Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai–Tibetan Plateau
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Ecogeographical adaptation in anurans is evident in an interactive change between body size and extremities (hereafter Hypothesis 1).
- (2)
- Geographic variation in anurans’ morphological traits is an outcome of combined environmental and biological effects (hereafter Hypothesis 2).
2. Materials and Methods
2.1. Data Collection
2.2. Environmental Variables
2.3. Statistical Analysis
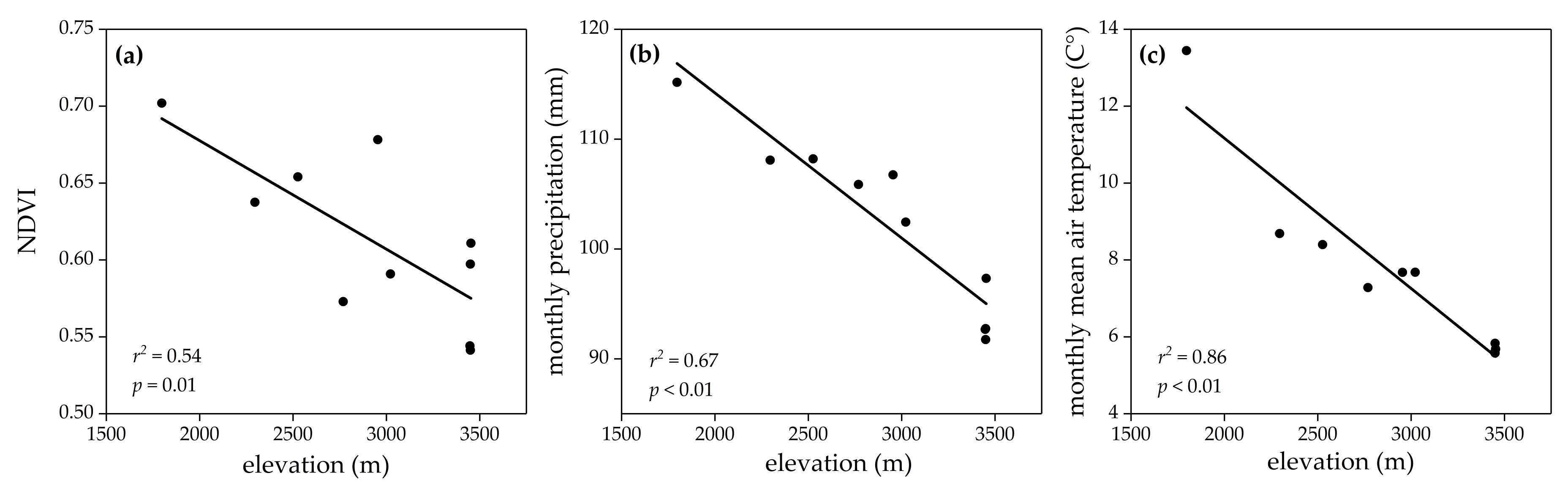
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Global warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; IPCC: Geneva, Switzerland, 2018. [Google Scholar]
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.-O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Orizaola, G.; Quintela, M.; Laurila, A. Climatic adaptation in an isolated and genetically impoverished amphibian population. Ecography 2020, 33, 730–737. [Google Scholar] [CrossRef]
- Fox, R.J.; Donelson, J.M.; Schunter, C.; Ravasi, T.; Gaitán-Espitia, J.D. Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R. Soc. B-Biol. Sci. 2019, 374, 20180174. [Google Scholar] [CrossRef]
- Parmesan, C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob. Chang. Biol. 2007, 13, 1860–1872. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Walls, S.C.; Bancroft, B.A.; Lawler, J.J.; Searle, C.L.; Gervasi, S.S. Direct and indirect effects of climate change on amphibian populations. Diversity 2010, 2, 281–313. [Google Scholar] [CrossRef]
- Hopkins, W.A. Amphibians as models for studying environmental change. ILAR J. 2007, 48, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Laugen, A.T.; Laurila, A.; Jönsson, K.I.; Söderman, F.; Merilä, J. Do common frogs (Rana temporaria) follow Bergmann’s rule? Evol. Ecol. Res. 2005, 7, 717–731. [Google Scholar]
- Olalla-Tárraga, M.Á.; Rodríguez, M.Á. Energy and interspecific body size patterns of amphibian faunas in Europe and North America: Anurans follow Bergmann’s rule, urodeles its converse. Glob. Ecol. Biogeogr. 2007, 16, 606–617. [Google Scholar] [CrossRef]
- Adams, D.C.; Church, J.O. Amphibians do not follow Bergmann’s rule. Evolution 2008, 62, 413–420. [Google Scholar] [CrossRef]
- Gouveia, S.F.; Correia, I. Geographical clines of body size in terrestrial amphibians: Water conservation hypothesis revisited. J. Biogeogr. 2016, 43, 2075–2084. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Meiri, S.; Jara, M.; Olalla-Tárraga, M.Á.; Hodgson, D.J. Global patterns of body size evolution are driven by precipitation in legless amphibians. Ecography 2019, 42, 1682–1690. [Google Scholar] [CrossRef]
- McNab, B.K. On the ecological significance of Bergmann’s rule. Ecology 1971, 52, 845–854. [Google Scholar] [CrossRef]
- Mousseau, T.A. Ectotherms follow the converse to Bergmann’s rule. Evolution 1997, 51, 630–632. [Google Scholar] [CrossRef] [Green Version]
- Meiri, S.; Dayan, T. On the validity of Bergmann’s rule. J. Biogeogr. 2003, 30, 331–351. [Google Scholar] [CrossRef]
- Rypel, A.L. The cold-water connection: Bergmann’s rule in North American freshwater fishes. Am. Nat. 2014, 183, 147–156. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse; Göttinger Studien: Göttingen, Germany, 1848; pp. 595–708. [Google Scholar]
