Heat Stress Responses in Birds: A Review of the Neural Components
Abstract
:Simple Summary
Abstract
1. Introduction
2. Development of the Avian Thermostat
2.1. Development of Temperature Sensing Neurons
2.2. A Brief Mention: The Mammalian Thermosensory Pathway
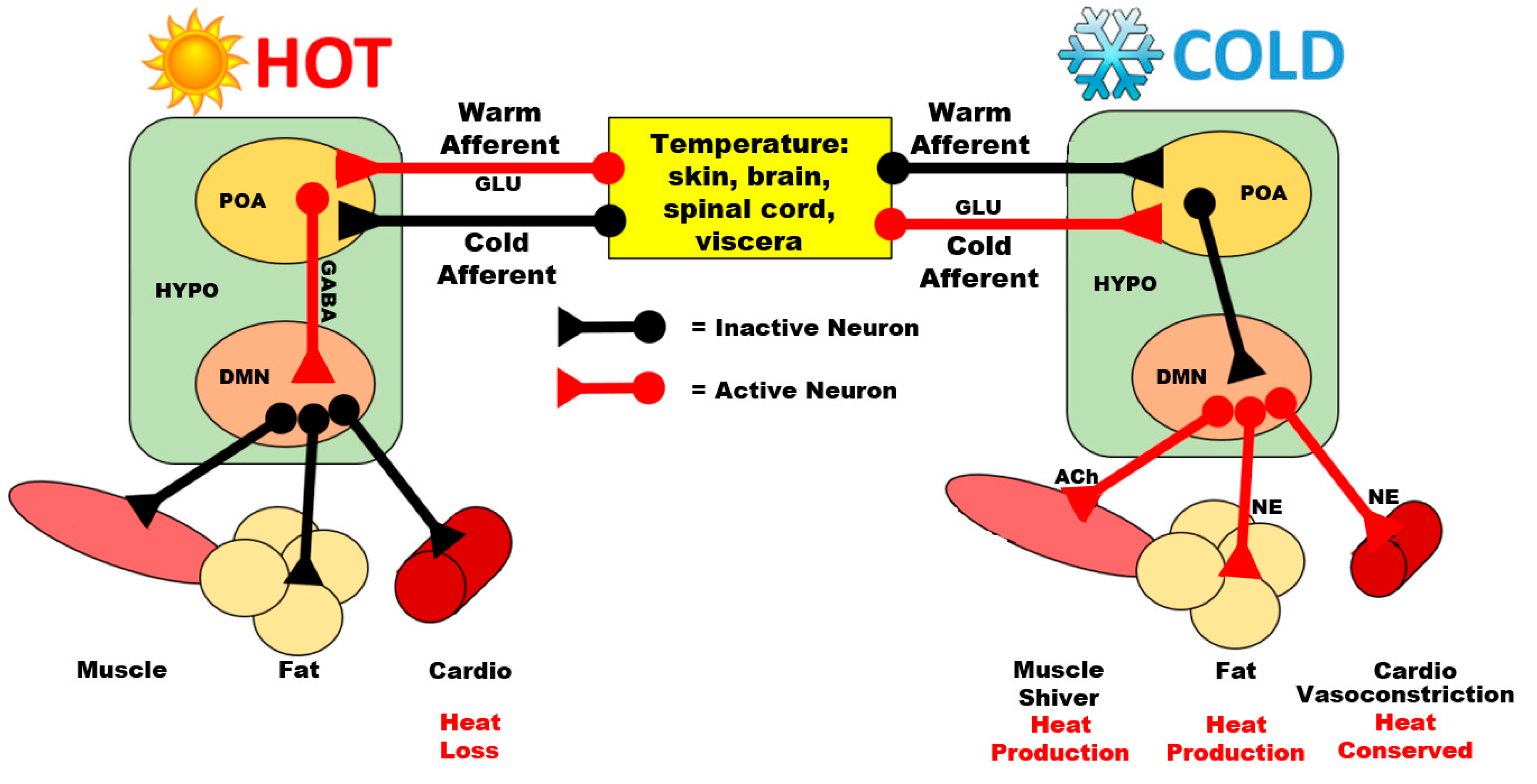
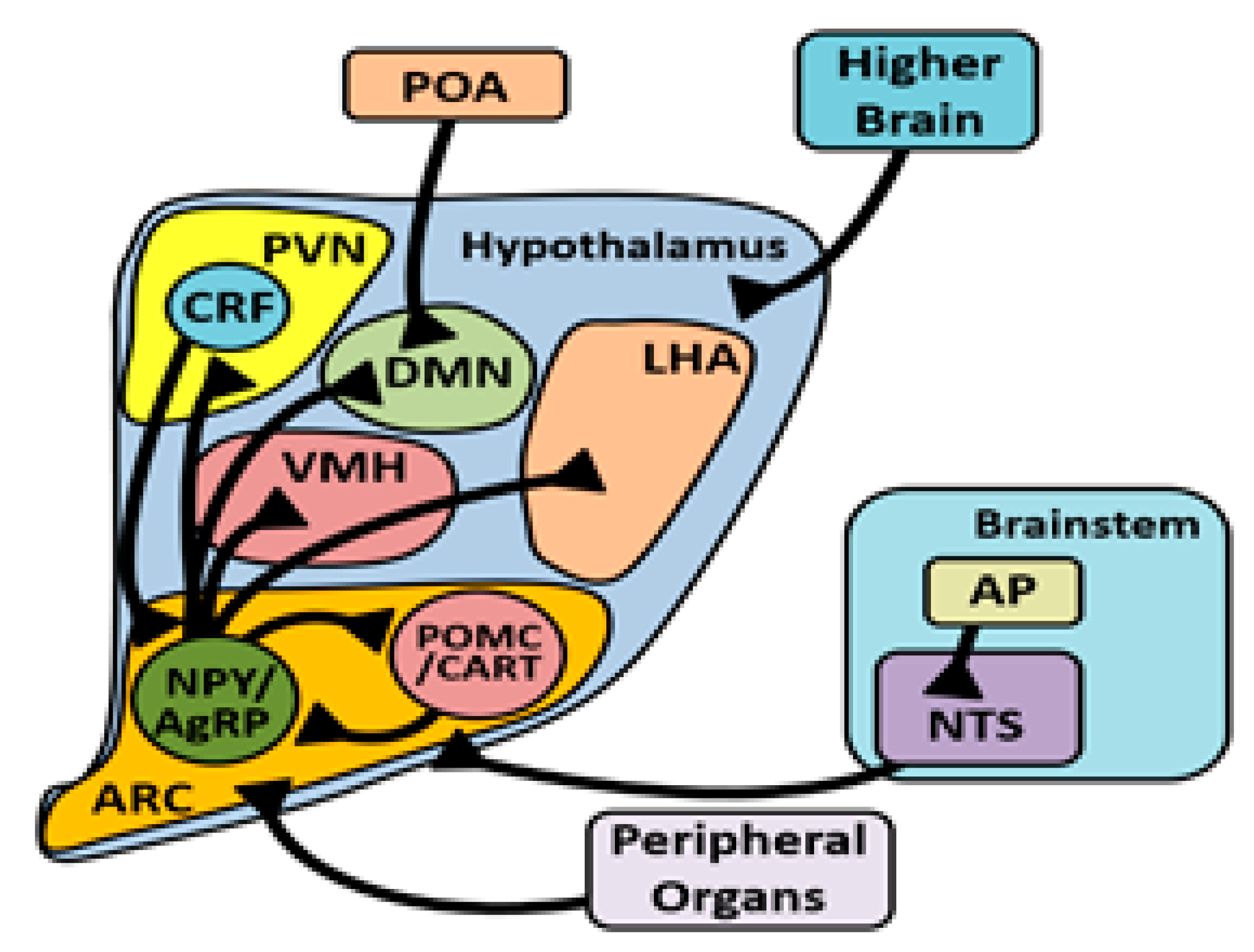
3. Hypothalamic Signaling during Heat Exposure
3.1. Preoptic Area
3.2. Nucleus of the Hippocampal Commissure
3.3. Paraventricular Nucleus
3.4. Arcuate Nucleus
4. Extrahypothalamic Endocrine Consequences
4.1. Corticotrophs and Corticosterone
4.2. Thyrotrophs and Thyroid Hormones
4.3. Lactotrophs and Prolactin
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nawab, A.; Ibtisham, F.; Li, G.; Kieser, B.; Wu, J.; Liu, W.; Zhao, Y.; Nawab, Y.; Li, K.; Xiao, M.; et al. Heat stress in poultry production: Mitigation strategies to overcome the future challenges facing the global poultry industry. J. Therm. Biol. 2018, 78, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Sajjanar, B.; Lonkar, V.; Kurade, N.; Kadam, A.; Nirmal, A.; Brahmane, M.; Bal, S. Assessing and mitigating the impact of heat stress on poultry. Adv. Anim. Vet. Sci. 2016, 4, 332–341. [Google Scholar] [CrossRef]
- Faria, D.; Junqueira, O.; Souza, P.; Titto, E. Performance, body temperature and egg quality of laying hens fed vitamins D and C under three environmental temperatures. Braz. J. Poult. Sci. 2001, 3, 49–56. [Google Scholar] [CrossRef]
- Bollengier-Lee, S.; Williams, P.E.; Whitehead, C.C. Optimal dietary concentration of vitamin E for alleviating the effect of heat stress on egg production in laying hens. Br. Poult. Sci. 1999, 40, 102–107. [Google Scholar] [CrossRef]
- Deng, W.; Dong, X.F.; Tong, J.M.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens1. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef]
- Ryder, A.A.; Feddes, J.J.R.; Zuidhof, M.J. Field Study to Relate Heat Stress Index to Broiler Performance. J. Appl. Poult. Res. 2004, 13, 493–499. [Google Scholar] [CrossRef]
- Mitchell, M.A.; Kettlewell, P.J. Physiological stress and welfare of broiler chickens in transit: Solutions not problems! Poult. Sci. 1998, 77, 1803–1814. [Google Scholar] [CrossRef]
- Mujahid, A.; Yoshiki, Y.; Akiba, Y.; Toyomizu, M. Superoxide radical production in chicken skeletal muscle induced by acute heat stress. Poult. Sci. 2005, 84, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Al-Fataftah, A.; Abu-Dieyeh, Z. Effect of chronic heat stress on broiler performance in Jordan. Int. J. Poult. Sci. 2007, 6, 64–70. [Google Scholar]
- Arjona, A.A.; Denbow, D.M.; Weaver, W.D., Jr. Effect of Heat Stress Early in Life on Mortality of Broilers Exposed to High Environmental Temperatures Just Prior to Marketing. Poult. Sci. 1988, 67, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Quinteiro-Filho, W.M.; Rodrigues, M.V.; Ribeiro, A.; Ferraz-de-Paula, V.; Pinheiro, M.L.; Sá, L.R.M.; Ferreira, A.J.P.; Palermo-Neto, J. Acute heat stress impairs performance parameters and induces mild intestinal enteritis in broiler chickens: Role of acute hypothalamic-pituitary-adrenal axis activation1. J. Anim. Sci. 2012, 90, 1986–1994. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Kikusato, M.; Maekawa, T.; Shirakawa, H.; Toyomizu, M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 401–406. [Google Scholar] [CrossRef]
- Flanagan, S.W.; Moseley, P.L.; Buettner, G.R. Increased flux of free radicals in cells subjected to hyperthermia: Detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998, 431, 285–286. [Google Scholar] [CrossRef] [Green Version]
- Zuo, L.; Christofi, F.L.; Wright, V.P.; Liu, C.Y.; Merola, A.J.; Berliner, L.J.; Clanton, T.L. Intra-and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am. J. Physiol. Cell. Physiol. 2000, 279, C1058–C1066. [Google Scholar] [CrossRef] [PubMed]
- Mashaly, M.M.; Hendricks, G.L.; Kalama, M.A.; Gehad, A.E.; Abbas, A.O.; Patterson, P.H. Effect of Heat Stress on Production Parameters and Immune Responses of Commercial Laying Hens1. Poult. Sci. 2004, 83, 889–894. [Google Scholar] [CrossRef]
- Allahverdi, A.; Feizi, A.; Takhtfooladi, H.A.; Nikpiran, H. Effects of heat stress on acid-base imbalance, plasma calcium concentration, egg production and egg quality in commercial layers. Glob. Vet. 2013, 10, 203–207. [Google Scholar]
- Obidi, J.; Onyeanusi, B.; Ayo, J.; Rekwot, P.; Abdullahi, S. Effect of timing of artificial insemination on fertility and hatchability of Shikabrown breeder hens. Int. J. Poult. Sci. 2008. [Google Scholar] [CrossRef] [Green Version]
- Onuora, G. Seasonal variation in semen quality in the guinea fowl. Niger. Vet. J. 1982, 11, 8–15. [Google Scholar]
- Giuseppe, P.; Giovanni, C. Biological rhythm in livestock. J. Vet. Sci. 2002, 3, 145–157. [Google Scholar] [CrossRef]
