Insight into the Prospects for Nanotechnology in Wheat Biofortification
Abstract
Simple Summary
Abstract
1. Introduction
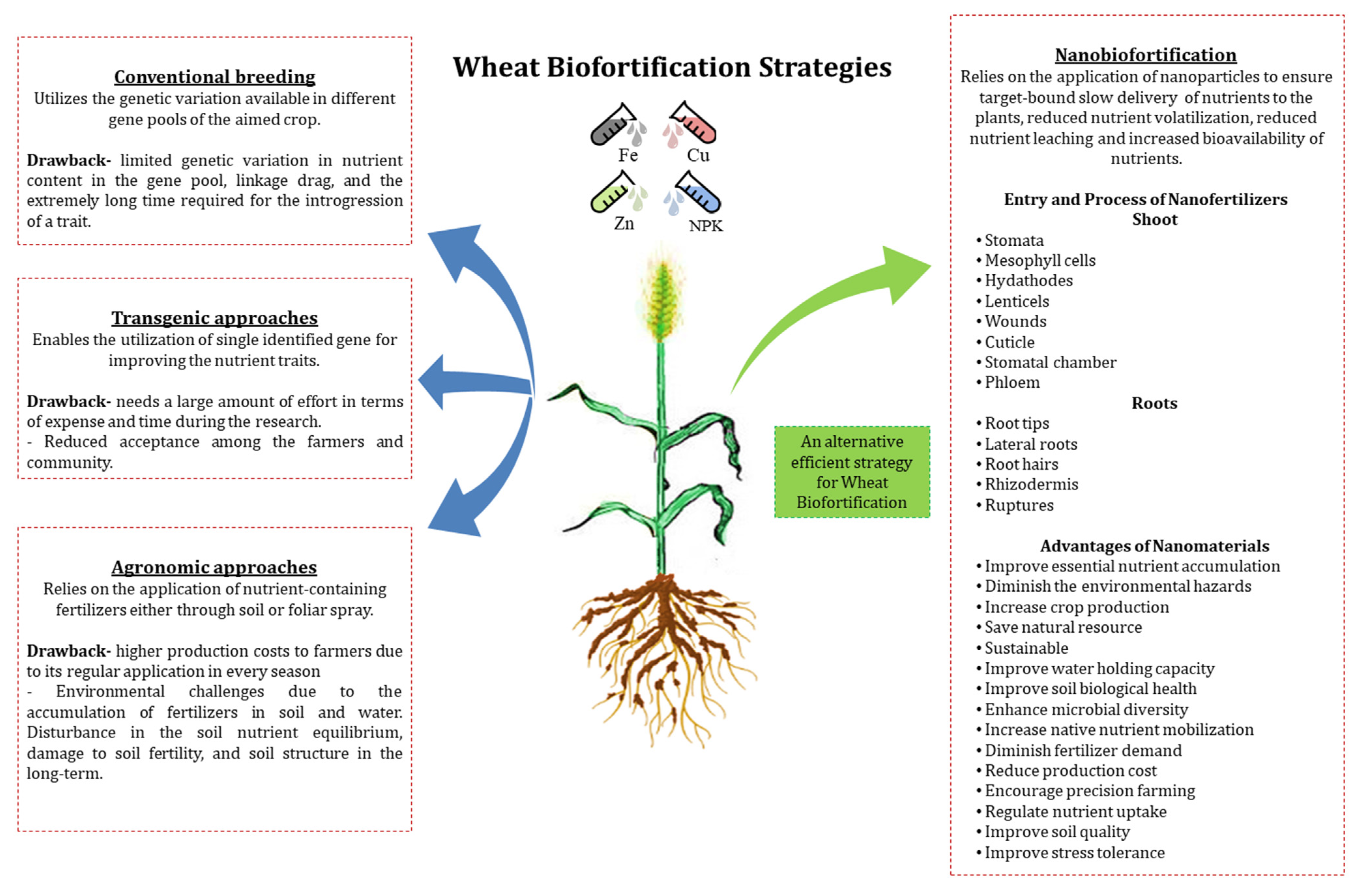
2. Advantages of Nanobiofortification over Agronomic Biofortification
3. The “Nano” Forms Used in Biofortification Programs and Their Types
4. Micronutrients Nanobiofortification in Wheat
5. Macronutrients Biofortification in Wheat
6. Entry of Nanoparticles into Wheat Plants and Their Effect on Nutritional Composition
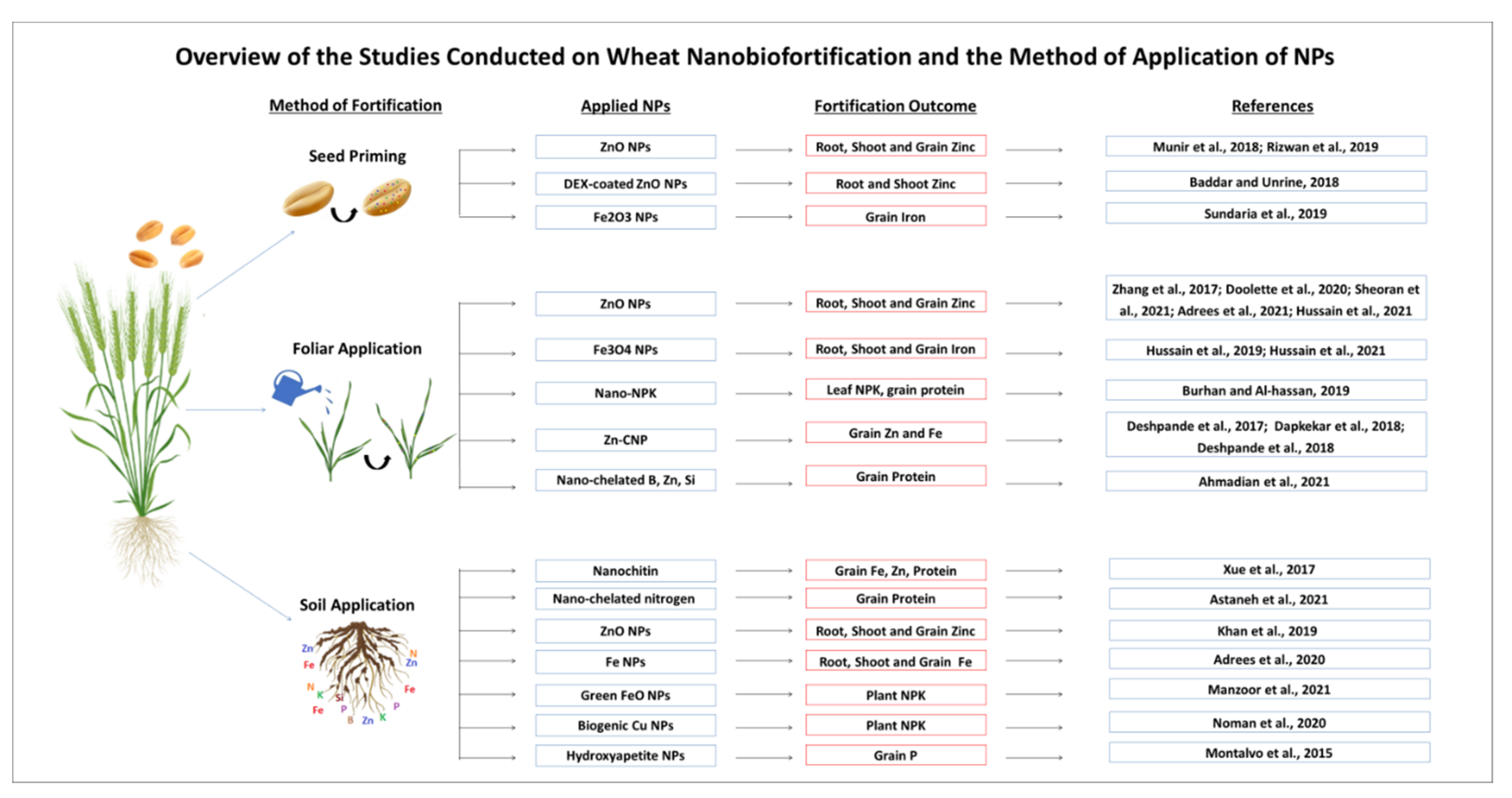
7. Wheat Micronutrients Nanobiofortification via Seed Priming
8. Wheat Micronutrients Nanobiofortification via Soil Fertilization
9. Wheat Micronutrients Nanobiofortification via Foliar Fertilization
10. Other Diverse Aspects of Wheat Nanobiofortification
11. Wheat Nanobiofortification in the Light of Cost Effectiveness and Human Health
12. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2021. Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Muthayya, S.; Rah, J.H.; Sugimoto, J.D.; Roos, F.F.; Kraemer, K.; Black, R.E. The Global Hidden Hunger Indices and Maps: An Advocacy Tool for Action. PLoS ONE 2013, 8, e67860. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Hamurcu, M.; Hakki, E.E.; Gezgin, S. 2—Role of molecular approaches in improving genetic variability of micronutrients and their utilization in breeding programs. In Wheat and Barley Grain Biofortification; Gupta, O.P., Pandey, V., Narwal, S., Sharma, P., Ram, S., Singh, G.P., Eds.; Woodhead Publishing: Duxford, UK, 2020; pp. 27–52. [Google Scholar]
