.J.; Harvey, E.; Bunce, M. Development and Evaluation of Fish EDNA Metabarcoding Assays Facilitate the Detection of Cryptic Seahorse Taxa (Family: Syngnathidae). Environ. DNA 2020, 2, 614–626. [Google Scholar] [CrossRef]
- Singer, G.A.C.; Fahner, N.A.; Barnes, J.G.; McCarthy, A.; Hajibabaei, M. Comprehensive biodiversity analysis via ultra-deep patterned flow cell technology: A case study of eDNA metabarcoding seawater. Sci. Rep. 2019, 9, 5991. [Google Scholar] [CrossRef] [Green Version]
- Collins, R.A.; Wangensteen, O.S.; O’Gorman, E.J.; Mariani, S.; Sims, D.W.; Genner, M.J. Persistence of Environmental DNA in Marine Systems. Commun. Biol. 2018, 1, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a Century of Global Decline in Oceanic Sharks and Rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Liu, K.-M.; Huang, L.-H.; Su, K.-Y.; Joung, S.-J. Vulnerability Assessment of Pelagic Sharks in the Western North Pacific by Using an Integrated Ecological Risk Assessment. Animals 2021, 11, 2161. [Google Scholar] [CrossRef] [PubMed]
- Chuang, P.-S.; Hung, T.-C.; Chang, H.-A.; Huang, C.-K.; Shiao, J.-C. The Species and Origin of Shark Fins in Taiwan’s Fishing Ports, Markets, and Customs Detention: A DNA Barcoding Analysis. PLoS ONE 2016, 11, e0147290. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-C.; Smith, D.G.; McCosker, J.E.; Hibino, Y.; Loh, K.-H.; Tighe, K.A.; Shao, K.-T. Annotated Checklist of Eels (Orders Anguilliformes and Saccopharyngiformes) from Taiwan. Zootaxa 2015, 4060, 140–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rimmer, M.A.; Glamuzina, B. A Review of Grouper (Family Serranidae: Subfamily Epinephelinae) Aquaculture from a Sustainability Science Perspective. Rev. Aquac. 2019, 11, 58–87. [Google Scholar] [CrossRef]
- Worm, B.; Barbier, E.B.; Beaumont, N.; Duffy, J.E.; Folke, C.; Halpern, B.S.; Jackson, J.B.; Lotze, H.K.; Micheli, F.; Palumbi, S.R. Impacts of Biodiversity Loss on Ocean Ecosystem Services. Science 2006, 314, 787–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Primer Sets | Sequence | Reference |
|---|---|---|
| 12S | Miya et al. 2015 [25] | |
| MiFish-U-F | GTCGGTAAAACTCGTGCCAGC | |
| MiFish-U-R | CATAGTGGGGTATCTAATCCCAGTTTG | |
| MiFish-E-F | GTTGGTAAATCTCGTGCCAGC | |
| MiFish-E-R | CATAGTGGGGTATCTAATCCTAGTTTG | |
| ND5 | Miya et al. 2015 [25] | |
| MiFish-tuna-ND5-F | ATGTCCTTCCTCCTTATCGGCTG | |
| MiFish-tuna-ND5-R | TTGCCAGTGGCAGCTACGATC | |
| COI | Shokralla et al. 2015 [26] | |
| Fish-miniA-F | ACIAAICAIAAAGAYATYGGC | |
| Fish-miniA-R | AARAAAATYATAACRAAIGCRTGIGC | |
| Fish-miniC-F | ACYAAICAYAAAGAYATIGGCAC | |
| Fish-miniC-R | GAARATCATAATGAAGGCATGIGC | |
| Fish-miniE-F | ACYAAICAYAAAGAYATIGGCAC | |
| Fish-miniE-R | CTTATRTTRTTTATICGIGGRAAIGC | |
| FishF1 | TCAACCAACCACAAAGACATTGGCAC | Becker et al. 2011 [27] |
| FishF2 | TCGACTAATCATAAAGATATCGGCAC | |
| FishR1 | TAGACTTCTGGGTGGCCAAAGAATCA | |
| FishR2 | ACTTCAGGGTGACCGAAGAATCAGAA | |
| Shark-COI-MINI-R | AAGATTACAAAAGCGTGGGC | Zahn et al. 2020 [28] |
| Shark-MiniV1-R | AAGATTATTACAAAAGCRTGRGC | |
| Shark-MiniV2-R | AAGATTATTACRAADGCRTGRGC |
| Items | Reads | 12S | COI | ND5 | All |