- Allen, J.A. The influence of physical conditions in the genesis of species. Radic. Rev. 1877, 1, 108–140. [Google Scholar]
- Feng, X.Y.; Chen, W.; Hu, J.H.; Jiang, J.P. Variation and sexual dimorphism of body size in the plateau brown frog along an altitudinal gradient. Asian Herpetol. Res. 2015, 6, 291–297. [Google Scholar] [CrossRef]
- Sinsch, U.; Pelster, B.; Ludwig, G. Large-scale variation of size- and age-related life-history traits in the common frog: A sensitive test case for macroecological rules. J. Zool. 2015, 297, 32–43. [Google Scholar] [CrossRef]
- Yu, T.L.; Jia, G.; Sun, H.Q.; Shi, W.H.; Li, X.L.; Wang, H.B.; Huang, M.R.; Ding, S.Y.; Chen, J.P.; Zhang, M. Altitudinal body size variation in Rana kukunoris: The effects of age and growth rate on the plateau brown frog from the eastern Tibetan Plateau. Ethol. Ecol. Evol. 2021, 1–13. [Google Scholar] [CrossRef]
- Olalla-Tárraga, M.Á.; Diniz-Filho, J.A.F.; Bastos, R.P.; Rodríguez, M.Á. Geographic body size gradients in tropical regions: Water deficit and anuran body size in the Brazilian Cerrado. Ecography 2009, 32, 581–590. [Google Scholar] [CrossRef]
- Martínez-Monzón, A.; Blain, H.A.; Cuenca-Bescós, G.; Rodríguez, M.Á. Climate and amphibian body size: A new perspective gained from the fossil record. Ecography 2018, 41, 1307–1318. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mestre, I.; Buchholz, D.R. Developmental plasticity mirrors differences among taxa in spadefoot toads linking plasticity and diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 19021–19026. [Google Scholar] [CrossRef] [Green Version]
- Alho, J.S.; Herczeg, G.; Laugen, A.T.; Räsänen, K.; Laurila, A.; Merilä, J. Allen’s rule revisited: Quantitative genetics of extremity length in the common frog along a latitudinal gradient. J. Evol. Biol. 2011, 24, 59–70. [Google Scholar] [CrossRef]
- Schäuble, C.S. Variation in body size and sexual dimorphism across geographical and environmental space in the frogs Limnodynastes tasmaniensis and L. peronii. Biol. J. Linn. Soc. 2004, 82, 39–56. [Google Scholar] [CrossRef]
- Karkach, A.S. Trajectories and models of individual growth. Demogr. Res. 2006, 15, 347–400. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Net primary productivity of terrestrial communities: Prediction from climatological data. Am. Nat. 1968, 102, 67–74. [Google Scholar] [CrossRef]
- Yom-Tov, Y.; Geffen, E. Geographic variation in body size: The effects of ambient temperature and precipitation. Oecologia 2006, 148, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Huston, M.A.; Wolverton, S. Regulation of animal size by eNPP, Bergmann’s rule and related phenomena. Ecol. Monogr. 2011, 81, 349–405. [Google Scholar] [CrossRef]
- Hjernquist, M.B.; Söderman, F.; Jönsson, K.I.; Herczeg, G.; Laurila, A.; Merilä, J. Seasonality determines patterns of growth and age structure over a geographic gradient in an ectothermic vertebrate. Oecologia 2012, 170, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Oromi, N.; Sanuy, D.; Sinsch, U. Altitudinal variation of demographic life-history traits does not mimic latitudinal variation in natterjack toads (Bufo calamita). Zoology 2012, 115, 30–37. [Google Scholar] [CrossRef]
- Liao, W.B.; Lu, X. Age structure and body size of the Chuanxi Tree Frog Hyla annectans chuanxiensis from two different elevations in Sichuan (China). Zool. Anz. 2010, 248, 255–263. [Google Scholar] [CrossRef]