- Watford, M.; Wu, G. Glutamine metabolism in uricotelic species: Variation in skeletal muscle glutamine synthetase, glutaminase, glutamine levels and rates of protein synthesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M. Central regulation of food intake in the neonatal chick. Anim. Sci. J. 2002, 73, 83–94. [Google Scholar] [CrossRef]
- Yalcin, S.; Settar, P.; Ozkan, S.; Cahaner, A. Comparative evaluation of three commercial broiler stocks in hot versus temperate climates. Poult. Sci. 1997, 76, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Price, E.R.; Dzialowski, E.M. Development of endothermy in birds: Patterns and mechanisms. J. Comp. Physiol. B Biochem. 2018, 188, 373–391. [Google Scholar] [CrossRef]
- Cahaner, A.; Leenstra, F. Effects of High Temperature on Growth and Efficiency of Male and Female Broilers from Lines Selected for High Weight Gain, Favorable Feed Conversion, and High or Low Fat Content. Poult. Sci. 1992, 71, 1237–1250. [Google Scholar] [CrossRef]
- Marchini, C.F.; Café, M.B.; Araújo, E.G.; Nascimento, M.R. Physiology, cell dynamics of small intestinal mucosa, and performance of broiler chickens under heat stress: A review. Rev. Colomb. Cienc. Pecu. 2016, 29, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Zaboli, G.; Huang, X.; Feng, X.; Ahn, D.U. How can heat stress affect chicken meat quality?—A review. Poult. Sci. 2019, 98, 1551–1556. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Sugiharto, S.; Turrini, Y.; Isroli, I.; Endang, W.; Endang, K. Dietary supplementation of probiotics in poultry exposed to heat stress—A review. Ann. Anim. Sci. 2017, 17, 591–604. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat. Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Nyoni, N.M.B.; Grab, S.; Archer, E.R.M. Heat stress and chickens: Climate risk effects on rural poultry farming in low-income countries. Clim. Dev. 2019, 11, 83–90. [Google Scholar] [CrossRef]
- Lara, L.J.; Rostagno, M.H. Impact of Heat Stress on Poultry Production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Babazadeh, D.; Naveed, M.; Arain, M.A.; Hassan, F.U.; Chao, S. Reconsidering betaine as a natural anti-heat stress agent in poultry industry: A review. Trop. Anim. Health Prod. 2017, 49, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Abbas, G.; Alagawany, M.; Kamboh, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Chao, S. Heat stress management in poultry farms: A comprehensive overview. J. Therm. Biol. 2019, 84, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, B.; Basta, D. Development of hypothalamic neuronal thermosensitivity in birds during the perinatal period. J. Therm. Biol. 2000, 25, 119–123. [Google Scholar] [CrossRef]
- Tzschentke, B.; Basta, D. Early development of neuronal hypothalamic thermosensitivity in birds: Influence of epigenetic temperature adaptation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 825–832. [Google Scholar] [CrossRef]
- Sallagundala, N.; Yakimova, K.; Tzschentke, B. Characterization of neuronal hypothalamic plasticity in chicken: A comparative analysis. In New Insights into Fundamental Physiology and Perinatal Adaptation in Domestic Fowl; Yahav, Y., Tzschentke, B., Eds.; Nottingham Univ Press: Nottingham, UK, 2006; pp. 99–108. [Google Scholar]
- Tzschentke, B.; Nichelmann, M. Influence of prenatal and postnatal acclimation on nervous and peripheral thermoregulation. Ann. N. Y. Acad. Sci. 1997, 813, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Tzschentke, B.; Rumpf, M. Embryonic development of endothermy. Respir. Physiol. Neurobiol. 2011, 178, 97–107. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zheng, J. High temperature sensitivity is intrinsic to voltage-gated potassium channels. Elife 2014, 3, e03255. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J. Thermosensation and pain. J. Neurobiol. 2004, 61, 3–12. [Google Scholar] [CrossRef]
- Tan, C.L.; Knight, Z.A. Regulation of Body Temperature by the Nervous System. Neuron 2018, 98, 31–48. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.D.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Trombley, P.Q.; van den Pol, A.N. Excitatory actions of GABA in developing rat hypothalamic neurones. J. Physiol. 1996, 494, 451–464. [Google Scholar] [CrossRef]
- Saito, S.; Banzawa, N.; Fukuta, N.; Saito, C.T.; Takahashi, K.; Imagawa, T.; Ohta, T.; Tominaga, M. Heat and Noxious Chemical Sensor, Chicken TRPA1, as a Target of Bird Repellents and Identification of Its Structural Determinants by Multispecies Functional Comparison. Mol. Biol. Evol. 2014, 31, 708–722. [Google Scholar] [CrossRef] [Green Version]
- Randall, W.C. Factors influencing the temperature regulation of birds. Am. J. Physiol.-Legacy Content 1943, 139, 56–63. [Google Scholar] [CrossRef]