|---|---|---|---|---|---|
| December-I | 6,995,762 | 64 | 52 | 15 | 81 * |
| December-II | 10,194,137 | 39 | 44 | 11 | 56 * |
| January-I | 22,323,200 | 103 | 68 | 17 | 126 * |
| January-II | 11,021,896 | 44 | 52 | 14 | 60 * |
| All | 50,534,995 | 112 * | 88 * | 18 * | 153 * |
| Class | Order | Family | Species | Class | Order | Family | Species |
|---|---|---|---|---|---|---|---|
| Actinopterygii | Actinopterygii | ||||||
| Anguilliformes | Congridae | 2 | Perciformes | Haemulidae | 2 | ||
| Muraenidae | 12 | Istiophoridae | 5 | ||||
| Beloniformes | Scomberesocidae | 1 | Kyphosidae | 1 | |||
| Clupeiformes | Engraulidae | 1 | Labridae | 2 | |||
| Gadiformes | Gadidae | 3 | Lutjanidae | 8 | |||
| Mugiliformes | Mugilidae | 4 | Malacanthidae | 1 | |||
| Ophidiiformes | Ophidiidae | 1 | Mullidae | 2 | |||
| Pleuronectiformes | Cynoglossidae | 1 | Nemipteridae | 1 | |||
| Paralichthyidae | 1 | Nomeidae | 1 | ||||
| Salmoniformes | Salmonidae | 1 | Pempheridae | 1 | |||
| Scorpaeniformes | Scorpaenidae | 2 | Pomacentridae | 7 | |||
| Siluriformes | Loricariidae | 2 | Scombridae | 12 | |||
| Tetraodontiformes | Diodontidae | 1 | Serranidae | 12 | |||
| Monacanthidae | 3 | Siganidae | 1 | ||||
| Tetraodontidae | 1 | Sparidae | 3 | ||||
| Perciformes | Acropomatidae | 2 | Stromateidae | 3 | |||
| Apogonidae | 2 | Trichiuridae | 5 | ||||
| Bramidae | 1 | Xiphiidae | 1 | ||||
| Caesionidae | 3 | Chondrichthyes | |||||
| Carangidae | 11 | Carcharhiniformes | Carcharhinidae | 7 | |||
| Centrolophidae | 3 | Sphyrnidae | 2 | ||||
| Channichthyidae | 1 | Triakidae | 5 | ||||
| Coryphaenidae | 1 | Lamniformes | Alopiidae | 2 | |||
| Emmelichthyidae | 1 | Myliobatiformes | Dasyatidae | 5 | |||
| Gempylidae | 2 | Urolophidae | 1 |
| Order | Family | Scientific Name | 12S | COI | December-I | December-II | January-I | January-II | Status |
|---|---|---|---|---|---|---|---|---|---|
| Carcharhiniformes | Carcharhinidae | Carcharhinus brevipinna | 1 | 0 | 0 | 1 | 0 | 1 | VU |
| Carcharhinus falciformis | 1 | 0 | 0 | 1 | 0 | 0 | VU | ||
| Carcharhinus macloti | 1 | 0 | 0 | 1 | 0 | 0 | NT | ||
| Carcharhinus obscurus | 1 | 1 | 0 | 1 | 1 | 1 | EN | ||
| Carcharhinus sealei | 0 | 1 | 1 | 1 | 1 | 1 | NT | ||
| Carcharhinus sorrah | 1 | 0 | 0 | 1 | 0 | 0 | NT | ||
| Prionace glauca | 1 | 1 | 0 | 1 | 1 | 1 | NT | ||
| Sphyrnidae | Sphyrna lewini | 1 | 0 | 0 | 1 | 0 | 0 | CR | |
| Sphyrna zygaena | 1 | 1 | 1 | 1 | 1 | 1 | VU | ||
| Triakidae | Galeorhinus galeus | 0 | 1 | 1 | 0 | 1 | 0 | CR | |
| Hemitriakis japanica | 0 | 1 | 1 | 0 | 1 | 0 | EN | ||
| Mustelus asterias | 0 | 1 | 1 | 0 | 1 | 0 | NT | ||
| Mustelus griseus | 0 | 1 | 1 | 1 | 0 | 0 | EN | ||
| Mustelus manazo | 0 | 1 | 1 | 1 | 0 | 0 | EN | ||
| Lamniformes | Alopiidae | Alopias pelagicus | 1 | 1 | 1 | 1 | 1 | 1 | EN |
| Alopias superciliosus | 1 | 1 | 1 | 1 | 1 | 1 | VU | ||
| Myliobatiformes | Dasyatidae | Himantura leoparda | 0 | 1 | 0 | 1 | 1 | 1 | VU |
| Maculabatis gerrardi | 1 | 1 | 1 | 1 | 1 | 1 | EN | ||
| Maculabatis pastinacoides | 1 | 1 | 0 | 0 | 1 | 0 | EN | ||
| Pastinachus gracilicaudus | 0 | 1 | 0 | 1 | 1 | 1 | EN | ||
