Immune Response in Crayfish Is Species-Specific and Exhibits Changes along Invasion Range of a Successful Invader
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Procedure
2.3. Immune Response Analyses
2.3.1. The Encapsulation Response Analyses
2.3.2. Hemolymph Sampling Procedure
2.3.3. Total Hemocyte Count
2.3.4. PO Activity and Total proPO
2.4. Body Condition Parameters
2.5. Statistical Analyses
2.5.1. Comparisons of Changes of the Signal Crayfish Immune Response along Invasion Range and Their Potential Drivers
2.5.2. Comparisons of Immune Response between the Invasive Signal Crayfish and the Native Narrow-Clawed Crayfish
3. Results
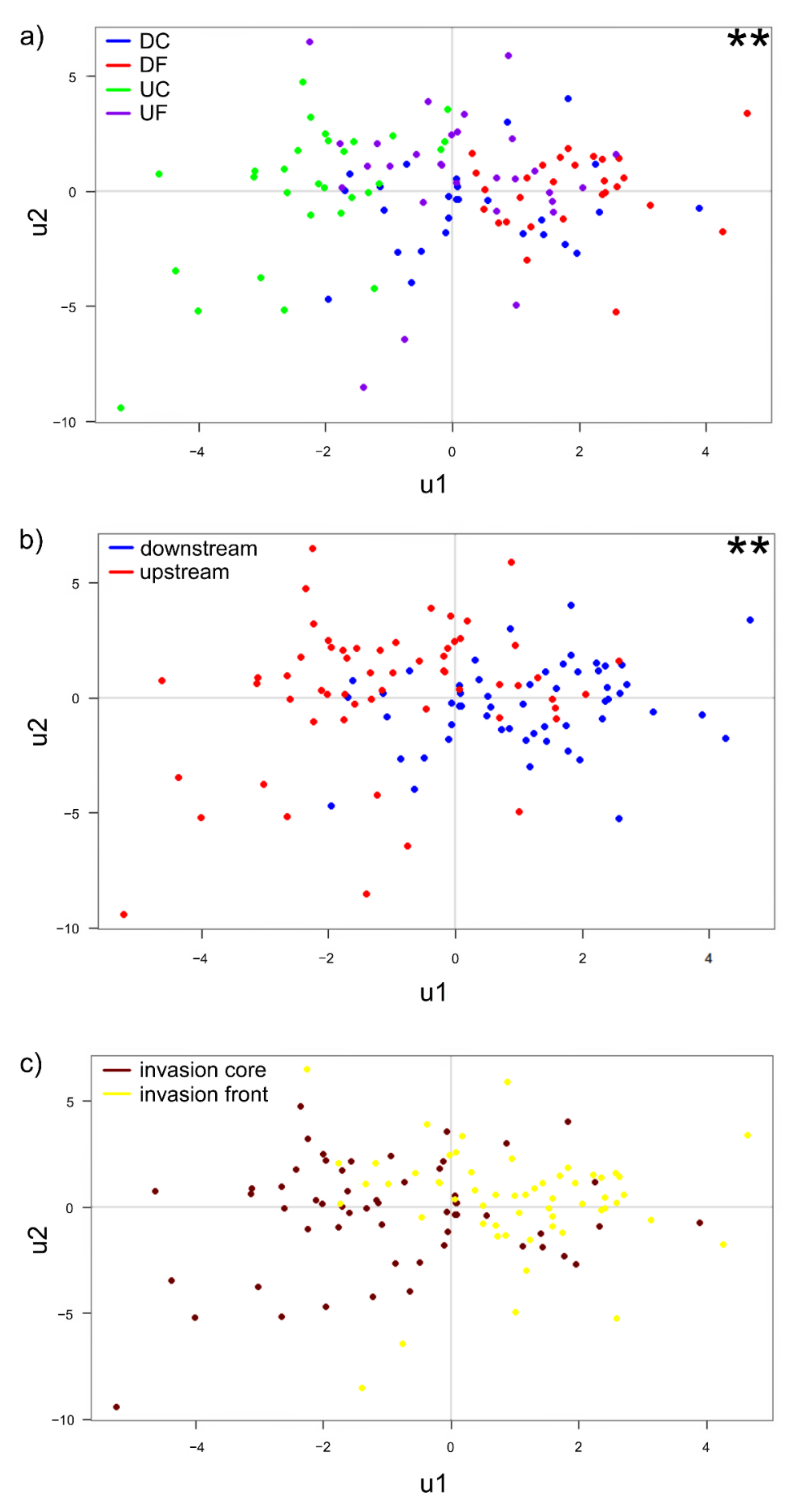
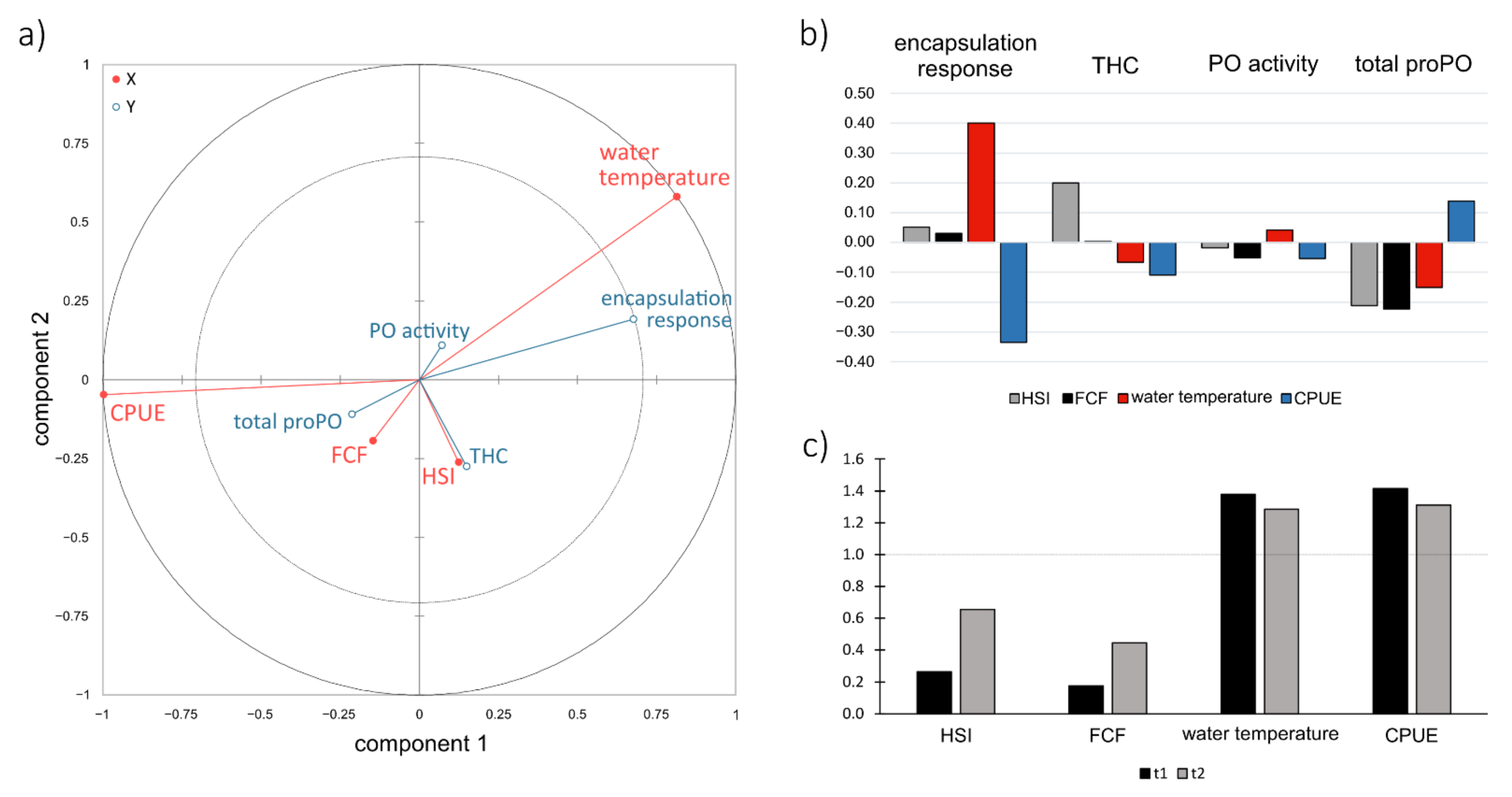
3.1. Comparisons of Changes of the Signal Crayfish Immune Response along Its Invasion Range and Their Potential Drivers
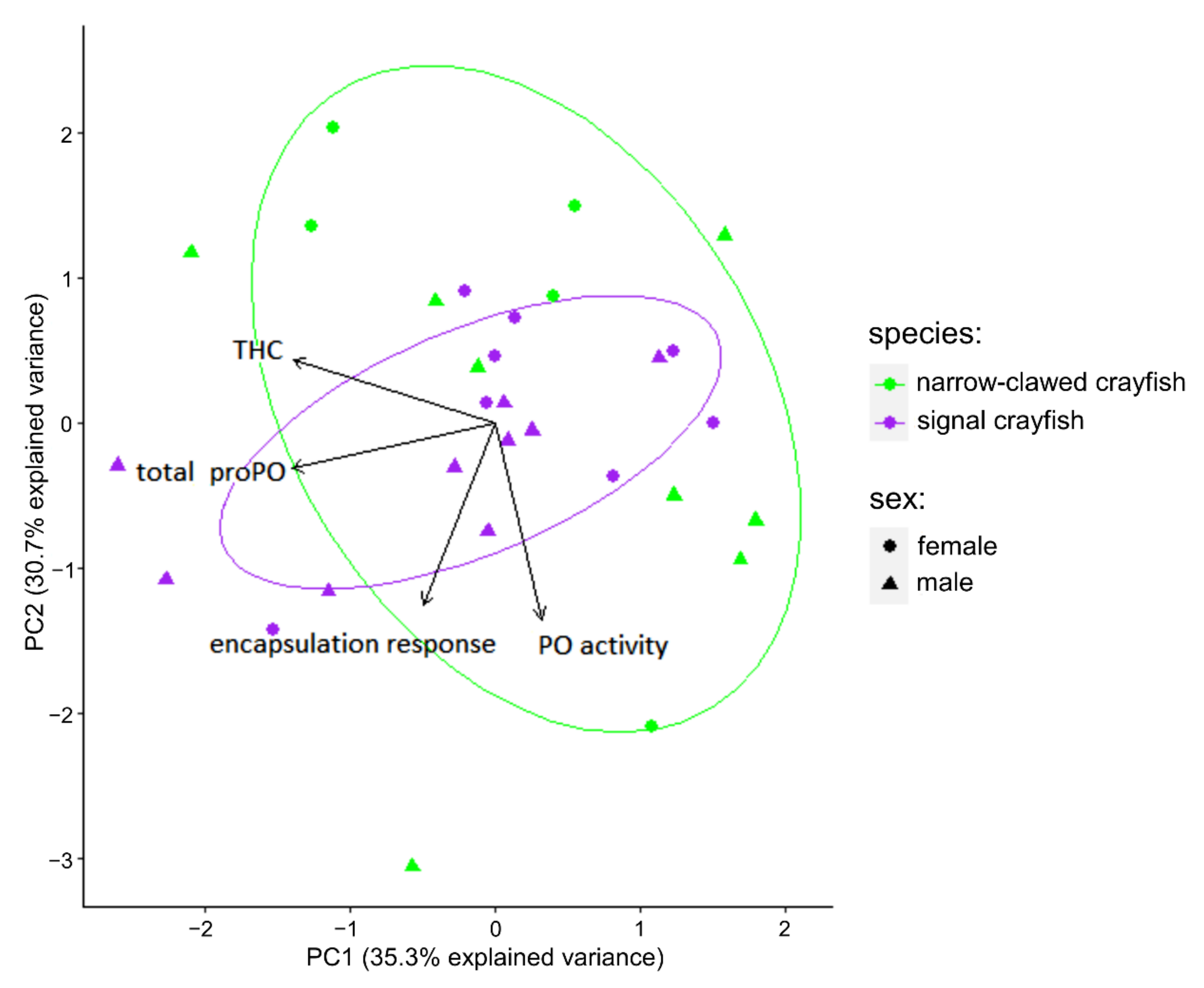
3.2. Comparisons of Immune Response between the Invasive Signal Crayfish and the Native Narrow-Clawed Crayfish
4. Discussion
4.1. Comparisons of Changes of the Signal Crayfish Immune Response along Its Invasion Range and Their Potential Drivers
4.2. Relationships between the Predictors and Immune Parameters
4.3. Comparisons of Immune Response between the Invasive Signal Crayfish and the Native Narrow-Clawed Crayfish
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mack, R.N.; Simberloff, D.; Lonsdale, W.M.; Evans, H.; Clout, M.; Bazzaz, F.A. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol. Appl. 2000, 10, 689–710. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Shackleton, R.T.; Shackleton, C.M.; Kull, C.A. The role of invasive alien species in shaping local livelihoods and human well-being: A review. J. Environ. Manag. 2018, 229, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Chinchio, E.; Crotta, M.; Romeo, C.; Drewe, J.A.; Guitian, J.; Ferrari, N. Invasive alien species and disease risk: An open challenge in public and animal health. PLoS Pathog. 2020, 16, e1008922. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Klasing, K.C. A role for immunology in invasion biology. Trends Ecol. Evol. 2004, 19, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.M.; Torchin, M.E.; Hatcher, M.J.; Kotanen, P.M.; Blumenthal, D.M.; Byers, J.E.; Coon, C.A.; Frankel, V.M.; Holt, R.D.; Hufbauer, R.A.; et al. Indirect effects of parasites in invasions. Funct. Ecol. 2012, 26, 1262–1274. [Google Scholar] [CrossRef]
- White, T.A.; Perkins, S.E. The ecoimmunology of invasive species. Funct. Ecol. 2012, 26, 1313–1323. [Google Scholar] [CrossRef]
- Vogel, H.; Schmidtberg, H.; Vilcinskas, A. Comparative transcriptomics in three ladybird species supports a role for immunity in invasion biology. Dev. Comp. Immunol. 2017, 67, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in Invertebrates: Measurement and Ecotoxicological Relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef]
- Phillips, B.L.; Brown, G.P.; Shine, R. Life-history evolution in range-shifting populations. Ecology 2010, 91, 1617–1627. [Google Scholar] [CrossRef]
- Brown, G.P.; Shine, R. Immune Response Varies with Rate of Dispersal in Invasive Cane Toads (Rhinella marina). PLoS ONE 2014, 9, e99734. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.P.; Kelehear, C.; Shilton, C.M.; Phillips, B.L.; Shine, R. Stress and immunity at the invasion front: A comparison across cane toad (Rhinella marina) populations. Biol. J. Linn. Soc. 2015, 116, 748–760. [Google Scholar] [CrossRef]
- Keane, R. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Zuk, M.; Stoehr, A.M. Immune defense and host life history. Am. Nat. 2002, 160 (Suppl. 4), S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.L.; Kelehear, C.; Pizzatto, L.; Brown, G.P.; Barton, D.; Shine, R. Parasites and pathogens lag behind their host during periods of host range advance. Ecology 2010, 91, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Colautti, R.I.; Ricciardi, A.; Grigorovich, I.A.; MacIsaac, H.J. Is invasion success explained by the enemy release hypothesis? Ecol. Lett. 2004, 7, 721–733. [Google Scholar] [CrossRef]
