Elastic Moduli of Avian Eggshell
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Collection and Effective Young’s Modulus
2.2. Scanning Electron Microscope (SEM)
2.3. Measuring Weight Percentage of CaCO3
2.4. X-ray Diffraction (XRD)
2.5. Electron Backscatter Diffraction (EBSD)
2.6. Nanoindentation Testing
3. Results and Discussion
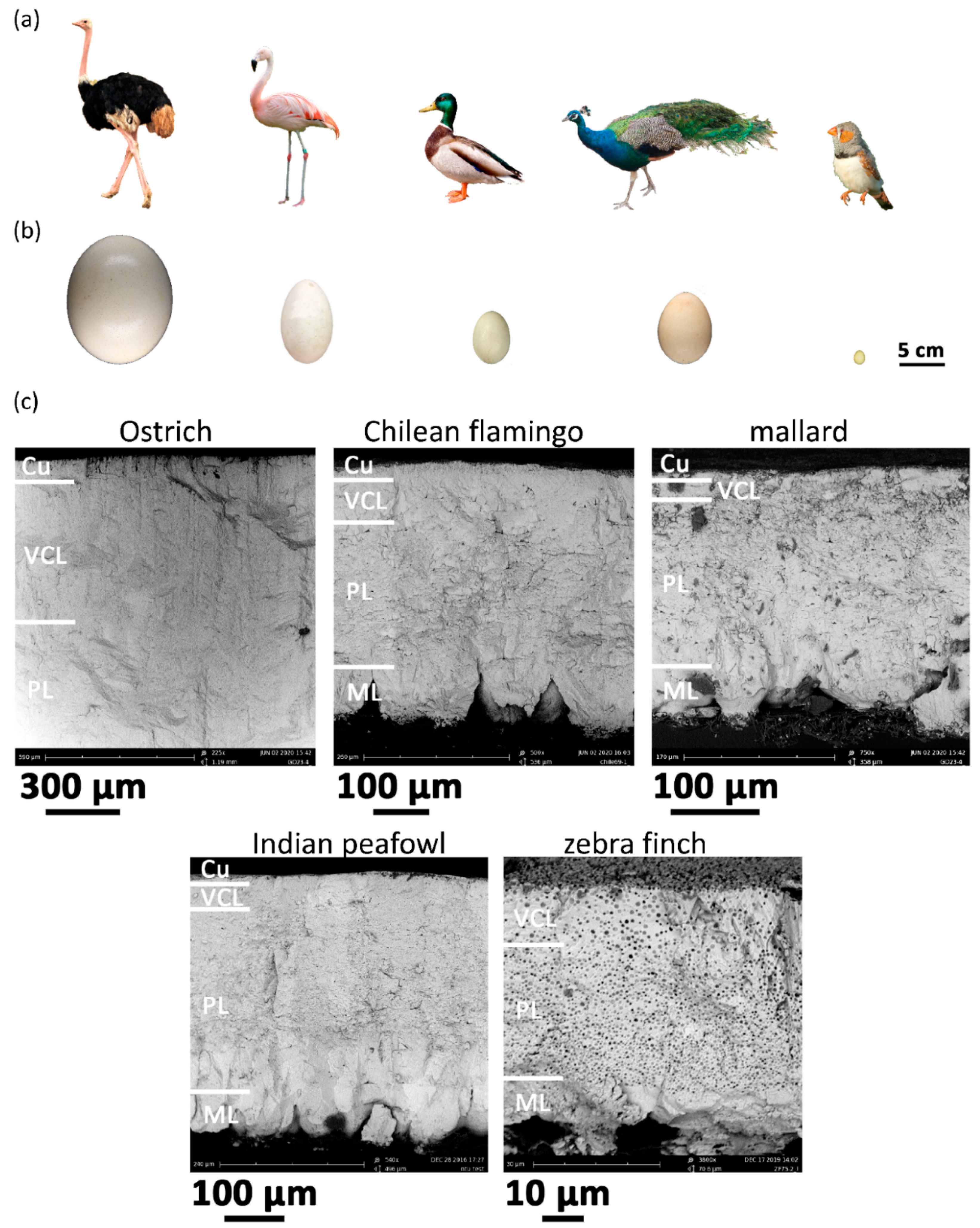
3.1. SEM Microphotograph of Avian Eggshell
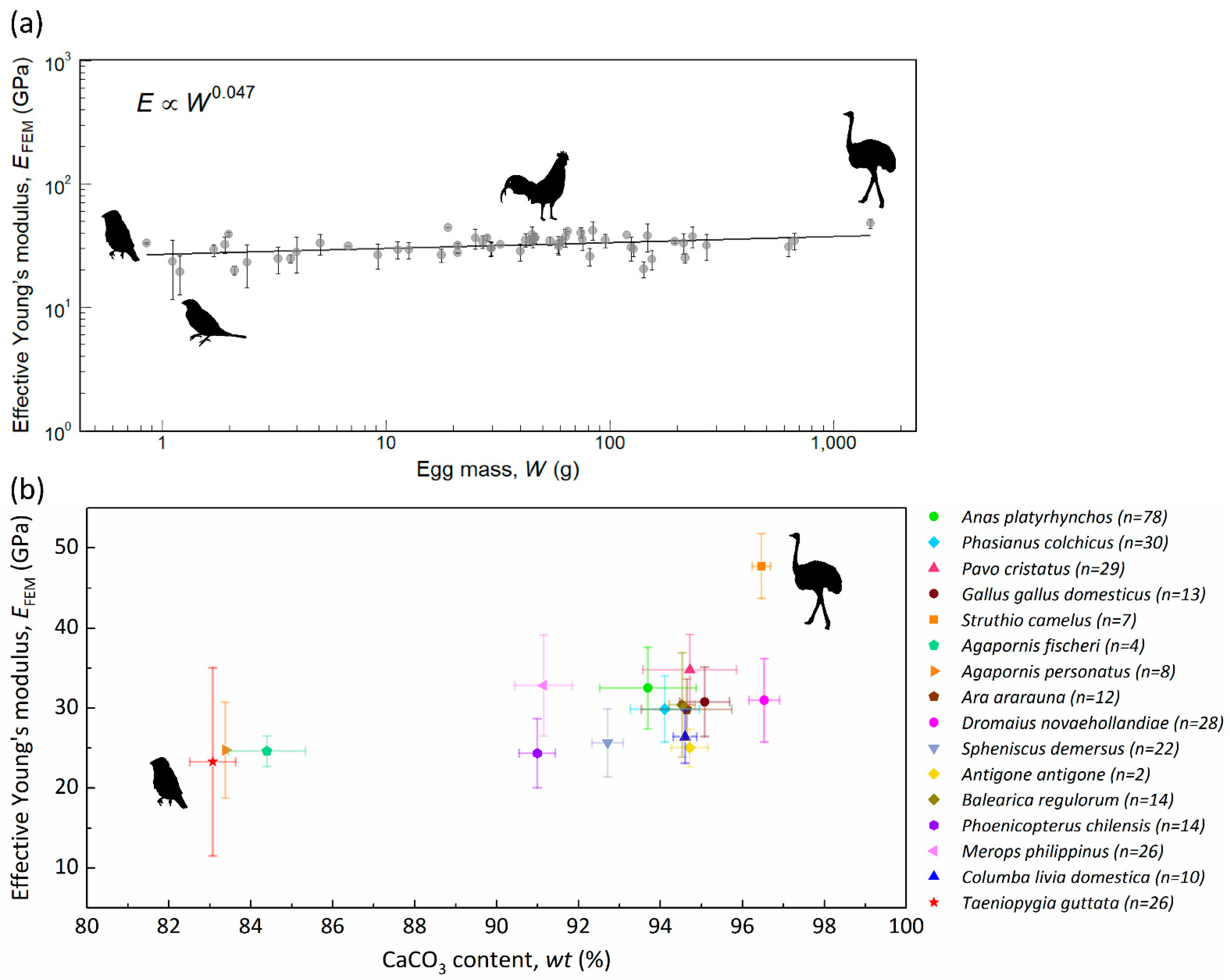
3.2. Effective Young’s Modulus and Weight Percentage of CaCO3
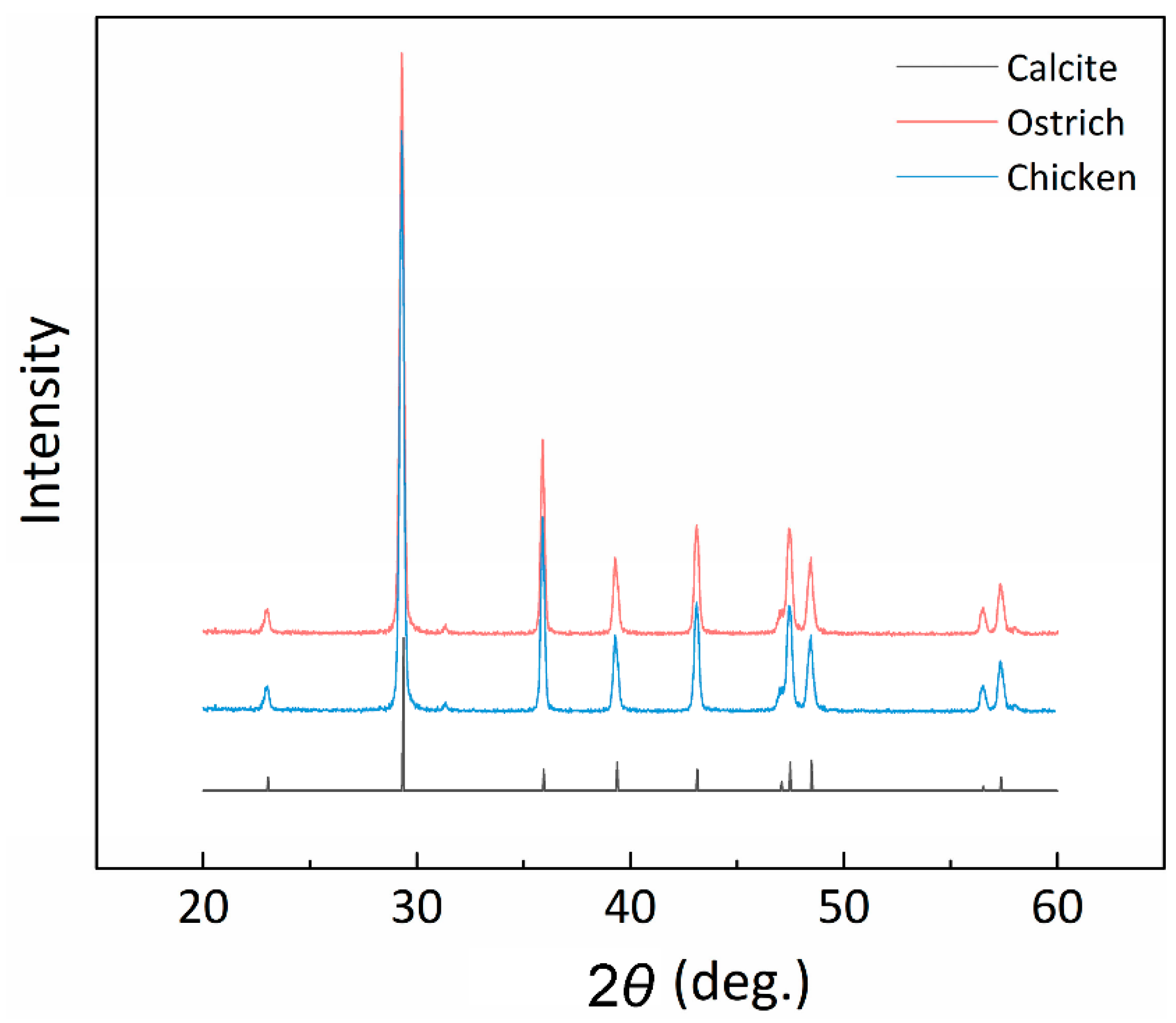
3.3. X-ray Diffraction
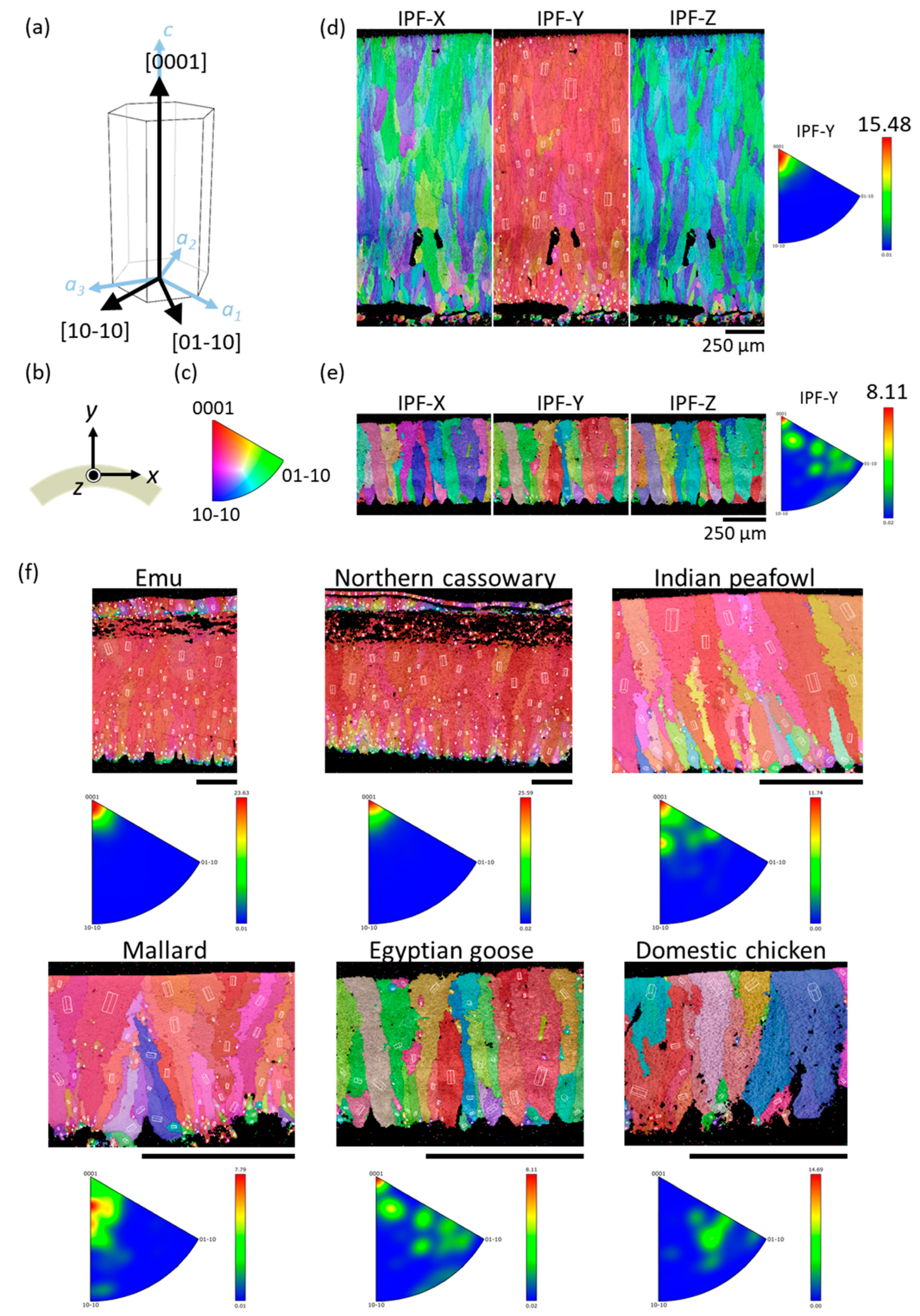
3.4. Crystallographic Analysis Using EBSD
3.5. Nanoindentation
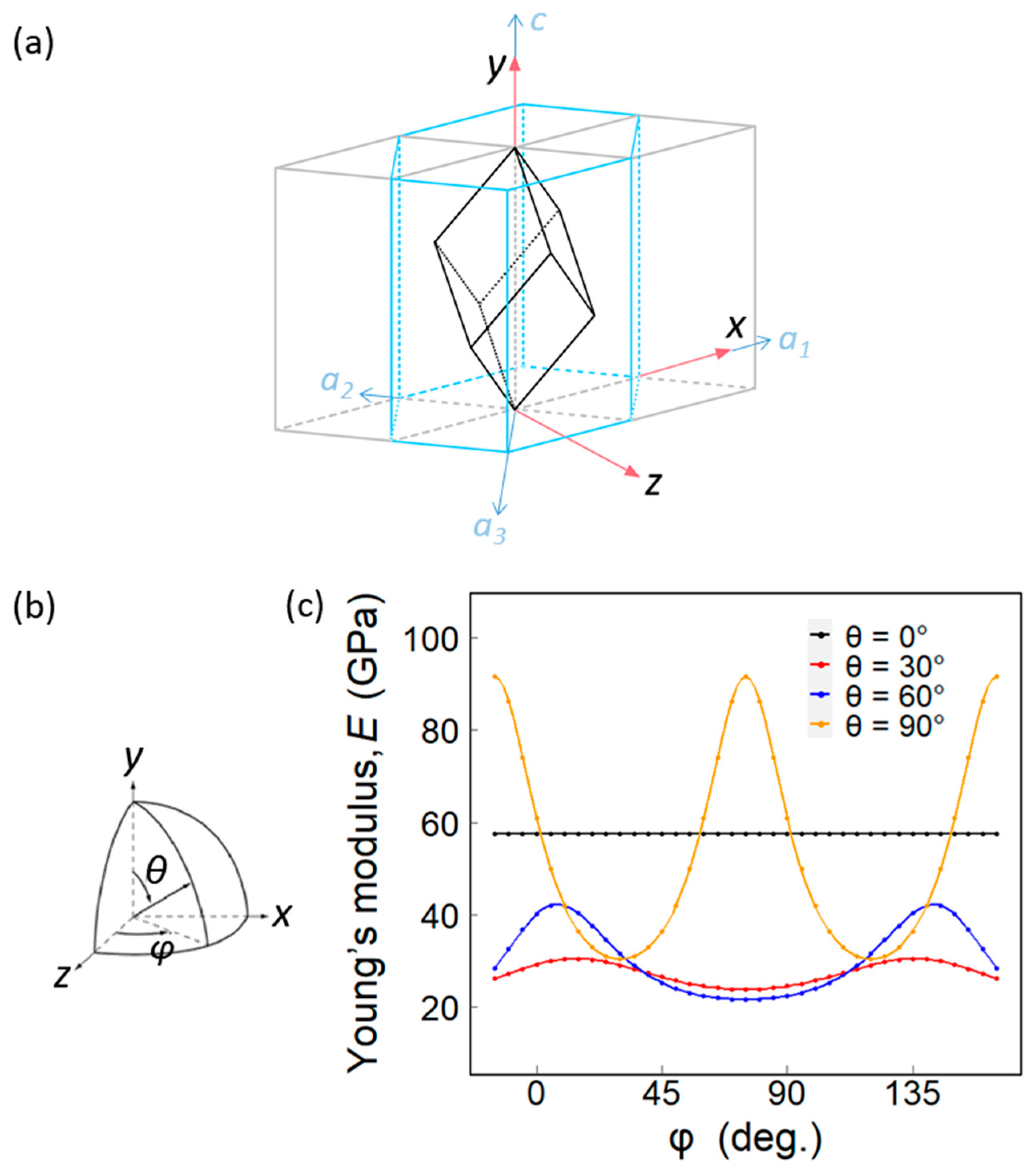
3.6. Theoretical Values
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamat, S.; Su, X.; Ballarini, R.; Heuer, A.H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature 2000, 405, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.E.; Fitzharris, T.P.; Barnett, B.D. Effects