- Monnet, J.M.; Cherry, M.I. Sexual size dimorphism in anurans. Proc. R. Soc. B Biol. Sci. 2002, 269, 2301–2307. [Google Scholar] [CrossRef] [Green Version]
- Blanckenhorn, W.U.; Dixon, A.F.; Fairbairn, D.J.; Foellmer, M.W.; Gibert, P.; Linde, K.V.D.; Meier, R.; Nylin, S.; Pitnick, S.; Schoff, C.; et al. Proximate causes of Rensch’s rule: Does sexual size dimorphism in arthropods result from sex differences in development time? Am. Nat. 2007, 169, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Reading, C.J. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia 2007, 151, 125. [Google Scholar] [CrossRef] [PubMed]
- Tracy, C.R.; Christian, K.A.; Tracy, C.R. Not just small, wet, and cold: Effects of body size and skin resistance on thermoregulation and arboreality of frogs. Ecology 2010, 91, 1477–1484. [Google Scholar] [CrossRef]
- Jeckel, A.M.; Saporito, R.A.; Grant, T. The relationship between poison frog chemical defenses and age, body size, and sex. Front. Zool. 2015, 12, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.L.; Peters, S.E. Isometric contractile properties of sexually dimorphic forelimb muscles in the marine toad Bufo marinus Linnaeus 1758: Functional analysis and implications for amplexus. J. Exp. Biol. 2006, 209, 3448–3456. [Google Scholar] [CrossRef] [Green Version]
- López, P.; Martín, J. Locomotor capacity and dominance in male lizards Lacerta monticola: A trade-off between survival and reproductive success? Biol. J. Linn. Soc. 2002, 77, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Ashton, K.G.; Tracy, M.C.; Queiroz, A.D. Is Bergmann’s rule valid for mammals? Am. Nat. 2000, 156, 390–415. [Google Scholar] [CrossRef]
- Moore, M.P.; Landberg, T.; Whiteman, H.H. Maternal investment mediates offspring life history variation with context-dependent fitness consequences. Ecology 2015, 96, 2499–2509. [Google Scholar] [CrossRef]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica, Amphibia; Science Press: Beijing, China, 2009; Volume 3. (In Chinese) [Google Scholar]
- Fei, L.; Ye, C.Y.; Jiang, J.P. Colored Atlas of Chinese Amphibians and Their Distributions; Sichuan Publishing House of Science & Technology: Chengdu, China, 2012. (In Chinese) [Google Scholar]
- Chen, W.; Tang, Z.H.; Fan, X.G.; Wang, Y.; Pike, D.A. Maternal investment increases with altitude in a frog on the Tibetan Plateau. J. Evol. Biol. 2013, 26, 2710–2715. [Google Scholar] [CrossRef] [Green Version]
- Watt, C.; Mitchell, S.; Salewski, V. Bergmann’s rule; A concept cluster? Oikos 2010, 119, 89–100. [Google Scholar] [CrossRef]
- Berven, K.A. The genetic basis of altitudinal variation in the wood frog Rana sylvatica. I. An experimental analysis of life history traits. Evolution 1982, 962–983. [Google Scholar] [CrossRef]
- Caruso, N.M.; Sears, M.W.; Adams, D.C.; Lips, K.R. Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Chang. Biol. 2014, 20, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.B.; Whittaker, R.J.; Malhi, Y. ET come home: Potential evapotranspiration in geographical ecology. Glob. Ecol. Biogeogr. 2011, 20, 1–18. [Google Scholar] [CrossRef]
- Rodríguez, M.Á.; Belmontes, J.A.; Hawkins, B.A. Energy, water and large-scale patterns of reptile and amphibian species richness in Europe. Acta Oecol. 2005, 28, 65–70. [Google Scholar] [CrossRef]