- Pandey, A.; Khan, M.K.; Hakki, E.E.; Thomas, G.; Hamurcu, M.; Gezgin, S.; Gizlenci, O.; Akkaya, M.S. Assessment of genetic variability for grain nutrients from diverse regions: Potential for wheat improvement. SpringerPlus 2016, 5, 1912. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.J.; Nestel, P.; Meenakshi, J.; Qaim, M.; Sachdev, H.; Bhutta, Z.A. Plant breeding to control zinc deficiency in India: How cost-effective is biofortification? Public Health Nutr. 2007, 10, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Athar, T.; Khan, M.K.; Pandey, A.; Yilmaz, F.G.; Hamurcu, M.; Hakki, E.E.; Gezgin, S. Biofortification and the involved modern approaches. J. Elem. 2020, 25, 717–731. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Pérez-Massot, E.; Banakar, R.; Gómez-Galera, S.; Zorrilla-López, U.; Sanahuja, G.; Arjó, G.; Miralpeix, B.; Vamvaka, E.; Farré, G.; Rivera, S.M. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes Nutr. 2013, 8, 29–41. [Google Scholar] [CrossRef]
- Hefferon, K.L. Can biofortified crops help attain food security? Curr. Mol. Biol. Rep. 2016, 2, 180–185. [Google Scholar] [CrossRef][Green Version]
- Lyons, G.; Ortiz-Monasterio, I.; Stangoulis, J.; Graham, R. Selenium concentration in wheat grain: Is there sufficient genotypic variation to use in breeding? Plant Soil 2005, 269, 369–380. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, S.; Kumar, R. Bio-nanotechnology and its role in agriculture and food industry. J. Mol. Genet. Med. 2018, 12, 1747-0862. [Google Scholar]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munne-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. Int. J. Exp. Plant Biol. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamun, M.R.; Hasan, M.R.; Ahommed, M.S.; Bacchu, M.S.; Ali, M.R.; Khan, M.Z.H. Nanofertilizers towards sustainable agriculture and environment. Environ. Technol. Innov. 2021, 23, 101658. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.; Imran, M. Effect of Zinc Oxide Nanoparticles on the Growth and Zn Uptake in Wheat (Triticum aestivum L.) by Seed Priming Method. Dig. J. Nanomater. Biostruct. (DJNB) 2018, 13, 315–323. [Google Scholar]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; Zia Ur Rehman, M.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Hafeez, M.; Rizwan, M.; Hussain, K.; Asrar, M.; Alyemeni, M.N.; Wijaya, L.; Ali, S. Foliar exposure of zinc oxide nanoparticles improved the growth of wheat (Triticum aestivum L.) and decreased cadmium concentration in grains under simultaneous Cd and water deficient stress. Ecotoxicol. Environ. Saf. 2021, 208, 111627. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Almutairi, K.F.; Alotaibi, M.; Shami, A.; Alhammad, B.A.; Battaglia, M.L. Nano-Fertilization as an Emerging Fertilization Technique: Why Can Modern Agriculture Benefit from Its Use? Plants 2020, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Roser, M. Diet Compositions. In Our World Data, 2017. Available online: https://ourworldindata.org/diet-compositions (accessed on 30 August 2021).