- Saito, S.; Tominaga, M. Evolutionary tuning of TRPA1 and TRPV1 thermal and chemical sensitivity in vertebrates. Temperature 2017, 4, 141–152. [Google Scholar] [CrossRef]
- Nakamura, K.; Morrison, S.F. A thermosensory pathway mediating heat-defense responses. Proc. Natl. Acad. Sci. USA 2010, 107, 8848–8853. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Morrison, S.F. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008, 11, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, T.; Kataoka, N.; Nakamura, Y.; Nakamura, K. The lateral parabrachial nucleus, but not the thalamus, mediates thermosensory pathways for behavioural thermoregulation. Sci. Rep. 2017, 7, 5031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKinley, M.J.; Johnson, A.K. The Physiological Regulation of Thirst and Fluid Intake. Physiology 2004, 19, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Booth, D.A. Mechanism of action of norepinephrine in eliciting an eating response on injection into the rat hypothalamus. J. Pharmacol. Exp. Ther. 1968, 160, 336–348. [Google Scholar] [PubMed]
- Kosunen, K.J.; Pakarinen, A.J.; Kuoppasalmi, K.; Adlercreutz, H. Plasma renin activity, angiotensin II, and aldosterone during intense heat stress. J. Appl. Physiol. 1976, 41, 323–327. [Google Scholar] [CrossRef]
- Johnson, A.K.; Cunningham, J.T.; Thunhorst, R.L. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin. Exp. Pharmacol. Physiol. 1996, 23, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.M.; Bazzino, P.; Hurh, S.J.; Konanur, V.R.; Roitman, J.D.; Roitman, M.F. Thirst recruits phasic dopamine signaling through subfornical organ neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 30744. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K. Central circuitries for body temperature regulation and fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1207–R1228. [Google Scholar] [CrossRef] [Green Version]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. TRPV1 Reporter Mice Reveal Highly Restricted Brain Distribution and Functional Expression in Arteriolar Smooth Muscle Cells. J. Neurosci. 2011, 31, 5067. [Google Scholar] [CrossRef]
- Molinas, A.J.R.; Desmoulins, L.D.; Hamling, B.V.; Butcher, S.M.; Anwar, I.J.; Miyata, K.; Enix, C.L.; Dugas, C.M.; Satou, R.; Derbenev, A.V.; et al. Interaction between TRPV1-expressing neurons in the hypothalamus. J. Neurophysiol. 2019, 121, 140–151. [Google Scholar] [CrossRef]
- Mezey, É.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655. [Google Scholar] [CrossRef]
- Brobeck, J.R. Food intake as a mechanism of temperature regulation. Yale J. Biol. Med. 1948, 20, 545–552. [Google Scholar] [CrossRef]
- Jeong, J.H.; Lee, D.K.; Liu, S.-M.; Chua, S.C., Jr.; Schwartz, G.J.; Jo, Y.-H. Activation of temperature-sensitive TRPV1-like receptors in ARC POMC neurons reduces food intake. PLoS Biol. 2018, 16, e2004399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharif-Naeini, R.; Ciura, S.; Bourque, C.W. TRPV1 Gene Required for Thermosensory Transduction and Anticipatory Secretion from Vasopressin Neurons during Hyperthermia. Neuron 2008, 58, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozyreva, T.; Evtushenko, A.; Voronova, I.; Khramova, G.; Kozaruk, V. Effect of activation of peripheral ion channel TRPM8 on gene expression of thermosensitive TRP ion channels in the hypothalamus. Comparison with the effect of cooling. Bull. Exp. Biol. Med. 2018, 166, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Broberger, C.; Visser, T.J.; Kuhar, M.J.; Hökfelt, T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology 1999, 70, 295–305. [Google Scholar] [CrossRef]
- Mercer, R.E.; Chee, M.J.; Colmers, W.F. The role of NPY in hypothalamic mediated food intake. Front. Neuroendocrinol. 2011, 32, 398–415. [Google Scholar] [CrossRef]
- Tebbe, J.J.; Mronga, S.; Schäfer, M.K.-H.; Rüter, J.; Kobelt, P.; Mönnikes, H. Stimulation of neurons in rat ARC inhibits gastric acid secretion via hypothalamic CRF1/2- and NPY-Y1 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1075–G1083. [Google Scholar] [CrossRef] [Green Version]
- Berk, M.L.; Butler, A.B. Efferent projections of the medial preoptic nucleus and medial hypothalamus in the pigeon. J. Comp. Neurol. 1981, 203, 379–399. [Google Scholar] [CrossRef]
- Kuenzel, W.J.; Medina, L.; Csillag, A.; Perkel, D.J.; Reiner, A. The avian subpallium: New insights into structural and functional subdivisions occupying the lateral subpallial wall and their embryological origins. Brain Res. 2011, 1424, 67–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruuskanen, S.; Hsu, B.-Y.; Nord, A. Endocrinology of thermoregulation in birds in a changing climate. Mol. Cell. Endocrinol. 2021, 519, 111088. [Google Scholar] [CrossRef] [PubMed]