- Blossey, B.; Nötzold, R. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Torchin, M.E.; Lafferty, K.D.; Dobson, A.P.; McKenzie, V.J.; Kuris, A.M. Introduced species and their missing parasites. Nature 2003, 421, 628–630. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.W.; Paterson, R.A.; Townsend, C.R.; Poulin, R.; Tompkins, D.M. Parasite spillback: A neglected concept in invasion ecology? Ecology 2009, 90, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Therry, L.; Nilsson-Örtman, V.; Bonte, D.; Stoks, R. Rapid evolution of larval life history, adult immune function and flight muscles in a poleward-moving damselfly. J. Evol. Biol. 2014, 27, 141–152. [Google Scholar] [CrossRef]
- Cornet, S.; Brouat, C.; Diagne, C.; Charbonnel, N. Eco-immunology and bioinvasion: Revisiting the evolution of increased competitive ability hypotheses. Evol. Appl. 2016, 9, 952–962. [Google Scholar] [CrossRef]
- Diagne, C.; Gilot-Fromont, E.; Cornet, S.; Husse, L.; Doucouré, S.; Dalecky, A.; Bâ, K.; Kane, M.; Niang, Y.; Diallo, M.; et al. Contemporary variations of immune responsiveness during range expansion of two invasive rodents in Senegal. Oikos 2017, 126, 435–446. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M.; Brzeziński, M.; Niemczynowicz, A.; Zalewski, A. High parasite infection level in non-native invasive species: It is just a matter of time. Ecography 2017, 41, 1283–1294. [Google Scholar] [CrossRef]
- Ochocki, B.; Miller, T. Rapid evolution of dispersal ability makes biological invasions faster and more variable. Nat. Commun. 2017, 8, 14315. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.; Evans, M.R. Ecological immunology: Life history trade-offs and immune defense in birds. Behav. Ecol. 2000, 11, 19–26. [Google Scholar] [CrossRef]
- Söderbäck, B. Interspecific dominance relationship and aggressive interactions in the freshwater crayfishes Astacus astacus (L.) and Pacifastacus leniusculus (Dana). Can. J. Zool. 1991, 69, 1321–1325. [Google Scholar] [CrossRef]
- Usio, N.; Kamiyama, R.; Saji, A.; Takamura, N. Size-dependent impacts of invasive alien crayfish on a littoral marsh community. Biol. Conserv. 2009, 142, 1480–1490. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Pintor, L.M.; Sih, A.; Bauer, M. Differences in aggression, activity and boldness between native introduced populations of an invasive crayfish. Oikos 2008, 117, 1629–1636. [Google Scholar] [CrossRef]
- Matsuzaki, S.S.; Usio, N.; Takamura, N.; Washitani, I. Contrasting impacts of invasive engineers on freshwater ecosystems: An experiment and meta-analysis. Oecologia 2009, 158, 673–686. [Google Scholar] [CrossRef]
- Hudina, S.; Hock, K.; Radović, A.; Klobučar, G.; Petković, J.; Jelić, M.; Maguire, I. Species-specific differences in dynamics of agonistic interactions may contribute to the competitive advantage of the invasive signal crayfish (Pacifastacus leniusculus) over the native narrow-clawed crayfish (Astacus leptodactylus). Mar. Freshw. Behav. Physiol. 2016, 49, 147–157. [Google Scholar] [CrossRef]
- Lodge, D.M.; Deines, A.; Gherardi, F.; Yeo, D.C.J.; Arcella, T.; Baldridge, A.K.; Barnes, M.A.; Chadderton, W.L.; Feder, J.L.; Gantz, C.A.; et al. Global Introductions of Crayfishes: Evaluating the Impact of Species Invasions on Ecosystem Services. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 449–472. [Google Scholar] [CrossRef]
- Twardochleb, L.; Olden, J.; Larson, E. A global meta-analysis of the ecological impacts of nonnative crayfish. Freshw. Sci. 2013, 32, 1367–1382. [Google Scholar] [CrossRef]
- Hudina, S.; Žganec, K.; Lucić, A.; Trgovčić, K.; Maguire, I. Recent invasion of the karstic river systems in Croatia through illegal introductions of the signal crayfish. Freshw. Crayfish 2013, 19, 21–27. [Google Scholar] [CrossRef]
- Hudina, S.; Kutleša, P.; Trgovčić, K.; Duplić, A. Dynamics of range expansion of the signal crayfish (Pacifastacus leniusculus) in a recently invaded region in Croatia. Aquat. Invasions 2017, 12, 67–75. [Google Scholar] [CrossRef]
