The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Blastocystis spp. Infection in Humans
4. Blastocystis spp. Infection in Animals
5. Blastocystis spp. in Food and Environmental Sources
6. Distribution of Blastocystis spp. by Country
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, K.S.W. New Insights on Classification, Identification, and Clinical Relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008, 21, 639–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfellani, M.A.; Stensvold, C.R.; Vidal-Lapiedra, A.; Onuoha, E.S.U.; Fagbenro-Beyioku, A.F.; Clark, C.G. Variable Geographic Distribution of Blastocystis Subtypes and Its Potential Implications. Acta Trop. 2013, 126, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Yao, J.; Chen, S.; He, T.; Chai, Y.; Zhou, Z.; Shi, X.; Liu, H.; Zhong, Z.; Fu, H.; et al. First Identification and Molecular Subtyping of Blastocystis sp. in Zoo Animals in Southwestern China. Parasites Vectors 2021, 14, 11. [Google Scholar] [CrossRef]
- Andersen, L.O.; Stensvold, C.R. Blastocystis in Health and Disease: Are We Moving from a Clinical to a Public Health Perspective? J. Clin. Microbiol. 2016, 54, 524–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stenzel, D.J.; Boreham, P.F.L. Blastocystis hominis Revisited. Clin. Microbiol. Rev. 1996, 105, 563–584. [Google Scholar] [CrossRef]
- Silberman, J.D.; Sogin, M.L.; Leipe, D.D.; Clark, C.G. Human Parasite Finds Taxonomic Home. Nature 1996, 380, 398. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Koyama, Y.; Tsuchiya, E.; Takami, K. Blastocystis Phylogeny among Various Isolates from Humans to Insects. Parasitol. Int. 2016, 65, 750–759. [Google Scholar] [CrossRef]
- Lepczyńska, M.; Białkowska, J.; Dzika, E.; Piskorz-Ogórek, K.; Korycińska, J. Blastocystis: How Do Specific Diets and Human Gut Microbiota Affect Its Development and Pathogenicity? Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Parija, S.; Jeremiah, S. Blastocystis: Taxonomy, Biology and Virulence. Trop. Parasitol. 2013, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Stensvold, C.R.; Suresh, G.K.; Tan, K.S.W.; Thompson, R.C.A.; Traub, R.J.; Viscogliosi, E.; Yoshikawa, H.; Clark, C.G. Terminology for Blastocystis Subtypes-a Consensus. Trends Parasitol. 2007, 23, 93–96. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Clark, C.G. Pre-Empting Pandora’s Box: Blastocystis Subtypes Revisited. Trends Parasitol. 2020, 36, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Hublin, J.S.Y.; Maloney, J.G.; Santin, M. Blastocystis in Domesticated and Wild Mammals and Birds. Res. Vet. Sci. 2020, 135, 260–282. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.D.; Sánchez, A.; Hernández, C.; Flórez, C.; Bernal, M.C.; Giraldo, J.C.; Reyes, P.; López, M.C.; García, L.; Cooper, P.J. Geographic Distribution of Human Blastocystis Subtypes in South America. Infect. Genet. Evol. 2016, 41, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, W.; Wang, X.; Zhang, Z.; Zhang, R.; Duan, Z. Blastocystis Infection and Subtype Distribution in Domestic Animals in the Qinghai-Tibetan Plateau Area (QTPA) in China: A Preliminary Study. Parasitol. Int. 2021, 81, 102272. [Google Scholar] [CrossRef] [PubMed]
- Noradilah, S.A.; Anuar, T.S.; Moktar, N.; Lee, I.L.; Salleh, F.M.; Azreen, S.N.A.M.; Husnie, N.S.M.M.; Azrul, S.M.; Abdullah, W.O.; Nordin, A.; et al. Molecular Epidemiology of Blastocystis sp. in Animals Reared by the Aborigines during Wet and Dry Seasons in Rural Communities, Pahang, Malaysia. Southeast Asian J. Trop. Med. Public Health 2017, 48, 1151–1160. [Google Scholar]
- Yoshikawa, H.; Yoshida, K.; Nakajima, A.; Yamanari, K.; Iwatani, S.; Kimata, I. Fecal-Oral Transmission of the Cyst Form of Blastocystis hominis in Rats. Parasitol. Res. 2004, 94, 391–396. [Google Scholar] [CrossRef]
- Anuar, T.S.; Ghani, M.K.A.; Azreen, S.N.; Salleh, F.M.; Moktar, N. Blastocystis Infection in Malaysia: Evidence of Waterborne and Human-to-Human Transmissions among the Proto-Malay, Negrito and Senoi Tribes of Orang Asli. Parasites Vectors 2013, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.; Chye, T.; Karmacharya, M.; Govind, S. Blastocystis sp.: Waterborne Zoonotic Organism, a Possibility? Parasites Vectors 2012, 5, 130. [Google Scholar] [CrossRef] [Green Version]
- Rajah Salim, H.; Kumar, G.S.; Vellayan, S.; Mak, J.W.; Khairul Anuar, A.; Init, I.; Vennila, G.D.; Saminathan, R.; Ramakrishnan, K. Blastocystis in Animal Handlers. Parasitol. Res. 1999, 85, 1032–1033. [Google Scholar] [CrossRef]
- Rivera, W.L. Phylogenetic Analysis of Blastocystis Isolates from Animal and Human Hosts in the Philippines. Vet. Parasitol. 2008, 156, 178–182. [Google Scholar] [CrossRef]
- Parkar, U.; Traub, R.J.; Vitali, S.; Elliot, A.; Levecke, B.; Robertson, I.; Geurden, T.; Steele, J.; Drake, B.; Thompson, R.C.A. Molecular Characterization of Blastocystis Isolates from Zoo Animals and Their Animal-Keepers. Vet. Parasitol. 2010, 169, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Noradilah, S.A.; Lee, I.L.; Anuar, T.S.; Salleh, F.M.; Abdul Manap, S.N.A.; Husnie, N.S.; Azrul, S.M.; Moktar, N. Blastocystis spp. Contaminated Water Sources in Aboriginal Settlements. Trop. Biomed. 2017, 34, 110–117. [Google Scholar] [PubMed]
- Leelayoova, S.; Siripattanapipong, S.; Thathaisong, U.; Naaglor, T.; Taamasri, P.; Piyaraj, P.; Mungthin, M. Drinking Water: A Possible Source of Blastocystis spp. Subtype 1 Infection in Schoolchildren of a Rural Community in Central Thailand. Am. J. Trop. Med. Hyg. 2008, 79, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.C.; da Silva, M.D.C.; Pereira, R.Â.S.; Pinto, L.C. Prevalence of Contamination by Intestinal Parasites in Vegetables (Lactuca sativa L. and Coriandrum sativum L.) Sold in Markets in Belém, Northern Brazil. J. Sci. Food Agric. 2020, 100, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Caradonna, T.; Marangi, M.; Del Chierico, F.; Ferrari, N.; Reddel, S.; Bracaglia, G.; Normanno, G.; Putignani, L.; Giangaspero, A. Detection and Prevalence of Protozoan Parasites in Ready-to-Eat Packaged Salads on Sale in Italy. Food Microbiol. 2017, 67, 67–75. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.I. Prevalence Intestinal Parasites in Leafy Vegetables in Riyadh, Saudi Arabia. Int. J. Trop. Med. 2010, 5, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, F.; Koltas, I.S. Evaluation of the Transmission Mode of B. hominis by Using PCR Method. Parasitol. Res. 2010, 107, 841–845. [Google Scholar] [CrossRef]
- World Health Organization. Microbial Fact Sheets. In World Health Organization Guidelines for Drinking-Water Quality (WHO GDWQ); Gutenberg: Triq Tal Barrani, Malta, 2011; pp. 231–305. [Google Scholar]
- Shrestha, K.; Acharya, K.P.; Shrestha, S. One Health: The Interface between Veterinary and Human Health. Int. J. One Health 2018, 4, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, J.S.; Jeggo, M. The One Health Approach-Why Is It So Important? Trop. Med. Infect. Dis. 2019, 4, 88. [Google Scholar] [CrossRef] [Green Version]
- Mackenzie, J.; McKinnon, M.; Jeggo, M. One Health: From Concept to Practice. In Confronting Emerging Zoonoses: The One Health Paradigm; Yamada, A., Kahn, L.H., Kaplan, B., Monath, T.P., Woodall, J., Conti, L., Eds.; Springer: Tokyo, Japan, 2014; pp. 163–189. [Google Scholar] [CrossRef]
- Adao, D.E.V.; Rivera, W.L. Recent Advances in Blastocystis sp. Research. Philipp. Sci. Lett. 2018, 11, 39–60. [Google Scholar] [CrossRef]
- Rauff-Adedotun, A.A.; Mohd Zain, S.N.; Farah Haziqah, M.T. Current Status of Blastocystis sp. in Animals from Southeast Asia: A Review. Parasitol. Res. 2020, 119, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Barua, P.; Khanum, H.; Haque, R.; Najib, F.; Kabir, M. Establishment of Blastocystis hominis In-Vitro Culture Using Fecal Samples from Infants in Slum Area of Mirpur, Dhaka, Bangladesh. Acta Med. Int. 2015, 2, 40. [Google Scholar] [CrossRef]
- Ben-Shimol, S.; Sagi, O.; Greenberg, D. Differences in Prevalence of Parasites in Stool Samples between Three Distinct Ethnic Pediatric Populations in Southern Israel, 2007–2011. Parasitol. Int. 2014, 63, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-X.; Zhou, Y.-M.; Xu, W.; Tian, L.-G.; Chen, J.-X.; Chen, S.-H.; Dang, Z.-S.; Gu, W.-P.; Yin, J.-W.; Serrano, E.; et al. Impact of Co-Infections with Enteric Pathogens on Children Suffering from Acute Diarrhea in Southwest China. Infect. Dis. Poverty 2016, 5, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Wei, Z.; Zhang, Y.; Zhang, Q.; Li, J.; Zhang, L.; Wang, R. Genetic Diversity of Blastocystis in Kindergarten Children in Southern Xinjiang, China. Parasites Vectors 2020, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.-Q.; Kang, J.-M.; Li, Y.-T.; Chen, H.-H.; Chu, Y.-H.; Yu, Y.-F.; Wu, X.-P.; Ai, L.; Chen, J.-X.; Tian, L.-G.; et al. Prevalence and Risk Factors of Blastocystis Infections among Primary School Students in Jiangjin District, Chongqing City. Chin. J. Schistosomiasis Control. 2020, 32, 489–497. [Google Scholar] [CrossRef]
- Liao, C.-W.; Chiu, K.-C.; Chiang, I.-C.; Cheng, P.-C.; Chuang, T.-W.; Kuo, J.-H.; Tu, Y.-H.; Fan, C.-K. Prevalence and Risk Factors for Intestinal Parasitic Infection in Schoolchildren in Battambang, Cambodia. Am. J. Trop. Med. Hyg. 2017, 96, 583–588. [Google Scholar] [CrossRef] [Green Version]
- Rayan, P.; Verghese, S.; McDonnell, P.A. Geographical Location and Age Affects the Incidence of Parasitic Infestations in School Children. Indian J. Pathol. Microbiol. 2010, 53, 504–508. [Google Scholar] [CrossRef]
- Yoshikawa, H.; Tokoro, M.; Nagamoto, T.; Arayama, S.; Asih, P.B.S.; Rozi, I.E.; Syafruddin, D. Molecular Survey of Blastocystis sp. from Humans and Associated Animals in an Indonesian Community with Poor Hygiene. Parasitol. Int. 2016, 65, 780–784. [Google Scholar] [CrossRef]
- Zulfa, F.; Sari, I.P.; Kurniawan, A. Association of Blastocystis Subtypes with Diarrhea in Children. J. Phys. Conf. Ser. 2017, 884, 012031. [Google Scholar] [CrossRef] [Green Version]
- Sari, I.P.; Benung, M.R.; Wahdini, S.; Kurniawan, A. Diagnosis and Identification of Blastocystis Subtypes in Primary School Children in Jakarta. J. Trop. Pediatr. 2018, 64, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Subahar, R.; Susanto, L.; Astuty, H.; Winita, R.; Sari, I.P. Intestinal Parasitic Infections and Hemoglobin Levels among Schoolchildren Participating in a Deworming Program in Jakarta, Indonesia: A Cross-Sectional Study. Open Access Maced. J. Med. Sci. 2020, 8, 589–594. [Google Scholar] [CrossRef]
- Sari, I.P.; Audindra, S.; Zhafira, A.S.; Rahma, A.A.; Syarira, C.V.; Wahdini, S. Nutritional Status of School-Aged Children with Intestinal Parasite Infection in South Jakarta, Indonesia. Open Access Maced. J. Med. Sci. 2021, 9, 95–100. [Google Scholar] [CrossRef]
- Ashtiani, M.T.H.; Monajemzadeh, M.; Saghi, B.; Shams, S.; Mortazavi, S.H.; Khaki, S.; Mohseni, N.; Kashi, L.; Nikmanesh, B. Prevalence of Intestinal Parasites among Children Referred to Children’s Medical Center during 18 Years (1991–2008), Tehran, Iran. Ann. Trop. Med. Parasitol. 2011, 105, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niaraki, S.R.; Hajialilo, E.; Delshad, A.; Alizadeh, S.A.; Alipour, M.; Heydarian, P.; Saraei, M. Molecular Epidemiology of Blastocystis spp. in Children Referred to Qods Hospital in Northwest of Iran. J. Parasit. Dis. 2020, 44, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Taee, N.; Faraji Goodarzi, M.; Ebrahimzadeh, F. Prevalence and Risk Factors of Intestinal Protozoan Infections in Children (2–15 Yr Old) from Lorestan Province, Western Iran. Trop. Biomed. 2018, 35, 259–266. [Google Scholar]
- Abdi, J.; Farhadi, M.; Aghaee, S. Prevalence of Intestinal Parasites among Children Attending the Daycare Centers of Ilam, Western Iran. J. Med. Sci. 2014, 14, 143–146. [Google Scholar] [CrossRef] [Green Version]
- Daryani, A.; Sharif, M.; Nasrolahei, M.; Khalilian, A.; Mohammadi, A.; Barzegar, G. Epidemiological Survey of the Prevalence of Intestinal Parasites among Schoolchildren in Sari, Northern Iran. Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Hazrati Tappeh, K.H.; Mostaghim, M.; Hanifian, H.; Khalkhali, H.; Mousavi, J. A Study on the Intestinal Parasitic Infections among Elementary School Students at a District (Silvana) In Urmia, West Azerbaijan. Int. J. Res. Appl. Basic Med. Sci. 2015, 1, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, R.; Nourian, A.; Hanilo, A.; Kamali, K. Prevalence of Intestinal Parasites among Primary School Students in Zanjan City (2013). J. Zanjan Univ. Med. Sci. Health Serv. 2016, 24, 121–130. [Google Scholar]
- Babakhani, M.; Safari, R.; Rajati, F.; Salimi, S.; Omidian doost, A. Prevalence and Risk Factors Associated with Intestinal Parasitic Infections among School Children in Gashky, West of Iran. Int. J. Pediatr. 2017, 5, 5263–5273. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Sadeghi, S.; Shahmoradi, B.; Ghahremani, E. Prevalence of Intestinal Protozoa Infections and Associated Risk Factors among Schoolchildren in Sanandaj City, Iran. Iran. J. Parasitol. 2017, 12, 108–116. [Google Scholar]
- Saki, J.; Amraee, D. Prevalence of Intestinal Parasites Among the Rural Primary School Students in the West of Ahvaz County, Iran, 2015. Jentashapir J. Health Res. 2017, 8, e40326. [Google Scholar] [CrossRef] [Green Version]
- Turki, H.; Hamedi, Y.; Heidari-Hengami, M.; Najafi-Asl, M.; Rafati, S.; Sharifi-Sarasiabi, K. Prevalence of Intestinal Parasitic Infection among Primary School Children in Southern Iran. J. Parasit. Dis. 2017, 41, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, N.K.; Al-Saadoon, M.A. Microsporidiosis among Children with Malignant Diseases in Basrah, Iraq. Pak. J. Med. Sci. 2012, 28, 621–624. [Google Scholar]
- Osman, M.; El Safadi, D.; Cian, A.; Benamrouz, S.; Nourrisson, C.; Poirier, P.; Pereira, B.; Razakandrainibe, R.; Pinon, A.; Lambert, C.; et al. Prevalence and Risk Factors for Intestinal Protozoan Infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among Schoolchildren in Tripoli, Lebanon. PLoS Negl. Trop. Dis. 2016, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Abd. Ghani, M.K.; Yusof, H. Blastocystis hominis: Kehadirannya Di Dalam Sampel Feses Kanak-Kanak Orang Asli Di Pos Lenjang, Pahang, Malaysia. Sains Malays. 2011, 40, 1123–1127. [Google Scholar]
- Abdulsalam, A.M.; Ithoi, I.; Al-Mekhlafi, H.M.; Ahmed, A.; Surin, J.; Mak, J.-W. Drinking Water Is a Significant Predictor of Blastocystis Infection among Rural Malaysian Primary Schoolchildren. Parasitology 2012, 139, 1014–1020. [Google Scholar] [CrossRef] [Green Version]
- Al-Harazi, T.; Ghani, M.K.A.; Othman, H. Prevalence of Intestinal Protozoan Infections among Orang Asli Schoolchildren in Pos Senderut, Pahang, Malaysia. J. Egypt. Soc. Parasitol. 2013, 43, 561–568. [Google Scholar] [CrossRef]
- Sinniah, B.; Hassan, A.K.R.; Sabaridah, I.; Soe, M.M.; Ibrahim, Z.; Ali, O. Prevalence of Intestinal Parasitic Infections among Communities Living in Different Habitats and Its Comparison with One Hundred and One Studies Conducted over the Past 42 Years (1970 to 2013) in Malaysia. Trop. Biomed. 2014, 31, 190–206. [Google Scholar]
- Nithyamathi, K.; Chandramathi, S.; Kumar, S. Predominance of Blastocystis sp. Infection among School Children in Peninsular Malaysia. PLoS ONE 2016, 11, e0136709. [Google Scholar] [CrossRef]
- Tang, S.G.H.; Kamel, A.G.M. Intestinal Protozoan Infections of Schoolchildren in an Aboriginal (Orang Asli) Settlement in Perak, Malaysia. Int. Med. J. 2020, 27, 31–35. [Google Scholar]
- Adli, M.N.; Mohamed Kamel, A.G. Blastocystosis amongst the Orang Asli (Aborigine) School Children of Sktar Kuala Kubu Bharu, Selangor, Malaysia. Int. Med. J. 2020, 27, 412–414. [Google Scholar]
- Mukhiya, R.K.; Rai, S.K.; Karki, A.B.; Prajapati, A. Intestinal Protozoan Parasitic Infection among School Children. J. Nepal Health Res. Counc. 2012, 10, 204–207. [Google Scholar] [PubMed]
- Al-Mohammed, H.I.; Amin, T.T.; Aboulmagd, E.; Hablus, H.R.; Zaza, B.O. Prevalence of Intestinal Parasitic Infections and Its Relationship with Socio-Demographics and Hygienic Habits among Male Primary Schoolchildren in Al-Ahsa, Saudi Arabia. Asian Pac. J. Trop. Med. 2010, 3, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Bakarman, M.A.; Hegazi, M.A.; Butt, N.S. Prevalence, Characteristics, Risk Factors, and Impact of Intestinal Parasitic Infections on School Children in Jeddah, Western Saudi Arabia. J. Epidemiol. Glob. Health 2019, 9, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntaravitun, P.; Dokmaikaw, A. Prevalence of Intestinal Protozoan Infections among Schoolchildren in Bang Khla District, Chachoengsao Province, Central Thailand. Asian Pac. J. Trop. Dis. 2017, 7, 523–526. [Google Scholar] [CrossRef]
- Sanprasert, V.; Srichaipon, N.; Bunkasem, U.; Srirungruang, S.; Nuchprayoon, S. Prevalence of Intestinal Protozoan Infections among Children in Thailand: A Large-Scale Screening and Comparative Study of Three Standard Detection Methods. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1123–1133. [Google Scholar] [PubMed]
- Thathaisong, U.; Siripattanapipong, S.; Mungthin, M.; Pipatsatitpong, D.; Tan-Ariya, P.; Naaglor, T.; Leelayoova, S. Identification of Blastocystis Subtype 1 Variants in the Home for Girls, Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2013, 88, 352–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipatsatitpong, D.; Leelayoova, S.; Mungthin, M.; Aunpad, R.; Naaglor, T.; Rangsin, R. Prevalence and Risk Factors for Blastocystis Infection among Children and Caregivers in a Child Care Center, Bangkok, Thailand. Am. J. Trop. Med. Hyg. 2015, 93, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punsawad, C.; Phasuk, N.; Bunratsami, S.; Thongtup, K.; Viriyavejakul, P.; Palipoch, S.; Koomhin, P.; Nongnaul, S. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors for Hookworm Infections among Primary Schoolchildren in Rural Areas of Nakhon Si Thammarat, Southern Thailand 11 Medical and Health Sciences 1117 Public Health and Health Services. BMC Public Health 2018, 18, 1118. [Google Scholar] [CrossRef]
- Assavapongpaiboon, B.; Bunkasem, U.; Sanprasert, V.; Nuchprayoon, S. A Cross-Sectional Study on Intestinal Parasitic Infections in Children in Suburban Public Primary Schools, Saraburi, the Central Region of Thailand. Am. J. Trop. Med. Hyg. 2018, 98, 763–767. [Google Scholar] [CrossRef]
- Boondit, J.; Pipatsatitpong, D.; Mungthin, M.; Taamasri, P.; Tan-Ariya, P.; Naaglor, T.; Leelayoova, S. Incidence and Risk Factors of Blastocystis Infection in Orphans at the Babies’ Home, Nonthaburi Province, Thailand. J. Med. Assoc. Thail. 2014, 97, S52–S59. [Google Scholar]
- Kitvatanachai, S.; Rhongbutsri, P. Intestinal Parasitic Infections in Suburban Government Schools, Lak Hok Subdistrict, Muang Pathum Thani, Thailand. Asian Pac. J. Trop. Med. 2013, 6, 699–702. [Google Scholar] [CrossRef] [Green Version]
- Popruk, S.; Thima, K.; Udonsom, R.; Rattaprasert, P.; Sukthana, Y. Does Silent Giardia Infection Need Any Attention? Open Trop. Med. J. 2011, 4, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Güdücüoǧlu, H.; Parlak, M.; Ciçek, M.; Yaman, G.; Oztürk, O.; Cikman, A.; Berktaş, M. [Investigation of Intestinal Parasites in Students of Mustafa Cengiz Primary School in Van].|Van Mustafa Cengiz Ilköǧretim Okulu Öǧrencilerinde Baǧirsak Parazitlerinin Araştirilmasi. Turk. Parazitolojii Derg. 2010, 34, 172–175. [Google Scholar]
- Hamamci, B.; Cetinkaya, U.; Delice, S.; Erçal, B.D.; Gücüyetmez, S.; Yazar, S. [Investigation of Intestinal Parasites among Primary School Students in Kayseri-Hacilar]. Kayseri-Hacilar’da İlköǧretim Okulu Öǧrencilerinde Baǧirsak Parazitlerinin Araştirilmasi. Turk. Parazitolojii Derg. 2011, 35, 96–99. [Google Scholar] [CrossRef]
- Sankur, F.; Ayturan, S.; Malatyali, E.; Ertabaklar, H.; Ertug, S. The Distribution of Blastocystis Subtypes among School-Aged Children in Mugla, Turkey. Iran. J. Parasitol. 2017, 12, 580–586. [Google Scholar] [PubMed]
- Calik, S.; Karaman, U.; Colak, C. Prevalence of Microsporidium and Other Intestinal Parasites in Children from Malatya, Turkey. Indian J. Microbiol. 2011, 51, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dogan, N.; Aydin, M.; Tuzemen, N.U.; Dinleyici, E.C.; Oguz, I.; Dogruman-Al, F. Subtype Distribution of Blastocystis spp. Isolated from Children in Eskisehir, Turkey. Parasitol. Int. 2017, 66, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Salehi Kahish, R.; Alghasi, A.; Hadadi, S.; Nasab, M.A.; Mafakherzadeh, A. The Prevalence of Blastocystis Infection in Pediatric Patients with Malignancy: A Single-Center Study in Ahvaz, Iran. Arch. Pediatr. Infect. Dis. 2021, 9, e104068. [Google Scholar] [CrossRef]
- Asghari, A.; Zare, M.; Hatam, G.; Shahabi, S.; Gholizadeh, F.; Motazedian, M. Molecular Identification and Subtypes Distribution of Blastocystis sp. Isolated from Children and Adolescent with Cancer in Iran: Evaluation of Possible Risk Factors and Clinical Features. Acta Parasitol. 2020, 65, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Salehi Kahyesh, R.; Alghasi, A.; Haddadi, S.; Sharhani, A. Intestinal Parasites Infection in Children with Cancer in Ahvaz, Southwest Iran. Interdiscip. Perspect. Infect. Dis. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Zabolinejad, N.; Berenji, F.; Eshkaftaki, E.B.; Badeii, Z.; Banihashem, A.; Afzalaqaei, M. Intestinal Parasites in Children with Lymphohematopoietic Malignancy in Iran, Mashhad. Jundishapur J. Microbiol. 2013, 6, e7765. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudvand, H.; Sepahvand, A.; Badparva, E.; Khatami, M.; Niazi, M.; Moayyedkazemi, A. Possible Association and Risk Factors of Blastocystis Infection and Colorectal Cancers in Western Iran. Arch. Clin. Infect. Dis. 2021, 16, e90861. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, G.; Zhao, W.; Yang, Z.; Shen, Y.; Sun, Y.; Liu, A.; Cao, J. Genotyping of Enterocytozoon Bieneusi and Subtyping of Blastocystis in Cancer Patients: Relationship to Diarrhea and Assessment of Zoonotic Transmission. Front. Microbiol. 2017, 8, 1835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandramathi, S.; Suresh, K.; Anita, Z.B.; Kuppusamy, U.R. Infections of Blastocystis hominis and Microsporidia in Cancer Patients: Are They Opportunistic? Trans. R. Soc. Trop. Med. Hyg. 2012, 106, 267–269. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Ahmed, M.A.; Ahmed, S.A.; Al-Semany, S.A.; Alghamdi, S.S.; Zaglool, D.A. Predominance and Association Risk of Blastocystis hominis Subtype i in Colorectal Cancer: A Case Control Study. Infect. Agent. Cancer 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Yersal, O.; Malatyali, E.; Ertabaklar, H.; Oktay, E.; Barutca, S.; Ertug, S. Blastocystis Subtypes in Cancer Patients: Analysis of Possible Risk Factors and Clinical Characteristics. Parasitol. Int. 2016, 65, 792–796. [Google Scholar] [CrossRef]