| Pateobatis jenkinsii | 0 | 1 | 0 | 0 | 1 | 1 | VU | ||
| Urolophidae | Urolophus aurantiacus | 1 | 0 | 0 | 1 | 0 | 0 | VU |
| Order | Family | Scientific Name | 12S | COI | December-I | December-II | January-I | January-II | Status |
|---|---|---|---|---|---|---|---|---|---|
| Anguilliformes | Congridae | Bathycongrus retrotinctus | 1 | 0 | 0 | 1 | 0 | 0 | R |
| Gnathophis nystromi | 0 | 1 | 0 | 1 | 0 | 1 | R | ||
| Muraenidae | Gymnothorax flavimarginatus | 1 | 1 | 1 | 1 | 1 | 1 | E | |
| Gymnothorax isingteena | 1 | 1 | 1 | 1 | 1 | 0 | U | ||
| Gymnothorax javanicus | 1 | 0 | 1 | 0 | 0 | 0 | E | ||
| Gymnothorax prionodon | 1 | 0 | 0 | 1 | 0 | 0 | E | ||
| Gymnothorax margaritophorus | 1 | 0 | 0 | 1 | 0 | 0 | E | ||
| Gymnothorax niphostigmus | 1 | 0 | 0 | 1 | 0 | 0 | T | ||
| Gymnothorax reevesii | 1 | 1 | 1 | 1 | 1 | 0 | E | ||
| Gymnothorax reticularis | 1 | 0 | 0 | 1 | 1 | 1 | U | ||
| Gymnothorax thyrsoideus | 1 | 0 | 0 | 1 | 1 | 1 | E | ||
| Gymnothorax undulatus | 1 | 1 | 1 | 1 | 1 | 0 | E | ||
| Enchelycore anatina | 1 | 0 | 0 | 1 | 0 | 0 | U | ||
| Strophidon sathete | 1 | 0 | 1 | 1 | 1 | 1 | R |
| Order | Family | Scientific Name | 12S | COI | December-I | December-II | January-I | January-II | Status |
|---|---|---|---|---|---|---|---|---|---|
| Perciformes | Serranidae | Aethaloperca rogaa | 1 | 1 | 1 | 1 | 1 | 0 | W |
| Cephalopholis boenak | 1 | 0 | 1 | 1 | 1 | 0 | W | ||
| Cephalopholis sexmaculata | 1 | 0 | 1 | 1 | 1 | 0 | W | ||
| Epinephelus awoara | 1 | 1 | 1 | 0 | 0 | 1 | W | ||
| Epinephelus coioides | 1 | 1 | 1 | 1 | 1 | 0 | C | ||
| Epinephelus fasciatomaculosus | 1 | 0 | 0 | 1 | 0 | 0 | W | ||
| Epinephelus fuscoguttatus | 1 | 1 | 1 | 1 | 1 | 1 | C | ||
| Epinephelus lanceolatus | 1 | 0 | 0 | 1 | 0 | 0 | C | ||
| Epinephelus bruneus | 1 | 0 | 1 | 1 | 0 | 1 | C | ||
| Epinephelus quoyanus | 1 | 0 | 1 | 1 | 0 | 0 | W | ||
| Epinephelus tukula | 1 | 0 | 0 | 1 | 0 | 0 | C | ||
| Variola louti | 1 | 0 | 0 | 1 | 0 | 0 | W |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-T.; Liao, C.-H.; Hsu, T.-H. Environmental DNA (eDNA) Metabarcoding in the Fish Market and Nearby Seafood Restaurants in Taiwan Reveals the Underestimation of Fish Species Diversity in Seafood. Biology 2021, 10, 1132. https://doi.org/10.3390/biology10111132
Lee H-T, Liao C-H, Hsu T-H. Environmental DNA (eDNA) Metabarcoding in the Fish Market and Nearby Seafood Restaurants in Taiwan Reveals the Underestimation of Fish Species Diversity in Seafood. Biology. 2021; 10(11):1132. https://doi.org/10.3390/biology10111132
Chicago/Turabian StyleLee, Hung-Tai, Cheng-Hsin Liao, and Te-Hua Hsu. 2021. "Environmental DNA (eDNA) Metabarcoding in the Fish Market and Nearby Seafood Restaurants in Taiwan Reveals the Underestimation of Fish Species Diversity in Seafood" Biology 10, no. 11: 1132. https://doi.org/10.3390/biology10111132
APA StyleLee, H.-T., Liao, C.-H., & Hsu, T.-H. (2021). Environmental DNA (eDNA) Metabarcoding in the Fish Market and Nearby Seafood Restaurants in Taiwan Reveals the Underestimation of Fish Species Diversity in Seafood. Biology, 10(11), 1132. https://doi.org/10.3390/biology10111132