- Dragičević, P.; Faller, M.; Kutleša, P.; Hudina, S. Update on the signal crayfish, Pacifastacus leniusculus (Dana, 1852) range expansion in Croatia: A 10-year report. BioInvasions Rec. 2020, 9, 793–807. [Google Scholar] [CrossRef]
- Rebrina, F.; Skejo, J.; Lucić, A.; Hudina, S. Trait variability of the signal crayfish (Pacifastacus leniusculus) in a recently invaded region reflects potential benefits and trade-offs during dispersal. Aquat. Invasions 2015, 10, 41–50. [Google Scholar] [CrossRef]
- Hudina, S.; Žganec, K.; Hock, K. Differences in aggressive behaviour along the expanding range of an invasive crayfish: An important component of invasion dynamics. Biol. Invasions 2015, 17, 3101–3112. [Google Scholar] [CrossRef]
- Groen, M.; Sopinka, N.M.; Marentette, J.R.; Reddon, A.R.; Brownscombe, J.; Fox, M.G.; Marsh-Rollo, S.E.; Balshine, S. Is there a role for aggression in round goby invasion fronts? Behaviour 2012, 149, 685–703. [Google Scholar] [CrossRef]
- Masson, L.; Brownscombe, J.W.; Fox, M.G. Fine scale spatio-temporal life history shifts in an invasive species at its expansion front. Biol. Invasions 2016, 18, 775–792. [Google Scholar] [CrossRef]
- Maguire, I.; Jelić, M.; Klobučar, G. Update on the distribution of freshwater crayfish in Croatia. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 31. [Google Scholar] [CrossRef]
- Sritunyalucksana, K.; Söderhäll, K. The proPO and clotting system in crustaceans. Aquaculture 2000, 191, 53–69. [Google Scholar] [CrossRef]
- Theopold, U.; Schmidt, O.; Söderhäll, K.; Dushay, M.S. Coagulation in arthropods: Defence, wound closure and healing. Trends Immunol. 2004, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Ferrandon, D.; Imler, J.-L.; Hetru, C.; Hoffmann, J.A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol. 2007, 7, 862–874. [Google Scholar] [CrossRef]
- Söderhäll, K. Editorial: Invertebrate immunity. Dev. Comp. Immunol. 1999, 23, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, M.; Córdoba-Aguilar, A.; Condé, R.; Lanz-Mendoza, H. Current immunity markers in insect ecological immunology: Assumed trade-offs and methodological issues. Bull. Entomol. Res. 2013, 103, 127–139. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Kanost, M.R.; Trenczek, T. Biological mediators of insect immunity. Annu. Rev. Entomol. 1997, 42, 611–643. [Google Scholar] [CrossRef]
- Johansson, M.W.; Söderhäll, K. Exocytosis of the prophenoloxidase activating system from crayfish haemocytes. J. Comp. Physiol. B 1985, 156, 175–181. [Google Scholar] [CrossRef]
- Söderhäll, K.; Cerenius, L. Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol. 1998, 10, 23–28. [Google Scholar] [CrossRef]
- Nappi, A.J.; Vass, E.; Frey, F.; Carton, Y. Superoxidase anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 1995, 68, 450–456. [Google Scholar]
- Cerenius, L.; Lee, B.L.; Söderhäll, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, J.; Gollas-Galván, T.; Vargas-Albores, F. Activation of the prophenoloxidase system of the brown shrimp (Penaeus californiensis Holmes). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1996, 113, 61–66. [Google Scholar] [CrossRef]
- Persson, M.; Cerenius, L.; Söderhäll, K. The influence of haemocyte number on the resistance of the freshwater crayfish, Pacifastacus leniusculus Dana, to the parasitic fungus Aphanomyces astaci. J. Fish Dis. 1987, 10, 471–477. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Muséum National d’Histoire Naturelle: Paris, France, 2006. [Google Scholar]
- Westman, K.; Pursiainen, M.; Vilkman, R. A new folding trap model which prevents crayfish from escaping. Freshw. Crayfish 1978, 4, 235–242. [Google Scholar] [CrossRef]
- Dana, E.D.; López-Santiago, J.; García-De-Lomas, J.; Garcia-Ocaña, D.M.; Gámez, V.; Ortega, F. Long-term management of the invasive Pacifastacus leniusculus (Dana, 1852) in a small mountain stream. Aquat. Invasions 2010, 5, 317–322. [Google Scholar] [CrossRef]
- Gruber, C.; Kortet, R.; Vainikka, A.; Hyvärinen, P.; Rantala, M.J.; Pikkarainen, A.; Jussila, J.; Makkonen, J.; Kokko, H.; Hirvonen, H. Variation in Resistance to the Invasive Crayfish Plague and Immune Defence in the Native Noble Crayfish. Ann. Zool. Fenn. 2014, 51, 371–389. [Google Scholar] [CrossRef]