- Laskowski, W.; Gorska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolinska, J. How Important are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef]
- Balk, J.; Connorton, J.M.; Wan, Y.; Lovegrove, A.; Moore, K.L.; Uauy, C.; Sharp, P.A.; Shewry, P.R. Improving wheat as a source of iron and zinc for global nutrition. Nutr. Bull. 2019, 44, 53–59. [Google Scholar] [CrossRef]
- Yashveer, S.; Singh, V.; Kaswan, V.; Kaushik, A.; Tokas, J. Green biotechnology, nanotechnology and bio-fortification: Perspectives on novel environment-friendly crop improvement strategies. Biotechnol. Genet. Eng. Rev. 2014, 30, 113–126. [Google Scholar] [CrossRef]
- Elemike, E.; Uzoh, I.; Onwudiwe, D.; Babalola, O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Feregrino-Perez, A.A.; Magaña-López, E.; Guzmán, C.; Esquivel, K. A general overview of the benefits and possible negative effects of the nanotechnology in horticulture. Sci. Hortic. 2018, 238, 126–137. [Google Scholar] [CrossRef]
- Solanki, P.; Bhargava, A.; Chhipa, H.; Jain, N.; Panwar, J. Nano-fertilizers and their smart delivery system. In Nanotechnologies in Food and Agriculture; Springer International Publishing: Basel, Switzerland, 2015; pp. 81–101. [Google Scholar]
- De La Torre-Roche, R.; Cantu, J.; Tamez, C.; Zuverza-Mena, N.; Hamdi, H.; Adisa, I.O.; Elmer, W.; Gardea-Torresdey, J.; White, J.C. Seed Biofortification by Engineered Nanomaterials: A Pathway To Alleviate Malnutrition? J. Agric. Food Chem. 2020, 68, 12189–12202. [Google Scholar] [CrossRef] [PubMed]
- León-Silva, S.; Arrieta-Cortes, R.; Fernández-Luqueño, F.; López-Valdez, F. Design and Production of Nanofertilizers. In Agricultural Nanobiotechnology: Modern Agriculture for a Sustainable Future; López-Valdez, F., Fernández-Luqueño, F., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 17–31. [Google Scholar]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef]
- Sekhon, B.S. Nanotechnology in agri-food production: An overview. Nanotechnol. Sci. Appl. 2014, 7, 31. [Google Scholar] [CrossRef]
- Naderi, M.; Danesh-Shahraki, A. Nanofertilizers and their roles in sustainable agriculture. Int. J. Agric. Crop Sci. (IJACS) 2013, 5, 2229–2232. [Google Scholar]
- Manjunatha, S.; Biradar, D.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–13. [Google Scholar]
- Adisa, I.O.; Pullagurala, V.L.R.; Peralta-Videa, J.R.; Dimkpa, C.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Recent advances in nano-enabled fertilizers and pesticides: A critical review of mechanisms of action. Environ. Sci. Nano 2019, 6, 2002–2030. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S.; Fugice, J.; Agyin-Birikorang, S.; Singh, U.; Hellums, D. Composite micronutrient nanoparticles and salts decrease drought stress in soybean. Agron. Sustain. Dev. 2017, 37, 5. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Mejias, J.H.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Front. Environ. Sci. 2021, 9, 52. [Google Scholar] [CrossRef]
- Golbashy, M.; Sabahi, H.; Allahdadi, I.; Nazokdast, H.; Hosseini, M. Synthesis of highly intercalated urea-clay nanocomposite via domestic montmorillonite as eco-friendly slow-release fertilizer. Arch. Agron. Soil Sci. 2017, 63, 84–95. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.; Wypych, F.; Petit, E.; Forano, C.; Prevot, V. Potential sustainable slow-release fertilizers obtained by mechanochemical activation of MgAl and MgFe layered double hydroxides and K2HPO4. Nanomaterials 2019, 9, 183. [Google Scholar] [CrossRef]
- Tarafder, C.; Daizy, M.; Alam, M.M.; Ali, M.R.; Islam, M.J.; Islam, R.; Ahommed, M.S.; Aly Saad Aly, M.; Khan, M.Z.H. Formulation of a Hybrid Nanofertilizer for Slow and Sustainable Release of Micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vega, A.-I.; Gomez-Quintero, T.; Nunez-Anita, R.-E.; Acosta-Torres, L.-S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 936041. [Google Scholar] [CrossRef]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures. Agronomy 2021, 11, 1239. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanomaterials: Classification and Properties. Nanosponges Synth. Appl. 2019, 1–26. [Google Scholar] [CrossRef]
- Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Mahmood, A.; Chattha, M.U.; Nawaz, M.; Subhani, M.N.; Kharal, M.; Khan, S. Biofortification of Wheat Cultivars to Combat Zinc Deficiency. Front. Plant Sci. 2017, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Elhaj Baddar, Z.; Unrine, J.M. Functionalized-ZnO-Nanoparticle Seed Treatments to Enhance Growth and Zn Content of Wheat (Triticum aestivum) Seedlings. J. Agric. Food Chem. 2018, 66, 12166–12178. [Google Scholar] [CrossRef]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; 2012. Available online: https://www.fao.org/3/ap106e/ap106e.pdf (accessed on 30 August 2021).