- Tong, Q.; Du, X.P.; Hu, Z.F.; Cui, L.Y.; Wang, H.B. Modelling the growth of the brown frog (Rana dybowskii). PeerJ 2018, 6, e4587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornthwaite, C.W.; Mather, J.R. The water balance. In Climatology; Centerton: Centerton, NJ, USA, 1955; Volume 8. [Google Scholar]
- Chevan, A.; Sutherland, M. Hierarchical partitioning. Am. Stat. 1991, 45, 90–96. [Google Scholar] [CrossRef]
- Walsh, C.; MacNally, R. The Hier. Part Package. Hierarchical Partitioning. R Package Version 1.0-6. 2020. Available online: https://cran.r-project.org/web/packages/hier.part/hier.part.pdf (accessed on 1 November 2020).
- MacNally, R. Multiple regression and inference in ecology and conservation biology: Further comments on identifying important predictor variables. Biodivers. Conserv. 2002, 11, 1397–1401. [Google Scholar] [CrossRef]
- Kasperson, J.X.; Kasperson, R.E.; Turner, B.; Hsieh, W.; Schiller, A. Vulnerability to global environmental change. In The Social Contours of Risk: Volume II: Risk Analysis, Corporations and the Globalization of Risk; Taylor and Francis: Oxfordshire, UK, 2012; pp. 245–285. [Google Scholar] [CrossRef]
- Brooke, C. Conservation and adaptation to climate change. Biol. Conserv. 2008, 22, 1471–1476. [Google Scholar] [CrossRef]
- Li, S.T.; Wu, X.; Li, D.Y.; Lou, S.L.; Mi, Z.P.; Liao, W.B. Body size variation of odorous frogs (Odorrana grahami) across altitudinal gradients. J. Herpetol. 2013, 23, 187–192. [Google Scholar]
- Nali, R.C.; Zamudio, K.R.; Haddad, C.F.; Prado, C.P. Size-dependent selective mechanisms on males and females and the evolution of sexual size dimorphism in frogs. Am. Nat. 2014, 184, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.E.; Aulner, D.A. Sexual dimorphism in forelimb muscles of the bullfrog, Rana catesbeiana: A functional analysis of isometric contractile properties. J. Exp. Biol. 2000, 203, 3639–3654. [Google Scholar] [CrossRef]
- Ryan, M.J.; Fox, J.H.; Wilczynski, W.; Rand, A.S. Sexual selection for sensory exploitation in the frog Physalaemus pustulosus. Nature 1990, 343, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Herrel, A.; Gonwouo, L.N.; Fokam, E.B.; Ngundu, W.I.; Bonneaud, C. Intersexual differences in body shape and locomotor performance in the aquatic frog, Xenopus tropicalis. J. Zool. 2012, 287, 311–316. [Google Scholar] [CrossRef]
- Louppe, V.; Courant, J.; Herrel, A. Differences in mobility at the range edge of an expanding invasive population of Xenopus laevis in the west of France. J. Exp. Biol. 2017, 220, 278–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, W.B.; Lu, X.; Jehle, R. Altitudinal variation in maternal investment and trade-offs between egg size and clutch size in the Andrew’s toad. J. Zool. 2014, 293, 84–91. [Google Scholar] [CrossRef]
- Ducret, V.; Videlier, M.; Moureaux, C.; Bonneaud, C.; Herrel, A. Do female frogs have higher resting metabolic rates than males? A case study with Xenopus allofraseri. J. Zool. 2020, 312, 221–226. [Google Scholar] [CrossRef]
- Atkinson, D. Ectotherm life history responses to developmental temperature. In Animals and Temperature: Phenotypic and Evolutionary Adaptation; Johnston, I.A., Bennett, A.F., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 183–204. [Google Scholar]