- Gruber, C.; Vainikka, A.; Hirvonen, H.; Rantala, M.J.; Kortet, R. Endogenous Seasonal Variation in the Encapsulation Response of the Noble Crayfish (Astacus astacus). Ann. Zool. Fenn. 2014, 51, 433–444. [Google Scholar] [CrossRef]
- Battistella, S.; Bonivento, P.; Amirante, G.A. Hemocytes and immunological reactions in crustaceans. Ital. J. Zool. 1996, 63, 337–343. [Google Scholar] [CrossRef]
- Johansson, M.W.; Keyser, P.; Sritunyalucksana, K.; Söderhäll, K. Crustacean haemocytes and haematopoiesis. Aquaculture 2000, 191, 45–52. [Google Scholar] [CrossRef]
- Pan, L.-Q.; Hu, F.-W.; Jing, F.-T.; Liu, H.-J. The effect of different acclimation temperatures on the prophenoloxidase system and other defence parameters in Litopenaeus vannamei. Fish Shellfish Immunol. 2008, 25, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Du, J.; Ou, J.; Li, W.; Wu, T.; Xiu, Y.; Meng, Q.; Ren, Q.; Gu, W.; Xue, H.; et al. Classification of circulating hemocytes from the red swamp crayfish Procambarus clarkii and their susceptibility to the novel pathogen Spiroplasma eriocheiris in vitro. Aquaculture 2012, 356–357, 371–380. [Google Scholar] [CrossRef]
- Becking, T.; Mrugała, A.; Delaunay, C.; Svoboda, J.; Raimond, M.; Viljamaa-Dirks, S.; Petrusek, A.; Grandjean, F.; Braquart-Varnier, C. Effect of experimental exposure to differently virulent Aphanomyces astaci strains on the immune response of the noble crayfish Astacus astacus. J. Invertebr. Pathol. 2015, 132, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tanner, C.A.; Burnett, L.E.; Burnett, K.G. The effects of hypoxia and pH on phenoloxidase activity in the Atlantic blue crab, Callinectes sapidus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Babu, V.S.; Lin, H.; Dai, Y.; Kou, H.; Chen, L.; Li, J.; Zhao, L.; Lin, L. The immune function of prophenoloxidase from red swamp crayfish (Procambarus clarkii) in response to bacterial infection. Fish Shellfish Immunol. 2019, 92, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Kortet, R. Male dominance and immunocompetence in a field cricket. Behav. Ecol. 2004, 15, 187–191. [Google Scholar] [CrossRef]
- Rantala, M.J.; Roff, D.A. Inbreeding and extreme outbreeding cause sex differences in immune defence and life history traits in Epirrita autumnata. Heredity 2007, 98, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Smilanich, A.M.; Dyer, L.A.; Gentry, G.L. The insect immune response and other putative defenses as effective predictors of parasitism. Ecology 2009, 90, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Ardia, D.R.; Gantz, J.E.; Schneider, B.C.; Strebel, S. Costs of immunity in insects: An induced immune response increases metabolic rate and decreases antimicrobial activity. Funct. Ecol. 2012, 26, 732–739. [Google Scholar] [CrossRef]
- Conte, F.; Voslarova, A.; Vecerek, V.; Elwood, R.W.; Coluccio, P.; Pugliese, M.; Passantino, A. Humane Slaughter of Edible Decapod Crustaceans. Animals 2021, 11, 1089. [Google Scholar] [CrossRef] [PubMed]
- Giulianini, P.G.; Bierti, M.; Lorenzon, S.; Battistella, S.; Ferrero, E.A. Ultrastructural and functional characterization of circulating hemocytes from the freshwater crayfish Astacus leptodactylus: Cell types and their role after in vivo artificial non-self challenge. Micron 2007, 38, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Malev, O.; Šrut, M.; Maguire, I.; Štambuk, A.; Ferrero, E.A.; Lorenzon, S.; Klobučar, G.I.V. Genotoxic, physiological and immunological effects caused by temperature increase, air exposure or food deprivation in freshwater crayfish Astacus leptodactylus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Jussila, J.; Jago, J.; Tsvetnenko, E.; Dunstan, B.; Evans, L.H. Total and differential haemocyte counts in western rock lobsters (Panulirus cygnus George) under postharvest stress. Mar. Fresh. Res. 1997, 48, 863–868. [Google Scholar] [CrossRef]
- Gollas-Galván, T.; Hernández-López, J.; Vargas-Albores, F. Prophenoloxidase from brown shrimp (Penaeus californiensis) hemocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1999, 122, 77–82. [Google Scholar] [CrossRef]