- Basavegowda, N.; Baek, K.H. Current and future perspectives on the use of nanofertilizers for sustainable agriculture: The case of phosphorus nanofertilizer. 3 Biotech 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.P.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant Growth Regul. 2018, 38, 122–131. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef]
- Zhu, J.; Li, J.; Shen, Y.; Liu, S.; Zeng, N.; Zhan, X.; White, J.C.; Gardea-Torresdey, J.; Xing, B. Mechanism of zinc oxide nanoparticle entry into wheat seedling leaves. Environ. Sci. Nano 2020, 7, 3901–3913. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Qayyum, M.F.; Nawaz, R.; Ahmad, A.; Asrar, M.; Ahmad, S.R.; Alsahli, A.A.; et al. Combined use of different nanoparticles effectively decreased cadmium (Cd) concentration in grains of wheat grown in a field contaminated with Cd. Ecotoxicol. Environ. Saf. 2021, 215, 112139. [Google Scholar] [CrossRef]
- Khan, Z.S.; Rizwan, M.; Hafeez, M.; Ali, S.; Javed, M.R.; Adrees, M. The accumulation of cadmium in wheat (Triticum aestivum) as influenced by zinc oxide nanoparticles and soil moisture conditions. Environ. Sci. Pollut. Res. Int. 2019, 26, 19859–19870. [Google Scholar] [CrossRef]
- Doolette, C.L.; Read, T.L.; Howell, N.R.; Cresswell, T.; Lombi, E. Zinc from foliar-applied nanoparticle fertiliser is translocated to wheat grain: A (65)Zn radiolabelled translocation study comparing conventional and novel foliar fertilisers. Sci. Total Environ. 2020, 749, 142369. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; Ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Shahid Chatha, S.A.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Qayyum, M.F.; Ok, Y.S.; Adrees, M.; Ibrahim, M.; Zia-ur-Rehman, M.; Farid, M.; Abbas, F. Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: A critical review. J. Hazard. Mater. 2017, 322, 2–16. [Google Scholar] [CrossRef]
- Jisha, K.C.; Vijayakumari, K.; Puthur, J.T. Seed priming for abiotic stress tolerance: An overview. Acta Physiol. Plant. 2013, 35, 1381–1396. [Google Scholar] [CrossRef]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Molecular processes induced in primed seeds—Increasing the potential to stabilize crop yields under drought conditions. J. Plant Physiol. 2016, 203, 116–126. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Ibrahim, E.A. Seed priming to alleviate salinity stress in germinating seeds. J. Plant Physiol. 2016, 192, 38–46. [Google Scholar] [CrossRef]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Gupta, N.; Dhingra, M. Effect of temperature regimes, seed priming and priming duration on germination and seedling growth on American cotton. J. Environ. Biol. 2018, 39, 83–91. [Google Scholar] [CrossRef]
- Yadav, R.; Saini, P.K.; Pratap, M.; Tripathi, S.K. Techniques of seed priming in field crops. Int. J. Chem. Stud. 2018, 6, 1588–1594. [Google Scholar]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. CuO and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1125. [Google Scholar] [CrossRef]
- Spielman-Sun, E.; Lombi, E.; Donner, E.; Howard, D.; Unrine, J.M.; Lowry, G.V. Impact of surface charge on cerium oxide nanoparticle uptake and translocation by wheat (Triticum aestivum). Environ. Sci. Technol. 2017, 51, 7361–7368. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef]
- Noman, M.; Ahmed, T.; Hussain, S.; Niazi, M.B.K.; Shahid, M.; Song, F. Biogenic copper nanoparticles synthesized by using a copper-resistant strain Shigella flexneri SNT22 reduced the translocation of cadmium from soil to wheat plants. J. Hazard. Mater. 2020, 398, 123175. [Google Scholar] [CrossRef]