- Von Bertalanffy, L. Quantitative laws in metabolism and growth. Q. Rev. Biol. 1957, 32, 217–231. [Google Scholar] [CrossRef]
- Field, R.; Hawkins, B.A.; Cornell, H.V.; Currie, D.J.; Diniz-Filho, J.A.F.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; et al. Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 2009, 36, 132–147. [Google Scholar] [CrossRef]
- Jetz, W.; Fine, P.V. Global gradients in vertebrate diversity predicted by historical area-productivity dynamics and contemporary environment. PLoS. Biol. 2012, 10, e1001292. [Google Scholar] [CrossRef]
- Regosin, J.V.; Windmiller, B.S.; Reed, J.M. Terrestrial habitat use and winter densities of the wood frog (Rana sylvatica). J. Herpetol. 2003, 37, 390–394. [Google Scholar] [CrossRef]
- Bull, E.L. Sexual differences in the ecology and habitat selection of Western Toads (Bufo boreas) in northeastern Oregon. Herpetol. Conserv. Biol. 2006, 1, 27–38. [Google Scholar]
- Rowley, J.J.; Alford, R.A. Movement patterns and habitat use of rainforest stream frogs in northern Queensland, Australia: Implications for extinction vulnerability. Wildl. Res. 2007, 34, 371–378. [Google Scholar] [CrossRef]
- Muths, E. Home range and movements of boreal toads in undisturbed habitat. Copeia 2003, 1, 160–165. [Google Scholar] [CrossRef]
- Sinsch, U.; Marangoni, F.; Oromi, N.; Leskovar, C.; Sanuy, D.; Tejedo, M. Proximate mechanisms determining size variability in natterjack toads. J. Zool. 2010, 281, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Morrison, C.; Hero, J.M. Geographic variation in life-history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef] [Green Version]



| Morphometric | Factor | Random Effect | Fixed Effect | ||
|---|---|---|---|---|---|
| SE | Z | SE | F | ||
| SVL | Population | <0.001 | 1.964 * | ||
| Sex | 0.006 | 39.918 ** | |||
| Age | 0.003 | 18.113 ** | |||
| LAHL | Population | 0.001 | 1.997 * | ||
| Sex | 0.006 | 9.089 ** | |||
| Age | 0.003 | 7.438 ** | |||
| HILL | Population | 0.001 | 1.980 * | ||
| Sex | 0.007 | 8.255 ** | |||
| Age | 0.003 | 10.346 ** | |||
| Sex (n) | Morphometric | Full Model (r2) | Full Model Variables (Slope, %I) | |||
|---|---|---|---|---|---|---|
| Age | NDVI | PREP | TEMP | |||
| Male (197) | SVL | 0.274 ** | +16.14 * | +9.21 * | +11.28 * | −63.37 * |
| LAHL | 0.208 ** | +7.16 * | +13.32 * | +31.73 * | −47.80 * | |
| HILL | 0.189 ** | +12.16 * | +11.85 * | +32.42 * | −43.57 * | |
| Female (58) | SVL | 0.223 ** | +32.24 * | +29.51 * | +24.92 | −13.32 |
| LAHL | 0.315 ** | +12.72 | +24.68 * | +53.93 * | −8.67 | |
| HILL | 0.345 ** | +9.77 | +34.42 * | +48.66 * | −10.16 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, K.W.; Yang, S.; Wang, X.; Tang, K.; Hu, J. Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai–Tibetan Plateau. Biology 2021, 10, 1081. https://doi.org/10.3390/biology10111081
Leung KW, Yang S, Wang X, Tang K, Hu J. Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai–Tibetan Plateau. Biology. 2021; 10(11):1081. https://doi.org/10.3390/biology10111081
Chicago/Turabian StyleLeung, Ka Wah, Shengnan Yang, Xiaoyi Wang, Ke Tang, and Junhua Hu. 2021. "Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai–Tibetan Plateau" Biology 10, no. 11: 1081. https://doi.org/10.3390/biology10111081