- Mülayim, S.; Aykur, M.; Dağcı, H.; Dalkılıç, S.; Aksoy, A.; Kaplan, M. Investigation of Isolated Blastocystis Subtypes from Cancer Patients in Turkey. Acta Parasitol. 2021, 66, 584–592. [Google Scholar] [CrossRef]
- Teng, X.J.; Chu, Y.H.; Zhai, C.C.; Yu, Y.F.; Cai, Y.C.; Chen, S.H.; Ai, L.; Tian, L.G.; Chen, J.X. The Epidemiological Characteristics and Infuencing Factors for Blastocystis hominis Infection among Human Immunodefciency Virus Seropositive Individuals in Tengchong of Yunnan Province. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2018, 36, 129–134. [Google Scholar]
- Tian, L.-G.; Wang, T.-P.; Chen, J.-X.; Cai, Y.-C.; Yin, X.-M.; Cheng, G.-J.; Wu, W.-D.; Steinmann, P.; Guo, J.; Tong, X.-M.; et al. Co-Infection of HIV and Parasites in China: Results from an Epidemiological Survey in Rural Areas of Fuyang City, Anhui Province, China. Front. Med. China 2010, 4, 192–198. [Google Scholar] [CrossRef]
- Tian, L.-G.; Chen, J.-X.; Wang, T.-P.; Cheng, G.-J.; Steinmann, P.; Wang, F.-F.; Cai, Y.-C.; Yin, X.-M.; Guo, J.; Zhou, L.; et al. Co-Infection of HIV and Intestinal Parasites in Rural Area of China. Parasites Vectors 2012, 5, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.-G.; Wang, T.-P.; Lv, S.; Wang, F.-F.; Guo, J.; Yin, X.-M.; Cai, Y.-C.; Dickey, M.K.; Steinmann, P.; Chen, J.-X. HIV and Intestinal Parasite Co-Infections among a Chinese Population: An Immunological Profile. Infect. Dis. Poverty 2013, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-X.; Yu, Y.-F.; Wu, X.-P.; Chu, Y.-H.; Teng, X.-J.; Wang, F.-F.; Chen, J.-X.; Tian, L.-G. Epidemiological Characteristics and Risk Factors of Blastocystis hominis Infection among Patients with Hiv/Aids in Fuyang City, Anhui Province. Chin. J. Schistosomiasis Control 2019, 31, 498–503. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Kang, F.-Y.; Chen, J.-X.; Tian, L.-G.; Geng, L.-L. Risk Factors for Blastocystis Infection in HIV/AIDS Patients with Highly Active Antiretroviral Therapy in Southwest China. Infect. Dis. Poverty 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu-Hua, H.; Hui-Hui, C.; Ke, Q.; Chao-Qun, N.; Guo-Hua, P.; Ying-Fang, Y.; Xian-Feng, Z.; Yan-Hong, C.; Dan, X.; Jia-Xu, C.; et al. Prevalence and Risk Factors of Blastocystis hominis Infections among AIDS Patients in Nanchang City. Chin. J. Schistosomiasis Control 2020, 32, 557–583. [Google Scholar] [CrossRef]
- Ramana, K.V.; Prakash, K.; Mohanty, S.K. A Study of Opportunistic Parasitic Infections and CD4 Counts in HIV-Seropositive Individuals in Narketpally, South India. Ann. Trop. Med. Public Health 2010, 3, 49–52. [Google Scholar] [CrossRef]
- Khalil, S.; Mirdha, B.R.; Sinha, S.; Panda, A.; Singh, Y.; Joseph, A.; Deb, M. Intestinal Parasitosis in Relation to Anti-Retroviral Therapy, CD4+ T-Cell Count and Diarrhea in HIV Patients. Korean J. Parasitol. 2015, 53, 705–712. [Google Scholar] [CrossRef]
- Berenji, F.; Sarvghad, M.R.; Fata, A.; Hosseininejad, Z.; Saremi, E.; Ganjbakhsh, M.; Jahanparvar, R.I. A Study of the Prevalence of Intestinal Parasitic Infection in HIV Positive Individuals in Mashhad, Northeast Iran. Jundishapur J. Microbiol. 2010, 3, 61–65. [Google Scholar]
- Yosefi, F.; Rahdar, M.; Alavi, S.M.; Samany, A. A Study on Prevalence of Gastrointestinal Parasitic Infections in HIV (+) Patients Referred to Ahvaz Razi Hospital in 2008–2009. Jundishapur J. Microbiol. 2012, 5, 424–426. [Google Scholar] [CrossRef] [Green Version]
- Agholi, M.; Hatam, G.R.; Motazedian, M.H. HIV/AIDS-Associated Opportunistic Protozoal Diarrhea. Aids Res. Hum. Retrovir. 2013, 29, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masoumi-Asl, H.; Khanaliha, K.; Bokharaei-Salim, F.; Esteghamati, A.; Kalantari, S.; Hosseinyrad, M. Enteric Opportunistic Infection and the Impact of Antiretroviral Therapy among HIV/AIDS Patients from Tehran, Iran. Iran. J. Public Health 2019, 48, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Anvari-Tafti, M.H.; Eslami, G.; Teimourzadeh-Baboli, A.; Ghafourzadeh, M. Food-Borne Protozoan Infection in HIV+/AIDS Patients and Healthy Individuals: A Case-Control Study in Iran. J. Food Qual. Hazards Control 2016, 3, 93–96. [Google Scholar]
- Piranshahi, A.R.; Tavalla, M.; Khademvatan, S. Genomic Analysis of Blastocystis hominis Isolates in Patients with HIV-Positive Using Locus SSU-RDNA. J. Parasit. Dis. 2018, 42, 28–33. [Google Scholar] [CrossRef]
- Paboriboune, P.; Phoumindr, N.; Borel, E.; Sourinphoumy, K.; Phaxayaseng, S.; Luangkhot, E.; Sengphilom, B.; Vansilalom, Y.; Odermatt, P.; Delaporte, E.; et al. Intestinal Parasitic Infections in HIV-Infected Patients, Lao People’s Democratic Republic. PLoS ONE 2014, 9, e91452. [Google Scholar] [CrossRef] [Green Version]
- Sherchan, J.B.; Ohara, H.; Sakurada, S.; Basnet, A.; Tandukar, S.; Sherchand, J.B.; Bam, D.S. Enteric Opportunistic Parasitic Infections among HIV-Seropositive Patients in Kathmandu, Nepal. Kathmandu Univ. Med. J. 2012, 10, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghimire, A.; Bhandari, S.; Tandukar, S.; Amatya, J.; Bhandari, D.; Sherchand, J.B. Enteric Parasitic Infection among HIV-Infected Patients Visiting Tribhuvan University Teaching Hospital, Nepal. BMC Res. Notes 2016, 9, 204. [Google Scholar] [CrossRef] [Green Version]
- Zorbozan, O.; Quliyeva, G.; Tunalı, V.; Özbilgin, A.; Turgay, N.; Gökengin, A.D. Intestinal Protozoa in Hiv-Infected Patients: A Retrospective Analysis. Turk. Parazitolojii Derg. 2018, 42, 187–190. [Google Scholar] [CrossRef]
- Davis, N.A.; Islamova, Z.I.; Giiasov, K.Z.; Badalova, N.S.; Takhtokhodzhaeva, G.R.; Latipov, R.R.; Osipova, S.O. Blastocystis hominis and Nonpathogenic Enteric Protozoa in Patients with Pulmonary Tuberculosis and Those with HIV Infection. Med. Parazitol. 2010, 3, 8–11. [Google Scholar]
- Taghipour, A.; Javanmard, E.; Mirjalali, H.; Haghighi, A.; Tabarsi, P.; Sohrabi, M.R.; Zali, M.R. Blastocystis Subtype 1 (Allele 4); Predominant Subtype among Tuberculosis Patients in Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, A.; Tabarsi, P.; Sohrabi, M.R.; Riahi, S.M.; Rostami, A.; Mirjalali, H.; Malih, N.; Haghighi, A. Frequency, Associated Factors and Clinical Symptoms of Intestinal Parasites among Tuberculosis and Non-Tuberculosis Groups in Iran: A Comparative Cross-Sectional Study. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 234–241. [Google Scholar] [CrossRef]
- Li, X.-X.; Chen, J.-X.; Wang, L.-X.; Tian, L.-G.; Zhang, Y.-P.; Dong, S.-P.; Hu, X.-G.; Liu, J.; Wang, F.-F.; Wang, Y.; et al. Intestinal Parasite Co-Infection among Pulmonary Tuberculosis Cases without Human Immunodeficiency Virus Infection in a Rural County in China. Am. J. Trop. Med. Hyg. 2014, 90, 106–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.-X.; Chen, J.-X.; Wang, L.-X.; Tian, L.-G.; Zhang, Y.-P.; Dong, S.-P.; Hu, X.-G.; Liu, J.; Wang, F.-F.; Wang, Y.; et al. Prevalence and Risk Factors of Intestinal Protozoan and Helminth Infections among Pulmonary Tuberculosis Patients without HIV Infection in a Rural County in P. R. China. Acta Trop. 2015, 149, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, A.; Azimi, T.; Javanmard, E.; Pormohammad, A.; Olfatifar, M.; Rostami, A.; Tabarsi, P.; Sohrabi, M.R.; Mirjalali, H.; Haghighi, A. Immunocompromised Patients with Pulmonary Tuberculosis; a Susceptible Group to Intestinal Parasites. Gastroenterol. Hepatol. Bed Bench 2018, 11, S134–S139. [Google Scholar] [PubMed]
- Azami, M.; Sharifi, M.; Hejazi, S.H.; Tazhibi, M. Intestinal Parasitic Infections in Renal Transplant Recipients. Braz. J. Infect. Dis. 2010, 14, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Idris, N.S.; Dwipoerwantoro, P.G.; Kurniawan, A.; Said, M. Intestinal Parasitic Infection of Immunocompromised Children with Diarrhea: Clinical Profile and Therapeutic Response. J. Infect. Dev. Ctries. 2010, 4, 309–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caner, A.; Zorbozan, O.; Tunalı, V.; Kantar, M.; Aydoğdu, S.; Aksoylar, S.; Gürüz, Y.; Turgay, N. Intestinal Protozoan Parasitic Infections in Immunocompromised Child Patients with Diarrhea. Jpn. J. Infect. Dis. 2020, 73, 187–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasti, S.; Hassanzadeh, M.; Hooshyar, H.; Momen-Heravi, M.; Mousavi, S.G.A.; Abdoli, A. Intestinal Parasitic Infections in Different Groups of Immunocompromised Patients in Kashan and Qom Cities, Central Iran. Scand. J. Gastroenterol. 2017, 52, 738–741. [Google Scholar] [CrossRef]
- Izadi, S.; Ghayour-Najafabadi, Z.; Yavari, M.; Mohaghegh, M.-A.; Wannigama, D.L.; Moslemzadeh, H.-R.; Azami, M.; Hejazi, S.-H. Intestinal Parasites Associated with Opportunistic Coccidial Infections among Immunocompromised Individuals in Central Iran: A Cross Sectional Study. Arch. Clin. Infect. Dis. 2019, 14, e79701. [Google Scholar] [CrossRef] [Green Version]
- Esteghamati, A.; Khanaliha, K.; Bokharaei-Salim, F.; Sayyahfar, S.; Ghaderipour, M. Prevalence of Intestinal Parasitic Infection in Cancer, Organ Transplant and Primary Immunodeficiency Patients in Tehran, Iran. Asian Pac. J. Cancer Prev. 2019, 20, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, L.; Ashrafi, K.; Atrkar Roushan, Z.; Mahmoudi, M.R.; Shenavar Masooleh, I.; Rahmati, B.; Saadat, F.; Mirjalali, H.; Sharifdini, M. Strongyloides Stercoralis and Other Intestinal Parasites in Patients Receiving Immunosuppressive Drugs in Northern Iran: A Closer Look at Risk Factors. Epidemiol. Health 2021, 43, e2021009. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.I. Intestinal Parasites Infection among Immunocompromised Patients in Riyadh, Saudi Arabia. Pak. J. Biol. Sci. 2010, 13, 390–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uysal, S.; Tunalı, V.; Akdur Öztürk, E.; Ardeniz, Ö.; Işıkgöz Taşbakan, M.; Pullukçu, H.; Özensoy Töz, S.; Turgay, N.; Arda, B. Incidence of Parasitic Diarrhea in Patients with Common Variable Immune Deficiency. Turk. Parazitolojii Derg. 2016, 40, 67–71. [Google Scholar] [CrossRef]
- Maçin, S.; Kaya, F.; Çağdaş, D.; Hizarcioglu-Gulsen, H.; Saltik-Temizel, I.N.; Tezcan, İ.; Demir, H.; Ergüven, S.; Akyön, Y. Detection of Parasites in Children with Chronic Diarrhea. Pediatr. Int. 2016, 58, 531–533. [Google Scholar] [CrossRef]
- Asadi, L.; Pourlak, T.; Ahmadi, B.; Aghamali, M.; Asgharzadeh, M.; Aghazadeh, M.; Zeinalzadeh, E.; Kafil, H.S. Etiological Agents of Pediatric Diarrhea in Ardebil, Northwestern Iran. Arch. Pediatr. Infect. Dis. 2018, 6, e11771. [Google Scholar] [CrossRef] [Green Version]
- Boughattas, S.; Behnke, J.M.; Al-Ansari, K.; Sharma, A.; Abu-Alainin, W.; Al-Thani, A.; Abu-Madi, M.A. Molecular Analysis of the Enteric Protozoa Associated with Acute Diarrhea in Hospitalized Children. Front. Cell. Infect. Microbiol. 2017, 7, 343. [Google Scholar] [CrossRef]
- Dahal, M.; Dahal, R.H.; Chaudhary, D.K. Prevalence of Cyclospora Cayetanensis and Other Enteropathogen among Children under the Age of 15 Years in Biratnagar, Nepal. Asian Pac. J. Trop. Dis. 2017, 7, 75–79. [Google Scholar] [CrossRef]
- Khalili, B.; Khani, M.R.; Taghipour, S. Blastocystis hominis Infection among Hospitalized Children Due to Diarrhea in Hajar Hospital, Shahre-Kord, Iran. Arch. Clin. Infect. Dis. 2012, 7, 52–55. [Google Scholar] [CrossRef] [Green Version]
- Kiani, H.; Haghighi, A.; Salehi, R.; Azargashb, E. Distribution and Risk Factors Associated with Intestinal Parasite Infections among Children with Gastrointestinal Disorders. Gastroenterol. Hepatol. Bed Bench 2016, 9, S80–S87. [Google Scholar]
- Awae, N.; Pipatsatitpong, D.; Mungthin, M.; Ruang-Areerate, T.; Leelayoova, S.; Aunpad, R.; Suwanvattana, P.; Thawornwan, U. Prevalence of and Risk Factors Associated with Parasitic, Bacterial and Viral Infections among Children with Gastrointestinal Illness in Bamrasnaradura Infectious Diseases Institute. Sci. Technol. Asia 2018, 23, 44–51. [Google Scholar] [CrossRef]
- Sigidaev, A.S.; Kozlov, S.S.; Tarasova, E.A.; Suvorova, M.A. Investigation of the Genetic Profile of Blastocystis Species in Saint Petersburg Residents with Gastrointestinal Tract Diseases in Different Age Groups. Med. Parazitol. 2013, 4, 19–23. [Google Scholar]
- Oyofo, B.A.; Subekti, D.; Tjaniadi, P.; Machpud, N.; Komalarini, S.; Setiawan, B.; Simanjuntak, C.; Punjabi, N.; Corwin, A.L.; Wasfy, M.; et al. Enteropathogens Associated with Acute Diarrhea in Community and Hospital Patients in Jakarta, Indonesia. Fems Immunol. Med. Microbiol. 2002, 34, 139–146. [Google Scholar] [CrossRef]
- Zhang, S.-X.; Yang, C.-L.; Gu, W.-P.; Ai, L.; Serrano, E.; Yang, P.; Zhou, X.; Li, S.-Z.; Lv, S.; Dang, Z.-S.; et al. Case-Control Study of Diarrheal Disease Etiology in Individuals over 5 Years in Southwest China. Gut Pathog. 2016, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Won, E.J.; Kim, S.H.; Kee, S.J.; Shin, J.H.; Suh, S.P.; Chai, J.Y.; Ryang, D.W.; Shin, M.G. Multiplex Real-Time PCR Assay Targeting Eight Parasites Customized to the Korean Population: Potential Use for Detection in Diarrheal Stool Samples from Gastroenteritis Patients. PLoS ONE 2016, 11, 0166957. [Google Scholar] [CrossRef] [PubMed]
- Jalallou, N.; Iravani, S.; Rezaeian, M.; Alinaghizade, A.; Mirjalali, H. Subtypes Distribution and Frequency of Blastocystis sp. Isolated from Diarrheic and Non-Diarrheic Patients. Iran. J. Parasitol. 2017, 12, 63–68. [Google Scholar] [PubMed]
- Najafi, A.; Mirzaei, A.; kermanjani, A.; Abdi, J.; Ghaderi, A.; Naserifar, R. Molecular Identification of Entamoeba Histolytica from Stool Samples of Ilam, Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 63, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Haghighi, A.; Rostami, A.; Azargashb, E.; Seyyed Tabaei, S.J.; Solgi, A.; Zebardast, N. Prevalence, Risk Factors and Symptoms Associated to Intestinal Parasite Infections among Patients with Gastrointestinal Disorders in Nahavand, Western Iran. Rev. Inst. Med. Trop. Sao Paulo 2016, 58, 42. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, A.; Haghighi, A.; Mojarad, E.N.; Zayeri, F.; Alebouyeh, M.; Khazan, H.; Kazemi, B.; Zali, M.R. Genetic Variability of Blastocystis sp. Isolated from Symptomatic and Asymptomatic Individuals in Iran. Parasitol. Res. 2012, 111, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, A.; Aboualsoltani, N.; Bazmani, A.; Khanmohammadi, M.; Aboualsoltani, F.; Fallah, E. PCR-Based Subtyping of Blastocystis Isolates from Symptomatic and Asymptomatic Patients in North-West of Iran. J. Pure Appl. Microbiol. 2013, 7, 2957–2963. [Google Scholar]
- Mirjalali, H.; Abbasi, M.R.; Naderi, N.; Hasani, Z.; Mirsamadi, E.S.; Stensvold, C.R.; Balaii, H.; Asadzadeh Aghdaei, H.; Zali, M.R. Distribution and Phylogenetic Analysis of Blastocystis sp. Subtypes Isolated from IBD Patients and Healthy Individuals in Iran. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2335–2342. [Google Scholar] [CrossRef]
- Kesuma, Y.; Firmansyah, A.; Bardosono, S.; Sari, I.P.; Kurniawan, A. Blastocystis ST-1 Is Associated with Irritable Bowel Syndrome-Diarrhea (IBS-D) in Indonesian Adolescences. Parasite Epidemiol. Control 2019, 6, e00112. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Khalil, S.; Mirdha, B.R.; Makharia, G.K.; Dattagupta, S.; Chaudhry, R. Molecular Characterization and Subtyping of Blastocystis species in Irritable Bowel Syndrome Patients from North India. PLoS ONE 2016, 11, 0147055. [Google Scholar] [CrossRef]
- Shafiei, Z.; Esfandiari, F.; Sarkari, B.; Rezaei, Z.; Fatahi, M.R.; Hosseini Asl, S.M.K. Parasitic Infections in Irritable Bowel Syndrome Patients: Evidence to Propose a Possible Link, Based on a Case-Control Study in the South of Iran. BMC Res. Notes 2020, 13, 264. [Google Scholar] [CrossRef]
- Khademvatan, S.; Masjedizadeh, R.; Rahim, F.; Mahbodfar, H.; Salehi, R.; Yousefi-Razin, E.; Foroutan, M. Blastocystis and Irritable Bowel Syndrome: Frequency and Subtypes from Iranian Patients. Parasitol. Int. 2017, 66, 142–145. [Google Scholar] [CrossRef]
- Sayal, R.A.; Hameed, S.; Faisal, M.M. Evaluation of IL-5 Concentration Level in Irritable Bowel Syndrome Patients That Suffering from Blastocystis Infection in Al-Najaf Province. Eur. J. Mol. Clin. Med. 2020, 7, 3807–3817. [Google Scholar]
- Surangsrirat, S.; Thamrongwittawatpong, L.; Piyaniran, W.; Naaglor, T.; Khoprasert, C.; Taamasri, P.; Mungthin, M.; Leelayoova, S. Assessment of the Association between Blastocystis Infection and Irritable Bowel Syndrome. J. Med. Assoc. Thai. 2010, 93 (Suppl. 6), S119–S124. [Google Scholar]
- Merza, A.S.; Mohammed Al-Saeed, A.T.; Najeeb, M.K. Comparison of The Efficiency of Different Techniques for The Detection Of Blastocystis hominis In Patients Attending Hospitals Of Duhok City, Kurdistan Region, Iraq. Biochem. Cell. Arch. 2020, 20, 4421–4425. [Google Scholar]
- Mutlag, S.K.; Ahmed, N.A.; Abbas, S.K. Investigation the Role of Blastocystis hominis Effect on The Levels of Il-10, Il-18 And Hematological Parameters. Biochem. Cell. Arch. 2019, 19, 3887–3892. [Google Scholar] [CrossRef]
- Sanpool, O.; Laoraksawong, P.; Janwan, P.; Intapan, P.M.; Sawanyawisuth, K.; Thanchomnang, T.; Changtrakul, Y.; Maleewong, W. Genetic Subtypes of Blastocystis Isolated from Thai Hospitalized Patients in Northeastern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 184–190. [Google Scholar] [PubMed]
- Koltas, I.S.; Elgun, G.; Eroglu, F.; Demirkazık, M. The Importance of Real-Time Polymerase Chain Reaction Method in Diagnosis of Intestinal Parasites in Cases with Diarrhea. Trop. Biomed. 2017, 34, 895–902. [Google Scholar]
- Aykur, M.; Calıskan Kurt, C.; Dirim Erdogan, D.; Biray Avcı, C.; Vardar, R.; Aydemir, S.; Girginkardeşler, N.; Gündüz, C.; Dagci, H. Investigation of Dientamoeba fragilis Prevalence and Evaluation of Sociodemographic and Clinical Features in Patients with Gastrointestinal Symptoms. Acta Parasitol. 2019, 64, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Usluca, S.; Inceboz, T.; Over, L.; Tuncay, S.; Yalçin, G.; Arcak, S.S.; Ozkoç, S.; Aksoy, U.; Akisü, C. [The Distribution of Intestinal Parasites Detected in The Dokuz Eylul University Medical Faculty Hospital between 2005 and 2008.]. Dokuz Eylül Universitesi Tip Fakültesi Araştirma ve Uygulama Hastanesi’nde 2005–2008 Yillari Arasinda Saptanan Baǧirsak Pa. Turk. Parazitolojii Derg. 2010, 34, 27–31. [Google Scholar]
- Cekin, A.H.; Cekin, Y.; Adakan, Y.; Tasdemir, E.; Koclar, F.G.; Yolcular, B.O. Blastocystosis in Patients with Gastrointestinal Symptoms: A Case–Control Study. BMC Gastroenterol. 2012, 12, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beiromvand, M.; Hashemi, S.J.; Arjmand, R.; Sadjadei, N.; Hardanipasand, L. Comparative Prevalence of Blastocystis in Patients with the Irritable Bowel Syndrome and Healthy Individuals: A Case Control Study. Jundishapur J. Microbiol. 2017, 10, e13572. [Google Scholar] [CrossRef] [Green Version]
- Feurle, G.E.; Moos, V.; Landt, O.; Corcoran, C.; Reischl, U.; Maiwald, M. Tropheryma whipplei in Feces of Patients with Diarrhea in 3 Locations on Different Continents. Emerg. Infect. Dis. 2021, 27, 932–935. [Google Scholar] [CrossRef]
- Hawash, Y.A.; Ismail, K.A.; Saber, T.; Eed, E.M.; Khalifa, A.S.; Alsharif, K.F.; Alghamdi, S.A. Dientamoeba fragilis Infection in Patients with Digestive and Non-Digestive Symptoms: A Case-Control Study. Korean J. Parasitol. 2020, 58, 129–134. [Google Scholar] [CrossRef]
- Alver, O.; Oral, B.; Töre, O. The Distribution of Intestinal Parasites Detected in the Uludag University Medical School Hospital between 2005 and 2008 Uludaǧ Üniversitesi Tip Fakültesi Hastanesine 2005-2008 Yillari Arasinda Başvuran Kişilerde Saptanan Baǧirsak Parazitlerinin Daǧi. Turk. Parazitolojii Derg. 2011, 35, 194–198. [Google Scholar] [CrossRef]
- Inceboz, T.; Usluca, S.; Over, L.; Yalçin, G.; Tuncay, S.; Ozkoç, S. The Epidemiology Research of Blastocystis hominis in the Dokuz Eylül University Medical Faculty Hospital between 2005 and 2009 Dokuz Eylül Üniversitesi Hastanesi’ne 2005-2009 Yillari Arasinda Başvuran Olgularda Blastocystis hominis Epidemiyolojisinin. Turk. Parazitolojii Derg. 2011, 35, 72–76. [Google Scholar] [CrossRef]
- Rostami Nejad, M.; Nazemalhosseini Mojarad, E.; Dabiri, H.; Nochi, Z.; Pourhoseingholi, M.A.; Sahebekhtiari, N.; Habibi, M.; Zali, M.R. A Case-Control Study of Blastocystis hominis among Iranian Population. East. Afr. J. Public Health 2010, 7, 101–104. [Google Scholar]
- Haider, S.S.; Baqai, R.; Qureshi, F.M.; Boorom, K. Blastocystis spp., Cryptosporidium spp., and Entamoeba histolytica Exhibit Similar Symptomatic and Epidemiological Patterns in Healthcare-Seeking Patients in Karachi. Parasitol. Res. 2012, 111, 1357–1368. [Google Scholar] [CrossRef]