- Conceição, E.P.S.; Madden, C.J.; Morrison, S.F. Neurons in the rat ventral lateral preoptic area are essential for the warm-evoked inhibition of brown adipose tissue and shivering thermogenesis. Acta Physiol. 2019, 225, e13213. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.B.G.; Saper, C.B. Median preoptic glutamatergic neurons promote thermoregulatory heat loss and water consumption in mice. J. Physiol. 2017, 595, 6569–6583. [Google Scholar] [CrossRef]
- Luo, F.; Mu, Y.; Gao, C.; Xiao, Y.; Zhou, Q.; Yang, Y.; Ni, X.; Shen, W.L.; Yang, J. Whole-brain patterns of the presynaptic inputs and axonal projections of BDNF neurons in the paraventricular nucleus. J. Genet. Genom. 2019, 46, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Givalois, L.; Naert, G.; Rage, F.; Ixart, G.; Arancibia, S.; Tapia-Arancibia, L. A single brain-derived neurotrophic factor injection modifies hypothalamo–pituitary–adrenocortical axis activity in adult male rats. Mol. Cell. Neurosci. 2004, 27, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Jeanneteau, F.D.; Lambert, W.M.; Ismaili, N.; Bath, K.G.; Lee, F.S.; Garabedian, M.J.; Chao, M.V. BDNF and glucocorticoids regulate corticotrophin-releasing hormone (CRH) homeostasis in the hypothalamus. Proc. Natl. Acad. Sci. USA 2012, 109, 1305–1310. [Google Scholar] [CrossRef] [Green Version]
- Nagarajan, G.; Jurkevich, A.; Kang, S.W.; Kuenzel, W.J. Anatomical and functional implications of corticotrophin-releasing hormone neurones in a septal nucleus of the avian brain: An emphasis on glial-neuronal interaction via V1a receptors in vitro. J. Neuroendocrinol. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Ubieta, R.; Uribe, R.M.; González, J.A.; García-Vázquez, A.; Pérez-Monter, C.; Pérez-Martínez, L.; Joseph-Bravo, P.; Charli, J.-L. BDNF up-regulates pre-pro-TRH mRNA expression in the fetal/neonatal paraventricular nucleus of the hypothalamus. Properties of the transduction pathway. Brain Res. 2007, 1174, 28–38. [Google Scholar] [CrossRef]
- Daghir, N.J. Poultry Production in Hot Climates; CABI: Wallingford, UK, 2008. [Google Scholar]
- Montagnese, C.M.; Székely, T.; Csillag, A.; Zachar, G. Distribution of vasotocin- and vasoactive intestinal peptide-like immunoreactivity in the brain of blue tit (Cyanistes coeruleus). Front. Neuroanat. 2015, 9, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scammell, T.; Gerashchenko, D.; Urade, Y.; Onoe, H.; Saper, C.; Hayaishi, O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc. Natl. Acad. Sci. USA 1998, 95, 7754–7759. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.A.; Teo, C.F.; Åkerblom, M.; Chen, C.; Tynan-La Fontaine, M.; Greiner, V.J.; Diaz, A.; McManus, M.T.; Jan, Y.N.; Jan, L.Y. Thermoregulation via Temperature-Dependent PGD(2) Production in Mouse Preoptic Area. Neuron 2019, 103, 309–322.e307. [Google Scholar] [CrossRef]
- Ohinata, K.; Takagi, K.; Biyajima, K.; Fujiwara, Y.; Fukumoto, S.; Eguchi, N.; Urade, Y.; Asakawa, A.; Fujimiya, M.; Inui, A.; et al. Central prostaglandin D(2) stimulates food intake via the neuropeptide Y system in mice. FEBS Lett. 2008, 582, 679–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahry, M.A.; Chowdhury, V.S.; Yang, H.; Tran, P.V.; Do, P.H.; Han, G.; Ikeda, H.; Cockrem, J.F.; Furuse, M. Central administration of neuropeptide Y differentially regulates monoamines and corticosterone in heat-exposed fed and fasted chicks. Neuropeptides 2017, 62, 93–100. [Google Scholar] [CrossRef]
- Eltahan, H.M.; Bahry, M.A.; Yang, H.; Han, G.; Nguyen, L.T.N.; Ikeda, H.; Ali, M.N.; Amber, K.A.; Furuse, M.; Chowdhury, V.S. Central NPY-Y5 sub-receptor partially functions as a mediator of NPY-induced hypothermia and affords thermotolerance in heat-exposed fasted chicks. Physiol. Rep. 2017, 5, e13511. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, T.; Saito, S.; Tomonaga, S.; Takagi, T.; Saito, E.-S.; Nakanishi, T.; Koutoku, T.; Tsukada, A.; Ohkubo, T.; Boswell, T.; et al. Effect of central administration of prolactin-releasing peptide on feeding in chicks. Physiol. Behav. 2004, 80, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.J.; Kang, S.W.; Kuenzel, W.J. Differential and temporal expression of corticotropin releasing hormone and its receptors in the nucleus of the hippocampal commissure and paraventricular nucleus during the stress response in chickens (Gallus gallus). Brain Res. 2019, 1714, 1–7. [Google Scholar] [CrossRef]
- Nagarajan, G.; Kang, S.W.; Kuenzel, W.J. Functional evidence that the nucleus of the hippocampal commissure shows an earlier activation from a stressor than the paraventricular nucleus: Implication of an additional structural component of the avian hypothalamo-pituitary-adrenal axis. Neurosci. Lett. 2017, 642, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bohler, M.; Gilbert, E.R.; Cline, M.A. Reduced food intake during exposure to high ambient temperatures is associated with molecular changes in the nucleus of the hippocampal commissure and the paraventricular and arcuate hypothalamic nuclei. Gen. Comp. Endocrinol. 2020, 298, 113576. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Ncho, C.M.; Choi, Y.-H. Regulation of gene expression in chickens by heat stress. J. Anim. Sci. Biotechnol. 2021, 12, 11. [Google Scholar] [CrossRef]