- Murtaza, G.; Javed, W.; Hussain, A.; Qadir, M.; Aslam, M. Soil-applied zinc and copper suppress cadmium uptake and improve the performance of cereals and legumes. Int. J. Phytoremediat. 2017, 19, 199–206. [Google Scholar] [CrossRef]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Sanabria, J.; Bindraban, P.S.; Singh, U.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Interactive effects of drought, organic fertilizer, and zinc oxide nanoscale and bulk particles on wheat performance and grain nutrient accumulation. Sci. Total Environ. 2020, 722, 137808. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Han, Y.; Tan, J.; Wang, Y.; Wang, G.; Wang, H. Effects of nanochitin on the enhancement of the grain yield and quality of winter wheat. J. Agric. Food Chem. 2017, 66, 6637–6645. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, N.; Bazrafshan, F.; Zare, M.; Amiri, B.; Bahrani, A. Nano-fertilizer prevents environmental pollution and improves physiological traits of wheat grown under drought stress conditions. Sci. Agropecu. 2021, 12, 41–47. [Google Scholar] [CrossRef]
- Ghafari, H.; Razmjoo, J. Effect of foliar application of nano-iron oxidase, iron chelate and iron sulphate rates on yield and quality of wheat. Int. J. Agron. Plant Prod. 2013, 4, 2997–3003. [Google Scholar]
- Sheoran, P.; Grewal, S.; Kumari, S.; Goel, S. Enhancement of growth and yield, leaching reduction in Triticum aestivum using biogenic synthesized zinc oxide nanofertilizer. Biocatal. Agric. Biotechnol. 2021, 32, 101938. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Lv, Z.; Cui, L.; Mao, H.; Kopittke, P.M. Using Synchrotron-Based Approaches To Examine the Foliar Application of ZnSO4 and ZnO Nanoparticles for Field-Grown Winter Wheat. J. Agric. Food Chem. 2018, 66, 2572–2579. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; van der Ent, A.; Cheng, M.; Jiang, H.; Lund Read, T.; Lombi, E.; Tang, C.; de Jonge, M.D.; Menzies, N.W.; et al. Absorption of foliar-applied Zn in sunflower (Helianthus annuus): Importance of the cuticle, stomata and trichomes. Ann. Bot. 2018, 123, 57–68. [Google Scholar] [CrossRef]
- Li, C.; Wang, P.; Lombi, E.; Cheng, M.; Tang, C.; Howard, D.L.; Menzies, N.W.; Kopittke, P.M. Absorption of foliar-applied Zn fertilizers by trichomes in soybean and tomato. J. Exp. Bot. 2018, 69, 2717–2729. [Google Scholar] [CrossRef] [PubMed]
- Read, T.L.; Doolette, C.L.; Li, C.; Schjoerring, J.K.; Kopittke, P.M.; Donner, E.; Lombi, E. Optimising the foliar uptake of zinc oxide nanoparticles: Do leaf surface properties and particle coating affect absorption? Physiol. Plant. 2020, 170, 384–397. [Google Scholar] [CrossRef]
- Dapkekar, A.; Deshpande, P.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc use efficiency is enhanced in wheat through nanofertilization. Sci. Rep. 2018, 8, 6832. [Google Scholar] [CrossRef]
- Deshpande, P.; Dapkekar, A.; Oak, M.; Paknikar, K.; Rajwade, J. Nanocarrier-mediated foliar zinc fertilization influences expression of metal homeostasis related genes in flag leaves and enhances gluten content in durum wheat. PLoS ONE 2018, 13, e0191035. [Google Scholar] [CrossRef]
- Behboudi, F.; Tahmasebi Sarvestani, Z.; Kassaee, M.Z.; Modares Sanavi, S.A.M.; Sorooshzadeh, A.; Ahmadi, S.B. Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 2018, 3, 22–39. [Google Scholar]
- Ahmadian, K.; Jalilian, J.; Pirzad, A. Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agric. Water Manag. 2021, 244, 106544. [Google Scholar] [CrossRef]
- Cakmak, I. Possible Roles of Zinc in Protecting Plant Cells from Damage by Reactive Oxygen Species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Nazaralian, S.; Majd, A.; Irian, S.; Najafi, F.; Ghahremaninejad, F.; Landberg, T.; Greger, M. Comparison of silicon nanoparticles and silicate treatments in fenugreek. Plant Physiol. Biochem. 2017, 115, 25–33. [Google Scholar] [CrossRef]