- Sakalar, Ç.; Kandemir, I.; Uyar, Y.; Kuk, S.; Gürbüz, E.; Yazar, S. Polymerase Chain Reaction Based Subtyping of Blastocystis spp. Isolates from Symptomatic Patients in Turkey|Türkiye’deki Semptomatik Hastalardan Elde Edilen Blastocystis spp. İzolatlarinin Polimeraz Zincir Reaksiyonu Ile Alt Tiplendirilmesi. Turk. Klin. J. Med. Sci. 2013, 33, 1064–1068. [Google Scholar] [CrossRef] [Green Version]
- Sharif, M.; Daryani, A.; Asgarian, F.; Nasrolahei, M. Intestinal Parasitic Infections among Intellectual Disability Children in Rehabilitation Centers of Northern Iran. Res. Dev. Disabil. 2010, 31, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Hazrati Tappeh, K.H.; Mohammadzadeh, H.; Nejad Rahim, R.; Barazesh, A.; Khashaveh, S.H.; Taherkhani, H. Prevalence of Intestinal Parasitic Infections among Mentally Disabled Children and Adults of Urmia, Iran. Iran. J. Parasitol. 2010, 5, 60–64. [Google Scholar]
- Khalili, B.; Imani, R.; Boostani, S. Intestinal Parasitic Infections in Chronic Psychiatric Patients in Sina Hospital Shahre-Kord, Iran. Jundishapur J. Microbiol. 2013, 6, 252–255. [Google Scholar] [CrossRef]
- Saeidinia, A.; Tavakoli, I.; Naghipour, M.R.; Rahmati, B.; Ghavami Lahiji, H.; Salkhori, O.; Ashrafi, K. Prevalence of Strongyloides stercoralis and Other Intestinal Parasites among Institutionalized Mentally Disabled Individuals in Rasht, Northern Iran. Iran. J. Parasitol. 2016, 11, 527–533. [Google Scholar]
- Shokri, A.; Sarasiabi, K.S.; Teshnizi, S.H.; Mahmoodi, H. Prevalence of Strongyloides stercoralis and Other Intestinal Parasitic Infections among Mentally Retarded Residents in Central Institution of Southern Iran. Asian Pac. J. Trop. Biomed. 2012, 2, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Rasti, S.; Arbabi, M.; Hooshyar, H. High Prevalence of Entamoeba histolytica and Enterobius vermicularis among Elderly and Mentally Retarded Residence in Golabchi Center, Kashan, Iran 2006–2007. Jundishapur J. Microbiol. 2012, 5, 585–589. [Google Scholar] [CrossRef]
- Mohammadi-Meskin, V.; Hamedi, Y.; Heydarihengami, M.; Eftekhar, E.; Shamseddin, J.; Sharifisarasiabi, K. Intestinal Parasitic Infections in Mental Retardation Center of Bandar Abbas, Southern Iran. Iran. J. Parasitol. 2019, 14, 318–325. [Google Scholar] [CrossRef]
- Sheikh, S.; Asghari, A.; Sadraei, J.; Pirestani, M.; Zare, M. Blastocystis sp. Subtype 9: As the First Reported Subtype in Patients with Schizophrenia in Iran. SN Compr. Clin. Med. 2020, 2, 633–639. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, Z.; Li, Q.; Lin, Y.; Yang, H.; Li, W. Prevalence and Subtype Diversity of Blastocystis in Human and Nonhuman Primates in North China. Parasitol. Res. 2020, 119, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-M.; Li, Y.-T.; Chen, R.; Yu, Y.-F.; Li, X.-T.; Wu, X.-P.; Chu, Y.-H.; Chen, J.-X.; Zhang, S.-X.; Tian, L.-G. Prevalence and Risk Factors of Blastocystis hominis Infection in Inpatients in Jiangjin District, Chongqing City. Chin. J. Schistosomiasis Control 2019, 31, 479–485. [Google Scholar] [CrossRef]
- Abdipour, Y.; Khazan, H.; Azargashb, E.; Mahmoudi, M.R.; Farahnak, A.; Rostami, A. Prevalence of Intestinal Parasitic Infections among Individuals Referred to the Medical Centers of Coastal Cities, Guilan Province, Northern Iran, 2015–2017. Iran. J. Public Health 2020, 49, 1157–1163. [Google Scholar]
- Bahrami, F.; Haghighi, A.; Zamini, G.; Khademerfan, M. Molecular Evidence for Zoonotic Transmission of Blastocystis Subtypes in Kurdistan Province, West of Iran. Ann. Parasitol. 2020, 66, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Gholipoor, Z.; Khazan, H.; Azargashb, E.; Youssefi, M.R.; Rostami, A. Prevalence and Risk Factors of Intestinal Parasite Infections in Mazandaran Province, North of Iran. Clin. Epidemiol. Glob. Health 2020, 8, 17–20. [Google Scholar] [CrossRef] [Green Version]
- Viesy, S.; Abdi, J.; Rezaei, Z. What Are Hidden Facts behind Intestinal Parasitic Infections in Ilam City? Infect. Disord. Drug Targets 2019, 19, 278–281. [Google Scholar] [CrossRef]
- Badparva, E.; Sadraee, J.; Kheirandish, F.; Frouzandeh, M. Genetic Diversity of Human Blastocystis Isolates in Khorramabad, Central Iran. Iran. J. Parasitol. 2014, 9, 44–49. [Google Scholar] [PubMed]
- Shaker, D.; Anvari, D.; Hosseini, S.A.; Fakhar, M.; Mardani, A.; Ziaei Hezarjaribi, H.; Gholami, S.; Gholami, S. Frequency and Genetic Diversity of Blastocystis Subtypes among Patients Attending to Health Centers in Mazandaran, Northern Iran. J. Parasit. Dis. 2019, 43, 537–543. [Google Scholar] [CrossRef]
- Haghighi, L.; Talebnia, S.E.; Mikaeili, F.; Asgari, Q.; Gholizadeh, F.; Zomorodian, K. Prevalence and Subtype Identification of Blastocystis Isolated from Human in Shiraz City, Southern Iran. Clin. Epidemiol. Glob. Health 2020, 8, 840–844. [Google Scholar] [CrossRef] [Green Version]
- Shaker, D.; Fakhar, M.; Ziaei, H.; Hosseini, S.A.; Gholami, S. Prevalence of Blastocystis hominis in Individuals Attending Sari Health Centers, 2014. J. Maz. Univ. Med. Sci. 2017, 27, 143–147. [Google Scholar]
- Tork, M.; Yazdani-Charati, J.; Sharif, M.; Dryani, A.; Nazar, I.; Pagheh, A.S.; Hosseini, S.-A. Association between Geographical Risk Factors and Intestinal Parasites in West of Mazandaran Province Using Geographic Information System. J. Maz. Univ. Med. Sci. 2017, 27, 124–132. [Google Scholar]
- Asfaram, S.; Daryani, A.; Sarvi, S.; Pagheh, A.S.; Hosseini, S.A.; Saberi, R.; Hoseiny, S.M.; Soosaraei, M.; Sharif, M. Geospatial Analysis and Epidemiological Aspects of Human Infections with Blastocystis hominis in Mazandaran Province, Northern Iran. Epidemiol. Health 2019, 41, e2019009. [Google Scholar] [CrossRef]
- Bafghi, A.F.; Hosseini, R.; Mollaei, H.R.; Barzegar, K. Geno-Typing and Comparison of Conventional and Molecular Diagnostic Techniques for Detection of Blastocystis on Health Centers in Kerman Iran. Epidemiol. Health 2021, 8, 10–16. [Google Scholar]
- Karimazar, M.; Rezaeian, S.; Ebrahimipour, M.; Ranjbarpour, A.; Madanipour, H.; Javan, S.; Najjari, M. Prevalence and Time-Trend Analysis of Intestinal Parasitic Infections in North-Central Iran, 2012–2016. Trop. Biomed. 2019, 36, 103–113. [Google Scholar]
- Abdul Ridha, A.A.F.; Faieq, Z.A. Epidemiology and Clinical Characteristics Associated with Blastocystis hominis in More Than Ten Years Infections in Wasit Province/Iraq. J. Phys. Conf. Ser. 2021, 1818, 012029. [Google Scholar] [CrossRef]
- Salehi, R.; Haghighi, A.; Stensvold, C.R.; Kheirandish, F.; Azargashb, E.; Raeghi, S.; Kohansal, C.; Bahrami, F. Prevalence and Subtype Identification of Blastocystis Isolated from Humans in Ahvaz, Southwestern Iran. Gastroenterol. Hepatol. Bed Bench 2017, 10, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Khademvatan, S.; Masjedizadeh, R.; Yousefi-Razin, E.; Mahbodfar, H.; Rahim, F.; Yousefi, E.; Foroutan, M. PCR-Based Molecular Characterization of Blastocystis hominis Subtypes in Southwest of Iran. J. Infect. Public Health 2018, 11, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sardarian, K.; Hajilooi, M.; Maghsood, A.; Moghimbeigi, A.; Alikhani, M. A Study of the Genetic Variability of Blastocystis hominis Isolates in Hamadan, West of Iran. Jundishapur J. Microbiol. 2012, 5, 555–559. [Google Scholar] [CrossRef] [Green Version]
- Hamidi, N.; Meamar, A.R.; Akhlaghi, L.; Rampisheh, Z.; Razmjou, E. Dientamoeba fragilis Diagnosis by Fecal Screening: Relative Effectiveness of Traditional Techniques and Molecular Methods. J. Infect. Dev. Ctries. 2018, 12, 52–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javadi, R.A.; Kazemi, F.; Fallahizadeh, S.; Arjmand, R. The Prevalence of Intestinal Parasitic Infections in Ahvaz, Southwest of Iran, during 2007–2017. Iran. J. Public Health 2019, 48, 2070–2073. [Google Scholar]
- Delshad, A.; Saraei, M.; Alizadeh, S.A.; Niaraki, S.R.; Alipour, M.; Hosseinbigi, B.; Bozorgomid, A.; Hajialilo, E. Distribution and Molecular Analysis of Blastocystis Subtypes from Gastrointestinal Symptomatic and Asymptomatic Patients in Iran. Afr. Health Sci. 2020, 20, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, P.; Mohaghegh, M.-A.; Ghorbani, M.; Mirzaii, M.; Abolhassani, M.; Mirbadie, S.-R. Investigating the Prevalence of Intestinal Parasites with an Emphasis on Strongyloides stercoralis Infection in Hospitalized Patients: A Regional Report from Iran. Ann. Parasitol. 2020, 66, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, Y.; Abbasi, F.; Shahabi, S.; Zaraei, A.; Mikaeili, F.; Sarkari, B. Comparative Genotyping of Blastocystis Infecting Cattle and Human in the South of Iran. Comp. Immunol. Microbiol. Infect. Dis. 2020, 72, 101529. [Google Scholar] [CrossRef] [PubMed]
- Salehi, M.; Mardaneh, J.; Niazkar, H.R.; Minooeianhaghighi, M.; Arshad, E.; Soleimani, F.; Mohammadzadeh, A. Prevalence and Subtype Analysis of Blastocystis hominis Isolated from Patients in the Northeast of Iran. J. Parasitol. Res. 2021, 2021, 8821885. [Google Scholar] [CrossRef] [PubMed]
- Greige, S.; El Safadi, D.; Khaled, S.; Gantois, N.; Baydoun, M.; Chemaly, M.; Benamrouz-Vanneste, S.; Chabé, M.; Osman, M.; Certad, G.; et al. First Report on the Prevalence and Subtype Distribution of Blastocystis sp. in Dairy Cattle in Lebanon and Assessment of Zoonotic Transmission. Acta Trop. 2019, 194, 23–29. [Google Scholar] [CrossRef]
- El Safadi, D.; Meloni, D.; Poirier, P.; Osman, M.; Cian, A.; Gaayeb, L.; Wawrzyniak, I.; Delbac, F.; El Alaoui, H.; Delhaes, L.; et al. Short Report: Molecular Epidemiology of Blastocystis in Lebanon and Correlation between Subtype 1 and Gastrointestinal Symptoms. Am. J. Trop. Med. Hyg. 2013, 88, 1203–1206. [Google Scholar] [CrossRef]
- Greige, S.; El Safadi, D.; Bécu, N.; Gantois, N.; Pereira, B.; Chabé, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and Subtype Distribution of Blastocystis sp. Isolates from Poultry in Lebanon and Evidence of Zoonotic Potential. Parasites Vectors 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Imam, A.; Altayyar, A.; Eltayeb, E.; Almushawa, Y. Frequency and Seasonality of Intestinal Parasitism in Qassim Region, Saudi Arabia. Pak. J. Med. Sci. 2012, 28, 10. [Google Scholar]
- Hassen Amer, O.; Ashankyty, I.M.; Haouas, N.A.S. Prevalence of Intestinal Parasite Infections among Patients in Local Public Hospitals of Hail, Northwestern Saudi Arabia. Asian Pac. J. Trop. Med. 2016, 9, 44–48. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, R.T.; El-Bali, M.A.; Mohamed, A.A.; Abdel-Fatah, M.A.; El-Malky, M.A.; Mowafy, N.M.; Zaghlool, D.A.; Bakri, R.A.; Al-Harthi, S.A. Subtyping of Blastocystis sp. Isolated from Symptomatic and Asymptomatic Individuals in Makkah, Saudi Arabia. Parasites Vectors 2017, 10, 174. [Google Scholar] [CrossRef] [Green Version]
- Laodim, P.; Intapan, P.M.; Sawanyawisuth, K.; Laummaunwai, P.; Maleewong, W. A Hospital-Based Study of Epidemiological and Clinical Data on Blastocystis hominis Infection. Foodborne Pathog. Dis. 2012, 9, 1077–1082. [Google Scholar] [CrossRef]
- Jantermtor, S.; Pinlaor, P.; Sawadpanich, K.; Pinlaor, S.; Sangka, A.; Wilailuckana, C.; Wongsena, W.; Yoshikawa, H. Subtype Identification of Blastocystis spp. Isolated from Patients in a Major Hospital in Northeastern Thailand. Parasitol. Res. 2013, 112, 1781–1786. [Google Scholar] [CrossRef]
- Polat, E.; Özdemir, S.; Sirekbasan, S. The Distribution of Intestinal Parasites in Patients Presenting to a University Hospital in Istanbul: A Seven-Year Retrospective Analysis. Turk. Parazitolojii Derg. 2020, 44, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, Y.E.; Yilmaz, H.; Cengiz, Z.T.; Ekici, A. Clinical Significance and Prevalence of Blastocystis hominis in Van, Turkey. Saudi Med. J. 2015, 36, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Sarzhanov, F.; Köster, P.C.; Dogruman-Al, F.; Bailo, B.; Dashti, A.; Demirel-Kaya, F.; Carmena, D. Detection of Enteric Parasites and Molecular Characterization of Giardia Duodenalis and Blastocystis sp. In Patients Admitted to Hospital in Ankara, Turkey. Parasitology 2021, 148, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Selek, M.B.; Bektöre, B.; Karagöz, E.; Baylan, O.; Özyurt, M. Distribution of Parasites Detected in Stool Samples of Patients Admitted to Our Parasitology Laboratory during a Three-Year Period between 2012 and 2014. Turk. Parazitolojii Derg. 2016, 40, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Yaman, O.; Hamamci, B.; Cetinkaya, U.; Kaya, M.; Ateş, S.; Gözkenç, N.; Ozcan, H.; Yazar, L.; Yazar, S. The Investigation of Intestinal Parasites in Foreign High School Students Yabanci Uyruklu Lise Öǧrencilerinde Intestinal Parazitlerin Araştirilmasi. Turk. Parazitolojii Derg. 2010, 34, 176–178. [Google Scholar]
- Zhan, T.-Z.; Liu, T.; Shi, H.-H.; He, S.-S.; Yan, H.; Liu, D.-Y. PCR-Based Genotype Classification of Blastocystis hominis Isolates from College Students of Guangxi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2014, 32, 209–211. [Google Scholar]
- Fallahi, S.; Rostami, A.; Mohammadi, M.; Ebrahimzadeh, F.; Pournia, Y. Practical Parasitology Courses and Infection with Intestinal Parasites in Students. J. Infect. Public Health 2016, 9, 654–660. [Google Scholar] [CrossRef]
- Srichaipon, N.; Nuchprayoon, S.; Charuchaibovorn, S.; Sukkapan, P.; Sanprasert, V. A Simple Genotyping Method for Rapid Differentiation of Blastocystis Subtypes and Subtype Distribution of Blastocystis spp. In Thailand. Pathogens 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Salemi, B.; Esteghamati, A.; Sayyahfar, S.; Bokharaei-Salim, F.; Keyvani, H.; Khanaliha, K. Frequency of Intestinal Parasitic Infection among Working Children in Tehran, Iran. Arch. Pediatr. Infect. Dis. 2019, 7, e93760. [Google Scholar] [CrossRef] [Green Version]
- Pintong, A.-R.; Sunyanusin, S.; Prasertbun, R.; Mahittikorn, A.; Mori, H.; Changbunjong, T.; Komalamisra, C.; Sukthana, Y.; Popruk, S. Blastocystis Subtype 5: Predominant Subtype on Pig Farms, Thailand. Parasitol. Int. 2018, 67, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Udonsom, R.; Prasertbun, R.; Mahittikorn, A.; Mori, H.; Changbunjong, T.; Komalamisra, C.; Pintong, A.-r.; Sukthana, Y.; Popruk, S. Blastocystis Infection and Subtype Distribution in Humans, Cattle, Goats, and Pigs in Central and Western Thailand. Infect. Genet. Evol. 2018, 65, 107–111. [Google Scholar] [CrossRef]
- Kheirandish, F.; Tarahi, M.J.; Ezatpour, B. Prevalence of Intestinal Parasites among Food Handlers in Western Iran | La Prevalencia de Parásitos Intestinales Entre Los Manipuladores de Alimentos En El Oeste de Iŕn. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Motazedian, M.H.; Najjari, M.; Ebrahimipour, M.; Asgari, Q.; Mojtabavi, S.; Mansouri, M. Prevalence of Intestinal Parasites among Food-Handlers in Shiraz, Iran. Iran. J. Parasitol. 2015, 10, 652–657. [Google Scholar]
- Sharif, M.; Daryani, A.; Kia, E.; Rezaei, F.; Nasiri, M.; Nasrolahei, M. Prevalence of Intestinal Parasites among Food Handlers of Sari, Northern Iran|Prevalência de Parasitas Intestinais Entre Manipuladores de Alimentos de Sari, Norte Do Iran. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Heydari-Hengami, M.; Hamedi, Y.; Najafi-Asl, M.; Sharifi-Sarasiabi, K. Prevalence of Intestinal Parasites in Food Handlers of Bandar Abbas, Southern Iran. Iran. J. Public Health 2018, 47, 111–118. [Google Scholar] [PubMed]
- Khodabakhsh Arbat, S.; Hooshyar, H.; Arbabi, M.; Eslami, M.; Abani, B.; Poor Movayed, R. Prevalence of Intestinal Parasites among Food Handlers in Kashan, Central Iran, 2017–2018. J. Parasit. Dis. 2018, 42, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Shahnazi, M.; Sadeghi, M.; Saraei, M.; Alipour, M.; Hajialilo, E. Prevalence of Parasitic Intestinal Infections Among Food Handlers in Qazvin, İran. Turk. Parazitolojii Derg. 2019, 43, 16–20. [Google Scholar] [CrossRef]
- Kheirandish, F.; Tarahi, M.J.; Haghighi, A.; Nazemalhosseini-Mojarad, E.; Kheirandish, M. Prevalence of Intestinal Parasites in Bakery Workers in Khorramabad, Lorestan Iran. Iran. J. Parasitol. 2011, 6, 76–83. [Google Scholar]
- Abdel-Dayem, M.; Al Zou’bi, R.; Hani, R.B.; Amr, Z.S. Microbiological and Parasitological Investigation among Food Handlers in Hotels in the Dead Sea Area, Jordan. J. Microbiol. Immunol. Infect. 2014, 47, 377–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, J.W.; Putnam, S.D.; Rockabrand, D.M.; Okla, G.E.; Mostafa, M.; Monteville, M.R.; Antosek, L.E.; Herbst, J.; Tribble, D.R.; Riddle, M.S.; et al. A Cross-Sectional Analysis of Clinical Presentations of and Risk Factors for Enteric Protozoan Infections in an Active Duty Population during Operation Iraqi Freedom. Trop. Dis. Travel Med. Vaccines 2015, 1, 2. [Google Scholar] [CrossRef] [Green Version]
- Kitvatanachai, S.; Rhongbutsri, P. Using Mini Parasep SF to Determine Intestinal Parasitic Infections Comparing to Conventional Methods in Gardener of Chanthaburi Province, Thailand. Asian Pac. J. Trop. Dis. 2017, 7, 596–600. [Google Scholar] [CrossRef]
- Sangwalee, W.; Rattanapitoon, N.; Thanchomnang, T. Intestinal Parasitic Infections and Risk Factors among Myanmar Migrant Workers in Northeast Thailand. Asian Pac. J. Trop. Med. 2021, 14, 17. [Google Scholar] [CrossRef]
- Abu-Madi, M.; Aly, M.; Behnke, J.M.; Clark, C.G.; Balkhy, H. The Distribution of Blastocystis Subtypes in Isolates from Qatar. Parasites Vectors 2015, 8, 465. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.; Boughattas, S.; Behnke, J.M.; Sharma, A.; Ismail, A. Coproscopy and Molecular Screening for Detection of Intestinal Protozoa. Parasites Vectors 2017, 10, 414. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.A.; Behnke, J.M.; Doiphode, S.H. Changing Trends in Intestinal Parasitic Infections among Long-Term-Residents and Settled Immigrants in Qatar. Parasites Vectors 2010, 3, 98. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.A.; Behnke, J.M.; Ismail, A.; Boughattas, S. Assessing the Burden of Intestinal Parasites Affecting Newly Arrived Immigrants in Qatar. Parasites Vectors 2016, 9, 619. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.A.; Behnke, J.M.; Boughattas, S.; Al-Thani, A.; Doiphode, S.H. A Decade of Intestinal Protozoan Epidemiology among Settled Immigrants in Qatar. BMC Infect. Dis. 2016, 16, 370. [Google Scholar] [CrossRef] [Green Version]
- Abu-Madi, M.A.; Behnke, J.M.; Ismail, A.; Al-Olaqi, N.; Al-Zaher, K.; El-Ibrahim, R. Comparison of Intestinal Parasitic Infection in Newly Arrived and Resident Workers in Qatar. Parasites Vectors 2011, 4, 211. [Google Scholar] [CrossRef] [Green Version]
- Wakid, M.H. Fecal Occult Blood Test and Gastrointestinal Parasitic Infection. J. Parasitol. Res. 2010, 2010, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.A.; Alam-Eldin, Y.H.; Eltaweel, N.A.; Elmorsy, S. Intestinal Parasites Detected During Pre-Employment Stool Examination at Tertiary Health Care Center In Makkah, Kingdom Of Saudi Arabia. J. Egypt. Soc. Parasitol. 2015, 45, 367–373. [Google Scholar] [CrossRef]
- Hsieh, M.-H.; Lin, W.-Y.; Dai, C.-Y.; Huang, J.-F.; Huang, C.-K.; Chien, H.-H.; Wang, C.-L.; Chung, W.-L.; Wu, J.-R.; Chen, E.-R.; et al. Intestinal Parasitic Infection Detected by Stool Examination in Foreign Laborers in Kaohsiung. Kaohsiung J. Med. Sci. 2010, 26, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, M.-H.; Lin, Y.-Y.; Hsu, Y.-K.; Yang, J.-F.; Hsu, Y.-C.; Chen, W.-C.; Dai, C.-Y.; Yu, M.-L.; Ho, C.-K. Intestinal Parasitic Infections in Foreigners Detected by Stool Examination in Taiwan. Open Infect. Dis. J. 2011, 5, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.H.; Lee, S.L.; Yang, C.C.; Lee, Y.M. Genetic Variability of Blastocystis hominis in Indonesian Immigrant Workers. J. Intern. Med. Taiwan 2014, 25, 199–205. [Google Scholar]
- Karaman, U.; Turan, A.; Depecik, F.; Geçit, I.; Ozer, A.; Karci, E.; Karadan, M. [Frequency of Intestinal Parasites among Administrators and Workers in Sanitary and Non-Sanitary Institutions. Sihhi ve Gayri Sihhi Müesseselerdeki İşletmeci ve Çalişanlari ve Baǧirsak Parazitlerinin Sikliǧi. Turk. Parazitolojii Derg. 2011, 35, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Sahimin, N.; Meor Termizi, F.H.; Rajamanikam, A.; Mohd Nazri, N.A.; Govind, S.K.; Mohd Zain, S.N. Prevalence and Subtypes of Blastocystis among Migrant Workers from Different Working Sectors in Peninsular Malaysia. Parasitol. Res. 2020, 119, 3555–3558. [Google Scholar] [CrossRef]
- Noor, R.; Saha, S.R.; Rahman, F.; Munshi, S.K.; Uddin, M.A.; Rahman, M.M. Frequency of Opportunistic and Other Intestinal Parasitic Infections in Patients Infected with Human Immunodeficiency Virus in Bangladesh. Tzu Chi Med. J. 2012, 24, 191–195. [Google Scholar] [CrossRef]
- Schär, F.; Inpankaew, T.; Traub, R.J.; Khieu, V.; Dalsgaard, A.; Chimnoi, W.; Chhoun, C.; Sok, D.; Marti, H.; Muth, S.; et al. The Prevalence and Diversity of Intestinal Parasitic Infections in Humans and Domestic Animals in a Rural Cambodian Village. Parasitol. Int. 2014, 63, 597–603. [Google Scholar] [CrossRef]
- Wang, W.; Owen, H.; Traub, R.J.; Cuttell, L.; Inpankaew, T.; Bielefeldt-Ohmann, H. Molecular Epidemiology of Blastocystis in Pigs and Their In-Contact Humans in Southeast Queensland, Australia, and Cambodia. Vet. Parasitol. 2014, 203, 264–269. [Google Scholar] [CrossRef]
- He, S.S.; Wu, L.Y.; Liu, X.Q.; Shi, H.H.; Chen, Z.; Zhang, H.; Pang, C.Y.; Li, Y.M. Investigation on the Infection of Blastocystis hominis in Populations in Bama Yao Autonomous County of Guangxi. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2013, 31, 76–77. [Google Scholar]
- Chen, S.; Zhang, Y.; Li, H.; Cai, Y.; Chen, J. Analysis on Parasitic Infection of Clinical Samples from Hospitals in Shanghai during 2011–2013. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2014, 32, 446–451. [Google Scholar]
- Umar, M.; Chen, X.; Osman, Y.; Simayi, A.; Hou, Y.; Maimaitiyiming, Y.; Xiao, N. Epidemiological Survey on Human Intestinal Protozoa in Xinjiang Uygur Autonomous Region in 2015. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi Chin. J. Parasitol. Parasit. Dis. 2016, 34, 361–365. [Google Scholar]