- Alderson, R.F.; Alterman, A.L.; Barde, Y.-A.; Lindsay, R.M. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron 1990, 5, 297–306. [Google Scholar] [CrossRef]
- Yu, S.; François, M.; Huesing, C.; Münzberg, H. The Hypothalamic Preoptic Area and Body Weight Control. Neuroendocrinology 2018, 106, 187–194. [Google Scholar] [CrossRef] [Green Version]
- Jurkevich, A.; Barth, S.W.; Grossmann, R. Sexual dimorphism of arg-vasotocin gene expressing neurons in the telencephalon and dorsal diencephalon of the domestic fowl. An immunocytochemical and in situ hybridization study. Cell Tissue Res. 1996, 287, 69–77. [Google Scholar] [CrossRef]
- Lei, L.; Hepeng, L.; Xianlei, L.; Hongchao, J.; Hai, L.; Sheikhahmadi, A.; Yufeng, W.; Zhigang, S. Effects of acute heat stress on gene expression of brain–gut neuropeptides in broiler chickens (Gallus gallus domesticus). J. Anim. Sci. 2013, 91, 5194–5201. [Google Scholar] [CrossRef]
- Mikami, S.-I. Immunocytochemistry of the Avian Hypothalamus and Adenohypophysis. Int. Rev. Cytol. 1986, 103, 189–248. [Google Scholar]
- Castro, M.G.; Estivariz, F.E.; Iturriza, F.C. The regulation of the corticomelanotropic cell activity in Aves–II. Effect of various peptides on the release of ACTH from dispersed, perfused duck pituitary cells. Comp. Biochem. Physiol. A Comp. Physiol. 1986, 83, 71–75. [Google Scholar] [CrossRef]
- Sharma, D.; Cornett, L.E.; Chaturvedi, C.M. Osmotic stress induced alteration in the expression of arginine vasotocin receptor VT2 in the pituitary gland and adrenal function of domestic fowl. Gen. Comp. Endocrinol. 2009, 160, 216–222. [Google Scholar] [CrossRef]
- Wang, S.; Bottje, W.G.; Kinzler, S.; Neldon, H.L.; Koike, T.I. Effect of heat stress on plasma levels of arginine vasotocin and mesotocin in domestic fowl (Gallus domesticus). Comp. Biochem. Physiol. 1989, 93, 721–724. [Google Scholar] [CrossRef]
- John, T.M.; George, J.C. Effects of arginine vasotocin on cardiorespiratory and thermoregulatory responses in the pigeon. Comp. Biochem. Pysiol. C Toxicol. 1992, 102, 353–359. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Tu, W.-L.; Cheng, C.-Y.; Wang, S.-H.; Tang, P.-C.; Chen, C.-F.; Chen, H.-H.; Lee, Y.-P.; Chen, S.-E.; Huang, S.-Y. Profiling of differential gene expression in the hypothalamus of broiler-type Taiwan country chickens in response to acute heat stress. Theriogenology 2016, 85, 483–494.e488. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Bahry, M.A.; Hui, Y.; Furuse, M.; Chowdhury, V.S. Acute heat stress up-regulates neuropeptide Y precursor mRNA expression and alters brain and plasma concentrations of free amino acids in chicks. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 187, 13–19. [Google Scholar] [CrossRef]
- Kuperman, Y.; Weiss, M.; Dine, J.; Staikin, K.; Golani, O.; Ramot, A.; Nahum, T.; Kühne, C.; Shemesh, Y.; Wurst, W.; et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell. Metab. 2016, 23, 1185–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, A.; Goebel, M.; Million, M.; Stenzel-Poore, M.P.; Kobelt, P.; Mönnikes, H.; Tacheé, Y.; Wang, L. Corticotropin-Releasing Factor-Overexpressing Mice Exhibit Reduced Neuronal Activation in the Arcuate Nucleus and Food Intake in Response to Fasting. Endocrinology 2009, 150, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Carsia, R.V.; Weber, H.; Perez, F.M., Jr. Corticotropin-Releasing Factor Stimulates the Release of Adrenocorticotropin from Domestic Fowl Pituitary Cells. Endocrinology 1986, 118, 143–148. [Google Scholar] [CrossRef]
- Salem, M.H.M.; Norton, H.W.; Nalbandov, A.V. The role of vasotocin and of CRF in ACTH release in the chicken. Gen. Comp. Endocrinol. 1970, 14, 281–289. [Google Scholar] [CrossRef]
- Mikhailova, M.V.; Blansett, J.; Jacobi, S.; Mayeux, P.R.; Cornett, L.E. Transmembrane domain IV of the Gallus gallus VT2 vasotocin receptor is essential for forming a heterodimer with the corticotrophin releasing hormone receptor. J. Biomed. Opt. 2008, 13, 031208. [Google Scholar] [CrossRef] [Green Version]
- Moawad, U.K.; Randa, M.H. Histocytological and histochemical features of the adrenal gland of Adult Egyptian native breeds of chicken (Gallus Gallus domesticus). Beni-Seuf Univ. J. Basic Appl. Sci. 2017, 6, 199–208. [Google Scholar] [CrossRef]