- Hernández-López, J.; Gollas-Galván, T.; Gómez-Jiménez, S.; Portillo-Clark, G.; Vargas-Albores, F. In the spiny lobster (Panulirus interruptus) the prophenoloxidase is located in plasma not in haemocytes. Fish Shellfish Immunol. 2003, 14, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Perazzolo, L.M.; Barracco, M.A. The prophenoloxidase activating system of the shrimp Penaeus paulensis and associated factors. Dev. Comp. Immunol. 1997, 21, 385–395. [Google Scholar] [CrossRef]
- Freitak, D.; Wheat, C.W.; Heckel, D.G.; Vogel, H. Immune system responses and fitness costs associated with consumption of bacteria in larvae of Trichoplusia ni. BMC Biol. 2007, 5, 56. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Bulletin of the Fisheries Research Board of Canada: Ottawa, ON, Canada, 1975. [Google Scholar]
- Rodríguez-González, H.; Hernández-Llamas, A.; Villarreal, H.; Saucedo, P.E.; García-Ulloa, M.; Rodríguez-Jaramillo, C. Gonadal development and biochemical composition of female crayfish Cherax quadricarinatus (Decapoda: Parastacidae) in relation to the Gonadosomatic Index at first maturation. Aquaculture 2006, 254, 637–645. [Google Scholar] [CrossRef]
- Streissl, F.; Hoödl, W. Growth, morphometrics, size at maturity, sexual dimorphism and condition index of Austropotamobius torrentium Schrank. Hydrobiologia 2002, 477, 201–208. [Google Scholar] [CrossRef]
- Peig, J.; Green, A.J. The paradigm of body condition: A critical reappraisal of current methods based on mass and length. Funct. Ecol. 2010, 24, 1323–1332. [Google Scholar] [CrossRef]
- Lucić, A.; Hudina, S.; Faller, M.; Cerjanec, D. A comparative study of the physiological condition of native and invasive crayfish in Croatian rivers. Biologia 2012, 67, 172–179. [Google Scholar] [CrossRef]
- Dobrović, A.; Maguire, I.; Boban, M.; Grbin, D.; Hudina, S. Reproduction dynamics of the marbled crayfish Procambarus virginalis Lyko, 2017 from an anthropogenic lake in northern Croatia. Aquat. Invasions 2021, 16, 482–498. [Google Scholar] [CrossRef]
- Dempster, T.; Sanchez-Jerez, P.; Fernandez-Jover, D.; Bayle-Sempere, J.; Nilsen, R.; Bjørn, P.A.; Uglem, I. Proxy measures of fitness suggest coastal fish farms can act as population sources and not ecological traps for wild gadoid fish. PLoS ONE 2011, 6, e15646. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Henningsson, M.; Sundbom, E.; Armelius, B.A.; Erdberg, P. PLS model building: A multivariate approach to personality test data. Scand. J. Psychol. 2001, 42, 399–409. [Google Scholar] [CrossRef]
- Jain, P.; Vineis, P.; Liquet, B.; Vlaanderen, J.; Bodinier, B.; van Veldhoven, K.; Kogevinas, M.; Athersuch, T.J.; Font-Ribera, L.; Villanueva, C.M.; et al. A multivariate approach to investigate the combined biological effects of multiple exposures. J. Epidemiol. Community Health 2018, 72, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Lew, S.; Glińska-Lewczuk, K.; Lew, M. The effects of environmental parameters on the microbial activity in peat-bog lakes. PLoS ONE 2019, 14, e0224441. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistic Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 15 June 2021).
- Sanchez, G. R Package Plsdepot PLS Regression 1. 2012, 1–13. Available online: https://cran.r-project.org/web/packages/plsdepot/plsdepot.pdf (accessed on 23 July 2021).
- Vu, V.Q.; A ggplot2 Based Biplot. In R Package Version 0.55; 2011. Available online: http://github.com/vgv/ggbiplot (accessed on 15 June 2021).
- Jiravanichpaisal, P.; Söderhäll, K.; Söderhäll, I. Effect of water temperature on the immune response and infectivity pattern of white spot syndrome virus (WSSV) in freshwater crayfish. Fish Shellfish Immunol. 2004, 17, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C. Effects of ecological factors on dominance and immune defence in crayfish. Ph.D. Dissertation, University of Helsinki, Helsinki, Finland, 25 September 2015. Available online: https://helda.helsinki.fi/handle/10138/156367 (accessed on 19 July 2021).