- Jiang, L.-Z. Epidemic Status of Human Key Parasitic Diseases in Tongcheng City, Anhui Province. Chin. J. Schistosomiasis Control 2018, 30, 68–71. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Zhu, Y.-K.; Chen, W.-Q.; Deng, Y.; Lin, X.-M.; Li, P.; Zhang, H.-W. Survey and Analysis of Epidemic Status of Principal Human Parasitosis in Ecological Region of Huaiyang Hills of Henan Province in 2015. Chin. J. Schistosomiasis Control 2017, 29. [Google Scholar] [CrossRef]
- Gong, B.; Liu, X.; Wu, Y.; Xu, N.; Xu, M.; Yang, F.; Tong, L.; Zhou, K.; Cao, J.; Liu, A.; et al. Prevalence and Subtype Distribution of Blastocystis in Ethnic Minority Groups on Both Sides of the China-Myanmar Border, and Assessment of Risk Factors|révalence et Distribution Des Sous-Types de Blastocystis Dans Les Groupes de Minorités Ethniques Des. Parasite 2019, 26, 46. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhang, S.; Ning, C.; Zhou, Y.; Teng, X.; Wu, X.; Chu, Y.; Yu, Y.; Chen, J.; Tian, L.; et al. Molecular Epidemiology and Risk Factors of Blastocystis sp. Infections among General Populations in Yunnan Province, Southwestern China. Risk Manag. Healthc. Policy 2020, 13, 1791–1801. [Google Scholar] [CrossRef]
- Ma, L.; Qiao, H.; Wang, H.; Li, S.; Zhai, P.; Huang, J.; Guo, Y. Molecular Prevalence and Subtypes of Blastocystis sp. in Primates in Northern China. Transbound. Emerg. Dis. 2020, 67, 2789–2796. [Google Scholar] [CrossRef]
- Seyer, A.; Karasartova, D.; Ruh, E.; Güreser, A.S.; Turgal, E.; Imir, T.; Taylan-Ozkan, A. Epidemiology and Prevalence of Blastocystis spp. in North Cyprus. Am. J. Trop. Med. Hyg. 2017, 96, 1164–1170. [Google Scholar] [CrossRef] [Green Version]
- Padukone, S.; Mandal, J.; Rajkumari, N.; Bhat, B.; Swaminathan, R.; Parija, S. Detection of Blastocystis in Clinical Stool Specimens Using Three Different Methods and Morphological Examination in Jones’ Medium. Trop. Parasitol. 2018, 8, 33–40. [Google Scholar] [CrossRef]
- Lappan, R.; Classon, C.; Kumar, S.; Singh, O.P.; De Almeida, R.V.; Chakravarty, J.; Kumari, P.; Kansal, S.; Sundar, S.; Blackwell, J.M. Meta-Taxonomic Analysis of Prokaryotic and Eukaryotic Gut Flora in Stool Samples from Visceral Leishmaniasis Cases and Endemic Controls in Bihar State India. PLoS Negl. Trop. Dis. 2019, 13, e0007444. [Google Scholar] [CrossRef] [Green Version]
- Wiria, A.E.; Hamid, F.; Wammes, L.J.; Prasetyani, M.A.; Dekkers, O.M.; May, L.; Kaisar, M.M.M.; Verweij, J.J.; Guigas, B.; Partono, F.; et al. Infection with Soil-Transmitted Helminths Is Associated with Increased Insulin Sensitivity. PLoS ONE 2015, 10, e0127746. [Google Scholar] [CrossRef] [Green Version]
- Yulfi, H.; Rozi, M.F.; Andriyani, Y.; Darlan, D.M. Prevalence of Cryptosporidium Spp. and Blastocystis hominis in Faecal Samples among Diarrheic HIV Patients in Medan, Indonesia. Med. Glas. 2021, 18, 1–7. [Google Scholar] [CrossRef]
- Sungkar, S.; Pohan, A.P.N.; Ramadani, A.; Albar, N.; Azizah, F.; Nugraha, A.R.A.; Wiria, A.E. Heavy Burden of Intestinal Parasite Infections in Kalena Rongo Village, a Rural Area in South West Sumba, Eastern Part of Indonesia: A Cross Sectional Study. BMC Public Health 2015, 15, 1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, E.; Tuda, J.; Imada, M.; Akao, N.; Fujita, K. The High Prevalence of Asymptomatic Toxocara Infection among Schoolchildren in Manado, Indonesia. Southeast Asian J. Trop Med. Public Health 2005, 36, 1399–1406. [Google Scholar] [PubMed]
- Turgay, N.; Unver-Yolasiǧmaz, A.; Oyur, T.; Bardak-Özcem, S.; Töz, S. Monthly Distribution of Intestinal Parasites Detected in a Part of Western Turkey between May 2009–April 2010-Results of Acid Fast and Modified Trichrome Staining Methods İzmir ve Çevresinde Bir Yilda (Mayis 2009-Nisan 2010) Saptanan Baǧirsak Parazit. Turk. Parazitolojii Derg. 2012, 36, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.R.; Hasani, H.; Tsiami, A.; Ashrafi, K.; Johnson, P.; Sharifdini, M.; Karanis, P. Intestinal Protozoan and Helminthic Infections among Hemodialysis and Cancer Patients. Parasitol. Res. 2020, 119, 3053–3059. [Google Scholar] [CrossRef] [PubMed]
- Khoshnood, S.; Rafiei, A.; Saki, J.; Alizadeh, K. Prevalence and Genotype Characterization of Blastocystis hominis among the Baghmalek People in Southwestern Iran in 2013–2014. Jundishapur J. Microbiol. 2015, 8, e23930. [Google Scholar] [CrossRef] [Green Version]
- Pestehchian, N.; Nazari, M.; Haghighi, A.; Salehi, M.; Yosefi, H.; Khosravi, N. Prevalence of Intestinal Parasitic Infection among Inhabitants and Tribes of Chelgerd, Iran, 2008–2009. J. Clin. Diagn. Res. 2015, 9, LC01–LC04. [Google Scholar] [CrossRef]
- Sadeghi, H.; Bakht, M.; Saghafi, H.; Shahsavari, T. Prevalence of Intestinal Parasites in a Population in Eghbalieh City from Qazvin Province, Iran. J. Parasit. Dis. 2015, 39, 126–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, H.; Borji, H. A Survey of Intestinal Parasites in a Population in Qazvin, North of Iran. Asian Pac. J. Trop. Dis. 2015, 5, 231–233. [Google Scholar] [CrossRef]
- Badparva, E.; Kheirandish, F.; Ebrahimzade, F. Prevalence of Intestinal Parasites in Lorestan Province, West of Iran. Asian Pac. J. Trop. Dis. 2014, 4, S728–S732. [Google Scholar] [CrossRef]
- Mahni, M.B.; Rezaeian, M.; Kia, E.B.; Raeisi, A.; Khanaliha, K.; Tarighi, F.; Kamranrashani, B. Prevalence of Intestinal Parasitic Infections in Jiroft, Kerman Province, Iran. Iran. J. Parasitol. 2016, 11, 232–238. [Google Scholar]
- Tork, M.; Sharif, M.; Charati, J.Y.; Nazar, I.; Hosseini, S.A. Prevalence of Intestinal Parasitic Infections and Associated Risk Factors in West of Mazandaran Province, Iran. J. Maz. Univ. Med. Sci. 2016, 25, 81–88. [Google Scholar]
- Jafari, R.; Sharifi, F.; Bagherpour, B.; Safari, M. Prevalence of Intestinal Parasites in Isfahan City, Central Iran, 2014. J. Parasit. Dis. 2016, 40, 679–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmati, N.; Razmjou, E.; Hashemi-Hafshejani, S.; Motevalian, A.; Akhlaghi, L.; Meamar, A.R. Prevalence and Risk Factors of Human Intestinal Parasites in Roudehen, Tehran Province, Iran. Iran. J. Parasitol. 2017, 12, 364–373. [Google Scholar] [PubMed]
- Riabi, T.R.; Haghighi, A.; Mirjalali, H.; Gol, S.M.A.; Karamati, S.A.; Ghasemian, M.; Monfared, A.B.; Aghamohammadi, E.; Zojaji, H. Study of Prevalence, Distribution and Clinical Significance of Blastocystis Isolated from Two Medical Centers in Iran. Gastroenterol. Hepatol. Bed Bench 2017, 10, S102–S107. [Google Scholar] [CrossRef]
- Mardani Kataki, M.; Tavalla, M.; Beiromvand, M. Higher Prevalence of Blastocystis hominis in Healthy Individuals than Patients with Gastrointestinal Symptoms from Ahvaz, Southwestern Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 160–164. [Google Scholar] [CrossRef]
- Abbaszadeh Afshar, M.J.; Barkhori Mehni, M.; Rezaeian, M.; Mohebali, M.; Baigi, V.; Amiri, S.; Amirshekari, M.B.; Hamidinia, R.; Samimi, M. Prevalence and Associated Risk Factors of Human Intestinal Parasitic Infections: A Population-Based Study in the Southeast of Kerman Province, Southeastern Iran. BMC Infect. Dis. 2020, 20, 12. [Google Scholar] [CrossRef] [Green Version]
- Sobati, H. Epidemiological Study of Parasitic Infections in Bumusa Island, Hormozgan. Iran. J. Parasitol. 2020, 15, 425–434. [Google Scholar] [CrossRef]
- Shirvani, G.; Fasihi-Harandi, M.; Raiesi, O.; Bazargan, N.; Zahedi, M.J.; Sharifi, I.; Kalantari-Khandani, B.; Nooshadokht, M.; Shabandoust, H.; Mohammadi, M.A.; et al. Prevalence and Molecular Subtyping of Blastocystis from Patients with Irritable Bowel Syndrome, Inflammatory Bowel Disease and Chronic Urticaria in Iran. Acta Parasitol. 2020, 65, 90–96. [Google Scholar] [CrossRef]
- Barati, M.; Taghipour, A.; Bakhshi, B.; Shams, S.; Pirestani, M. Prevalence of Intestinal Parasitic Infections and Campylobacter spp. among Children with Gastrointestinal Disorders in Tehran, Iran. Parasite Epidemiol. Control 2021, 13. [Google Scholar] [CrossRef]
- Bairami Kuzehkanani, A.; Rezaei, S.; Babaei, Z.; Niyyati, M.; Hashemi, S.N.; Rezaeian, M. Enteric Protozoan Parasites in Rural Areas of Bandar-Abbas, Southern Iran: Comparison of Past and Present Situation. Iran. J. Public Health 2011, 40, 80–85. [Google Scholar]
- Sarkari, B.; Hosseini, G.; Motazedian, M.H.; Fararouei, M.; Moshfe, A. Prevalence and Risk Factors of Intestinal Protozoan Infections: A Population-Based Study in Rural Areas of Boyer-Ahmad District, Southwestern Iran. BMC Infect. Dis. 2016, 16, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Sharifdini, M.; Ghanbarzadeh, L.; Barikani, A.; Saraei, M. Prevalence of Intestinal Parasites among Rural Inhabitants of Fouman, Guilan Province, Northern Iran with Emphasis on Strongyloides stercoralis. Iran. J. Parasitol. 2020, 15, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pagheh, A.S.; Sharif, M.; Daryani, A.; Yazdani-Charati, J.; Nazar, E.; Asfaram, S.; Hosseini, S.-A.; Tork, M.; Soosaraie, M.; Syadatpanah, A.; et al. A Cross-Sectional Analysis of Intestinal Parasitic Infections among the General Population in North of Iran. J. Infect. Dev. Ctries. 2018, 12, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beiromvand, M.; Panabad, E.; Rafiei, A. Status of Intestinal Parasitic Infections among Rural and Urban Populations, Southwestern Iran. Asian Pac. J. Trop. Med. 2019, 12, 130–136. [Google Scholar] [CrossRef]
- Taherkhani, K.; Barikani, A.; Shahnazi, M.; Saraei, M. Prevalence of Intestinal Parasites among Rural Residents of Takestan in North-West of Iran. Iran. J. Parasitol. 2019, 14, 657–663. [Google Scholar] [CrossRef]
- Kim, M.-J.; Won, E.J.; Kim, S.H.; Shin, J.H.; Chai, J.-Y. Molecular Detection and Subtyping of Human Blastocystis and the Clinical Implications: Comparisons between Diarrheal and Non-Diarrheal Groups in Korean Populations. Korean J. Parasitol. 2020, 58, 321–326. [Google Scholar] [CrossRef]
- Sayasone, S.; Mak, T.K.; Vanmany, M.; Rasphone, O.; Vounatsou, P.; Utzinger, J.; Akkhavong, K.; Odermatt, P. Helminth and Intestinal Protozoa Infections, Multiparasitism and Risk Factors in Champasack Province, Lao People’s Democratic Republic. PLoS Negl. Trop. Dis. 2011, 5, e1037. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Jollivet, C.; Morand, S.; Thongmalayvong, B.; Somphavong, S.; Siew, C.-C.; Ting, P.-J.; Suputtamongkol, S.; Saensombath, V.; Sanguankiat, S.; et al. Intestinal Parasitic Infections and Environmental Water Contamination in a Rural Village of Northern Lao PDR. Korean J. Parasitol. 2017, 55, 523–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanpool, O.; Laymanivong, S.; Thanchomnang, T.; Rodpai, R.; Sadaow, L.; Phosuk, I.; Maleewong, W.; Intapan, P.M. Subtype Identification of Human Blastocystis spp. Isolated from Lao People’s Democratic Republic. Acta Trop. 2017, 168, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Araj, G.F.; Musharraheh, U.M.; Haydar, A.; Ghawi, A.; Itani, R.; Saliba, R. Trends and Prevalence of Intestinal Parasites at a Tertiary Care Center in Lebanon over a Decade. J. Med. Liban. 2011, 59, 143–148. [Google Scholar]
- Khaled, S.; Gantois, N.; Ayoubi, A.; Even, G.; Sawant, M.; El Houmayraa, J.; Nabot, M.; Benamrouz-Vanneste, S.; Chabé, M.; Certad, G.; et al. Blastocystis sp. Prevalence and Subtypes Distribution amongst Syrian Refugee Communities Living in North Lebanon. Microorganisms 2021, 9, 184. [Google Scholar] [CrossRef]
- Sinniah, B.; Sabaridah, I.; Soe, M.M.; Sabitha, P.; Awang, I.P.R.; Ong, G.P.; Hassan, A.K.R. Determining the Prevalence of Intestinal Parasites in Three Orang Asli (Aborigines) Communities in Perak, Malaysia. Trop. Biomed. 2012, 29, 200–206. [Google Scholar]
- Mohammad, N.A.; Al-Mekhlafi, H.M.; Anuar, T.S. Subtype Distribution of Blastocystis Isolated from Humans and Associated Animals in an Indigenous Community with Poor Hygiene in Peninsular Malaysia. Trop. Biomed. 2018, 35, 849–860. [Google Scholar]
- Noradilah, S.A.; Moktar, N.; Anuar, T.S.; Lee, I.L.; Salleh, F.M.; Manap, S.N.A.A.; Mohtar, N.S.H.M.; Azrul, S.M.; Abdullah, W.O.; Nordin, A.; et al. Microscopy-Based Techniques: A Reliable Method for Detecting Blastocystis sp. Infection. Sylwan 2017, 161, 149–153. [Google Scholar]
- Mohammad, N.A.; Al-Mekhlafi, H.M.; Moktar, N.; Anuar, T.S. Prevalence and Risk Factors of Blastocystis Infection among Underprivileged Communities in Rural Malaysia. Asian Pac. J. Trop. Med. 2017, 10, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Noradilah, S.A.; Moktar, N.; Anuar, T.S.; Lee, I.L.; Salleh, F.M.; Manap, S.N.A.A.; Mohtar, N.S.H.M.; Azrul, S.M.; Abdullah, W.O.; Nordin, A.; et al. Molecular Epidemiology of Blastocystosis in Malaysia: Does Seasonal Variation Play an Important Role in Determining the Distribution and Risk Factors of Blastocystis Subtype Infections in the Aboriginal Community? Parasites Vectors 2017, 10, 1–12. [Google Scholar] [CrossRef]
- Noradilah, S.A.; Moktar, N.; Lee, I.L.; Salleh, F.M.; Anuar, T.S. Comparison of Direct Examination and in Vitro Culture for the Detection of Blastocystis sp. In Orang Asli Stool Samples. Sains Malays. 2018, 47, 2099–2104. [Google Scholar] [CrossRef]
- Mohammad, N.A.; Al-Mekhlafi, H.M.; Anuar, T.S. Genetic Diversity of Blastocystis Isolates from Symptomatic and Asymptomatic Orang Asli in Pahang, Malaysia. Southeast Asian J. Trop. Med. Public Health 2018, 49, 189–197. [Google Scholar]
- Muslim, A.; Sofian, S.M.; Shaari, S.A.; Hoh, B.-P.; Lim, Y.A.-L. Prevalence, Intensity and Associated Risk Factors of Soil Transmitted Helminth Infections: A Comparison between Negritos (Indigenous) in Inland Jungle and Those in Resettlement at Town Peripheries. PLoS Negl. Trop. Dis. 2019, 13, e0007331. [Google Scholar] [CrossRef]
- Lee, I.L.; Tan, T.C.; Tan, P.C.; Nanthiney, D.R.; Biraj, M.K.; Surendra, K.M.; Suresh, K.G. Predominance of Blastocystis sp. Subtype 4 in Rural Communities, Nepal. Parasitol. Res. 2012, 110, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.J.; Rivera, W.L. Comparison of Direct Fecal Smear Microscopy, Culture, and Polymerase Chain Reaction for the Detection of Blastocystis sp. in Human Stool Samples. Asian Pac. J. Trop. Med. 2013, 6, 780–784. [Google Scholar] [CrossRef] [Green Version]
- Belleza, M.L.B.; Reyes, J.C.B.; Tongol-Rivera, P.N.; Rivera, W.L. Subtype Analysis of Blastocystis sp. Isolates from Human and Canine Hosts in an Urban Community in the Philippines. Parasitol. Int. 2016, 65, 291–294. [Google Scholar] [CrossRef]
- Adao, D.E.; Dela Serna, A.O.; Belleza, M.L.; Bolo, N.R.; Rivera, W.L. Subtype Analysis of Blastocystis sp. Isolates from Asymptomatic Individuals in an Urban Community in the Philippines. Ann. Parasitol. 2016, 62, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Belleza, M.L.B.; Cadacio, J.L.C.; Borja, M.P.; Solon, J.A.A.; Padilla, M.A.; Tongol-Rivera, P.N.; Rivera, W.L. Epidemiologic Study of Blastocystis Infection in an Urban Community in the Philippines. J. Environ. Public Health 2015, 2015, 894297. [Google Scholar] [CrossRef]
- Weerakoon, K.G.; Gordon, C.A.; Williams, G.M.; Cai, P.; Gobert, G.N.; Olveda, R.M.; Ross, A.G.; Olveda, D.U.; McManus, D.P. Co-Parasitism of Intestinal Protozoa and Schistosoma Japonicum in a Rural Community in the Philippines. Infect. Dis. Poverty 2018, 7, 121. [Google Scholar] [CrossRef] [Green Version]
- AlDahhasi, W.; Toulah, F.; Wakid, M. Evaluation of Common Microspcpic Technique for Detection of Blastocystis hominis. J. Egypt. Soc. Parasitol. 2020, 50, 33–40. [Google Scholar] [CrossRef]
- Hawash, Y.A.; Dorgham, L.S.; Amir, E.-A.M.; Sharaf, O.F. Prevalence of Intestinal Protozoa among Saudi Patients with Chronic Renal Failure: A Case-Control Study. J. Trop. Med. 2015, 2015, 563478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqumber, M.A. Association between Papio Hamadryas Populations and Human Gastrointestinal Infectious Diseases in Southwestern Saudi Arabia. Ann. Saudi Med. 2014, 34, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kaewpitoon, N.; Kaewpitoon, S.; Meererksom, T.; Chan-Aran, S.; Sangwalee, W.; Norkaew, J.; Chuatanam, J.; Kujapan, J.; Padchasuwan, N.; Tongtawee, T. Detection of Opisthorchis Viverrini Infection among the ASEAN Population in Thailand Using a Verbal Screening Test and Fecal Concentrator Kit. Iran. J. Parasitol. 2018, 13, 258–266. [Google Scholar] [PubMed]
- Kaewjai, C.; Prommano, O.; Tonsomboon, A. Detection of Blastocystis hominis Using Simple Direct Smear Compared with Jones’ Medium Cultivation in Thatu Subdistrict, Chiang Khan Distric, Loei Province. Thammasat Med. J. 2020, 20, 8–14. [Google Scholar]
- Popruk, N.; Prasongwattana, S.; Mahittikorn, A.; Palasuwan, A.; Popruk, S.; Palasuwan, D. Prevalence and Subtype Distribution of Blastocystis Infection in Patients with Diabetes Mellitus in Thailand. Int. J. Environ. Res. Public Health 2020, 17, 8877. [Google Scholar] [CrossRef] [PubMed]
- Prommi, A.; Prombutara, P.; Watthanakulpanich, D.; Adisakwattana, P.; Kusolsuk, T.; Yoonuan, T.; Poodeepiyasawat, A.; Homsuwan, N.; Prummongkol, S.; Tanita, M.; et al. Intestinal Parasites in Rural Communities in Nan Province, Thailand: Changes in Bacterial Gut Microbiota Associated with Minute Intestinal Fluke Infection. Parasitology 2020, 147, 972–984. [Google Scholar] [CrossRef]
- Yowang, A.; Tsaousis, A.D.; Chumphonsuk, T.; Thongsin, N.; Kullawong, N.; Popluechai, S.; Gentekaki, E. High Diversity of Blastocystis Subtypes Isolated from Asymptomatic Adults Living in Chiang Rai, Thailand. Infect. Genet. Evol. 2018, 65, 270–275. [Google Scholar] [CrossRef] [Green Version]
- Punsawad, C.; Phasuk, N.; Bunratsami, S.; Thongtup, K.; Siripakonuaong, N.; Nongnaul, S. Prevalence of Intestinal Parasitic Infection and Associated Risk Factors among Village Health Volunteers in Rural Communities of Southern Thailand. BMC Public Health 2017, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Palasuwan, A.; Palasuwan, D.; Mahittikorn, A.; Chiabchalard, R.; Combes, V.; Popruk, S. Subtype Distribution of Blastocystis in Communities along the Chao Phraya River, Thailand. Korean J. Parasitol. 2016, 54, 455–460. [Google Scholar] [CrossRef] [Green Version]
- Kitvatanachai, S.; Taylor, A.; Rhongbutsri, P.; Taylor, W.R.J. Modified Harada-Mori and Simple Wet Mount to Determine Hookworm Infections in Yo Island Urban Area, Songkhla, Southern Thailand. Trop. Med. Health 2019, 47, 27. [Google Scholar] [CrossRef]
- Boonjaraspinyo, S.; Boonmars, T.; Kaewsamut, B.; Ekobol, N.; Laummaunwai, P.; Aukkanimart, R.; Wonkchalee, N.; Juasook, A.; Sriraj, P. A Cross-Sectional Study on Intestinal Parasitic Infections in Rural Communities, Northeast Thailand. Korean J. Parasitol. 2013, 51, 727–734. [Google Scholar] [CrossRef]
- Suntaravitun, P.; Dokmaikaw, A. Prevalence of Intestinal Parasites and Associated Risk Factors for Infection among Rural Communities of Chachoengsao Province, Thailand. Korean J. Parasitol. 2018, 56, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongthamarin, K.; Trairattanapa, T.; Kijanukul, S.; Kritsilpe, T.; Poobunjirdkul, S.; Chuengdee, W.; Mungthi, M.; Leelayoova, S.; Naaglor, T.; Taamasri, P.; et al. Blastocystis Incidence, Spontaneous Clearance, Persistence and Risk Factors in a Rural Community in Thailand: A Prospective Cohort Study. Asian Pac. J. Trop. Med. 2020, 13, 123–130. [Google Scholar] [CrossRef]
- Popruk, S.; Udonsom, R.; Koompapong, K.; Mahittikorn, A.; Kusolsuk, T.; Ruangsittichai, J.; Palasuwan, A. Subtype Distribution of Blastocystis in Thai-Myanmar Border, Thailand. Korean J. Parasitol. 2015, 53, 13–19. [Google Scholar] [CrossRef]
- Karasartova, D.; Gureser, A.S.; Zorlu, M.; Turegun-Atasoy, B.; Taylan-Ozkan, A.; Dolapci, M. Blastocystosis in Post-Traumatic Splenectomized Patients. Parasitol. Int. 2016, 65, 802–805. [Google Scholar] [CrossRef] [PubMed]
- Karadag, G.; Tamer, G.S.; Dervisoglu, E. Investigation of Intestinal Parasites in Dialysis Patients. Saudi Med. J. 2013, 34, 714–718. [Google Scholar] [PubMed]
- Dogruman-Al, F.; Simsek, Z.; Boorom, K.; Ekici, E.; Sahin, M.; Tuncer, C.; Kustimur, S.; Altinbas, A. Comparison of Methods for Detection of Blastocystis Infection in Routinely Submitted Stool Samples, and Also in IBS/IBD Patients in Ankara, Turkey. PLoS ONE 2010, 5, e15484. [Google Scholar] [CrossRef]
- Köksal, F.; Başlanti, I.; Samasti, M. A Retrospective Evaluation of the Prevalence of Intestinal Parasites in Istanbul, Turkey İstanbul’da Baǧirsak Parazitlerinin Sikliǧi Bakimindan Retrospektif Bir Deǧerlendirme. Turk. Parazitolojii Derg. 2010, 34, 166–171. [Google Scholar]
- Çetinkaya, Ü.; Yazar, S.; Kuk, S.; Ateş, S.; Hamamci, B.; Gedikbaş, T.; Şahin, İ. The Distribution of Intestinal Parasites Determined between 2009–2010 in Erciyes University, Medical Faculty, Parasitology Laboratory Erciyes Üniversitesi Tıp Fakültesi Tıbbi Parazitoloji Anabilim Dalı Laboratuvarında 2009-2010 Yılları Arasında Saptanan. Kafkas Univ. Vet. Fak. Derg. 2012, 18, A93–A96. [Google Scholar] [CrossRef]
- Düzyol, D.; Kilimcioǧlu, A.A.; Ozyurt, B.C.; Ozkan, H.; Girginkardeşler, N. Incidence of Intestinal Parasites Detected in the Department of Parasitology in Celal Bayar University Hospital between 2006 and 2010. Celal Bayar Üniversitesi Hastanesi Parazitoloji Polikliniǧi’nde 2006-2010 Yillari Arasinda Saptanan Baǧirsak Parazit. Turk. Parazitolojii Derg. 2012, 36, 147–151. [Google Scholar] [CrossRef]
- Kurt, Ö.; Akyar, I.; Görgün, S.; Kocagöz, T.; Özbilgin, A. Feconomics®: A Simple, Novel and Fast Technique for Stool Concentration in Parasitology Laboratory. Kafkas Univ. Vet. Fak. Derg. 2012, 18, A161–A165. [Google Scholar] [CrossRef]