- Humayun, K.A.; Aoyama, M.; Sugita, S. Morphological and histological studies on the adrenal gland of the chicken (Gallus domesticus). J. Poult. Sci. 2012, 49, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Garriga, C.; Hunter, R.R.; Amat, C.; Planas, J.M.; Mitchell, M.A.; Moretó, M. Heat stress increases apical glucose transport in the chicken jejunum. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R195–R201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Aqil, A.; Zulkifli, I. Changes in heat shock protein 70 expression and blood characteristics in transported broiler chickens as affected by housing and early age feed restriction. Poult. Sci. 2009, 88, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- el-Halawani, M.; Waibel, P.; Appel, J.; Good, A. Effects of temperature stress on catecholamines and corticosterone of male turkeys. Am. J. Physiol.-Legacy Content 1973, 224, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Jeronen, E.; Isometsä, P.; Hissa, R.; Pyörnilä, A. Effect of acute temperature stress on the plasma catecholamine, corticosterone and metabolite levels in the pigeon. Comp. Biochem. Pysiol. C Toxicol. 1976, 55, 17–22. [Google Scholar] [CrossRef]
- Koch, K.A.; Wingfield, J.C.; Buntin, J.D. Glucocorticoids and Parental Hyperphagia in Ring Doves (Streptopelia risoria). Horm. Behav. 2002, 41, 9–21. [Google Scholar] [CrossRef]
- Landys, M.M.; Ramenofsky, M.; Guglielmo, C.G.; Wingfield, J.C. The low-affinity glucocorticoid receptor regulates feeding and lipid breakdown in the migratory Gambel’s white-crowned sparrow Zonotrichia leucophrys gambelii. J. Exp. Biol. 2004, 207, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Wall, J.P.; Cockrem, J.F. Effects of corticosterone treatment on responses to fasting in Japanese quail. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.S.; Van Kampen, M. Energy relationships in growing chickens given daily injections of corticosterone. Br. Poult. Sci. 1984, 25, 477–485. [Google Scholar] [CrossRef]
- Covasa, M.; Forbes, J.M. Selection of foods by broiler chickens following corticosterone administration. Br. Poult. Sci. 1995, 36, 489–501. [Google Scholar] [CrossRef]
- Liu, L.; Song, Z.; Jiao, H.; Lin, H. Glucocorticoids Increase NPY Gene Expression via Hypothalamic AMPK Signaling in Broiler Chicks. Endocrinology 2014, 155, 2190–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Lu, Z.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress alters hypothalamus integrity, the serum indexes and attenuates expressions of hypothalamic appetite genes in broilers. J. Therm. Biol. 2019, 81, 110–117. [Google Scholar] [CrossRef]
- Beckford, R.C.; Ellestad, L.E.; Proszkowiec-Weglarz, M.; Farley, L.; Brady, K.; Angel, R.; Liu, H.-C.; Porter, T.E. Effects of heat stress on performance, blood chemistry, and hypothalamic and pituitary mRNA expression in broiler chickens. Poult. Sci. 2020, 99, 6317–6325. [Google Scholar] [CrossRef] [PubMed]
- Buyse, J.; Decuypere, E.; Sharp, P.J.; Huybrechts, L.M.; Kühn, E.R.; Whitehead, C. Effect of corticosterone on circulating concentrations of corticosterone, prolactin, thyroid hormones and somatomedin C and on fattening in broilers selected for high or low fat content. J. Endocrinol. 1987, 112, 229–237. [Google Scholar] [CrossRef]
- Angelier, F.; Parenteau, C.; Ruault, S.; Angelier, N. Endocrine consequences of an acute stress under different thermal conditions: A study of corticosterone, prolactin, and thyroid hormones in the pigeon (Columbia livia). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 196, 38–45. [Google Scholar] [CrossRef]
- May, J.D.; Deaton, J.W.; Reece, F.N.; Branton, S.L. Effect of Acclimation and Heat Stress on Thyroid Hormone Concentration1. Poult. Sci. 1986, 65, 1211–1213. [Google Scholar] [CrossRef]
- Sotelo-Rivera, I.; Jaimes-Hoy, L.; Cote-Vélez, A.; Espinoza-Ayala, C.; Charli, J.L.; Joseph-Bravo, P. An Acute Injection of Corticosterone Increases Thyrotrophin-Releasing Hormone Expression in the Paraventricular Nucleus of the Hypothalamus but Interferes with the Rapid Hypothalamus Pituitary Thyroid Axis Response to Cold in Male Rats. J. Neuroendocrinol. 2014, 26, 861–869. [Google Scholar] [CrossRef]
- Servatius, R.J.; Ottenweller, J.E.; Natelson, B.H. A comparison of the effects of repeated stressor exposures and corticosterone injections on plasma cholesterol, thyroid hormones and corticosterone levels in rats. Life Sci. 1994, 55, 1611–1617. [Google Scholar] [CrossRef]
- Silberman, D.M.; Wald, M.; Genaro, A.M.a. Effects of chronic mild stress on lymphocyte proliferative response. Participation of serum thyroid hormones and corticosterone. Int. Immunopharmacol. 2002, 2, 487–497. [Google Scholar] [CrossRef]
- Pant, K.; Chandola-Saklani, A. A role for thyroid hormones in the development of premigratory disposition in redheaded bunting, Emberiza bruniceps. J. Comp. Physiol. B Biochem. 1993, 163, 389–394. [Google Scholar] [CrossRef]