- Ma, X.; Zhu, F.; Jin, Q. Antibiotics and chemical disease-control agents reduce innate disease resistance in crayfish. Fish Shellfish Immunol. 2018, 86, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Strauss, A.; White, A.; Boots, M. Invading with biological weapons: The importance of disease-mediated invasions. Funct. Ecol. 2012, 26, 1249–1261. [Google Scholar] [CrossRef]
- Prüter, H.; Franz, M.; Twietmeyer, S.; Böhm, N.; Middendorff, G.; Portas, R.; Melzheimer, J.; Kolberg, H.; von Samson-Himmelstjerna, G.; Greenwood, A.D.; et al. Increased immune marker variance in a population of invasive birds. Sci. Rep. 2020, 10, 21764. [Google Scholar] [CrossRef]
- Kelehear, C.; Shine, R. Tradeoffs between dispersal and reproduction at an invasion front of cane toads in tropical Australia. Sci. Rep. 2020, 10, 486. [Google Scholar] [CrossRef]
- Selechnik, D.; West, A.J.; Brown, G.P.; Fanson, K.V.; Addison, B.; Rollins, L.A.; Shine, R. Effects of invasion history on physiological responses to immune system activation in invasive Australian cane toads. PeerJ 2017, 5, e3856. [Google Scholar] [CrossRef] [PubMed]
- Selechnik, D.; Richardson, M.F.; Shine, R.; Brown, G.P.; Rollins, L.A. Immune and environment-driven gene expression during invasion: An eco-immunological application of RNA-Seq. Ecol. Evol. 2019, 9, 6708–6721. [Google Scholar] [CrossRef]
- Brown, G.P.; Phillips, B.L.; Dubey, S.; Shine, R. Invader immunology: Invasion history alters immune system function in cane toads (Rhinella marina) in tropical Australia. Ecol. Lett. 2015, 18, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.P.; Holden, D.; Shine, R.; Phillips, B.L. Invasion history alters the behavioural consequences of immune system activation in cane toads. J. Anim. Ecol. 2018, 87, 716–726. [Google Scholar] [CrossRef]
- Assis, V.R.; Gardner, S.T.; Smith, K.M.; Gomes, F.R.; Mendonça, M.T. Stress and immunity: Field comparisons among populations of invasive cane toads in Florida. J. Exp. Zool. A Ecol. Integr. Physiol. 2020, 333, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Dragičević, P.; Bielen, A.; Petrić, I.; Vuk, M.; Žučko, J.; Hudina, S. Microbiome of the successful freshwater invader, the signal crayfish, and its changes along the invasion range. Microbiol. Spectr. 2021, 9, e0038921. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.-C. Effects of pH, temperature and salinity on immune parameters of the freshwater prawn Macrobrachium rosenbergii. Fish Shellfish Immunol. 2000, 10, 387–391. [Google Scholar] [CrossRef]
- Le Moullac, G.; Haffner, P. Environmental factors affecting immune responses in Crustacea. Aquaculture 2000, 191, 121–131. [Google Scholar] [CrossRef]
- Wei, K.; Yang, J. Copper-induced oxidative damage to the prophenoloxidase-activating system in the freshwater crayfish Procambarus clarkii. Fish Shellfish Immunol. 2016, 52, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Korkut, G.G.; Söderhäll, I.; Söderhäll, K.; Noonin, C. The effect of temperature on bacteria-host interactions in the freshwater crayfish, Pacifastacus leniusculus. J. Invertebr. Pathol. 2018, 157, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qi, C.; Jia, Y.; Gu, Z.; Li, E. Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 2020, 524, 735256. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, Y.; Lu, K.; Xiong, H.; Zhang, Y.; Wei, W. Herbicide atrazine exposure induce oxidative stress, immune dysfunction and WSSV proliferation in red swamp crayfish Procambarus clarkia. Chemosphere 2021, 283, 131227. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC of the European Parliament and of the Council. OJ L 2008, 348, 84–97. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008L0105 (accessed on 19 August 2021).
- Gomez-Jimenez, S.; Uglow, R.F.; Gollas-Galvan, T. The effects of cooling and emersion on total haemocyte count and phenoloxidase activity of the spiny lobster Panulirus interruptus. Fish Shellfish Immunol. 2000, 10, 631–635. [Google Scholar] [CrossRef]