- Yilmaz, H.; Taş-Cengiz, Z.; Ceylan, A.; Ekici, A. The Distribution of Intestinal Parasites in People Admitted to the Yüzüncü Yil University Parasitology Laboratory of Health Research and Training Hospital, in 2009 Yüzüncü Yil Üniversitesi Araştirma ve Uygulama Hastanesi Parazitoloji Laboratuarina 20. Turk. Parazitolojii Derg. 2012, 36, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Gülmez, D.; Saribaş, Z.; Akyön, Y.; Ergüven, S. The Results of Hacettepe University Faculty of Medicine Parasitology Laboratory in 2003–2012: Evaluation of 10 Years Hacettepe Üniversitesi Tip Fakültesi Parazitoloji Laboratuvari 2003-2012 Yillari Sonuçlari: 10 Yillik Deǧerlendirme. Turk. Parazitolojii Derg. 2013, 37, 97–101. [Google Scholar] [CrossRef]
- Kirkoyun Uysal, H.; Akgül, O.; Purisa, S.; Oner, Y.A. Twenty-Five Years of Intestinal Parasite Prevalence in İstanbul University, İstanbul Faculty of Medicine: A Retrospective Study İstanbul Üniversitesi İstanbul Tip Fakültesi’nde 25 Yillik İntestinal Parazit Prevalansi: Retrospektif Bir Çalişma. Turk. Parazitolojii Derg. 2014, 38, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Öncel, K. Distribution of Intestinal Parasites Detected in Şanlıurfa Mehmet Akif Inan Education and Research Hospital Between October 2015 and October 2016. Turk. Parazitolojii Derg. 2018, 42, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malatyali, E.; Ertabaklar, H.; Ertuğ, S. Subtype Distribution of Blastocystis spp. With DNA Barcoding and Evaluation of Diagnostic Methods|DNA Barkotlama Yöntemiyle Blastocystis Alt Tiplerinin Belirlenmesi ve Tanı Yöntemlerinin Değerlendirilmesi. Mikrobiyol. Bul. 2019, 53, 308–318. [Google Scholar] [CrossRef]
- Taş Cengiz, Z.; Yılmaz, H.; Beyhan, Y.E.; Çiçek, M. A Comprehensive Retrospective Study: Intestinal Parasites in Human in Van Province. Turk. Parazitolojii Derg. 2019, 43, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Gulhan, B.; Aydin, M.; Demirkazik, M.; Koltas, I.S.; Cikman, A.; Turkmen, K.; Duran, T. Subtype Distribution and Molecular Characterization of Blastocystis from Hemodialysis Patients in Turkey. J. Infect. Dev. Ctries. 2020, 14, 1448–1454. [Google Scholar] [CrossRef]
- Koltas, I.S.; Eroglu, F. Subtype Analysis of Blastocystis Isolates Using SSU RRNA-DNA Sequencing in Rural and Urban Population in Southern Turkey. Exp. Parasitol. 2016, 170, 247–251. [Google Scholar] [CrossRef]
- AbuOdeh, R.; Ezzedine, S.; Samie, A.; Stensvold, C.R.; ElBakri, A. Prevalence and Subtype Distribution of Blastocystis in Healthy Individuals in Sharjah, United Arab Emirates. Infect. Genet. Evol. 2016, 37, 158–162. [Google Scholar] [CrossRef]
- Toychiev, A.; Navruzov, B.; Pazylova, D.; Davis, N.; Badalova, N.; Osipova, S. Intestinal Protozoa and Helminths in Ulcerative Colitis and the Influence of Anti-Parasitic Therapy on the Course of the Disease. Acta Trop. 2021, 213, 105755. [Google Scholar] [CrossRef]
- Hatipoğlu, S.; Lök, U.; Gülaçtı, U.; Çelik, T. Pre-Operative Stool Analysis for Intestinal Parasites and Fecal Occult Blood in Patients with Acute Appendicitis Akut Apandisit Hastalarinda Gaitada Gizli Kan ve Intestinal Parazitler Için Ameliyat Öncesi Gaita Analizi. Turk. J. Trauma Emerg. Surg. 2016, 22, 471–476. [Google Scholar] [CrossRef] [Green Version]
- Angal, L.; Mahmud, R.; Samin, S.; Yap, N.-J.; Ngui, R.; Amir, A.; Ithoi, I.; Kamarulzaman, A.; AL Lim, Y. Determining Intestinal Parasitic Infections (IPIs) in Inmates from Kajang Prison, Selangor, Malaysia for Improved Prison Management. BMC Infect. Dis. 2015, 15, 467. [Google Scholar] [CrossRef] [Green Version]
- Chandramathi, S.; Suresh, K.; Kuppusamy, U.R. Elevated Levels of Urinary Hyaluronidase in Humans Infected with Intestinal Parasites. Ann. Trop. Med. Parasitol. 2010, 104, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Riabi, T.; Mirjalali, H.; Haghighi, A.; Rostami Nejad, M.; Pourhoseingholi, M.A.; Poirier, P.; Delbac, F.; Wawrzyniak, I.; Zali, M.R. Genetic Diversity Analysis of Blastocystis Subtypes from Both Symptomatic and Asymptomatic Subjects Using a Barcoding Region from the 18S RRNA Gene. Infect. Genet. Evol. 2018, 61, 119–126. [Google Scholar] [CrossRef]
- Vezir, S.; Kaya, F.; Vezir, E.; Karaosmanoğlu, N.; Adiloğlu, A.K. Evaluation of Intestinal Parasites in Patients with Chronic Spontaneous Urticaria in a Territory Hospital in Turkey. J. Infect. Dev. Ctries. 2019, 13, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Aydin, M.; Yazici, M.; Demirkazik, M.; Koltas, I.; Cikman, A.; Gulhan, B.; Duran, T.; Yilmaz, A.; Kara, M. Molecular Characterization and Subtyping of Blastocystis in Urticarial Patients in Turkey. Asian Pac. J. Trop. Med. 2019, 12, 450–456. [Google Scholar] [CrossRef]
- Yildiz, S.; Dogan, I.; Dogruman-Al, F.; Nalbantoglu, U.; Ustek, D.; Sarzhanov, F.; Yildirim, S. Association of Enteric Protist Blastocystis spp. and Gut Microbiota with Hepatic Encephalopathy. J. Gastrointest. Liver Dis. 2016, 25, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Alinaghizade, A.; Mirjalali, H.; Mohebali, M.; Stensvold, C.R.; Rezaeian, M. Inter- and Intra-Subtype Variation of Blastocystis Subtypes Isolated from Diarrheic and Non-Diarrheic Patients in Iran. Infect. Genet. Evol. 2017, 50, 77–82. [Google Scholar] [CrossRef]
- Thergarajan, G.; Kumar, S.; Bhassu, S.; Omar, S.F.B.S.; Rampal, S. Effect of Blastocystis sp. In Dengue Patients—Increase in the Treatment Cost and Exacerbation of Symptoms. PLoS ONE 2019, 14, e0211034. [Google Scholar] [CrossRef]
- Roy, M.; Singha, B.; Dhar, D.; Roychoudhury, S. Prevalence of Giardia Intestinalis with Other Co-Infecting Parasites in Barak Valley, Assam, India: A Molecular Approach. J. Parasit. Dis. 2019, 43, 426–442. [Google Scholar] [CrossRef]
- Barazesh, A.; Fouladvand, M.; Tahmasebi, R.; Heydari, A.; Fallahi, J. The Prevalence of Intestinal Parasites in Hemodialysis Patients in Bushehr, Iran. Hemodial. Int. 2015, 19, 447–451. [Google Scholar] [CrossRef] [Green Version]
- Pipatsatitpong, D.; Rangsin, R.; Leelayoova, S.; Naaglor, T.; Mungthin, M. Incidence and Risk Factors of Blastocystis Infection in an Orphanage in Bangkok, Thailand. Parasites Vectors 2012, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Koltas, I.S.; Akyar, I.; Elgun, G.; Kocagoz, T. Feconomics; a New and More Convenient Method, the Routine Diagnosis of Intestinal Parasiticinfections. Parasitol. Res. 2014, 113, 2503–2508. [Google Scholar] [CrossRef] [PubMed]
- Ozbagcivan, O.; Akarsu, S.; Avci, C.; Inci, B.B.; Fetil, E. Examination of the Microbial Spectrum in the Etiology of Erythema Nodosum: A Retrospective Descriptive Study. J. Immunol. Res. 2017, 2017, 8139591. [Google Scholar] [CrossRef] [Green Version]
- Tunalı, V.; Öztürk, E.A.; Ünver, A.; Turgay, N. The Prevalance of Blastocystosis among Patients with Gastrointestinal and Dermatologic Complaints and Effects of Blastocystis spp. Density on Symptomatology. Turk. Parazitolojii Derg. 2018, 42, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Jafari, R.; Gorgizadeh, H.; Soosaraei, M.; Shokri, A.; Bandalizadeh, Z.; Ahmadi, H.-Y.; Banimostafavi, E.S.; Fakhar, M. Initial Evidences of Salt and Pepper Retinal Lesions (Sprl) in Patients with Intestinal Protozoan Infections in Iran. Infect. Disord. Drug Targets 2021, 21, 60–67. [Google Scholar] [CrossRef]
- Teh, C.L.; Wan, S.A.; Ling, G.R. Severe Infections in Systemic Lupus Erythematosus: Disease Pattern and Predictors of Infection-Related Mortality. Clin. Rheumatol. 2018, 37, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Malatyalı, E.; Sankur, F.; Akın, M.N.; Ertabaklar, H.; Ertuğ, S. Subtype Distribution of Blastocystis in Pregnant Women and Analysis of Possible Risk Factors. Turk. Parazitolojii Derg. 2020, 44, 221–225. [Google Scholar] [CrossRef]
- Tai, W.-P.; Hu, P.-J.; Wu, J.; Lin, X.-C. Six Ulcerative Colitis Patients with Refractory Symptoms Co-Infective with Blastocystis Hominis in China. Parasitol. Res. 2011, 108, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.H.; Hu, X.F.; Liu, T.L.; Hu, R.S.; Yu, Z.Q.; Yang, W.B.; Wu, Y.L.; Yu, S.K.; Song, J.K. Molecular Characterization of Blastocystis sp. in Captive Wild Animals in Qinling Mountains. Parasitol. Res. 2017, 116, 2327–2333. [Google Scholar] [CrossRef]
- Li, X.-D.; Zou, Y.; Pan, J.; Liang, Q.-L.; Zeng, Z.; Meng, Y.-M.; Wang, X.-L.; Wang, H.-N.; Zhu, X.-Q. Prevalence and Subtypes of Blastocystis Sp. Infection in Zoo Animals in Three Cities in China. Parasitol. Res. 2020, 119, 465–471. [Google Scholar] [CrossRef]
- Ma, Y.T.; Liu, Q.; Xie, S.C.; Li, X.D.; Ma, Y.Y.; Li, T.S.; Gao, W.W.; Zhu, X.Q. Prevalence and Subtypes of Blastocystis in Alpacas, Vicugna Pacos in Shanxi Province, China. Korean J. Parasitol. 2020, 58, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, C.; Selvaraj, J.; Gomathinayagam, S.; Thangapandiyan, M.; Ravikumar, G.; Roy, P.; Balachandran, C. Blastocystis sp. from Food Animals in India. J. Parasit. Dis. 2014, 38, 440–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemalatha, C.; Chandrawathani, P.; Suresh Kumar, G.; Premaalatha, B.; Geethamalar, S.; Lily Rozita, M.; Farah Haziqah, M.T.; Sabapathy, D.; Ramlan, M. The Diagnosis of Blastocystis sp. From Animals an Emerging Zoonosis. Malays. J. Vet. Res. 2014, 5, 15–22. [Google Scholar]
- Abd Razak, N.A.; Yusof, A.M.; Mohammad, M. Identification of Blastocystis sp. Infection from Cattle, Goat and Sheep Isolated from Farms in Pahang, Malaysia. Int. J. Allied Health Sci. 2019, 3, 810. [Google Scholar]
- Kamaruddin, S.K.; Mat Yusof, A.; Mohammad, M. Prevalence and Subtype Distribution of Blastocystis sp. in Cattle from Pahang, Malaysia. Trop. Biomed. 2020, 37, 127–141. [Google Scholar]
- Aynur, Z.E.; Güçlü, Ö.; Yıldız, İ.; Aynur, H.; Ertabaklar, H.; Bozdoğan, B.; Ertuğ, S. Molecular Characterization of Blastocystis in Cattle in Turkey. Parasitol. Res. 2019, 118, 1055–1059. [Google Scholar] [CrossRef]
- Hastutiek, P.; Yuniarti, W.M.; Djaeri, M.; Lastuti, N.D.R.; Suprihati, E.; Suwanti, L.T. Prevalence and Diversity of Gastrointestinal Protozoa in Madura Cattle at Bangkalan Regency, East Java, Indonesia. Vet. World 2019, 12, 198–204. [Google Scholar] [CrossRef]
- Susana, Y.; Suwanti, L.T.; Suprihati, E. Identification and Prevalence of Gastrointestinal Parasites in Beef Cattle in Siak Sri Indrapura, Riau, Indonesia. Indones. J. Trop. Infect. Dis. 2019, 7, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Suwanti, L.T.; Susana, Y.; Hastutiek, P.; Suprihati, E.; Lastuti, N.D.R. Blastocystis spp. Subtype 10 Infected Beef Cattle in Kamal and Socah, Bangkalan, Madura, Indonesia. Vet. World 2020, 13, 231–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badparva, E.; Sadraee, J.; Kheirandish, F. Genetic Diversity of Blastocystis Isolated from Cattle in Khorramabad, Iran. Jundishapur J. Microbiol. 2015, 8, 4–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rostami, M.; Fasihi-Harandi, M.; Shafiei, R.; Aspatwar, A.; Derakhshan, F.K.; Raeghi, S. Genetic Diversity Analysis of Blastocystis Subtypes and Their Distribution among the Domestic Animals and Pigeons in Northwest of Iran. Infect. Genet. Evol. 2020, 86, 104591. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Sumiyoshi, T.; Ohtaki, T.; Matsumoto, J. Prevalence and Molecular Subtyping of Blastocystis from Dairy Cattle in Kanagawa, Japan. Parasitol. Int. 2018, 67, 702–705. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tao, W.; Gong, B.; Yang, H.; Li, Y.; Song, M.; Lu, Y.; Li, W. First Report of Blastocystis Infections in Cattle in China. Vet. Parasitol. 2017, 246, 38–42. [Google Scholar] [CrossRef]
- Wang, J.; Gong, B.; Yang, F.; Zhang, W.; Zheng, Y.; Liu, A. Subtype Distribution and Genetic Characterizations of Blastocystis in Pigs, Cattle, Sheep and Goats in Northeastern China’s Heilongjiang Province. Infect. Genet. Evol. 2018, 57, 171–176. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.-H.; Seo, M.-G.; Kim, H.-Y.; Kim, J.W.; Lee, Y.-R.; Kim, J.H.; Kwon, O.-D.; Kwak, D. Occurrence and Genetic Diversity of Blastocystis in Korean Cattle. Vet. Parasitol. 2018, 258, 70–73. [Google Scholar] [CrossRef]
- AbuOdeh, R.; Ezzedine, S.; Madkour, M.; Stensvold, C.R.; Samie, A.; Nasrallah, G.; AlAbsi, E.; ElBakri, A. Molecular Subtyping of Blastocystis from Diverse Animals in the United Arab Emirates. Protist 2019, 170, 125679. [Google Scholar] [CrossRef]
- Mirzapour, A.; Kiani, H.; Mobedi, I.; Spotin, A.; Seyyed Tabaei, S.J.; Rahimi, M. Frequency of Intestinal Parasites among Zoo Animal by Morphometric Criteria and First Report of the Bivitellobilharzia Nairi from Elephant (Elephasmaximus Maximus) in Iran. Iran. J. Parasitol. 2018, 13, 611–617. [Google Scholar]
- Mohammad, N.A.; Al-Mekhlafi, H.M.; Moktar, N.; Anuar, T.S. Molecular Detection and Subtyping of Blastocystis in Javan Rusa (Cervus timorensis) and Sika Deer (Cervus nippon) from Peninsular Malaysia. Thai J. Vet. Med. 2018, 48, 295–301. [Google Scholar]
- Mohd Zain, S.N.; Farah Haziqah, M.T.; Woh, P.Y.; Fazly Ann, Z.; Vickneshwaran, M.; Mohd Khalid, M.K.N.; Arutchelvan, R.; Suresh, K. Morphological and Molecular Detection of Blastocystis in Wildlife from Tioman Island, Malaysia. Trop. Biomed. 2017, 34, 249–255. [Google Scholar] [PubMed]
- Wang, J.; Gong, B.; Liu, X.; Zhao, W.; Bu, T.; Zhang, W.; Liu, A.; Yang, F. Distribution and Genetic Diversity of Blastocystis Subtypes in Various Mammal and Bird Species in Northeastern China. Parasites Vectors 2018, 11, 522. [Google Scholar] [CrossRef] [Green Version]
- Ni, H.-B.; Gong, Q.-L.; Zhang, N.-Z.; Zhao, Q.; Tao, W.-F.; Qiu, H.-Y.; Fei, Y.-C.; Zhang, X.-X. Molecular Detection of Blastocystis in Black Bears and Sika Deer in Northern China. Parasitol. Res. 2021, 120, 1481–1487. [Google Scholar] [CrossRef]
- Li, J.; Karim, M.R.; Li, D.; Rahaman Sumon, S.M.M.; Siddiki, S.H.M.F.; Rume, F.I.; Sun, R.; Jia, Y.; Zhang, L. Molecular Characterization of Blastocystis sp. in Captive Wildlife in Bangladesh National Zoo: Non-Human Primates with High Prevalence and Zoonotic Significance. Int. J. Parasitol. Parasites Wildl. 2019, 10, 314–320. [Google Scholar] [CrossRef]
- Kim, K.T.; Noh, G.; Lee, H.; Kim, S.H.; Jeong, H.; Kim, Y.; Jheong, W.H.; Oem, J.K.; Kim, T.H.; Kwon, O.D.; et al. Genetic Diversity and Zoonotic Potential of Blastocystis in Korean Water Deer, Hydropotes inermis argyropus. Pathogens 2020, 9, 955. [Google Scholar] [CrossRef]
- Song, J.K.; Yin, Y.L.; Yuan, Y.J.; Tang, H.; Ren, G.J.; Zhang, H.J.; Li, Z.X.; Zhang, Y.M.; Zhao, G.H. First Genotyping of Blastocystis sp. in Dairy, Meat, and Cashmere Goats in Northwestern China. Acta Trop. 2017, 176, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Wang, K.; Gu, Y. Occurrence of Blastocystis sp. and Pentatrichomonas hominis in Sheep and Goats in China. Parasites Vectors 2018, 11, 93. [Google Scholar] [CrossRef] [Green Version]
- Ghimire, T.R.; Bhattarai, N. A Survey of Gastrointestinal Parasites of Goats in a Goat Market in Kathmandu, Nepal. J. Parasit. Dis. 2019, 43, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.C.; Tan, P.C.; Sharma, R.; Sugnaseelan, S.; Suresh, K.G. Genetic Diversity of Caprine Blastocystis from Peninsular Malaysia. Parasitol. Res. 2013, 112, 85–89. [Google Scholar] [CrossRef]
- Adao, D.E.V.; Ducusin, R.J.T.; Padilla, M.A.; Rivera, W.L. Molecular Characterization of Blastocystis Isolates Infecting Farm Animals in Victoria and Pila, Laguna, Philippines. Philipp. Agric. Sci. 2016, 99, 304–310. [Google Scholar]
- Adhikari, J.N.; Adhikari, R.B.; Bhattarai, B.P.; Thapa, T.B.; Ghimire, T.R. Short Communication: A Small-Scale Coprological Survey of the Endoparasites in the Himalayan Goral Naemorhedus goral (Hardwick, 1825) in Nepal. Biodiversitas 2021, 22, 1285–1290. [Google Scholar] [CrossRef]
- Song, J.K.; Hu, R.S.; Fan, X.C.; Wang, S.S.; Zhang, H.J.; Zhao, G.H. Molecular Characterization of Blastocystis from Pigs in Shaanxi Province of China. Acta Trop. 2017, 173, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, Y.; Jiang, Y.; Xing, J.; Tao, D.; Qi, M. First Report of Blastocystis Infection in Pigs from Large Farms in Xinjiang, China. J. Eukaryot. Microbiol. 2020, 67, 642–647. [Google Scholar] [CrossRef]
- Han, J.Q.; Li, Z.; Zou, Y.; Pu, L.H.; Zhu, X.Q.; Zou, F.C.; Huang, C.Q. Prevalence, Molecular Characterization and Risk Factors of Blastocystis sp. from Farmed Pigs in Yunnan Province, Southwestern China. Acta Parasitol. 2020, 65, 1005–1010. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, W.B.; Zou, F.C.; Lin, R.Q.; Zhu, X.Q.; Hou, J.L. Molecular Detection and Subtype Distribution of Blastocystis in Farmed Pigs in Southern China. Microb. Pathog. 2021, 151, 104751. [Google Scholar] [CrossRef]
- Arpitha, G.M.; Sreekumar, C.; Latha, B.R.; Vijaya Bharathi, M. Prevalence and Staining Characteristics of Blastocystis Isolates from Food Animals in Tamil Nadu. Vet. Parasitol. Reg. Stud. Rep. 2018, 11, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, D.; Suwanti, L.T.; Lastuti, N.D.R.; Mufasirin; Suprihati, E.; Yuniarti, W.M.; Widisuputri, N.K.A. Deteksi Molekuler Blastocystis sp. Pada Babi Terinfeksi Di Kabupaten Tabanan Dan Badung, Provinsi Bali, Indonesia. J. Vet. 2020, 21, 227–233. [Google Scholar] [CrossRef]
- Widisuputri, N.K.A.; Suwanti, L.T.; Plumeriastuti, H. A Survey for Zoonotic and Other Gastrointestinal Parasites in Pig in Bali Province, Indonesia. Indones. J. Trop. Infect. Dis. 2020, 8, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Paik, S.; Jung, B.Y.; Lee, H.; Hwang, M.H.; Han, J.E.; Rhee, M.H.; Kim, T.H.; Kwon, O.D.; Kwak, D. Molecular Detection and Subtyping of Blastocystis in Korean Pigs. Korean J. Parasitol. 2019, 57, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Adao, D.E.V.; Ronquillo, I.D.J.; Dela Cruz, Y.K.M.; Pagoso, E.J.A.; Rivera, W.L. Molecular Characterization of Giardia Duodenalis and Blastocystis sp. in Livestock from Animal Farms in Bulacan, Philippines. Southeast Asian J. Trop. Med. Public Health 2019, 50, 450–460. [Google Scholar]
- De La Cruz, C.P.P.; Gorospe, M.M.; Paller, V.G.V. Blastocystis Infection among Backyard-Raised Pigs in Bay, Province of Laguna, the Philippines. Asian J. Microbiol. Biotechnol. Environ. Sci. 2016, 18, 487–494. [Google Scholar]
- Evidor, F.M.R.; Rivera, W.L. Genetic Subtypes of Blastocystis sp. Isolated from Fecal Materials in the Large Intestines of Slaughtered Swine. Philipp. J. Vet. Med. 2016, 53, 59–64. [Google Scholar]
- Murao, L.A.E.; Cadiz, A.E.; De St, Y.; Ladera, L.; Montajes, K.P.; Catherine, M.; Pierre, B.O.; Gilles, G.; Oponda, N.B.; Lagat, F.T.; et al. Exploratory Investigation on the Occurrence, Spatial Distribution, and Risk Factors of Selected Zoonotic Enteropathogens in Davao City Backyard Farms. 2018. Available online: http://ojs.upmin.edu.ph/index.php/banwa-b/article/view/339 (accessed on 30 August 2021).
- Sanyanusin, S.; Mori, H.; Prasertbun, R.; Pintong, A.; Komalamisra, C.; Changbunjong4, T.; Popruk, S.; Mahittikorn, A. Molecular Detection and Genotyping of Blastocystis and Enterocytozoon bieneusi in Humans and Pigs in Nakhon Pathom Province, Thailand. Jt. Int. Trop. Med. Meet. Proc. 2017, 6, 1–6. [Google Scholar]
- Alfellani, M.A.; Taner-Mulla, D.; Jacob, A.S.; Imeede, C.A.; Yoshikawa, H.; Stensvold, C.R.; Clark, C.G. Genetic Diversity of Blastocystis in Livestock and Zoo Animals. Protist 2013, 164, 497–509. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Seo, M.-G.; Oem, J.-K.; Kim, Y.-S.; Lee, S.-Y.; Kim, J.; Jeong, H.; Jheong, W.-H.; Kim, Y.; Lee, W.-J.; et al. Molecular Detection and Subtyping of Blastocystis Detected in Wild Boars (Sus scrofa) in South Korea. J. Wildl. Dis. 2020, 56, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, K.; Sarkari, B.; Mansouri, M.; Motazedian, M.H. Zoonotic Intestinal Protozoan of the Wild Boars, Sus scrofa, in Persian Gulf’s Coastal Area (Bushehr Province), Southwestern Iran. Vet. World 2016, 9, 1047–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, M.; Song, J.K.; Yang, F.; Zou, M.; Wang, P.X.; Wang, D.; Zhang, H.J.; Zhao, G.H.; Lin, Q. First Genotyping of Blastocystis in Yaks from Qinghai Province, Northwestern China. Parasites Vectors 2019, 12, 171. [Google Scholar] [CrossRef]
- Li, W.; Liu, X.; Gu, Y.; Liu, J.; Luo, J. Prevalence of Cryptosporidium, Giardia, Blastocystis, and Trichomonads in Domestic Cats in East China. J. Vet. Med. Sci. 2019, 81, 890–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patagi, A.L.; Suwanti, L.T.; Chairul, A.; Yuniarti, W.M.; Sarmanu; Suprihati, E. Prevalance of Gastrointestinal Protozoa of Cats in Animal Hospital and Animal Clinic in Surabaya. J. Parasite Sci. 2018, 2, 61–66. [Google Scholar]
- Khademvatan, S.; Abdizadeh, R.; Rahim, F.; Hashemitabar, M.; Ghasemi, M.; Tavalla, M. Stray Cats Gastrointestinal Parasites and Its Association with Public Health in Ahvaz City, South Western of Iran. Jundishapur J. Microbiol. 2014, 7. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, I.; Bozorg-Ghalati, F.; Gazzonis, A.L.; Manfredi, M.T.; Motazedian, M.H.; Mohammadpour, N. First Molecular Subtyping and Phylogeny of Blastocystis sp. Isolated from Domestic and Synanthropic Animals (Dogs, Cats and Brown Rats) in Southern Iran. Parasites Vectors 2020, 13, 365. [Google Scholar] [CrossRef]
- Kwak, D.; Seo, M.-G. Genetic Analysis of Zoonotic Gastrointestinal Protozoa and Microsporidia in Shelter Cats in South Korea. Pathogens 2020, 9, 894. [Google Scholar] [CrossRef]
- Farah Haziqah, M.T.; Chandrawathani, P.; Douadi, B.; Suresh, K.; Wilson, J.J.; Mohd Khalid, M.K.N.; Rajamanikam, A.; Lewis, J.W.; Mohd Zain, S.N. Impact of PH on the Viability and Morphology of Blastocystis Isolates. Trop. Biomed. 2018, 35, 501–510. [Google Scholar]
- Mohammad Rahimi, H.; Nemati, S.; Mirjalali, H.; Sharifdini, M.; Zali, M.R. Molecular Characterization and Identification of Blastocystis and Its Subtypes from Raccoon (Procyon lotor) in North of Iran. Parasitol. Res. 2020, 119, 2741–2745. [Google Scholar] [CrossRef]