- Al-Amri, N.; Tombuloglu, H.; Slimani, Y.; Akhtar, S.; Barghouthi, M.; Almessiere, M.; Alshammari, T.; Baykal, A.; Sabit, H.; Ercan, I.; et al. Size effect of iron (III) oxide nanomaterials on the growth, and their uptake and translocation in common wheat (Triticum aestivum L.). Ecotoxicol. Environ. Saf. 2020, 194, 110377. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Andrews, J.; Fugice, J.; Singh, U.; Bindraban, P.S.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Facile Coating of Urea With Low-Dose ZnO Nanoparticles Promotes Wheat Performance and Enhances Zn Uptake Under Drought Stress. Front. Plant Sci. 2020, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. Sci. Total Environ. 2017, 589, 11–24. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2018, 66, 6462–6473. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M.R.; Ghodszad, L.; Lajayer, B.A. Dilemma of hydroxyapatite nanoparticles as phosphorus fertilizer: Potentials, challenges and effects on plants. Environ. Technol. Innov. 2020, 19, 100869. [Google Scholar] [CrossRef]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Burhan, M.; Al-Hassan, S. Impact of nano npk fertilizers to correlation between productivity, quality and flag leaf of some bread wheat varieties. Iraqi J. Agric. Sci. 2019, 50, 1–7. [Google Scholar]
- Gomez, A.; Narayan, M.; Zhao, L.; Jia, X.; Bernal, R.A.; Lopez-Moreno, M.L.; Peralta-Videa, J.R. Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J. Hazard. Mater. 2021, 401, 123385. [Google Scholar] [CrossRef]
- Achari, G.A.; Kowshik, M. Recent Developments on Nanotechnology in Agriculture: Plant Mineral Nutrition, Health, and Interactions with Soil Microflora. J. Agric. Food Chem. 2018, 66, 8647–8661. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Elbasiouny, H.; Elbehiry, F.; Elsakhawy, T.; Omara, A.E.; Amer, M.; Bayoumi, Y.; Shalaby, T.A.; Eid, Y.; et al. Nano-biofortification of different crops to immune against COVID-19: A review. Ecotoxicol. Environ. Saf. 2021, 222, 112500. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Riaz, M.; Adrees, M.; Hussain, A.; Zahir, Z.A.; Rinklebe, J. Effects of nanoparticles on trace element uptake and toxicity in plants: A review. Ecotoxicol. Environ. Saf. 2021, 221, 112437. [Google Scholar] [CrossRef]
- DeMan, J.M.; Finley, J.W.; Hurst, W.J.; Lee, C.Y. Principles of Food Chemistry; Springer: Gaithersburg, Maryland, 1999; Volume 478. [Google Scholar]
- Robsa, S. Review paper on the Role of Somatic Hybridization in Crop Improvement. Int. J. Res. Stud. Agric. Sci. 2018, 4, 1–8. [Google Scholar]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Kumar, S.; Srivastava, A.K. Nanodiagnostics for plant pathogens. Environ. Chem. Lett. 2017, 15, 7–13. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Ashraful Alam, M.; Syed, M.A.; Hossain, J.; Sarkar, S.; Saha, S.; Bhadra, P.; et al. Consequences and Mitigation Strategies of Abiotic Stresses in Wheat (Triticum aestivum L.) under the Changing Climate. Agronomy 2021, 11, 241. [Google Scholar] [CrossRef]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef]
| Reference | Type of Application | Place of Trial | Growth Medium | On Application of Nanomaterial | On Application of Conventional Fertilizers | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Applied Nanomaterial | Amount Applied | Nutrient Content in Grain without Application of Nanomaterial | Nutrient Content in Grain on Addition of Nanomaterial | Applied Conventional Fertilizer | Amount Applied | Nutrient Content in Grain on Addition of Conventional Fertilizers | ||||