- Landys-Ciannelli, M.M.; Ramenofsky, M.; Piersma, T.; Jukema, J.; Group, C.R.; Wingfield, J.C. Baseline and Stress-Induced Plasma Corticosterone during Long-Distance Migration in the Bar-Tailed Godwit, Limosa lapponica. Physiol. Biochem. Zool. 2002, 75, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piersma, T.; Reneerkens, J.; Ramenofsky, M. Baseline Corticosterone Peaks in Shorebirds with Maximal Energy Stores for Migration: A General Preparatory Mechanism for Rapid Behavioral and Metabolic Transitions? Gen. Comp. Endocrinol. 2000, 120, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klandorf, H.; Sharp, P.J.; Macleod, M.G. The relationship between heat production and concentrations of plasma thyroid hormones in the domestic hen. Gen. Comp. Endocrinol. 1981, 45, 513–520. [Google Scholar] [CrossRef]
- Collin, A.; Buyse, J.; van As, P.; Darras, V.M.; Malheiros, R.D.; Moraes, V.M.B.; Reyns, G.E.; Taouis, M.; Decuypere, E. Cold-induced enhancement of avian uncoupling protein expression, heat production, and triiodothyronine concentrations in broiler chicks. Gen. Comp. Endocrinol. 2003, 130, 70–77. [Google Scholar] [CrossRef]
- DuRant, S.E.; Carter, A.W.; Denver, R.J.; Hepp, G.R.; Hopkins, W.A. Are thyroid hormones mediators of incubation temperature-induced phenotypes in birds? Biol. Lett. 2014, 10, 20130950. [Google Scholar] [CrossRef] [Green Version]
- McNabb, F.M.A.; Weirich, R.T.; McNabb, R.A. Thyroid function in embryonic and perinatal Japanese quail. Gen. Comp. Endocrinol. 1981, 43, 218–226. [Google Scholar] [CrossRef]
- McNabb, F.; King, D. Thyroid hormones in growth, metabolism and development. In The Endocrinology of Growth, Development and Metabolism in Vertebrate; Pang, P.K.T., Schreibman, M.T., Eds.; Academic Press: New York, NY, USA, 1993; pp. 393–417. [Google Scholar]
- Wittmann, J.; Kugler, W.; Kolb, H. Influence of L-thyroxine and thiourea on metabolism and lung respiration in embryonic chicks. In Respiration and Metabolism of Embryonic Vertebrates; Springer: Berlin, Germany, 1984; pp. 311–318. [Google Scholar]
- Welcker, J.; Chastel, O.; Gabrielsen, G.W.; Guillaumin, J.; Kitaysky, A.S.; Speakman, J.R.; Tremblay, Y.; Bech, C. Thyroid hormones correlate with basal metabolic rate but not field metabolic rate in a wild bird species. PLoS ONE 2013, 8, e56229. [Google Scholar] [CrossRef] [Green Version]
- Hameed, S.; Patterson, M.; Dhillo, W.S.; Rahman, S.A.; Ma, Y.; Holton, C.; Gogakos, A.; Yeo, G.S.H.; Lam, B.Y.H.; Polex-Wolf, J.; et al. Thyroid Hormone Receptor Beta in the Ventromedial Hypothalamus Is Essential for the Physiological Regulation of Food Intake and Body Weight. Cell Rep. 2017, 19, 2202–2209. [Google Scholar] [CrossRef]
- Kong, W.M.; Martin, N.M.; Smith, K.L.; Gardiner, J.V.; Connoley, I.P.; Stephens, D.A.; Dhillo, W.S.; Ghatei, M.A.; Small, C.J.; Bloom, S.R. Triiodothyronine Stimulates Food Intake via the Hypothalamic Ventromedial Nucleus Independent of Changes in Energy Expenditure. Endocrinology 2004, 145, 5252–5258. [Google Scholar] [CrossRef]
- Coppola, A.; Liu, Z.-W.; Andrews, Z.B.; Paradis, E.; Roy, M.-C.; Friedman, J.M.; Ricquier, D.; Richard, D.; Horvath, T.L.; Gao, X.-B.; et al. A Central Thermogenic-like Mechanism in Feeding Regulation: An Interplay between Arcuate Nucleus T3 and UCP2. Cell. Metab. 2007, 5, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Dahlke, F.; Gonzales, E.; Gadelha, A.C.; Maiorka, A.; Borges, S.A.; Rosa, P.S.; Faria Filho, D.E.; Furlan, R.L. Feathering, triiodothyronine and thyroxineplasma levels and body temperature of two broiler lines raised under different temperatures. Cienc. Rural 2005, 35, 664–670. [Google Scholar] [CrossRef]
- Chiang, W.; Booren, A.; Strasburg, G. The effect of heat stress on thyroid hormone response and meat quality in turkeys of two genetic lines. Meat. Sci. 2008, 80, 615–622. [Google Scholar] [CrossRef]
- Decuypere, E.; Kuhn, E. Alterations in thyroid hormone physiology induced by temperature and feeding in newly hatched chickens. Acta Physiol. Polonica 1988, 39. [Google Scholar]
- Geraert, P.A.; Padilha, J.C.; Guillaumin, S. Metabolic and endocrine changes induced by chronic heat exposure in broiler chickens: Biological and endocrinological variables. Br. J. Nutr. 1996, 75, 205–216. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Raimbault, S.; Dridi, S.; Denjean, F.; Lachuer, J.; Couplan, E.; Bouillaud, F.; Bordas, A.; Duchamp, C.; Taouis, M.; Ricquier, D. An uncoupling protein homologue putatively involved in facultative muscle thermogenesis in birds. Biochem. J. 2001, 353, 441–444. [Google Scholar] [CrossRef]
- Vianna, C.R.; Hagen, T.; ZHANG, C.-Y.; Bachman, E.; Boss, O.; Gereben, B.; Moriscot, A.S.; Lowell, B.B.; Bicudo, J.E.P.; Bianco, A.C. Cloning and functional characterization of an uncoupling protein homolog in hummingbirds. Physiol. Genom. 2001, 5, 137–145. [Google Scholar] [CrossRef]