- Liao, S.; Lin, X.; Sun, Y.; Qi, N.; Lv, M.; Wu, C.; Li, J.; Hu, J.; Yu, L.; Cai, H.; et al. Occurrence and Genotypes of Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. in Household, Shelter, Breeding, and Pet Market Dogs in Guangzhou, Southern China. Sci. Rep. 2020, 10, 17736. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cuttell, L.; Bielefeldt-Ohmann, H.; Inpankaew, T.; Owen, H.; Traub, R.J. Diversity of Blastocystis Subtypes in Dogs in Different Geographical Settings. Parasites Vectors 2013, 6, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohaghegh, M.A.; Vafaei, M.R.; Ghomashlooyan, M.; Azami, M.; Falahati, M.; Azadi, Y.; Yousefi, H.A.; Jabalameli, Z.; Hejazi, S.H. A Wide Diversity of Zoonotic Intestinal Parasites in Domestic and Stray Dogs in Rural Areas of Kermanshah Province, Iran. Trop. Biomed. 2018, 35, 82–90. [Google Scholar]
- Mirbadie, S.R.; Najafi Nasab, A.; Mohaghegh, M.A.; Norouzi, P.; Mirzaii, M.; Spotin, A. Molecular Phylodiagnosis of Echinococcus granulosus Sensu Lato and Taenia hydatigena Determined by Mitochondrial Cox1 and SSU-RDNA Markers in Iranian Dogs: Indicating the First Record of Pig Strain (G7) in Definitive Host in the Middle East. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 88–95. [Google Scholar] [CrossRef]
- Deng, L.; Yao, J.-X.; Liu, H.-F.; Zhou, Z.-Y.; Chai, Y.-J.; Wang, W.-Y.; Zhong, Z.-J.; Deng, J.-L.; Ren, Z.-H.; Fu, H.-L.; et al. First Report of Blastocystis in Giant Pandas, Red Pandas, and Various Bird Species in Sichuan Province, Southwestern China. Int. J. Parasitol. Parasites Wildl. 2019, 9, 298–304. [Google Scholar] [CrossRef]
- Zanzani, S.A.; Gazzonis, A.L.; Epis, S.; Manfredi, M.T. Study of the Gastrointestinal Parasitic Fauna of Captive Non-Human Primates (Macaca fascicularis). Parasitol. Res. 2016, 115, 307–312. [Google Scholar] [CrossRef]
- Li, T.C.; Li, Z.; Zhang, Y.L.; Chen, W.J.; Dong, X.L.; Yang, J.F.; Li, H.X.; Zou, F.C. Assessment of the Subtypes and the Zoonotic Risk of Blastocystis sp. of Experimental Macaques in Yunnan Province, Southwestern China. Parasitol. Res. 2020, 119, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Casim, L.F.; Bandal, M.Z.; Gonzales, J.C.B.; Valdez, E.M.M., Jr.; Chavez, G.C.S.; Paller, V.G.V. Enteroparasites of Captive Long-Tailed Macaques (Macaca fascicularis) from National Wildlife Research and Rescue Center, Diliman, Quezon City, Philippines. Asian J. Conserv. Biol. 2015, 4, 54–61. [Google Scholar]
- Vaisusuk, K.; Saijuntha, W.; Sedlak, S.; Thanchomnang, T.; Pilap, W.; Suksavate, W.; Stensvold, C.R.; Tantrawatpan, C. Blastocystis Subtypes Detected in Long-Tailed Macaques in Thailand—Further Evidence of Cryptic Host Specificity. Acta Trop. 2018, 184, 78–82. [Google Scholar] [CrossRef]
- Labes, E.M.; Hegglin, D.; Grimm, F.; Nurcahyo, W.; Harrison, M.E.; Bastian, M.L.; Deplazes, P. Intestinal Parasites of Endangered Orangutans (Pongo pygmaeus) in Central and East Kalimantan, Borneo, Indonesia. Parasitology 2010, 137, 123–135. [Google Scholar] [CrossRef] [Green Version]
- Dalimi, A.; Motamedi, G.; Hablolvarid, M.H.; Abdoli, A. Alimentary Tract Parasites of Vervet Monkeys (Cercopithecus aethiops): A Potential Reservoir for Human Transmission. Arch. Razi Inst. 2016, 71, 277–281. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Y.; Qiao, H.; Li, S.; Wang, H.; Zhang, N.; Zhang, X. Cockroach as a Vector of Blastocystis sp. Is Risk for Golden Monkeys in Zoo. Korean J. Parasitol. 2020, 58, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Adrus, M.; Zainudin, R.; Ahamad, M.; Jayasilan, M.A.; Abdullah, M.T. Gastrointestinal Parasites of Zoonotic Importance Observed in the Wild, Urban, and Captive Populations of Non-Human Primates in Malaysia. J. Med. Primatol. 2019, 48, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Farah Haziqah, M.T.; Chandrawathani, P.; Mohd, Z.S.N.; Suresh, K.G.; Hemalatha, C.; Premaalatha, B. A Preliminary Study of Blastocystis sp. Isolated from Chicken in Perak and Selangor, Malaysia. Malays. J. Vet. Res. 2014, 5, 21–25. [Google Scholar]
- Farah Haziqah, M.T.; Chandrawathani, P.; Suresh, K.G.; Wilson, J.-J.; Mohd Khairul Nizam, M.K.; Rajamanikam, A.; Bathmanaban, P.; Siti, N.M.Z. Prevalence, Ultrastructure and Subtypes of Blastocystis In Chickens (Gallus gallus) From Peninsular Malaysia. Southeast Asian J. Trop. Med. Public Health 2018, 49, 921–932. [Google Scholar]
- Zhang, X.; Qiao, J.Y.; Wu, X.M.; Ma, Q.Y.; Hu, H.; Wang, J.; Che, L.F. Ascaris Spp. and Capillaria Caudinflata Infections in Captive-Bred Crested Ibis (Nipponia nippon) in China. Zoo Biol. 2014, 34, 80–84. [Google Scholar] [CrossRef]
- Asghari, A.; Sadraei, J.; Pirestani, M.; Mohammadpour, I. First Molecular Identification and Subtype Distribution of Blastocystis sp. Isolated from Hooded Crows (Corvus cornix) and Pigeons (Columba livia) in Tehran Province, Iran. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 25–30. [Google Scholar] [CrossRef]
- Chandrasekaran, H.; Govind, S.K.; Panchadcharam, C.; Bathmanaban, P.; Raman, K.; Thergarajan, G. High Lipid Storage in Vacoular Forms of Subtype 6 Blastocystis sp. in Ostrich. Parasites Vectors 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Xiao, X.; Zhou, S.-H.; Jiang, N.; Tian, D.-Z.; Zhou, Z.-M.; Zhang, M.; Ke, H.; Jiang, X.-C.; Lv, W.-L.; Gao, Q.-H. First Record of Leptospira and Blastocystis Infections in Captive Flying Squirrels (Trogopterus xanthipes) from Enshi County, China. Acta Trop. 2019, 197, 105065. [Google Scholar] [CrossRef]
- Chai, Y.; Deng, L.; Liu, H.; Yao, J.; Zhong, Z.; Fu, H.; Shen, L.; Zhou, Z.; Deng, J.; Hu, Y.; et al. First Subtyping of Blastocystis sp. from Pet Rodents in Southwestern China. Int. J. Parasitol. Parasites Wildl. 2020, 11, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, Y.; Jiang, Y.; Wang, W.; Chao, L.; Sun, R.; Li, J.; Karim, M.R.; Qi, M. Molecular Identification and Subtyping of Blastocystis sp. In Laboratory Rats in China. Parasite 2020, 27, 35. [Google Scholar] [CrossRef] [PubMed]
- Prasetyo, R.H. Survey of House Rat Intestinal Parasites from Surabaya District, East Java, Indonesia That Can Cause Opportunistic Infections in Humans. Southeast Asian J. Trop. Med. Public Health 2016, 47, 194–198. [Google Scholar]
- Katsumata, M.; Yoshikawa, H.; Tokoro, M.; Mizuno, T.; Nagamoto, T.; Hendarto, J.; Asih, P.B.S.; Rozi, I.E.; Kimata, I.; Takami, K.; et al. Molecular Phylogeny of Blastocystis Isolates from Wild Rodents Captured in Indonesia and Japan. Parasitol. Res. 2018, 117, 2841–2846. [Google Scholar] [CrossRef] [PubMed]
- Seifollahi, Z.; Sarkari, B.; Motazedian, M.H.; Asgari, Q.; Ranjbar, M.J.; Abdolahi Khabisi, S. Protozoan Parasites of Rodents and Their Zoonotic Significance in Boyer-Ahmad District, Southwestern Iran. Vet. Med. Int. 2016, 2016, 3263868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Premaalatha, B.; Chandrawathani, P.; Priscilla, F.X.; Farah Haziqah, M.; Jamnah, O.; Zaini, C.M.; Ramlan, M. A Survey of Endoparasite and Ectoparasite Infections of Wild Rats Caught in Areas of Ipoh and Kuala Lumpur, Malaysia. Malays. J. Vet. Res. 2017, 8, 29–34. [Google Scholar]
- Farah Haziqah, M.T.; Mohd Zain, S.N.; Chandrawathani, P.; Premaalatha, B.; Mohd Khairul Nizam, M.K.; Arutchelvan, R.; Suresh, K. Genetic Diversity of Rodent Blastocystis sp. From Peninsular Malaysia. Trop. Biomed. 2018, 35, 586–592. [Google Scholar]
- Kalani, H.; Daryani, A.; Fakhar, M.; Sharif, M.; Faridnia, R. A Survey on Intestinal Parasites in Swiss Webster Mice. J. Maz. Univ. Med. Sci. 2013, 22, 63–69. [Google Scholar]
- Chamavit, P.; Sahaisook, P.; Niamnuy, N. The Majority of Cockroaches from the Samutprakarn Province of Thailand Are Carriers of Parasitic Organisms. EXCLI J. 2011, 10, 218–222. [Google Scholar]
- Dokmaikaw, A.; Suntaravitun, P. Prevalence of Parasitic Contamination of Cockroaches Collected from Fresh Markets in Chachoengsao Province, Thailand. Kobe J. Med. Sci. 2019, 65, E118–E123. [Google Scholar]
- Oğuz, B.; Özdal, N.; Orunç Kilinç, Ö.; Değer, M.S. First Investigation on Vectorial Potential of Blattella Germanica in Turkey | Türkiye’de Blattella Germanica’nın Vektörlük Potansiyeli Üzerine Ilk Araştırma. Ank. Univ. Vet. Fak. Derg. 2017, 64, 141–144. [Google Scholar] [CrossRef]
- Motevalli-Haghi, S.F.; Shemshadian, A.; Nakhaei, M.; Faridnia, R.; Dehghan, O.; Malekzadeh Shafaroudi, M.; Nejadi Kelarijani, M.; Nikookar, S.H.; Kalani, H.; Fakhar, M. First Report of Lophomonas Spp. in German Cockroaches (Blattella Germanica) Trapped in Hospitals, Northern Iran. J. Parasit. Dis. 2021. [Google Scholar] [CrossRef]
- Farah Haziqah, M.T.; Nur Asyiqin, M.N.; Mohd Khalid, M.K.N.; Suresh, K.; Rajamanikam, A.; Chandrawathani, P.; Mohd Zain, S.N. Current Status of Blastocystis in Cockroaches. Trop. Biomed. 2017, 34, 741–745. [Google Scholar]
- Natalia, F.; Suwanti, L.T.; Suprihati, E.; Kusnoto; Koesdarto, S.; Srianto, P. Morphological Detection of the Intestinal Parasite Blastocystis sp. in Fresh and Cultured Feces of Pet Sugar Glider (Petaurus breviceps) (Mammalia: Marsupialia: Petauridae) in Surabaya, Indonesia. Philipp. J. Vet. Med. 2018, 55, 91–96. [Google Scholar]
- Li, T.-S.; Zou, Y.; Ma, Y.-T.; Ma, Y.-Y.; Chen, H.; Liang, X.-X.; Cong, W.; Sun, X.-L.; Zhu, X.-Q. Molecular Characterization of Eimeria spp. and Blastocystis in Rabbits in Shandong Province, China. Parasitol. Res. 2020, 119, 1547–1551. [Google Scholar] [CrossRef]
- Javanmard, E.; Rahimi, H.M.; Niyyati, M.; Aghdaei, H.A.; Sharifdini, M.; Mirjalali, H.; Zali, M.R.; Karanis, P. Molecular Analysis of Blastocystis sp. And Its Subtypes from Treated Wastewater Routinely Used for Irrigation of Vegetable Farmlands in Iran. J. Water Health 2019, 17, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Ithoi, I.; Jali, A.; Mak, J.W.; Wan Sulaiman, W.Y.; Mahmud, R. Occurrence of Blastocystis in Water of Two Rivers from Recreational Areas in Malaysia. J. Parasitol. Res. 2011, 2011, 123926. [Google Scholar] [CrossRef] [Green Version]
- Richard, R.L.; Ithoi, I.; Majid, M.A.A.; Wan Sulaiman, W.Y.; Tan, T.C.; Nissapatorn, V.; Lim, Y.A.L. Monitoring of Waterborne Parasites in Two Drinking Water Treatment Plants: A Study in Sarawak, Malaysia. Int. J. Environ. Res. Public Health 2016, 13, 641. [Google Scholar] [CrossRef] [Green Version]
- Noradilah, S.A.; Lee, I.L.; Anuar, T.S.; Salleh, F.M.; Manap, S.N.A.A.; Mohtar, N.S.H.M.; Azrul, S.M.; Abdullah, W.O.; Moktar, N. Occurrence of Blastocystis sp. in Water Catchments at Malay Villages and Aboriginal Settlement during Wet and Dry Seasons in Peninsular Malaysia. PeerJ 2016, 4, e2541. [Google Scholar] [CrossRef] [Green Version]
- Banaticla, J.E.G.; Rivera, W.L. Detection and Subtype Identification of Blastocystis Isolates from Wastewater Samples in the Philippines. J. Water Health 2011, 9, 128–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaman, Ü.; Kolören, Z.; Seferoğlu, O.; Ayaz, E.; Demirel, E. Presence of Parasites in Environmental Waters in Samsun and Its Districts. Turk. Parazitolojii Derg. 2017, 41, 19–21. [Google Scholar] [CrossRef]
- Koloren, Z.; Gulabi, B.B.; Karanis, P. Molecular Identification of Blastocystis sp. Subtypes in Water Samples Collected from Black Sea, Turkey. Acta Trop. 2018, 180, 58–68. [Google Scholar] [CrossRef]
- Kolören, Z.; Karaman, Ü. Investigation of Blastocystis Subspecies in Water Samples Collected from Ordu Province. Turk. Parazitolojii Derg. 2019, 43, 111–117. [Google Scholar] [CrossRef]
- Isazadeh, M.; Mirzaii-Dizgah, I.; Shaddel, M.; Homayouni, M.M. The Prevalence of Parasitic Contamination of Fresh Vegetables in Tehran, Iran. Turk. Parazitolojii Derg. 2020, 44, 143–148. [Google Scholar] [CrossRef]
- Al Nahhas, S.; Aboualchamat, G. Investigation of Parasitic Contamination of Salad Vegetables Sold by Street Vendors in City Markets in Damascus, Syria. Food Waterborne Parasitol. 2020, 21, e00090. [Google Scholar] [CrossRef]
- Han, T.-H.; Park, S.-H.; Chung, J.-Y.; Jeong, H.-W.; Jung, J.; Lee, J.-I.; Hwang, Y.-O.; Kim, I.-Y.; Lee, J.-H.; Jung, K. Detection of Pathogenic Viruses in the Ambient Air in Seoul, Korea. Food Environ. Virol. 2018, 10, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, P.A.; Jaimes, J.E.; Ramírez, J.D. A Summary of Blastocystis Subtypes in North and South America. Parasites Vectors 2019, 12, 376. [Google Scholar] [CrossRef]
- Zanetti, A.D.S.; Malheiros, A.F.; de Matos, T.A.; Longhi, F.G.; Moreira, L.M.; Silva, S.L.; Castrillon, S.K.I.; Ferreira, S.M.B.; Ignotti, E.; Espinosa, O.A. Prevalence of Blastocystis sp. Infection in Several Hosts in Brazil: A Systematic Review and Meta-Analysis. Parasit. Vectors 2020, 13, 30. [Google Scholar] [CrossRef] [PubMed]
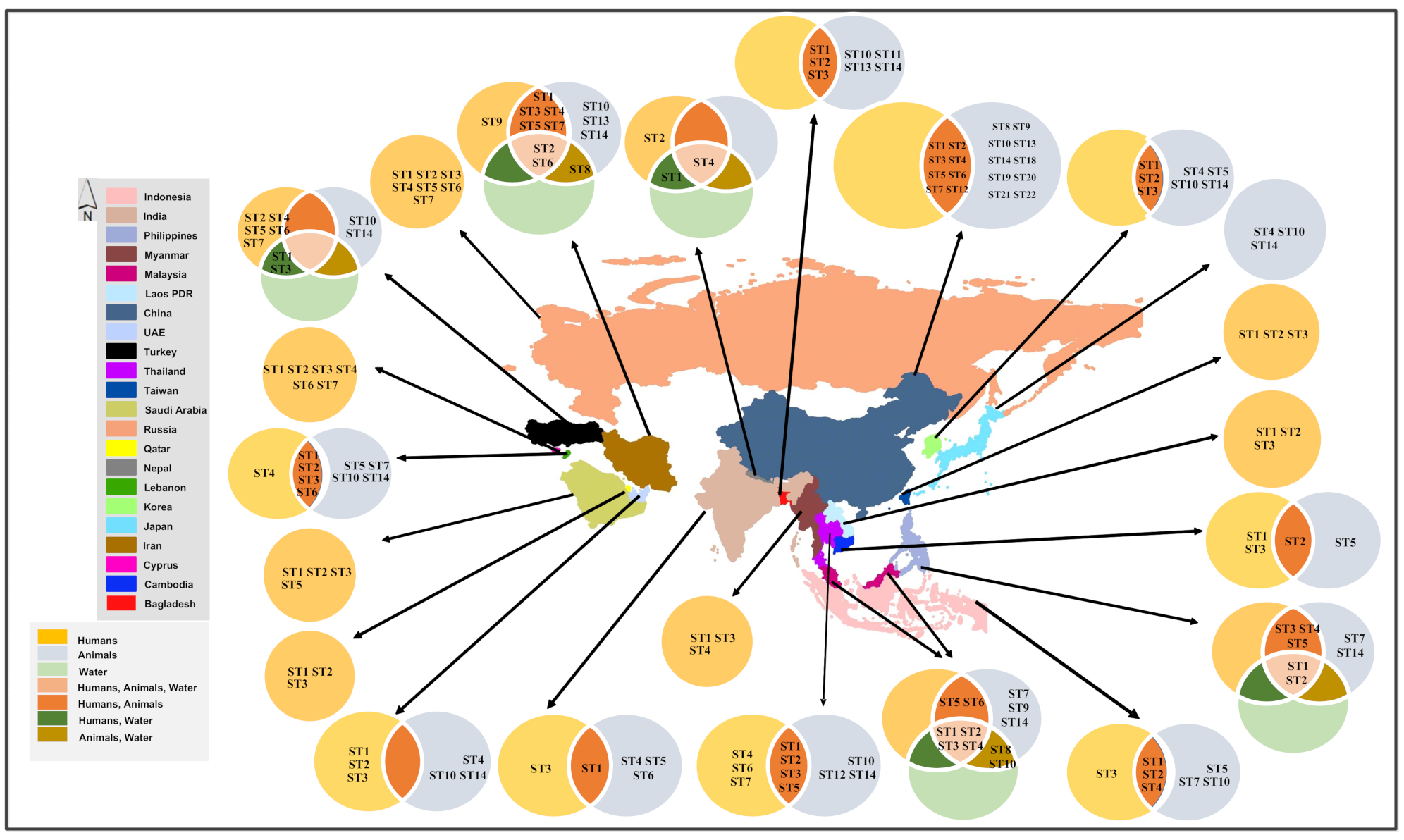
| Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|
| Bangladesh | 5679 | 795 (14.0) | NA | CM, 1VC | Barua et al. [34] |
| Israel | 45,978 | 5422 (11.8) | NA | CM, IVC | Ben-Shimol et al. [35] |
| China | 170 | 1 (0.5) | NA | MOL | Zhang et al. [36] |
| China | 609 | 87 (14.3) | ST1, ST2, ST3 | MOL | Qi et al. [37] |
| China | 466 | 71 (15.2) | ST1, ST3, ST6, ST7 | IVC, MOL | Ning et al. [38] |
| Cambodia | 308 | 15 (4.9) | NA | CM | Liao et al. [39] |
| India | 195 | 32 (16.4) | NA | CN | Rayan et al. [40] |
| Indonesia | 492 | 147 (29.9) | ST1, ST2, ST3 | IVC, MOL | Yoshikawa et al. [41] |
| Indonesia | 99 | 33 (33.3) | ST1, ST2, ST3 | MOL | Zulfa et al. [42] |
| Indonesia | 141 | 58 (41.1) | ST1, ST3, ST4 | IVC, MOL | Sari et al. [43] |
| Indonesia | 219 | 15 (6.8) | NA | CM | Subahar et al. [44] |
| Indonesia | 157 | 44 (28.0) | NA | CM | Sari et al. [45] |
| Iran | 124,366 | 3986 (3.2) | NA | CM | Ashtiani et al. [46] |
| Iran | 864 | 36 (4.1) | ST1, ST2, ST3 | CM, IVC, MOL | Niaraki et al. [47] |
| Iran | 366 | 11 (3.1) | NA | CM | Mahmoudvand et al. [48] |
| Iran | 650 | 37 (5.7) | NA | CM | Abdi et al. [49] |
| Iran | 1100 | 149 (13.5) | NA | CM | Daryani et al. [50] |
| Iran | 350 | 15 (4.3) | NA | CM | Hazrati Tappeh et al. [51] |
| Iran | 854 | 26 (3.0) | NA | CM | Norouzi et al. [52] |
| Iran | 200 | 35 (17.5) | NA | CM | Babakhani et al. [53] |
| Iran | 400 | 85 (21.3) | NA | CM | Bahmani et al. [54] |
| Iran | 306 | 9 (2.9) | NA | CM | Saki and Amraee [55] |
| Iran | 1465 | 31 (2.1) | NA | CM | Turki et al. [56] |
| Iraq | 107 | 4 (3.7) | NA | CM | Mahdi and Al-Saadoon [57] |
| Lebanon | 249 | 157 (63.0) | ST1, ST2, ST3 | MOL | Osman et al. [58] |
| Malaysia | 71 | 66 (93.0) | NA | CM | Abd. Ghani and Yusof [59] |
| Malaysia | 300 | 77 (25.7) | NA | IVC | Abdulsalam et al. [60] |
| Malaysia | 307 | 65 (21.2) | NA | CM | Al-Harazi et al. [61] |
| Malaysia | 342 | 4 (1.2) | NA | CM | Sinniah et al. [62] |
| Malaysia | 1760 | 186 (10.6) | ST1, ST2, ST3, ST4, ST5 | IVC, MOL | Nithyamathi et al. [63] |
| Malaysia | 116 | 2 (1.7) | NA | CM | Tang and Kamel [64] |
| Malaysia | 92 | 77 (83.7) | NA | CM | Adli et al. [65] |
| Nepal | 342 | 4 (1.2) | NA | CM | Mukhiya et al. [66] |
| Saudi Arabia | 1289 | 11 (0.9) | NA | CM | Al-Mohammed et al. [67] |
| Saudi Arabia | 581 | 10 (1.7) | NA | CM | Bakarman et al. [68] |
| Thailand | 203 | 9 (4.4) | NA | CM | Suntaravitun and Dokmaikaw [69] |
| Thailand | 1909 | 626 (32.8) | NA | CM | Sanprasert et al. [70] |
| Thailand | 370 | 118 (31.9) | ST1, ST2, ST6 | MOL | Thathaisong et al. [71] |
| Thailand | 233 | 29 (12.5) | ST1, ST2, ST3 | IVC, MOL | Pipatsatitpong et al. [72] |
| Thailand | 299 | 10 (3.3) | NA | CM | Punsawad et al. [73] |
| Thailand | 263 | 46 (17.5) | NA | CM, IVC | Assavapongpaiboon et al. [74] |
| Thailand | 331 | 44 (13.3) | ST1, ST3 | IVC, MOL | Boondit et al. [75] |
| Thailand | 202 | 3 (1.5) | NA | CM | Kitvatanachai and Rhongbutsri [76] |
| Thailand | 274 | 37 (13.5) | NA | CM | Popruk et al. [77] |
| Turkey | 195 | 28 (14.4) | NA | CM | Güdücüoğlu et al. [78] |
| Turkey | 328 | 77 (23.5) | NA | CM | Hamamci et al. [79] |
| Turkey | 468 | 35 (7.4) | ST1, ST2, ST3, ST7 | CM, IVC, MOL | Sankur et al. [80] |
| Turkey | 1181 | 7 (0.6) | NA | CM | Calik et al. [81] |
| Turkey | 219 | 97 (44.3) | ST1, ST2, ST3, ST4 | MOL | Dogan et al. [82] |
| Host | Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Cancer patients (children) | Iran | 52 | 11 (21.2) | NA | CM | Salehi Kahish et al. [83] |
| Cancer patients (children) | Iran | 200 | 24 (12.0) | ST1, ST2, ST3, ST7 | MOL | Asghari et al. [84] |
| Cancer patients (children) | Iran | 52 | 11 (21.2) | NA | CM | Salehi Kahyesh et al. [85] |
| Cancer patients (children) | Iran | 89 | 5 (5.6) | NA | CM | Zabolinejad et al. [86] |
| Cancer patients | Iran | 67 | 16 (23.9) | NA | CM, MOL | Mahmoudvand et al. [87] |
| Cancer patients | China | 381 | 27 (7.1) | ST1, ST3 | MOL | Zhang et al. [88] |
| Cancer patients | Malaysia | 61 | 13 (21.3) | NA | IVC | Chandramathi et al. [89] |
| Cancer patients | Saudi Arabia | 138 | 38 (27.5) | ST1, ST2, ST5 | MOL | Mohamed et al. [90] |
| Cancer patients | Turkey | 232 | 25 (10.8) | ST1, ST2, ST3 | CM, IVC, MOL | Yersal et al. [91] |
| Cancer patients | Turkey | 201 | 29 (14.4) | ST1, ST2, ST3 | CM, MOL | Mulayim et al. [92] |
| HIV/AIDS cases | China | 324 | 12 (3.7) | ST1, ST3, ST4, ST7, ST12 | MOL | Teng et al. [93] |
| HIV/AIDS cases | China | 720 | 154 (21.4) | NA | IVC | Tian et al. [94] |
| HIV/AIDS cases | China | 302 | 49 (16.2) | NA | IVC | Tian et al. [95] |
| HIV/AIDS cases | China | 79 | 11 (13.9) | NA | IVC | Tian et al. [96] |
| H IV/AIDS cases | China | 398 | 27 (6.8) | NA | MOL | Zhang et al. [97] |
| HIV/AIDS cases | China | 311 | 12 (3.9) | ST1, ST3, ST4, ST7 | MOL | Zhang et al. [98] |
| HIV/AIDS cases | China | 505 | 21 (4.2) | NA | MOL | Zhu-Hua et al. [99] |
| HIV/AIDS cases | India | 452 | 13 (2.9) | NA | CM | Ramana et al. [100] |
| HIV/AIDS cases | India | 200 | 14 (7.0) | NA | CM | Khalil et al. [101] |
| HIV/AIDS cases | Iran | 31 | 7 (22.6) | NA | CM | Berenji et al. [102] |
| HIV/AIDS cases | Iran | 60 | 10 (16.7) | NA | CM | Yosefi et al. [103] |
| HIV/AIDS cases | Iran | 356 | 14 (3.9) | NA | CM | Agholi et al. [104] |
| HIV/AIDS cases | Iran | 102 | 2 (1.9) | NA | CM | Masoumi-Asl et al. [105] |
| HIV/AIDS cases | Iran | 73 | 2 (2.7) | NA | CM | Anvari-Tafti et al. [106] |
| HIV/AIDS cases | Iran | 268 | 51 (19.0) | ST1, ST2, ST3, ST4 | MOL | Piranshahi et al. [107] |
| HIV/AIDS cases | Laos | 137 | 36 (26.3) | NA | CM | Paboriboune et al. [108] |
| HIV/AIDS cases | Nepal | 146 | 9 (6.2) | NA | CM | Sherchan et al. 2012 [109] |
| HIV/AIDS cases | Nepal | 112 | 1 (0.9) | NA | CM | Ghimire et al. [110] |
| HIV/AIDS cases | Turkey | 65 | 7 (10.8) | NA | CM | Zorbozan et al. [111] |
| HIV/AIDS cases | Uzbekistan | 500 | 211 (42.2) | NA | Davis et al. [112] | |
| Tuberculosis | Iran | 161 | 19 (11.8) | ST1, ST2, ST3 | CM, MOL | Taghipour et al. [113] |
| Tuberculosis | Iran | 161 | 19 (11.8) | NA | CM | Taghipour et al. [114] |
| Pulmonary tuberculosis | Uzbekistan | 300 | 161 (53.6) | NA | Davis et al. [112] | |
| Pulmonary tuberculosis | China | 369 | 23 (6.2) | NA | CM, 1VC | Li et al. [115] |
| Pulmonary tuberculosis | China | 369 | 23 (6.2) | NA | CM, 1VC | Li et al. [116] |
| pulmonary tuberculosis | Iran | 50 | 9 (18.0) | NA | CM | Taghipour et al. [117] |
| Renal transplant recipients | Iran | 150 | 7 (4.7) | NA | CM | Azami et al. [118] |
| Immunocompromised children with diarrhea | Indonesia | 42 | 23 (54.8) | NA | IVC | Idris et al. [119] |
| Immunocompromised children with diarrhea | Turkey | 62 | 6 (9.7) | NA | CM | Caner et al. [120] |
| Immunocompromised patients | Iran | 265 | 11 (4.2) | NA | CM | Rasti et al. [121] |
| Immunocompromised patients | Iran | 204 | 62 (30.4) | NA | CM | Izadi et al. [122] |
| Immunodeficient patients | Iran | 190 | 32 (16.8) | NA | CM | Esteghamati et al. [123] |
| Immunosuppressive drugs recipient | Iran | 494 | 49 (10.3) | NA | CM | Mirzaei et al. [124] |
| Immunocompromised patients | Saudi Arabia | 136 | 7 (5.2) | NA | CM | Al-Megrin et al. [125] |
| Common variable immune deficiency (CVID) syndrome patients | Turkey | 37 | 3 (8.1) | NA | CM | Uysal et al. [126] |
| Host | Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Children with diarrhea | China | 850 | 26 (3.1) | NA | MOL | Zhang et al. [36] |
| Children with diarrhea | Indonesia | 57 | 36 (63.1) | ST1, ST2, ST3, ST4 | MOL | Zulfa et al. [42] |
| Children with diarrhea | Turkey | 60 | 4 (6.7) | NA | CM | Maçin et al. [127] |
| Children with diarrhea | Iran | 400 | 8 (2.0) | NA | CM | Asadi et al. [128] |
| Children with diarrhea | Qatar | 580 | 27 (4.7) | NA | MOL | Boughattas et al. [129] |
| Children with diarrhea | Nepal | 588 | 5 (0.9) | NA | CM | Dahal et al. [130] |
| Children with diarrhea | Iran | 160 | 37 (23.1) | NA | CM | Khalili et al. [131] |
| Children with GID | Iran | 500 | 81 (16.2) | NA | CM | Kiani et al. [132] |
| Children with GID | Thailand | 82 | 13 (15.9) | NA | CM, IVC | Awae et al. [133] |
| Children with GID | Russia | 1273 | 62 (4.9) | ST1, ST2, ST3, ST4, ST7 | CM, MOL | Sigidaev et al. [134] |
| Children with GID | Turkey | 84 | 18 (21.4) | ST1, ST3, ST4 | MOL | Dogan et al. [82] |
| Patients with diarrhea | Indonesia | 389 | 22 (5.7) | NA | CM | Oyofo et al. [135] |
| Patients with diarrhea | China | 271 | 13 (4.8) | NA | MOL | Zhang et al. [136] |
| Patients with diarrhea | Korea | 117 | 8 (6.8) | NA | MOL | Won et al. [137] |
| Patients with diarrhea | Iran | 134 | 28 (20.9) | ST1, ST2, ST3 | CM, MOL | Jalallou et al. [138] |
| Patients with diarrhea | Iran | 2023 | 1357 (67.1) | NA | CM | Najafi et al. [139] |
| Patients with GID | Iran | 1301 | 350 (26.9) | NA | CM | Kiani et al. [140] |
| Patients with GID | Iran | 287 | 65 (22.7) | ST1, ST2, ST3, ST5 | IVC, MOL | Moosavi et al. [141] |
| Patients with GID | Iran | 23* | 23 | ST1 | CM, MOL | Shahbazi et al. [142] |
| IBD patients | Iran | 71 | 9 (12.7) | ST1, ST3 | IVC, MOL | Mirjalali et al. [143] |
| Adolescents with IBS | Indonesia | 137 | 50 (36.5) | ST1, ST2, ST3 | MOL | Kesuma et al. [144] |
| IBS patients | India | 150 | 50 (33.3) | ST1, ST3 | CM, IVC, MOL | Das et al. [145] |
| IBS patients | Iran | 100 | 15 (15.0) | NA | CM | Shafiei et al. [146] |