- Cheng, W.; Chen, J.-C. Effects of intrinsic and extrinsic factors on the haemocyte profile of the prawn, Macrobrachium rosenbergii. Fish Shellfish Immunol. 2001, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.; Reeson, A.F. Density-dependent prophylaxis: Evidence from Lepidoptera-baculovirus interactions? Ecol. Entomol. 1998, 23, 100–101. [Google Scholar] [CrossRef]
- Cotter, S.C.; Hails, R.S.; Cory, J.S.; Wilson, K. Density-dependent prophylaxis and condition-dependent immune function in Lepidopteran larvae: A multivariate approach. J. Anim. Ecol. 2004, 73, 283–293. [Google Scholar] [CrossRef]
- Kong, H.; Cheng, Y.; Luo, L.; Sappington, T.W.; Jiang, X.; Zhang, L. Density-dependent prophylaxis in crowded Beet Webworm, Loxostege sticticalis (Lepidoptera: Pyralidae) larvae to a parasitoid and a fungal pathogen. Int. J. Pest Manag. 2013, 59, 174–179. [Google Scholar] [CrossRef]
- Silva, F.W.S.; Viol, D.L.; Faria, S.V.; Lima, E.; Valicente, F.H.; Elliot, S.L. Two’s a crowd: Phenotypic adjustments and prophylaxis in Anticarsia gemmatalis larvae are triggered by the presence of conspecifics. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.W.S.; Elliot, S.L. Temperature and population density: Interactional effects of environmental factors on phenotypic plasticity, immune defenses, and disease resistance in an insect pest. Ecol. Evol. 2016, 6, 3672–3683. [Google Scholar] [CrossRef]
- Ferguson, L.V. Thermal Biology of Insect Immunity and Host-microbe Interactions. Ph.D. Dissertation, The University of Western Ontario, London, ON, Canada, 14 February 2017. Available online: https://ir.lib.uwo.ca/etd/4406 (accessed on 27 July 2021).
- Wojda, I. Temperature stress and insect immunity. J. Therm. Biol. 2017, 68, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Seehausen, M.L.; Naumann, P.-H.; Béliveau, C.; Martel, V.; Cusson, M. Impact of rearing temperature on encapsulation and the accumulation of transcripts putatively involved in capsule formation in a parasitized lepidopteran host. J. Insect Physiol. 2018, 107, 244–249. [Google Scholar] [CrossRef]
- Kong, H.; Liu, Z.; Yang, P.; Yuan, L.; Jing, W.; Dong, C.; Zheng, M.; Tian, Z.; Hou, Q.; Zhu, S. Effects of Larval Density on Plutella xylostella Resistance to Granulosis Virus. Insects 2020, 11, 857. [Google Scholar] [CrossRef]
- Lin, X.; Söderhäll, I. Crustacean hematopoiesis and the astakine cytokines. Blood 2011, 117, 6417–6424. [Google Scholar] [CrossRef]
- Söderhäll, I. Crustacean hematopoiesis. Dev. Comp. Immunol. 2016, 58, 129–141. [Google Scholar] [CrossRef]
- Smith, V.; Söderhäll, K. Induction of degranulation and lysis of haemocytes in the freshwater crayfish, Astacus astacus by components of the prophenoloxidase activating system in vitro. Cell Tissue Res. 1983, 233, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Söderhäll, I.; Bangyeekhun, E.; Mayo, S.; Söderhäll, K. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev. Comp. Immunol. 2003, 27, 661–672. [Google Scholar] [CrossRef]
- Lorenzon, S.; Martinis, M.; Ferrero, E.A. Ecological Relevance of Hemolymph Total Protein Concentration in Seven Unrelated Crustacean Species from Different Habitats Measured Predictively by a Density-Salinity Refractometer. J. Mar. Sci. 2011, 2011, 153654. [Google Scholar] [CrossRef]
- Sladkova, S.V.; Kholodkevich, S.V. Total protein in hemolymph of crawfish Pontastacus leptodactylus as a parameter of the functional state of animals and a biomarker of quality of habitat. J. Evol. Biochem. Physiol. 2011, 47, 160–167. [Google Scholar] [CrossRef]
- Sadd, B.M.; Siva-Jothy, M.T. Self-harm caused by an insect’s innate immunity. Proc. Biol. Sci. 2006, 273, 2571–2574. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 2004, 198, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. The invertebrate midintestinal gland (“hepatopancreas”) is an evolutionary forerunner in the integration of immunity and metabolism. Cell Tissue Res. 2014, 358, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 2019, 280, 1405–1444. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, D.K.; Sharma, P.K. Condition factor and organosomatic indices of parasitized Rattus rattus as indicators of host health. J. Parasit. Dis. 2017, 41, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Monceau, K.; Dechaume-Moncharmont, F.