| Deshpande et al. 2017 | Foliar | PVC columns | Sand | Zn-CNP | ≈20 mg g−1, ≈25mL | ≈15–19 µg g−1 DW Zn | ≈21–25 µg g−1 DW Zn | NC | ||
| Xue et al. 2017 | Soil | Pot | Soil | Nanochitin | 0.006 g kg−1 | 184.3 g kg−1 protein 58.6 mg kg−1 Fe 42.13 mg kg−1 Zn | 204.1 g kg−1 protein 65.39 mg kg−1 Fe 51.45 mg kg−1 Zn | NC | ||
| Zhang et al. 2017 | Foliar | Field | Soil | ZnO NP | 2 g L−1 at a rate of 1.2 kg ha−1 | 18.4 mg kg−1 (Year 1) Zn 23.6 mg kg−1 (Year 2) Zn | 26.5 mg kg−1 (Year 1) Zn 34.6 mg kg−1 (Year 2) Zn | ZnSO4 | 7 g L−1 at a rate of 4.2 kg ha−1 | 21.1 mg/kg (Year 1) Zn 29.5 mg kg−1 (Year 2) Zn |
| Dapkekar et al. 2018 | Foliar | Field | Soil | Zn-CNP | 40 mg L−1 | 39.5 µg g−1 | 53.3 µg g−1 | ZnSO4 | 400 mg L−1 | 59.40 µg g−1 Zn |
| Munir et al. 2018 | Seed Priming | Pot | Soil | ZnO NPs | 100 mg L−1 | ≈12 mg kg−1 DW Zn | ≈20 mg kg−1 DW Zn | NC | ||
| Burhan and Al-Hassan et al. 2019 | Foliar | Field | Soil | Nano NPK | 750:90:600 mg L−1 | - | 13.5 % protein | traditional NPK | 400 kg ha−1 urea, 200 kg ha−1 tri super phosphate, 100 kg ha−1 K2SO4 | 10.68 % protein |
| Hussain et al. 2019 | Foliar | Pot | Soil | Fe3O4 NP | 20 mg kg−1 | ≈40 mg kg−1 DW Fe | ≈120 mg kg−1 DW Fe | NC | ||
| Hussain et al. 2019 | Soil | Pot | Soil | Fe3O4 NP | 20 mg kg−1 | ≈40 mg kg−1 DW Fe | ≈90 mg kg−1 DW Fe | NC | ||
| Khan et al. 2019 | Soil | Field | Soil | ZnO NP | 100 mg kg−1 | ≈20 mg kg−1 DW Zn | ≈45 mg kg−1 DW Zn | NC | ||
| Rizwan et al. 2019 | Seed Priming | Pot | Soil | ZnO NPs | 100 mg L−1 | ≈15 mg kg−1 DW Zn | ≈30 mg kg−1 DW Zn | NC | ||
| Rizwan et al. 2019 | Seed Priming | Pot | Soil | Fe NPs | 20 mg L−1 | ≈15 mg kg−1 DW Fe | ≈30 mg kg−1 DW Fe | NC | ||
| Sundaria et al. 2019 | Seed Priming | Pot Greenhouse | Soil | Fe2O3 NP | 600 ppm | ≈30–40 mg kg−1 DW Fe | ≈40–45 mg kg−1 DW Fe | NC | ||
| Adrees et al. 2020 | Soil | Pot | Soil | Fe2O3 NP | 100 mg kg−1 | ≈20 mg kg−1 DW Fe | ≈45 mg kg−1 DW Fe | NC | ||
| Adrees et al. 2021 | Foliar | Pot | Soil | ZnO NP | 100 mg L−1 | ≈22 mg kg−1 DW Zn | ≈45 mg kg−1 DW Zn | NC | ||
| Astaneh et al. 2021 | Soil | Field | Soil | Nano-chelated nitrogen | 240 kg ha−1 | - | 69% protein 80 mg P 38 mg K | Urea | 240 kg ha−1 | 17% protein 54 mg P 27 mg K |
| Hussain et al. 2021 | Foliar | Field | Soil | Fe3O4 NP | 5 mg L−1 | ≈30 mg kg−1 DW Fe | ≈45 mg kg−1 DW Fe | NC | ||
| Hussain et al. 2021 | Foliar | Field | Soil | ZnO NP | 25 mg L−1 | ≈18 mg kg−1 DW Zn | ≈25 mg kg−1 DW Zn | NC | ||
| Sheoran et al. 2021 | Foliar | Pot | Soil | ZnO NP | 120 ppm | 17.48 mg g−1 FW Protein | 22.71 mg g−1 FW Protein | Chemical Zn | - | 19.91 mg g−1 FW Protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.K.; Pandey, A.; Hamurcu, M.; Gezgin, S.; Athar, T.; Rajput, V.D.; Gupta, O.P.; Minkina, T. Insight into the Prospects for Nanotechnology in Wheat Biofortification. Biology 2021, 10, 1123. https://doi.org/10.3390/biology10111123
Khan MK, Pandey A, Hamurcu M, Gezgin S, Athar T, Rajput VD, Gupta OP, Minkina T. Insight into the Prospects for Nanotechnology in Wheat Biofortification. Biology. 2021; 10(11):1123. https://doi.org/10.3390/biology10111123
Chicago/Turabian StyleKhan, Mohd. Kamran, Anamika Pandey, Mehmet Hamurcu, Sait Gezgin, Tabinda Athar, Vishnu D. Rajput, Om Prakash Gupta, and Tatiana Minkina. 2021. "Insight into the Prospects for Nanotechnology in Wheat Biofortification" Biology 10, no. 11: 1123. https://doi.org/10.3390/biology10111123
APA StyleKhan, M. K., Pandey, A., Hamurcu, M., Gezgin, S., Athar, T., Rajput, V. D., Gupta, O. P., & Minkina, T. (2021). Insight into the Prospects for Nanotechnology in Wheat Biofortification. Biology, 10(11), 1123. https://doi.org/10.3390/biology10111123