| IBS patients | Iran | 122 | 24 (19.7) | ST1, ST3, ST4, ST5 | MOL | Khademvatan et al. [147] |
| IBS patients | Iraq | 78 | 38 (48.7) | NA | CM, IVC | Sayal et al. [148] |
| IBS patients | Thailand | 66 | 11 (16.7) | NA | IVC | Surangsrirat et al. [149] |
| Patients with GID | Iraq | 579 | 98 (16.9) | NA | CM, IVC | Merza et al. [150] |
| Patients with GID | Iraq | 249 | 92 (36.9) | NA | CM | Mutlag et al. [151] |
| Patients with GID | Thailand | 5 | 5 (100.0) | ST3, ST6, ST7 | CM, IVC, MOL | Sanpool et al. [152] |
| Patients with diarrhea | Turkey | 272 | 16 (5.9) | NA | CM, MOL | Koltas et al. [153] |
| Patients with GID | Turkey | 490 | 89 (18.2) | NA | CM, IVC | Aykur et al. [154] |
| Patients with GID | Turkey | 14,246 | 689 (4.8) | NA | CM | Usluca et al. [155] |
| Patients with GID | Turkey | 2334 | 134 (5.7) | NA | CM | Cekin et al. [156] |
| Patients with GID | Iran | 152 | 16 (10.5) | ST1, ST2, ST3 | CM, IVC, MOL | Beiromvand et al. [157] |
| Patients with diarrhea | Singapore | 193 | 1 (0.5) | NA | CM, MOL | Feurle et al. [158] |
| Patients with GID | Saudi Arabia | 114 | 15 (13.2) | NA | CM | Hawash et al. [159] |
| Patients with GID | Turkey | 5624 | 136 (2.4) | NA | CM | Alver et al. [160] |
| Patients with GID | Turkey | 17756 | 778 (4.4) | NA | CM | İnceboz et al. [161] |
| Patients with GID | Iran | 670 | 38 (5.7) | NA | IVC | Rostami Nejad et al. [162] |
| Patients with GID | Pakistan | 339 | 59 (17.4) | NA | CM | Haider et al. [163] |
| Patients with GID | Turkey | 29 * | 29 | ST1, ST2, ST3, ST4 | CM, MOL | Sakalar et al. [164] |
| Host | Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Mentally disabled children | Iran | 362 | 20 (5.5) | NA | CM | Sharif et al. [165] |
| Mentally disabled children and adults | Iran | 225 | 9 (4.0) | NA | CM | Hazrati Tappeh et al. [166] |
| Psychiatric patients | Iran | 65 | 15 (23.1) | NA | CM | Khalili et al. [167] |
| Mentally disabled individuals | Iran | 173 | 29 (16.8) | NA | CM | Saeidinia et al. [168] |
| Mentally disabled individuals | Iran | 133 | 12 (9.0) | NA | CM | Shokri et al. [169] |
| Mentally disabled individuals and elderly people | Iran | 243 | 81 (33.3) | NA | CM | Rasti et al. [170] |
| Mentally disabled individuals | Iran | 126 | 38 (30.2) | NA | CM | Mohammadi-Meskin et al. [171] |
| Mental retardation center personnel | Iran | 37 | 12 (32.4) | NA | CM | Mohammadi-Meskin et al. [171] |
| Schizophrenic male patients | Iran | 58 | 32 (55.2) | ST1, ST3, ST9 | CM, MOL | Sheikh et al. [172] |
| Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|
| China | 126 | 3 (2.4) | ST5 | MOL | Zhu et al. [173] |
| China | 198 | 21 (10.6) | ST1, ST3, ST6, ST7 | MOL | Kang et al. [174] |
| Iran | 670 | 23 (3.4) | NA | IVC | Rostami Nejad et al. [162] |
| Iran | 1232 | 154 (12.6) | NA | CM | Abdipour et al. [175] |
| Iran | 1383 | 239 (17.3) | ST1, ST2, ST3 | CM, MOL | Bahrami et al. [176] |
| Iran | 984 | 13 (1.3) | NA | CM | Gholipoor et al. [177] |
| Iran | 417 | 39 (9.4) | NA | CM | Viesy et al. [178] |
| Iran | 511 | 33 (6.5) | ST2, ST3, ST5 | MOL | Badparva et al. [179] |
| Iran | 420 | 60 (14.3) | ST2, ST3 | CM, MOL | Shaker et al. [180] |
| Iran | 802 | 39 (4.9) | ST1, ST2, ST3, ST7 | MOL | Haghighi et al. [181] |
| Iran | 420 | 60 (14.3) | NA | CM | Shaker et al. [182] |
| Iran | 1120 | 65 (5.8) | NA | CM | Tork et al. [183] |
| Iran | 4788 | 247 (5.2) | NA | CM | Asfaram et al. [184] |
| Iran | 210 | 66 (31.4) | ST1, ST2, ST3, ST4, ST5, ST6 | MOL | Bafghi et al. [185] |
| Iran | 133 | 35 (26.3) | ST1, ST2, ST3, ST5 | IVC, MOL | Moosavi et al. [141] |
| Iran | 4427 | 407 (9.2) | NA | Karimazar et al. [186] | |
| Iraq | 300 | 146 (48.7) | NA | CM | Abdul Ridha and Faieq, [187] |
| Iran | 618 | 146 (23.6) | ST1, ST2, ST3 | CM, IVC, MOL | Salehi et al. [188] |
| Iran | 481 | 69 (14.4) | ST1, ST2, ST3, ST4, ST5 | MOL | Khademvatan et al. [189] |
| Iran | 250 | 41 (16.4) | ST1, ST2, ST3 | CM, IVC, MOL | Sardarian et al. [190] |
| Iran | 200 | 63 (31.5) | NA | CM, IVC | Hamidi et al. [191] |
| Iran | 5000 | 784 (1.6) | NA | CM | Javadi et al. [192] |
| Iran | 864 | 68 (7.9) | ST1, ST2, ST3 | CM, IVC, MOL | Delshad et al. [193] |
| Iran | 566 | 10 (1.8) | NA | CM | Norouzi et al. [194] |
| Iran | 100 | 13 (13.0) | ST1, ST2, ST6 | CM, MOL | Sharifi et al. [195] |
| Iran | 1878 | 152 (8.1) | ST1, ST2, ST3, ST7 | CM, MOL | Salehi et al. [196] |
| Lebanon | 40 | 23 (57.5) | ST1, ST2, ST3 | MOL | Greige et al. [197] |
| Lebanon | 220 | 42 (19.1) | ST1, ST2, ST3, ST4 | CM, MOL | El Safadi et al. [198] |
| Lebanon | 50 | 27 (54.0) | ST1, ST2, ST3 | MOL | Greige et al. [199] |
| Saudi Arabia | 23,278 | 5 (0.02) | NA | CM | Imam et al. [200] |
| Saudi Arabia | 130 | 3 (2.3) | NA | CM | Hassen Amer et al. [201] |
| Saudi Arabia | 1262 | 133 (10.5) | ST1, ST2, ST3 | IVC, MOL | Mohamed et al. [202] |
| Thailand | 14,325 | 199 (1.4) | NA | CM | Laodim et al. [203] |
| Thailand | 562 | 56 (9.9) | ST1, ST3, ST6, ST7 | IVC, MOL | Jantermtor et al. [204] |
| Thailand | 15 | 15 (100.0) | ST1, ST3, ST6, ST7 | CM, IVC, MOL | Sanpool et al. [152] |
| Turkey | 192 | 6 (3.1) | NA | CM | Cekin et al. [156] |
| Turkey | 20,948 | 13,245 (63.2) | NA | CM | Polat et al. [205] |
| Turkey | 50,185 | 275 (0.5) | NA | CM | Beyhan et al. [206] |
| Turkey | 4030 | 476 (11.1) | ST1, ST2, ST3 | CM, MOL | Sarzhanov et al. [207] |
| Turkey | 6757 | 160 (2.4) | NA | CM | Selek et al. [208] |
| Host | Country/Region | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Adolescents | Indonesia | 70 | 20 (28.6) | ST1, ST3 | MOL | Kesuma et al. [144] |
| High school students foreign | Turkey | 192 | 63 (32.8) | NA | Yaman et al. [209] | |
| College students | China | 53 * | 53 | ST1, ST3, ST4, ST6, ST7 | IVC, MOL | Zhan et al. [210] |
| College students of practical parasitology courses | Iran | 175 | 9 (5.1) | NA | CM | Fallahi et al. [211] |
| Students who did not take any practical parasitology courses | Iran | 135 | 5 (3.7) | NA | CM | Fallahi et al. [211] |
| University students | Thailand | 1025 | 416 (40.6) | ST1, ST2, ST3 | CM, IVC, MOL | Srichaipon et al. [212] |
| Working children | Iran | 175 | 57 (32.6) | NA | CM | Salemi et al. [213] |
| Caregivers in a childcare center | Thailand | 25 | 6 (24.0) | ST1, ST2, ST3 | IVC, MOL | Pipatsatitpong et al. [72] |
| Cattle breeders | Lebanon | 40 | 21 (52.5) | ST1, ST2, ST3 | MOL | Greige et al. [197] |
| Chicken slaughterhouse staff | Lebanon | 50 | 28 (56.0) | ST1, ST2, ST3, ST6 | MOL | Greige et al. [199] |
| Pig handler and individuals who lived near pig farms | Thailand | 154 | 10 (6.5) | ST1, ST3, ST5 | MOL | Pintong et al. [214] |
| Pig handler and individuals who lived near pig farms | Thailand | 117 | 15 (12.8) | ST1, ST2, ST3 | MOL | Udonsom et al. [215] |
| Food handlers | Iran | 210 | 3 (1.4) | NA | CM | Kheirandish et al. [216] |
| Food handlers | Iran | 1021 | 40 (3.9) | NA | CM | Motazedian et al. [217] |
| Food handlers | Iran | 1041 | 29 (2.8) | NA | CM | Sharif et al. [218] |
| Food handlers | Iran | 800 | 194 (24.3) | NA | CM | Heydari-Hengami et al. [219] |
| Food handlers | Iran | 1018 | 7 (7.2) | NA | CM | Khodabakhsh Arbat et al. [220] |
| Food handlers | Iran | 1530 | 44 (2.9) | NA | CM | Shahnazi et al. [221] |
| food handlers | Iran | 816 | 18 (2.2) | NA | CM | Kheirandish et al. [222] |
| Food handlers | Jordan | 901 | 6 (0.7) | NA | CM | Abdel-Dayem et al. [223] |
| Military personnel | Iraq | 437 | 36 (8.2) | NA | CM | Downs et al. [224] |
| Gardeners | Thailand | 253 | 23 (9.1) | NA | CM | Kitvatanachai and Rhongbutsri, [225] |
| Immigrant workers | Thailand | 600 | 6 (1.0) | NA | CM | Sangwalee et al. [226] |
| Immigrant workers | Qatar | 608 | 432 (71.1) | ST1, ST2, ST3 | MOL | Abu-Madi et al. [227] |
| Immigrant workers | Qatar | 735 | 479 (65.2) | NA | CM, MOL | Abu-Madi et al. [228] |
| Settled immigrant | Qatar | 9208 | 398 (4.3) | NA | CM | Abu-Madi et al. [229] |
| Newly arrived immigrants | Qatar | 2486 | 137 (5.5) | NA | MOL | Abu-Madi et al. [230] |
| Settled immigrants | Qatar | 29,286 | 1010 (3.5) | NA | MOL | Abu-Madi et al. [231] |
| Resident workers | Qatar | 772 | 39 (5.1) | NA | CM | Abu-Madi et al. [232] |
| Workers | Saudi Arabia | 1238 | 245 (19.8) | NA | CM | Wakid [233] |
| New employees in a tertiary health care center | Saudi Arabia | 2490 | 314 (12.6) | NA | Ahmed et al. [234] | |
| Foreign laborers | Taiwan | 7360 | 190 (2.6) | NA | CM | Hsieh et al. [235] |
| Foreigners | Taiwan | 2875 | 33 (1.1) | NA | CM | Hsieh et al. [236] |
| Indonesian immigrant workers | Taiwan | 128 | 28 (21.9) | ST1, ST2, ST3 | CM, MOL | Chen et al. [237] |
| Sanitary and Non-sanitary Institutions’ workers | Turkey | 2443 | 175 (7.2) | NA | CM | Karaman et al. [238] |
| Migrant workers | Malaysia | 220 | 68 (30.9) | ST1, ST2, ST3 | IVC, MOL | Sahimin et al. [239] |
| Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|
| Bangladesh | 140 | 51 (36.4) | NA | CM | Noor et al. [240] |
| Cambodia | 218 | 40 (18.4) | NA | CM | Schär et al. [241] |
| Cambodia | 210 | 116 (55.2) | ST1, ST2, ST3 | MOL | Wang et al. [242] |
| China | 497 | 215 (43.3) | NA | CM | He et al. [243] |
| China | 5939 | 494 (8.3) | NA | MOL | Chen et al. [244] |
| China | 26,886 | 2 (0.01) | NA | CM | Umar et al. [245] |
| China | 1023 | 1 (0.1) | NA | CM | Jiang [246] |
| China | 6710 | 19 (0.3) | NA | CM | Zhang et al. [247] |
| China | 303 | 67 (22.1) | NA | IVC | Tian et al. [95] |
| China | 294 | 64 (21.8) | NA | IVC | Tian et al. [96] |
| China | 149 | 9 (6.0) | NA | MOL | Zhang et al. [136] |
| China | 366 | 28 (7.6) | NA | CM, 1VC | Li et al. [115] |
| China | 289 | 13 (4.5) | ST1, ST3, ST4 | MOL | Gong et al. [248] |
| China | 507 | 48 (9.5) | ST1, ST2, ST3, ST4 | MOL | Deng et al. [249] |
| China | 1118 | 390 (34.9) | ST2, ST5 | MOL | Ma et al. [250] |
| Cyprus | 230 | 64 (27.8) | ST1, ST2, ST3, ST4, ST6, ST7 | MOL | Seyer et al. [251] |
| India | 279 | 105 (37.6) | NA | MOL | Padukone et al. [252] |
| India | 200 | 16 (8.0) | NA | CM | Khalil et al. [101] |
| India | 100 | 15 (15.0) | ST1, ST3 | CM, IVC, MOL | Das et al. [145] |
| India | 23 | 13 (56.5) | NA | MOL | Lappan et al. [253] |
| Indonesia | 646 | 15 (2.3) | NA | CM | Wiria et al. [254] |
| Indonesia | 54 | 5 (9.3) | NA | IVC | Yulfi et al. [255] |
| Indonesia | 424 | 146 (34.4) | NA | CM | Sungkar et al. [256] |
| Indonesia | 53 | 9 (17.0) | NA | CM | Hayashi et al. [257] |
| Iran | 5073 | 368 (7.3) | NA | CM | Turgay et al. [258] |
| Iran | 399 | 16 (4.0) | NA | CM | Mahmoudi et al. [259] |
| Iran | 130 | 40 (30.1) | ST1, ST2, ST3 | CM, IVC, MOL | Beiromvand et al. [157] |
| Iran | 20 | 3 (15.0) | NA | CM | Berenji et al. [102] |
| Iran | 166 | 35 (21.1) | ST1, ST2, ST3 | IVC, MOL | Mirjalali et al. [143] |
| Iran | 181 | 17 (9.4) | NA | CM | Taghipour et al. [114] |
| Iran | 225 | 5 (2.2) | NA | CM | Azami et al. [118] |
| Iran | 166 | 35 (21.1) | ST1, ST2, ST3 | CM, MOL | Jalallou et al. [138] |
| Iran | 147 | 0 (0.0) | NA | CM | Anvari-Tafti et al. [106] |
| Iran | 122 | 21 (17.2) | ST1, ST3, ST4, ST5 | MOL | Khademvatan et al. [147] |
| Iran | 100 | 6 (6.0) | NA | CM | Shafiei et al. [146] |
| Iran | 67 | 6 (9.0) | NA | CM, MOL | Mahmoudvand et al. [87] |
| Iran | 250 | 41 (16.4) | ST1, ST2, ST3 | CM, IVC, MOL | Sardarian et al. [190] |
| Iran | 1410 | 47 (3.3) | ST3, ST4, ST5, ST7 | CM, MOL | Khoshnood et al. [260] |
| Iran | 655 | 180 (27.5) | NA | CM | Pestehchian et al. [261] |
| Iran | 5743 | 54 (0.9) | NA | CM | Sadeghi et al. [262] |
| Iran | 5739 | 30 (0.5) | NA | CM | Sadeghi and Borji [263] |
| Iran | 2838 | 139 (5.0) | NA | CM | Badparva et al. 2014 [264] |
| Iran | 1060 | 145 (13.7) | NA | CM | Mahni et al. [265] |
| Iran | 880 | 55 (6.3) | NA | CM | Tork et al. [266] |
| Iran | 652 | 48 (7.4) | NA | CM | Jafari et al. [267] |
| Iran | 561 | 159 (28.4) | NA | CM | Hemmati et al. [268] |
| Iran | 554 | 93 (16.8) | NA | CM, IVC | Riabi et al. [269] |
| Iran | 345 | 85 (24.6) | ST1, ST2, ST3 | CM, IVC, MOL | Mardani Kataki et al. [270] |
| Iran | 861 | 114 (13.2) | NA | CM | Abbaszadeh Afshar et al. [271] |
| Iran | 732 | 63 (6.3) | NA | CM | Sobati [272] |
| Iran | 184 | 45 (24.5) | ST1, ST2, ST3 | MOL | Shirvani et al.[273] |
| Iran | 283 | 20 (7.1) | NA | CM | Barati et al.[274] |
| Iran | 2838 | 129 (4.5) | NA | CM | Badparva et al. [264] |
| Iran | 565 | 144 (25.5) | NA | CM | Bairami Kuzehkanani et al. [275] |
| Iran | 1025 | 182 (17.8) | NA | CM | Sarkari et al. [276] |
| Iran | 1500 | 13 (0.9) | NA | CM | Sharifdini et al. [277] |
| Iran | 4788 | 277 (5.8) | NA | CM | Pagheh et al. [278] |
| Iran | 1008 | 46 (4.6) | NA | CM | Beiromvand et al. [279] |
| Iran | 2280 | 81 (3.6) | NA | CM | Taherkhani et al. [280] |
| Iraq | 78 | 1 (1.3) | NA | CM, IVC | Sayal et al. [148] |
| Korea | 324 | 29 (9.0) | ST1, ST2, ST3 | MOL | Kim et al. [281] |
| Laos | 669 | 91 (13.6) | NA | CM | Sayasone et al. [282] |
| Laos | 305 | 45 (14.8) | NA | CM | Ribas et al. [283] |
| Laos | 60 | 32 (51.7) | ST1, ST2, ST3, ST7 | CM, IVC, MOL | Sanpool et al. [284] |
| Lebanon | 7477 | 178 (2.3) | NA | CM | Araj et al. [285] |
| Lebanon | 306 | 195 (63.7) | ST1, ST2, ST3, ST10 | MOL | Khaled et al. [286] |
| Malaysia | 77 | 4 (5.2) | NA | CM | Sinniah et al. [287] |
| Malaysia | 500 | 102 (20.4) | NA | CM | Anuar et al. [17] |
| Malaysia | 243 | 45 (18.5) | ST1, ST2, ST3 | MOL | Mohammad et al. [288] |
| Malaysia | 466 | 191 (41.0) | NA | CM, IVC, MOL | Noradilah et al. [289] |
| Malaysia | 253 | 103 (40.7) | NA | IVC | Mohammad et al. [290] |
| Malaysia | 473 | 191 (40.4) | ST1, ST2, ST3, ST4 | MOL | Noradilah et al. [291] |
| Malaysia | 466 | 191 (41.0) | NA | CM, IVC, MOL | Noradilah et al. [292] |
| Malaysia | 253 | 45 (17.8) | ST1, ST2, ST3 | MOL | Mohammad et al. [293] |
| Malaysia | 416 | 18 (4.3) | NA | CM | Muslim et al. [294] |
| Myanmar | 172 | 16 (9.3) | ST1, ST3, ST4 | MOL | Gong et al. [248] |
| Nepal | 241 | 63 (26.1) | ST1, ST2, ST4 | IVC, MOL | Lee et al. [295] |
| Philippines | 110 | 36 (32.7) | NA | IVC | Santos and Rivera [296] |
| Philippines | 1271 | 166 (13.0) | ST1, ST2, ST3, ST4, ST5 | IVC, MOL | Belleza et al. [297] |
| Philippines | 35 | 29 (82.9) | ST1, ST3, ST4 | MOL | Adao et al. [298] |
| Philippines | 1271 | 165 (13.0) | NA | IVC | Belleza et al. [299] |
| Philippines | 412 | 242 (58.7) | NA | MOL | Weerakoon et al. [300] |
| Saudi Arabia | 140 | 96 (68.6) | NA | CM | AlDahhasi et al. [301] |
| Saudi Arabia | 80 | 12 (15.0) | ST1, ST2, ST5 | MOL | Mohamed et al. [90] |
| Saudi Arabia | 50 | 4 (8.0) | NA | CM | Hawash et al. [302] |
| Saudi Arabia | 90 | 2 (2.2) | NA | CM | Hawash et al. [159] |
| Saudi Arabia | 795 | 131 (16.5) | NA | CM | Alqumber [303] |
| Saudi Arabia | 795 | 209 (26.3) | NA | CM | Alqumber [303] |
| Thailand | 249 | 1 (0.4) | NA | CM | Kaewpitoon et al. [304] |
| Thailand | 60 | 6 (10.0) | NA | IVC | Surangsrirat et al. [149] |
| Thailand | 475 | 58 (12.2) | NA | CM, IVC | Kaewjai et al. [305] |
| Thailand | 230 | 25 (10.8) | ST1, ST3, ST4 | MOL | Popruk et al. [306] |
| Thailand | 1047 | 29 (2.8) | NA | CM | Prommi et al. [307] |
| Thailand | 178 | 41 (23.0) | ST1, ST2 ST3, ST4, ST6, ST7 | MOL | Yowang et al. [308] |
| Thailand | 324 | 13 (4.0) | NA | CM | Punsawad et al. [309] |
| Thailand | 220 | 13 (5.9) | ST2, ST3, ST6 | MOL | Palasuwan et al. [310] |
| Thailand | 247 | 2 (0.8) | NA | CM | Kitvatanachai et al. [311] |
| Thailand | 253 | 4 (1.6) | NA | CM | Boonjaraspinyo et al. [312] |
| Thailand | 224 | 1 (0.4) | NA | CM | Suntaravitun and Dokmaikaw [313] |
| Thailand | 733 | 57 (7.8) | NA | IVC | Wongthamarin et al. [314] |
| Thailand | 207 | 77 (37.2) | ST1, ST2, ST3, ST4 | MOL | Popruk et al. [315] |
| Turkey | 30 | 4 (13.0) | NA | CM, MOL | Karasartova et al. [316] |
| Turkey | 150 | 16 (10.7) | NA | CM | Karadag et al. [317] |
| Turkey | 105 | 30 (28.6) | NA | IVC | Dogruman-Al et al. [318] |
| Turkey | 27,664 | 581 (2.1) | NA | CM | Koksal et al. [319] |
| Turkey | 176 | 30 (17.0) | NA | CM | Alver et al. [160] |
| Turkey | 16,445 | 2602 (15.8) | NA | CM | Çetinkaya et al. [320] |
| Turkey | 17,711 | 1353 (7.6) | NA | CM | Düzyol et al. [321] |
| Turkey | 251 | 54 (21.5) | NA | CM | Kurt et al. [322] |
| Turkey | 6267 | 968 (15.4) | NA | CM | Yılmaz et al. [323] |
| Turkey | 87,100 | 640 (0.7) | NA | Gülmez et al. [324] | |
| Turkey | 111,889 | 306 (0.3) | NA | CM | Kirkoyun Uysal et al. [325] |
| Turkey | 7353 | 1884 (63.6) | NA | CM | Öncel [326] |
| Turkey | 200 | 93 (46.5) | ST1, ST2, ST3, ST7 | MOL | Malatyalı et al. [327] |
| Turkey | 69,633 | 18,460 (26.5) | NA | CM | Taş Cengiz et al. [328] |
| Turkey | 104 | 10 (9.6) | ST1, ST2, ST3, ST6 | MOL | Gulhan et al. [329] |
| Turkey | 56 | 28 (50.0) | ST1, ST2, ST3, ST4, ST5, ST6, ST7 | MOL | Koltas and Eroglu [330] |
| United Arab Emirates | 133 | 59 (44.4) | ST1, ST2, ST3 | MOL | AbuOdeh et al. [331] |
| Uzbekistan | 300 | 31 (10.3) | NA | CM | Toychiev et al. [332] |
| Uzbekistan | 550 | 99 (18.0) | NA | CM | Davis et al. [112] |
| Host | Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Acute appendicitis patients | Turkey | 136 | 8 (5.9) | NA | CM | Hatipoğlu et al. [333] |
| Adult male prison inmates | Malaysia | 294 | 43 (14.6) | ST1, ST3, ST6 | CM, IVC, MOL | Angal et al. [334] |
| Adults with intestinal parasitic infection | Malaysia | 35 | 17 (48.0) | NA | IVC | Chandramathi et al. [335] |
| Asymptomatic Blastocystis positive patients | Iran | 25 * | 25 | ST1, ST2, ST3, ST7 | MOL | Rezaei Riabi et al. [336] |
| Asymptomatic Blastocystis positive patients | Iran | 34 * | 34 | ST2, ST3 | CM, MOL | Shahbazi et al. [142] |
| Chronic spontaneous urticaria (adults) | Turkey | 38 | 7 (18.4) | NA | CM | Vezir et al. [337] |
| Chronic spontaneous urticaria (children) | Turkey | 76 | 13 (17.1) | NA | CM | Vezir et al. [337] |
| Urticarial patients | Turkey | 133 | 16 (12.0) | ST1, ST2, ST3 | CM, MOL | Aydin et al. [338] |
| Cirrhotic patients | Turkey | 37 | 8 (21.6) | ST1, ST2, ST3 | MOL | Yildiz et al. [339] |
| Diarrheic and non-diarrheic patients | Iran | 400 | 58 (14.5) | ST1, ST2, ST3 | IVC, MOL | Alinaghizade et al. [340] |
| Dengue patients | Malaysia | 89 | 21 (23.6) | ST1, ST3, ST4, ST6 | IVC, MOL | Thergarajan et al. [341] |
| Dialysis patients | Turkey | 142 | 34 (23.9) | NA | CM | Karadag et al. [317] |
| Giardia intestinalis positive patients | India | 258 | 21 (8.1) | NA | CM | Roy et al. [342] |
| Hemodialysis patients | Iran | 88 | 8 (9.0) | NA | CM | Barazesh et al. [343] |
| Immunocompromised and control | Iran | 641 | 57 (8.9) | NA | CM | Mahmoudi et al. [259] |
| Orphanage (orphansand childcare workers) | Thailand | 343 | 94 (27.4) | NA | CM, IVC | Pipatsatitpong et al. [344] |
| Patients suspected to have intestinalparasites | Turkey | 918 | 38 (4.2) | NA | CM | Koltas et al. [345] |
| Patients with chronic renal failure | Saudi Arabia | 50 | 8 (16.0) | NA | CM | Hawash et al. [302] |
| Patients with chronic viral Hepatitis C | Russia | 327 | 108 (33.0) | ST3, ST5, ST6 | CM, MOL | Sigidaev et al. [134] |
| Patients with Erythema Nodosum | Turkey | 81 | 2 (2.5) | NA | Ozbagcivan et al. [346] | |
| Patients with gastrointestinal and/or dermatologic symptoms | Turkey | 37,108 | 2537 (6.8) | NA | CM | Tunalı et al. [347] |
| Patients with intestinal protozoan infections | Iran | 75 | 5 (6.7) | NA | CM | Jafari et al. [348] |
| Patients with systemic lupus erythematosus (SLE) | Malaysia | 187 | 1 (0.5) | NA | not stated | Teh et al. 2018 [349] |
| Post-traumatic splenectomized patients | Turkey | 30 | 12 (40.0) | ST1, ST3 | CM, MOL | Karasartova et al. [316] |
| Pregnant women | Turkey | 100 | 14 (14.0) | ST1, ST2, ST3 | CM, IVC, MOL | Malatyalı et al. [350] |
| Symptomatic Blastocystis positive patients | Iran | 30 * | 30 | ST1, ST2, ST3, ST6 | MOL | Rezaei Riabi et al. [336] |
| Ulcerative colitis patients with refractory symptoms | China | 49 | 6 (12.2) | NA | CM | Tai et al. [351] |
| Ulcerative colitis patients responsive to treatment | China | 73 | 1 (1.4) | NA | CM | Tai et al. [351] |
| Visceral Leishmaniasis cases | India | 23 | 14 (60.9) | NA | MOL | Lappan et al. [253] |
| Host | Country | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Artiodactyla | ||||||
| Alpaca | China | 14 | 12 (85.7) | ST10, ST14, ST18 | MOL | Zhao et al. [352] |
| Alpaca | China | 27 | 4 (14.8) | ST10, ST14 | MOL | Li et al. [353] |
| Alpaca | China | 366 | 87 (23.8) | ST5, ST10, ST14 | MOL | Ma et al. [354] |
| Alpaca | China | 11 | 4 (36.4) | ST10, ST14 | MOL | Deng et al. [3] |
| Blesbuck | China | 2 | 1 (50.0) | ST5 | MOL | Li et al. [353] |
| Buffalo | India | 1 | 1 (100.0) | NA | CM | Sreekumar et al. [355] |
| Buffalo | Nepal | 19 | 4 (21.1) | ST4 | IVC, MOL | Lee et al. [18] |
| Bushbuck | China | 18 | 8 (61.5) | ST10, ST14 | MOL | Zhao et al. [352] |
| Camel | China | 10 | 5 (50.0) | ST1, ST10 | MOL | Zhao et al. [352] |
| Camel | China | 40 | 14 (35.0) | ST2, ST10, ST14 | MOL | Zhang et al. [14] |
| Cattle | Lebanon | 254 | 161 (63.4) | ST1, ST2, ST3, ST5, ST7, ST10, ST14 | MOL | Greige et al. [197] |
| Cattle | Malaysia | 29 | 10 (34.5) | NA | IVC | Hemalatha et al. [356] |
| Cattle | Malaysia | 3 | 1 (33.3) | ST10 | MOL | Mohammad et al. [288] |
| Cattle | Malaysia | 110 | 6 (5.4) | NA | IVC | Abd Razak et al. [357] |
| Cattle | Malaysia | 80 | 35 (43.8) | ST1, ST3, ST4, ST5, ST10, ST14 | MOL | Kamaruddin et al. [358] |
| Cattle | Nepal | 6 | 1 (16.7) | Unknown | IVC, MOL | Lee et al. [18] |
| Cattle | Thailand | 42 | 21 (50.0) | ST10, ST12 | MOL | Udonsom et al. [215] |
| Cattle | Turkey | 80 | 9 (11.3) | ST10, ST14 | MOL | Aynur et al. [359] |
| Cattle | Indonesia | 500 | 72 (14.4) | NA | CM | Hastutiek et al. [360] |
| Cattle | Indonesia | 100 | 100 (100.0) | NA | CM | Susana et al. [361] |
| Cattle | Indonesia | 108 | 108 (100.0) | ST10 | CM, IVC, MOL | Suwanti et al. [362] |