-X.; Moreau, J.; Lucas, C.; Capoduro, R.; Motreuil, S.; Moret, Y. Personality, immune response and reproductive success: An appraisal of the pace-of-life syndrome hypothesis. J. Anim. Ecol. 2017, 86, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Riley, E.M.; Buchanan, K.L. Optimal immune responses: Immunocompetence revisited. Trends Ecol. Evol. 2005, 20, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Lawniczak, M.K.; Barnes, A.I.; Linklater, J.R.; Boone, J.M.; Wigby, S.; Chapman, T. Mating and immunity in invertebrates. Trends Ecol. Evol. 2007, 22, 48–55. [Google Scholar] [CrossRef]
- Bangyeekhun, E. Parasite on Crayfish: Characterisation of Their Pathogenesis, Host Interactions and Diversity. Ph.D. Dissertation, Uppsala University, Uppsala, Sweden, 21 November 2002. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-2770 (accessed on 19 August 2021).
- Cerenius, L.; Bangyeekhun, E.; Keyser, P.; Soderhall, I.; Soderhall, K. Host prophenoloxidase expression in freshwater crayfish is linked to increased resistance to the crayfish plague fungus, Aphanomyces astaci. Cell. Microbiol. 2003, 5, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Pavić, D.; Čanković, M.; Sviličić Petrić, I.; Makkonen, J.; Hudina, S.; Maguire, I.; Vladušić, T.; Šver, L.; Hrašćan, R.; Orlić, K.; et al. Non-destructive method for detecting Aphanomyces astaci, the causative agent of crayfish plague, on the individual level. J. Invertebr. Pathol. 2020, 169, 107274. [Google Scholar] [CrossRef]
- Cerenius, L.; Söderhäll, K. Crayfish immunity–Recent findings. Dev. Comp. Immunol. 2018, 80, 94–98. [Google Scholar] [CrossRef] [PubMed]
| Location | X (WGS84) | Y (WGS84) |
|---|---|---|
| upstream front (UF) | 45.320915 | 15.518373 |
| upstream core (UC) | 45.371918 | 15.521505 |
| downstream core (DC) | 45.411808 | 15.609231 |
| downstream front (DF) | 45.451355 | 15.567030 |
| MODEL | Df | Sum Sq | Mean Sq | F Value | p Value |
|---|---|---|---|---|---|
| sites along invasion range | |||||
| sites | 3 | 625 | 208.24 | 4.37 | 0.006 ** |
| sex | 1 | 12 | 12.32 | 0.26 | 0.61 |
| sites:sex | 3 | 133 | 44.22 | 0.93 | 0.43 |
| residuals | 106 | 5052 | 47.66 | ||
| downstream-upstream | |||||
| downstream-upstream | 1 | 410 | 409.9 | 8.35 | 0.004 ** |
| sex | 1 | 12 | 12.2 | 0.25 | 0.62 |
| downstream-upstream:sex | 1 | 0 | 0 | 0 | 0.99 |
| residuals | 110 | 5399 | 49.1 | ||
| core-front | |||||
| core-front | 1 | 112 | 111.5 | 2.2 | 0.14 |
| sex | 1 | 2 | 2.38 | 0.05 | 0.82 |
| core-front:sex | 1 | 149 | 148.95 | 2.95 | 0.08 |
| residuals | 110 | 5559 | 50.5 | ||
| MODEL | Df | Sum Sq | Mean Sq | F Value | p Value |
|---|---|---|---|---|---|
| species | 1 | 23.6 | 23.59 | 9.91 | 0.006 ** |
| sex | 1 | 13.35 | 13.35 | 5.61 | 0.25 |
| species:sex | 1 | 0.1 | 0.1 | 0.04 | 0.83 |
| residuals | 26 | 61.91 | 2.38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragičević, P.; Grbin, D.; Maguire, I.; Blažević, S.A.; Abramović, L.; Tarandek, A.; Hudina, S. Immune Response in Crayfish Is Species-Specific and Exhibits Changes along Invasion Range of a Successful Invader. Biology 2021, 10, 1102. https://doi.org/10.3390/biology10111102
Dragičević P, Grbin D, Maguire I, Blažević SA, Abramović L, Tarandek A, Hudina S. Immune Response in Crayfish Is Species-Specific and Exhibits Changes along Invasion Range of a Successful Invader. Biology. 2021; 10(11):1102. https://doi.org/10.3390/biology10111102
Chicago/Turabian StyleDragičević, Paula, Dorotea Grbin, Ivana Maguire, Sofia Ana Blažević, Lucija Abramović, Anita Tarandek, and Sandra Hudina. 2021. "Immune Response in Crayfish Is Species-Specific and Exhibits Changes along Invasion Range of a Successful Invader" Biology 10, no. 11: 1102. https://doi.org/10.3390/biology10111102
APA StyleDragičević, P., Grbin, D., Maguire, I., Blažević, S. A., Abramović, L., Tarandek, A., & Hudina, S. (2021). Immune Response in Crayfish Is Species-Specific and Exhibits Changes along Invasion Range of a Successful Invader. Biology, 10(11), 1102. https://doi.org/10.3390/biology10111102