- Bowen, S.J.; Washburn, K.W.; Huston, T.M. Involvement of the Thyroid Gland in the Response of Young Chickens to Heat Stress1. Poult. Sci. 1984, 63, 66–69. [Google Scholar] [CrossRef]
- Hwang-Bo, J.; Muramatsu, T.; Okumura, J. Research Note: Age Dependency of Triiodothyronine-Induced Thermogenesis in Young Chicks: Inhibition by Propylthiouracil. Poult. Sci. 1990, 69, 1599–1601. [Google Scholar] [CrossRef] [PubMed]
- Hwang-Bo, J.; Muramatsu, T.; Okumura, J. Research Note: Relative Biopotency of Triiodothyronine and of Thyroxine for Inducing Oxygen Consumption in Young Chicks. Poult. Sci. 1990, 69, 1027–1029. [Google Scholar] [CrossRef]
- Debonne, M.; Baarendse, P.J.J.; Van Den Brand, H.; Kemp, B.; Bruggeman, V.; Decuypere, E. Involvement of the hypothalamic-pituitary-thyroid axis and its interaction with the hypothalamic-pituitary-adrenal axis in the ontogeny of avian thermoregulation: A review. Worlds Poult. Sci. J. 2008, 64, 309–321. [Google Scholar] [CrossRef] [Green Version]
- López, M.; Varela, L.; Vázquez, M.J.; Rodríguez-Cuenca, S.; González, C.R.; Velagapudi, V.R.; Morgan, D.A.; Schoenmakers, E.; Agassandian, K.; Lage, R.; et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat. Med. 2010, 16, 1001–1008. [Google Scholar] [CrossRef]
- Boswell, T.; Sharp, P.; Hall, M.; Goldsmith, A. Migratory fat deposition in European quail: A role for prolactin? J. Endocrinol. 1995, 146, 71–79. [Google Scholar] [CrossRef]
- Bartov, I.; Jensen, L.S.; Veltmann, J.R. Effect of Corticosterone and Prolactin on Fattening in Broiler Chicks. Poult. Sci. 1980, 59, 1328–1334. [Google Scholar] [CrossRef]
- Hooley, R.D.; Findlay, J.K.; Stephenson, R.G.A. Effect of Heat Stress on Plasma Concentrations of Prolactin and Luteinizing Hormone in Ewes. Aust. J. Biol. Sci. 1979, 32, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Ronchi, B.; Stradaioli, G.; Verini Supplizi, A.; Bernabucci, U.; Lacetera, N.; Accorsi, P.A.; Nardone, A.; Seren, E. Influence of heat stress or feed restriction on plasma progesterone, oestradiol-17β, LH, FSH, prolactin and cortisol in Holstein heifers. Livest. Prod. Sci. 2001, 68, 231–241. [Google Scholar] [CrossRef]
- Amaral, B.C.D.; Connor, E.E.; Tao, S.; Hayen, J.; Bubolz, J.; Dahl, G.E. Heat stress abatement during the dry period influences prolactin signaling in lymphocytes. Domest. Anim. Endocrinol. 2010, 38, 38–45. [Google Scholar] [CrossRef]
- Collier, R.J.; Beede, D.K.; Thatcher, W.W.; Israel, L.A.; Wilcox, C.J. Influences of Environment and Its Modification on Dairy Animal Health and Production1. J. Dairy Sci. 1982, 65, 2213–2227. [Google Scholar] [CrossRef]
- Faichney, O.J.; Barry, T.N. Effects of Mild Heat Exposure and Suppression of Prolactin Secretion on Gastro-intestinal Tract Function and Temperature Regulation in Sheep. Aust. J. Biol. Sci. 1986, 39, 85–98. [Google Scholar] [CrossRef]
- Salah, M.S.; AlShaikh, M.A.; Al-Saiadi, M.Y.; Mogawer, H.H. Effect of prolactin inhibition on thermoregulation, water and food intakes in heat-stressed fat-tailed male lambs. Anim. Sci. 1995, 60, 87–91. [Google Scholar] [CrossRef]
- Rozenboim, I.; Mobarky, N.; Heiblum, R.; Chaiseha, Y.; Kang, S.W.; Biran, I.; Rosenstrauch, A.; Sklan, D.; El Halawani, M.E. The Role of Prolactin in Reproductive Failure Associated with Heat Stress in the Domestic Turkey1. Biol. Reprod. 2004, 71, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Elnagar, S.A.; Scheideler, S.E.; Beck, M.M. Reproductive hormones, hepatic deiodinase messenger ribonucleic acid, and vasoactive intestinal polypeptide-immunoreactive cells in hypothalamus in the heat stress-induced or chemically induced hypothyroid laying hen12. Poult. Sci. 2010, 89, 2001–2009. [Google Scholar] [CrossRef]
- Alamer, M. The role of prolactin in thermoregulation and water balance during heat stress in domestic ruminants. Asian J. Anim. Vet. Adv. 2011, 6, 1153–1169. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohler, M.W.; Chowdhury, V.S.; Cline, M.A.; Gilbert, E.R. Heat Stress Responses in Birds: A Review of the Neural Components. Biology 2021, 10, 1095. https://doi.org/10.3390/biology10111095
Bohler MW, Chowdhury VS, Cline MA, Gilbert ER. Heat Stress Responses in Birds: A Review of the Neural Components. Biology. 2021; 10(11):1095. https://doi.org/10.3390/biology10111095
Chicago/Turabian StyleBohler, Mark W., Vishwajit S. Chowdhury, Mark A. Cline, and Elizabeth R. Gilbert. 2021. "Heat Stress Responses in Birds: A Review of the Neural Components" Biology 10, no. 11: 1095. https://doi.org/10.3390/biology10111095
APA StyleBohler, M. W., Chowdhury, V. S., Cline, M. A., & Gilbert, E. R. (2021). Heat Stress Responses in Birds: A Review of the Neural Components. Biology, 10(11), 1095. https://doi.org/10.3390/biology10111095