| Cattle | Iran | 198 | 19 (9.6) | ST3, ST5, ST6 | MOL | Badparva et al. [363] |
| Cattle | Iran | 75 | 25 (33.3) | ST5, ST10 | CM, MOL | Sharifi et al. [195] |
| Cattle | Iran | 40 | 14 (35.0) | ST3, ST10, ST14 | CM, MOL | Rostami et al. [364] |
| Cattle | Japan | 133 | 72 (54.1) | ST10, ST14 | MOL | Masuda et al. [365] |
| Cattle | China | 526 | 54 (10.3) | ST4, ST5, ST10, ST14 | MOL | Zhu et al. [366] |
| Cattle | China | 147 | 14 (9.5) | ST3, ST10, ST14 | MOL | Wang et al. [367] |
| Cattle | China | 57 | 15 (26.3) | ST10, ST14 | MOL | Zhang et al. [14] |
| Cattle | Korea | 1512 | 101 (6.7) | ST1, ST5, ST10, ST14 | MOL | Lee et al. [368] |
| Cattle | United Arab Emirates | 22 | 5 (22.7) | ST10 | MOL | AbuOdeh et al. [369] |
| Deer (Caspian red deer) | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Deer (Javan rusa) | Malaysia | 50 | 14 (28.0) | ST10 | MOL | Mohammad et al. [371] |
| Deer (Mousedeer) | Malaysia | 4 | 1 (25.0) | Unknown (Clade IV) | IVC, MOL | Mohd Zain et al. [372] |
| Deer (Sambar deer) | Malaysia | 14 | 4 (28.6) | NA | IVC | Hemalatha et al. [356] |
| Deer (Sika deer) | Malaysia | 50 | 16 (32.0) | ST10 | MOL | Mohammad et al. [371] |
| Deer (Red deer) | China | 5 | 2 (40.0) | ST10 | MOL | Li et al. [353] |
| Deer (Red deer/Wapiti) | China | 3 | 1 (33.3) | ST10 | MOL | Zhao et al. [352] |
| Deer (Reindeer) | China | 104 | 7 (6.7) | ST10, ST13 | MOL | Wang et al. [373] |
| Deer (Fallow deer) | China | 2 | 1 (50.0) | ST10 | MOL | Zhao et al. [352] |
| Deer (White-lipped deer) | China | 1 | 1 (100.0) | ST10 | MOL | Zhao et al. [352] |
| Deer (Sika deer) | China | 8 | 3 (37.5) | ST10 | MOL | Zhao et al. [352] |
| Deer (Sika deer) | China | 82 | 12 (14.6) | ST10, ST14 | MOL | Wang et al. [373] |
| Deer (Sika deer) | China | 11 | 1 (9.1) | ST1 | MOL | Deng et al. [3] |
| Deer (Sika deer) | China | 760 | 6 (0.8) | ST10, ST14 | MOL | Ni et al. [374] |
| Deer (Spotted deer) | Bangladesh | 30 | 1 (3.3) | ST14 | MOL | Li et al. [375] |
| Deer (Water deer) | Korea | 125 | 51 (40.8) | ST4, ST14 | MOL | Kim et al. [376] |
| Eland | China | 9 | 6 (66.7) | ST10, ST14 | MOL | Zhao et al. [352] |
| Gayal | Bangladesh | 4 | 1 (25.0) | ST14 | MOL | Li et al. [375] |
| Giraffe | China | 10 | 2 (20.0) | ST12 | MOL | Zhao et al. [352] |
| Goat | China | 789 | 458 (58.0) | ST1, ST3, ST4, ST5, ST10, ST14 | MOL | Song et al. [377] |
| Goat | China | 781 | 2 (0.3) | ST1 | MOL | Li et al. [378] |
| Goat | China | 59 | 28 (47.5) | ST10, ST14 | MOL | Zhang et al. [14] |
| Goat | Nepal | 400 | 3 (0.8) | NA | CM | Ghimire and Bhattarai [379] |
| Goat | Malaysia | 236 | 73 (30.9) | ST1, ST3, ST6, ST7 | MOL | Tan et al. [380] |
| Goat | Malaysia | 31 | 8 (25.8) | ST4, ST8, ST10 | MOL | Noradilah et al. [15] |
| Goat | Malaysia | 65 | 14 (21.5) | NA | IVC | Abd Razak et al. [357] |
| Goat | Malaysia | 20 | 13 (65.0) | NA | IVC | Hemalatha et al. [356] |
| Goat | Nepal | 29 | 1 (3.4) | ST4 | IVC, MOL | Lee et al. [18] |
| Goat | Philippines | 6 | 1 (16.7) | ST14 | IVC, MOL | Adao et al. [381] |
| Goat | Thailand | 38 | 36 (94.7) | ST10, ST12, ST14 | MOL | Udonsom et al. [215] |
| Goral (Himalayan) | Nepal | 19 | 1 (5.3) | NA | CM | Adhikari et al. [382] |
| Guanaco | China | 20 | 14 (70.0) | ST10, ST22 | MOL | Zhao et al. [352] |
| Guar | Malaysia | 10 | 3 (30.0) | NA | IVC | Hemalatha et al. [356] |
| Oryx | China | 2 | 1 (50.0) | ST10 | MOL | Zhao et al. [352] |
| Oryx | China | 11 | 1 (9.1) | ST5 | MOL | Li et al. [353] |
| Pig | Cambodia | 73 | 33 (45.2) | ST5 | MOL | Wang et al. [242] |
| Pig | China | 560 | 419 (74.8) | ST1, ST3, ST5, ST10 | MOL | Song et al. [383] |
| Pig | China | 68 | 6 (8.8) | ST5 | MOL | Wang et al. [367] |
| Pig | China | 801 | 174 (21.7) | ST1, ST3, ST5 | MOL | Wang et al. [384] |
| Pig | China | 866 | 433 (50.0) | ST1, ST3, ST5 | MOL | Han et al. [385] |
| Pig | China | 396 | 170 (42.9) | ST1, ST5 | MOL | Zou et al. [386] |
| Pig | India | 1 | 1 (100.0) | NA | CM | Sreekumar et al. [355] |
| Pig | India | 90 | 85 (94.4) | NA | CM | Arpitha et al. [387] |
| Pig | Indonesia | 93 | 81 (87.1) | ST1, ST2, ST5, ST7 | IVC, MOL | Yoshikawa et al. [41] |
| Pig | Indonesia | 100 | 63 (63.0) | NA | CM | Mahendra et.al. [388] |
| Pig | Indonesia | 100 | 69 (69.0) | NA | CM | Widisuputri et al. [389] |
| Pig | Korea | 646 | 390 (60.4) | ST1, ST2, ST3, ST5 | MOL | Paik et al. [390] |
| Pig | Nepal | 11 | 4 (36.4) | ST4 | IVC, MOL | Lee et al. [18] |
| Pig | Philippines | 49 | 36 (73.5) | ST1, ST2, ST3, ST5 | MOL | Adao et al. [391] |
| Pig | Philippines | 99 | 20 (20.2) | ST1, ST5, ST7 | IVC, MOL | Adao et al. [381] |
| Pig | Philippines | 122 | 47 (38.5) | NA | CM, IVC | De La Cruz et al. [392] |
| Pig | Philippines | 100 | 14 (14.0) | ST1, ST5 | IVC, MOL | Evidor and Rivera [393] |
| Pig | Philippines | 101 | 2 (2.0) | NA | CM | Murao et al. [394] |
| Pig | Thailand | 102 | 32 (31.4) | ST1, ST3, ST12, ST14 | MOL | Sanyanusin et al. [395] |
| Pig | Thailand | 90 | 32 (35.6) | ST1, ST3, ST5 | MOL | Pintong et al. [214] |
| Pig | Thailand | 87 | 40 (46.0) | ST1, ST5 | MOL | Udonsom et al. [215] |
| Pig | Malaysia | 10 | 10 (100.0) | NA | IVC | Hemalatha et al. [356] |
| Pig | Vietnam | 12 | 12 (100.0) | ST5 | MOL | Alfellani et al. [396] |
| Sheep | Iran | 150 | 29 (19.3) | ST7, ST10 | CM, MOL | Rostami et al. [364] |
| Sheep | China | 832 | 50 (6.0) | ST5, ST10, ST14 | MOL | Li et al. [378] |
| Sheep | China | 109 | 6 (5.5) | ST1, ST5, ST10, ST14 | MOL | Wang et al. [367] |
| Sheep | China | 38 | 16 (42.1) | ST2, ST10, ST14 | MOL | Zhang et al. [14] |
| Sheep | China | 78 | 42 (53.8) | ST2, ST10, ST14 | MOL | Zhang et al. [14] |
| Sheep | United Arab Emirates | 11 | 7 (63.6) | ST10, ST14 | MOL | AbuOdeh et al. [369] |
| Sheep | Malaysia | 38 | 22 (57.9) | NA | IVC | Hemalatha et al. [356] |
| Sheep | Malaysia | 20 | 2 (10.0) | NA | IVC | Abd Razak et al. [357] |
| Small ruminants | India | 107 | 15 (14.0) | NA | CM | Arpitha et al. [387] |
| Takin | China | 49 | 28 (57.1) | ST10, ST12, ST14 | MOL | Zhao et al. [352] |
| Waterbuck | China | 3 | 3 (100.0) | ST12, ST14 | MOL | Zhao et al. [352] |
| Waterbuck | China | 2 | 1 (50.0) | ST21 | MOL | Zhao et al. [352] |
| Waterbuck | Bangladesh | 7 | 1 (14.3) | ST10 | MOL | Li et al. [375] |
| Wild boar | South Korea | 433 | 45 (10.4) | ST5 | MOL | Lee et al. [397] |
| Wild Boar | Iran | 25 | 11 (44.0) | NA | CM | Yaghoobi et al. [398] |
| Wild Boar | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Yak | China | 1027 | 278 (27.1) | ST10, ST12, ST14 | MOL | Ren et al. [399] |
| Yak | China | 102 | 39 (38.2) | ST2, ST10, ST14 | MOL | Zhang et al. [14] |
| Yak | China | 6 | 3 (50.0) | ST10, ST14 | MOL | Zhao et al. [352] |
| Perissodactyla | ||||||
| Horse | China | 32 | 9 (28.1) | ST2, ST10 | MOL | Zhang et al. [14] |
| Horse | China | 4 | 1 (25.0) | ST10 | MOL | Zhao et al. [352] |
| Wild Ass | China | 5 | 2 (40.0) | ST10, ST12 | MOL | Zhao et al. [352] |
| Pony | China | 6 | 1 (16.7) | ST10 | MOL | Zhao et al. [352] |
| Zebra | China | 7 | 1 (14.3) | ST10 | MOL | Li et al. [353] |
| Proboscidea | ||||||
| Elephant | Bangladesh | 3 | 1 (33.3) | ST11 | MOL | Li et al. [375] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Artic fox | China | 213 | 4 (1.9) | ST1, ST4, ST7 | MOL | Wang et al. [373] |
| Bear | China | 12 | 3 (25.0) | ST17 | MOL | Deng et al. [3] |
| Bear | China | 312 | 45 (14.4) | ST1 | MOL | Ni et al. [374] |
| Cat | China | 346 | 2 (0.6) | ST1 | MOL | Li et al. [400] |
| Cat | Indonesia | 90 | 48 (53.3) | NA | MOL | Patagi et al. [401] |
| Cat | Iran | 140 | 20 (14.3) | NA | CM | Khademvatan et al. [402] |
| Cat | Iran | 119 | 21 (17.7) | ST1, ST3, ST4, ST10, ST14 | MOL | Mohammadpour et al. [403] |
| Cat | South Korea | 158 | 1 (0.6) | ST4 | MOL | Kwak and Seo [404] |
| Cat | Malaysia | 60 | 12 (20.0) | ST1 | MOL | Farah Haziqah et al. [405] |
| Cat | Turkey | 3 | 3 (100.0) | ST3 | MOL | Eroglu and Koltas [19] |
| Common raccoon | Iran | 30 | 5 (6.7) | ST1, ST2, ST3 | MOL | Mohammad Rahimi et al. [406] |
| Dog | China | 136 | 4 (2.9) | ST1, ST4 | MOL | Wang et al. [373] |
| Dog | China | 651 | 35 (5.4) | ST1, ST3, ST10 | MOL | Liao et al. [407] |
| Dog | India | 80 | 19 (24.0) | ST1, ST4, ST5, ST6 | MOL | Wang et al. [408] |
| Dog | Iran | 301 | 59 (19.6) | NA | CM | Mohaghegh et al. [409] |
| Dog | Iran | 552 | 29 (5.2) | NA | CM | Mirbadie et al. [410] |
| Dog | Iran | 154 | 29 (18.8) | ST2, ST3, ST4, ST7, ST8, ST10 | MOL | Mohammadpour et al. [403] |
| Dog | Turkey | 4 | 4 (100.0) | ST1, ST2 | MOL | Eroglu and Koltas [19] |
| Dog | Philippines | 145 | 21 (14.5) | ST1, ST2, ST3, ST4, ST5 | IVC, MOL | Belleza et al. [297] |
| Dog | Malaysia | 84 | 40 (47.6) | ST1, ST3, ST4, ST8, ST10 | MOL | Noradilah et al. [15] |
| Dog | Thailand | 13 | 1 (7.7) | ST3 | MOL | Udonsom et al. [215] |
| Dog | Cambodia | 80 | 1 (1.3) | ST2 | MOL | Wang et al. [408] |
| Dog | China | 7 | 1 (14.3) | ST10 | MOL | Li et al. [353] |
| Leopard | China | 3 | 2 (66.7) | ST1, ST5 | MOL | Deng et al. [3] |
| Meerkat | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Meerkat | China | 2 | 1 (50.0) | ST5 | MOL | Li et al. [353] |
| Panda (Giant panda) | China | 81 | 10 (12.3) | ST1 | MOL | Deng et al. [411] |
| Panda (Red panda) | China | 23 | 2 (8.7) | ST1 | MOL | Deng et al. [411] |
| Raccoon dog | China | 40 | 3 (7.5) | ST3 | MOL | Wang et al. [373] |
| Tiger (Siberian tiger) | China | 13 | 1 (7.7) | ST10 | MOL | Li et al. [353] |
| Tiger (White tiger) | China | 9 | 1 (11.1) | ST10 | MOL | Li et al. [353] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Primates | ||||||
| Langur | Bangladesh | 5 | 3 (60.0) | ST1, ST13 | MOL | Li et al. [375] |
| Grey langur | Bangladesh | 2 | 1 (50.0) | ST1 | MOL | Li et al. [375] |
| White-cheeked gibbon | China | 4 | 1 (25.0) | ST1 | MOL | Ma et al. [250] |
| White-cheeked gibbon | China | 4 | 4 (100.0) | ST2, ST3 | MOL | Deng et al. [3] |
| Ring-tailed lemur | China | 6 | 2 (33.3) | ST2, ST4 | MOL | Li et al. [353] |
| Ring-tailed lemur | China | 16 | 7 (43.8) | ST3, ST5, ST9 | MOL | Ma et al. [250] |
| Ring-tailed lemur | China | 13 | 6 (46.2) | ST1, ST2 | MOL | Deng et al. [3] |
| Macaque | China | 97 | 85 (87.6) | ST1, ST2, ST3, ST5, ST7 | MOL | Zanzani et al. [412] |
| Macaque | China | 185 | 12 (7.0) | ST1, ST2, ST3 | MOL | Zhu et al. [173] |
| Macaque (experimental) | China | 505 | 235 (46.5) | ST1, ST2, ST3 | MOL | Li et al. [413] |
| Rhesus macaque | Bangladesh | 62 | 20 (32.3) | ST1, ST2, ST3 | MOL | Li et al. [375] |
| Rhesus macaque | China | 29 | 28 (96.6) | ST1, ST2, ST3, ST19 | MOL | Zhao et al. [352] |
| Rhesus macaque | China | 17 | 10 (58.8) | ST1 | MOL | Deng et al. [3] |
| Rhesus macaque | China | 18 | 6 (33.3) | ST2, ST3 | MOL | Ma et al. [250] |
| Japanese macaque | China | 33 | 6 (18.2) | ST2, ST3 | MOL | Ma et al. [250] |
| Macaque | Philippines | 50 | 5 (10.0) | NA | CM | Casim et al. [414] |
| Long-tailed macaque | Thailand | 628 | 263 (41.9) | ST1, ST2, ST3 | IVC, MOL | Vaisusuk et al. [415] |
| Crab-eating macaque | China | 13 | 3 (23.1) | ST2, ST3 | MOL | Ma et al. [250] |
| Orangutan | Indonesia | 262 | 36 (13.7) | NA | CM | Labes et al. [416] |
| Orangutan | Malaysia | 10 | 5 (50.0) | NA | IVC | Hemalatha et al. [356] |
| Vervet monkey | Iran | 40 | 3 (7.5) | NA | CM | Dalimi et al. [417] |
| Vervet monkey | Bangladesh | 7 | 3 (42.9) | ST2, ST3, ST13 | MOL | Li et al. [375] |
| Hamadryas baboon | Saudi Arabia | 823 | 349 (42.4) | NA | CM | Alqumber [303] |
| Hamadryas baboon | China | 23 | 13 (56.5) | ST1, ST3 | MOL | Zhao et al. [352] |
| Chimpanzee | China | 10 | 8 (80.0) | ST2 | MOL | Zhao et al. [352] |
| Chimpanzee | China | 15 | 3 (13.3) | ST1, ST5 | MOL | Ma et al. [250] |
| Francois’ leaf monkey | China | 1 | 1 (100.0) | ST2 | MOL | Zhao et al. [352] |
| Francois’ leaf monkey | China | 3 | 2 (66.7) | ST1 | MOL | Ma et al. [250] |
| Mandrill | China | 4 | 1 (25.0) | ST3 | MOL | Zhao et al. [352] |
| Mandrill | China | 15 | 9 (60.0) | ST1, ST4 | MOL | Ma et al. [250] |
| De Brazza’s monkey | China | 5 | 4 (80.0) | ST1, ST10 | MOL | Zhao et al. [352] |
| De Brazza’s monkey | China | 5 | 5 (100.0) | ST1, ST2 | MOL | Ma et al. [250] |
| Golden snub-nosed monkey | China | 46 | 41 (89.1) | ST1, ST13 | MOL | Zhao et al. [352] |
| Snub-nosed monkey | China | 22 | 9 (40.9) | ST1, ST2 | MOL | Ma et al. [250] |
| Golden monkey | China | 37 | 18 (48.6) | ST1, ST2, ST3 | MOL | Ma et al. [418] |
| Squirrel monkey | China | 93 | 19 (20.4) | ST17 | MOL | Deng et al. [3] |
| Common squirrel monkey | China | 30 | 9 (30.0) | ST1, ST5 | MOL | Ma et al. [250] |
| Red-faced spider monkey | China | 4 | 2 (50.0) | ST2, ST3 | MOL | Ma et al. [250] |
| Monkey | Philippines | 4 | 4 (100.0) | ST1, ST2, ST3 | MOL | Rivera [21] |
| Non-human primates | Malaysia | 308 | 5 (1.6) | NA | CM | Adrus et al. [419] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Duck | Philippines | 31 | 3 (9.6) | ST7, B. pythoni | IVC, MOL | Adao et al. [381] |
| Birds | Turkey | 5 | 5 (100.0) | ST1, ST2 | MOL | Eroglu and Koltas [19] |
| Chicken | China | 46 | 6 (13.0) | ST6, ST7 | MOL | Wang et al. [373] |
| Chicken | Philippines | 34 | 5 (14.7) | ST7, Mixed | IVC, MOL | Adao et al. [381] |
| Chicken | India | 24 | 20 (83.3) | NA | CM | Sreekumar et al. [355] |
| Chicken | India | 170 | 50 (29.4) | NA | CM | Arpitha et al. [387] |
| Chicken | Indonesia | 38 | 13 (34.2) | ST7 | IVC, MOL | Yoshikawa et al. [41] |
| Chicken | Lebanon | 223 | 71 (31.8) | ST6, ST7 | MOL | Greige et al. [199] |
| Chicken | Malaysia | 104 | 27 (26.0) | ST1, ST3, ST6, ST7, ST9 | MOL | Noradilah et al. [15] |
| Chicken | Malaysia | 15 | 1 (6.7) | ST6 | MOL | Mohammad et al. [288] |
| Chicken | Malaysia | 107 | 27 (25.2) | NA | IVC | Farah Haziqah et al. [420] |
| Chicken | Malaysia | 179 | 47 (26.3) | ST1, ST6, ST7, ST8 | IVC, MOL | Farah Haziqah et al. [421] |
| Crested ibis | China | 63 | 6 (9.5) | NA | CM | Zhang et al. [422] |
| Crow (Hooded) | Iran | 144 | 64 (44.4) | ST13, ST14 | IVC, MOL | Asghari et al. [423] |
| Duck | Malaysia | 20 | 8 (40.0) | ST1, ST2, ST3, ST7 | MOL | Noradilah et al. [15] |
| Green-naped lorikeet | China | 2 | 1 (50.0) | ST10 | MOL | Li et al. [353] |
| Ostrich | China | 9 | 3 (33.3) | ST5, ST10, ST20 | MOL | Zhao et al. [352] |
| Ostrich | China | 19 | 6 (31.6) | ST5 | MOL | Deng et al. [3] |
| Ostrich | Malaysia | 37 | 37 (100.0) | ST6 | IVC, MOL | Chandrasekaran et al. [424] |
| Ostrich | Malaysia | 37 | 37 (100.0) | NA | IVC | Hemalatha et al. [424] |
| Ostrich | China | 3 | 2 (66.7) | ST5 | MOL | Li et al. [353] |
| Green peafowl | China | 12 | 1 (8.3) | ST3 | MOL | Deng et al. [3] |
| Green peafowl | China | 15 | 1 (6.7) | ST8 | MOL | Deng et al. [411] |
| Indian peafowl | China | 20 | 3 (15.0) | ST7, ST8 | MOL | Li et al. [353] |
| Pigeon | China | 34 | 4 (11.8) | ST8 | MOL | Deng et al. [3] |
| Pigeon | China | 47 | 1 (2.1) | ST6 | MOL | Wang et al. [373] |
| Pigeon | Iran | 156 | 67 (42.9) | ST13 | IVC, MOL | Asghari et al. [423] |
| Poultry | Iran | 132 | 21 (15.9) | ST7, ST10, ST14 | CM, MOL | Rostami et al. [364] |
| Red crowned crane | China | 43 | 6 (14.0) | ST6, ST7 | MOL | Wang et al. [373] |
| Red-crowned crane | China | 2 | 1 (50.0) | ST14 | MOL | Li et al. [353] |
| Ruddy shelduck | China | 11 | 2 (18.2) | ST8 | MOL | Deng et al. [411] |
| Swan | Malaysia | 20 | 7 (35.0) | ST1, ST3 | MOL | Noradilah et al. [15] |
| Black swan | China | 38 | 4 (10.5) | ST8 | MOL | Deng et al. [411] |
| Turkey | India | 4 | 3 (75.0) | NA | CM | Sreekumar et al. [355] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Flying squirrel | China | 207 | 63 (30.4) | ST1, ST3, ST13 | MOL | Xiao et al. [425] |
| Eastern chipmunk | China | 171 | 8 (4.7) | ST4 | MOL | Chai et al. [426] |
| Eurasian red squirrel | China | 72 | 7 (9.7) | ST4 | MOL | Chai et al. [426] |
| Black great squirrel | China | 1 | 1 (100.0) | ST4 | MOL | Deng et al. [3] |
| Red giant flying squirrel | China | 1 | 1 (100.0) | ST4 | MOL | Deng et al. [3] |
| Indian palm squirrel | United Arab Emirates | 4 | 2 (50.0) | ST4 | MOL | AbuOdeh et al. [369] |
| Shrew-faced squirrel | United Arab Emirates | 1 | 1 (100.0) | ST17 | MOL | AbuOdeh et al. [369] |
| Chinese striped hamster | China | 98 | 12 (12.2) | ST4 | MOL | Chai et al. [426] |
| Chinchilla | China | 72 | 3 (4.2) | ST4, ST17 | MOL | Chai et al. [426] |
| Chinchilla | China | 6 | 4 (66.7) | ST17 | MOL | Deng et al. [3] |
| Guinea pig | China | 90 | 12 (13.3) | ST4 | MOL | Chai et al. [426] |
| Patagonian mara | China | 15 | 3 (20.0) | ST4 | MOL | Li et al. [353] |
| Rat (Mus musculus) | China | 108 | 4 (3.7) | ST4 | MOL | Wang et al. [373] |
| Laboratory rats | China | 355 | 29 (8.2) | ST4, ST7 | MOL | Li et al. [427] |
| Rat (Rattus exulans) | Indonesia | 77 | 10 (13.0) | ST4 | IVC, MOL | Yoshikawa et al. [41] |
| Rat | Indonesia | 98 | 6 (6.0) | NA | CM | Prasetyo [428] |
| Rat (Rattus exulans) | Indonesia | 67 | 11 (16.4) | ST4 | MOL | Katsumata et al. [429] |
| Rodents | Iran | 52 | 3 (5.8) | NA | CM | Seifollahi et al. [430] |
| Rat (Rattus norvegicus) | Iran | 127 | 20 (15.8) | ST1, ST3, ST4 | MOL | Mohammadpour et al. [403] |
| Rat (Rattus norvegicus) | Malaysia | 95 | 48 (51.0) | NA | CM | Premaalatha et al. [431] |
| Rat (Rattus norvegicus) | Malaysia | 290 | 133 (45.9) | ST1, ST4, ST5, ST7 | IVC, MOL | Farah Haziqah et al. [432] |
| Wild rats (Rattus novercious) | Japan | 48 | 12 (25.0) | ST4 | MOL | Katsumata et al. [429] |
| Swiss-Webster mice | Iran | 50 | 1 (2.0) | NA | CM | Kalani et al. [433] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Squamata | ||||||
| Cobra snake | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Albino python | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Water monitor lizard | Malaysia | 6 | 1 (1.6) | Unknown (Clade VIII) | IVC, MOL | Mohd Zain et al. [372] |
| Testudines | ||||||
| African spurred tortoise | United Arab Emirates | 19 | 5 (26.3) | Unknown | MOL | AbuOdeh et al. [369] |
| Greek tortoise | United Arab Emirates | 2 | 1 (50.0) | Unknown | MOL | AbuOdeh et al. [369] |
| Iguana | United Arab Emirates | 1 | 1 (100.0) | Unknown | MOL | AbuOdeh et al. [369] |
| Host | Country | No. of Samples Examined | Number of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Blattodea | ||||||
| Cockroach | China | 116 | 96 (82.8) | ST2 | MOL | Ma et al. [418] |
| Cockroach | Thailand | 920 | 9 (1.0) | NA | CM | Chamavit et al. [434] |
| Cockroach | Thailand | 450 | 18 (4.0) | NA | CM | Dokmaikaw and Suntaravitun [435] |
| Cockroach (Blatella germanica) | Turkey | 138 | 57 (41.0) | NA | CM | Oguz et al. [436] |
| Cockroach (Blatella germanica) | Iran | 496 | 5 (1.0) | NA | CM | Motevalli-Haghi et al. [437] |
| Cockroach (Periplaneta americana) | Malaysia | 151 | 61 (40.4) | ST3 | IVC, MOL | Farah Haziqah et al. [438] |
| Diprotodontia | ||||||
| Gray kangaroo | China | 11 | 8 (72.7) | ST10 | MOL | Zhao et al. [352] |
| Red-necked wallaby | China | 15 | 2 (13.3) | ST11 | MOL | Li et al. [353] |
| Sugar glider | Indonesia | 100 | 100 (100.0) | NA | CM, IVC | Natalia et al. [439] |
| Lagomorpha | ||||||
| New Zealand white rabbit | China | 215 | 7 (3.3) | ST4 | MOL | Wang et al. [373] |
| Rabbit | China | 616 | 6 (1.0) | NA | MOL | Li et al. [440] |
| Rabbit | United Arab Emirates | 3 | 1 (33.3) | ST14 | MOL | AbuOdeh et al. [369] |
| Eulipotyphla | ||||||
| Hedgehog | Iran | 1 | 1 (100.0) | NA | CM | Mirzapour et al. [370] |
| Country | Food/Environmental Source | No. of Samples Examined | No. of Positive Samples (%) | Subtypes (STs) Identified | Method(s) | References |
|---|---|---|---|---|---|---|
| Iran | Treated wastewater | 12 | 5 (41.7) | ST2, ST6, ST8 | F, MOL | Javanmard et al. [441] |
| Malaysia | River water | 480 | 133 (27.7) | NA | MB, IVC | Ithoi et al. [442] |
| Malaysia | Drinking water treatment plants | 85 | 22 (25.9) | NA | IMS, CM | Richard et al. [443] |
| Malaysia | River water | 14 | 14 (100.0) | ST1, ST2, ST3 | MF, MOL | Noradilah et al. [444] |
| Various water sources | ST1, ST2, ST3, ST4, ST8, ST10 | |||||
| Malaysia | River water | 7 | 3 (42.9) | NA | MF, IVC | Noradilah et al. [23] |
| Village water sources | 16 | 1 (6.3) | ||||
| Nepal | River water | 4 | 4 (100.0) | ST1, ST4 | C, MOL | Lee et al. [18] |
| Philippines | Wastewater (influent) | 31 | 7 (23.0) | ST1, ST2 | C, IVC, MOL | Banaticla and Rivera, [445] |
| Wastewater (effluent) | 31 | 2 (7.0) | ST1, ST2 | |||
| Turkey | Tap water | 25 | 3 (12.0) | ST1 | MOL | Eroglu and Koltas, [19] |
| Turkey | Streams and drinking water | 228 | 47 (20.6) | NA | CM | Karaman et al. [446] |
| Turkey | River water | 195 | 9 (4.6) | ST1, ST3 | C, MOL | Koloren et al. [447] |
| Sea water | 48 | 1 (2.1) | ST1 | |||
| Turkey | Surface water | 75 | 4 (5.3) | ST1, ST3 | C, MOL | Kolören and Karaman [448] |
| Saudi Arabia | Leafy vegetables | 470 | 13 (2.8) | NA | S, CM | Al-Megrin [27] |
| Iran | Fresh vegetables | 240 | 10 (4.2) | NA | S, CM | Isazadeh et al. [449] |
| Syria | Fresh vegetables | 128 | 13 (10.2) | NA | MOL | Al Nahhas and Aboualchamat [450] |
| Korea | Ambient air | 71 | 1 (1.4) | NA | MOL | Han et al. [451] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauff-Adedotun, A.A.; Meor Termizi, F.H.; Shaari, N.; Lee, I.L. The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia. Biology 2021, 10, 990. https://doi.org/10.3390/biology10100990
Rauff-Adedotun AA, Meor Termizi FH, Shaari N, Lee IL. The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia. Biology. 2021; 10(10):990. https://doi.org/10.3390/biology10100990
Chicago/Turabian StyleRauff-Adedotun, Adedolapo Aminat, Farah Haziqah Meor Termizi, Nurshafarina Shaari, and Ii Li Lee. 2021. "The Coexistence of Blastocystis spp. in Humans, Animals and Environmental Sources from 2010–2021 in Asia" Biology 10, no. 10: 990. https://doi.org/10.3390/biology10100990