of varying chamber construction and embryo pre-incubation age on survival and growth of chick embryos in shell-less culture. Anat. Rec. 1981, 199, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, C.V. The physics of gas exchange across the avian eggshell. Am. Zool. 1980, 20, 329–338. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Messens, W.; Grijspeerdt, K.; Herman, L. Eggshell penetration by Salmonella: A review. Worlds Poult. Sci. J. 2005, 61, 71–86. [Google Scholar] [CrossRef]
- Gantois, I.; Ducatelle, R.; Pasmans, F.; Haesebrouck, F.; Gast, R.; Humphrey, T.J.; Van Immerseel, F. Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 2009, 33, 718–738. [Google Scholar] [CrossRef]
- Graveland, J.; Drent, R.H. Calcium availability limits breeding success of passerines on poor soils. J. Anim. Ecol. 1997, 66, 279–288. [Google Scholar] [CrossRef]
- Miller, D.S.; Kinter, W.B.; Peakall, D.B. Enzymatic basis for DDE-induced eggshell thinning in a sensitive bird. Nature 1976, 259, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, D.; Jiang, W.; Goldbaum, D.; Saleem, A.; Basu, K.; Pacella, M.S.; Bohm, C.F.; Chromik, R.R.; Hincke, M.T.; Rodriguez-Navarro, A.B.; et al. Nanostructure, osteopontin, and mechanical properties of calcitic avian eggshell. Sci. Adv. 2018, 4, eaar3219. [Google Scholar] [CrossRef]
- Deeming, D.C. Avian Incubation: Behaviour, Environment, and Evolution; Oxford University Press: Oxford, UK, 2002; pp. xiv, 421p. [Google Scholar]
- Yen, A.; Wu, H.-J.; Chen, P.-Y.; Yu, H.-T.; Juang, J.-Y. Egg incubation mechanics of giant birds. Biology 2021, 10, 738. [Google Scholar] [CrossRef]
- Ugural, A.C. Advanced Mechanics of Materials and Applied Elasticity, 6th ed.; Pearson: Boston, MA, USA, 2019; pp. xvii, 680p. [Google Scholar]
- Hahn, E.N.; Sherman, V.R.; Pissarenko, A.; Rohrbach, S.D.; Fernandes, D.J.; Meyers, M.A. Nature’s technical ceramic: The avian eggshell. J. R. Soc. Interface 2017, 14, 20160804. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.Y.; Chen, P.Y.; Yang, D.C.; Wu, S.P.; Yen, A.; Hsieh, H.I. The avian egg exhibits general allometric invariances in mechanical design. Sci. Rep. 2017, 7, 14205. [Google Scholar] [CrossRef] [PubMed]
- Kemps, B.; De Ketelaere, B.; Bamelis, F.; Govaerts, T.; Mertens, K.; Kamers, B.; Tona, K.; Decuypere, E.; De Baerdemaeker, J. Development of a methodology for the calculation of young’s modulus of eggshell using vibration measurements. Biosyst. Eng. 2004, 89, 215–221. [Google Scholar] [CrossRef]
- De Ketelaere, B.; Govaerts, T.; Coucke, P.; Dewil, E.; Visscher, J.; Decuypere, E.; De Baerdemaeker, J. Measuring the eggshell strength of 6 different genetic strains of laying hens: Techniques and comparisons. Br. Poult. Sci. 2002, 43, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Amer Eissa, A.H. Comparative eggshell stability assessment using three different non-destructive sensing instruments and breakage force strength. J. Food Eng. 2009, 93, 444–452. [Google Scholar] [CrossRef]
- Perianu, C.; Ketelaere, B.D.; Pluymers, B.; Desmet, W. Finite element approach for simulating the dynamic mechanical behaviour of a chicken egg. Biosyst. Eng. 2010, 106, 79–85. [Google Scholar] [CrossRef]
- Taylor, D.; Walsh, M.; Cullen, A.; O’Reilly, P. The fracture toughness of eggshell. Acta Biomater. 2016, 37, 21–27. [Google Scholar] [CrossRef]
- Gill, F.; Donsker, D.; Rasmussen, P. IOC World Bird List (v11.2). 2021. [Google Scholar] [CrossRef]
- Spatz, H.C.; O’Leary, E.; Vincent, J. Young’s moduli and shear moduli in cortical bone. Proc. R. Soc. B 1996, 263, 287–294. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Chiang, P.-L.; Kao, Y.-C.; Hsu, F.-L.; Juang, J.-Y. Cracking failure of curved hollow tree trunks. R. Soc. Open Sci. 2020, 7, 200203. [Google Scholar] [CrossRef]
- Huang, Y.S.; Hsu, F.L.; Lee, C.M.; Juang, J.Y. Failure mechanism of hollow tree trunks due to cross-sectional flattening. R. Soc. Open Sci. 2017, 4, 160972. [Google Scholar] [CrossRef] [PubMed]
- Flores-Johnson, E.A.; Carrillo, J.G.; Zhai, C.; Gamboa, R.A.; Gan, Y.; Shen, L. Microstructure and mechanical properties of hard Acrocomia mexicana fruit shell. Sci. Rep. 2018, 8, 9668. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, O.P.; Torres, F.G.; Arroyo, J.; Gonzales, K.N.; Fernández-García, M.; López, D. Mechanical properties of calcite- and aragonite-based structures by nanoindentation tests. Bioinspired Biomim. Nanobiomat. 2020, 9, 112–121. [Google Scholar] [CrossRef]
- Meyers, M.A.; McKittrick, J.; Chen, P.Y. Structural biological materials: Critical mechanics-materials connections. Science 2013, 339, 773–779. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.; Lee, Y.N. Electron backscatter diffraction (EBSD) analysis of maniraptoran eggshells with important implications for microstructural and taphonomic interpretations. Palaeontology 2019, 62, 777–803. [Google Scholar] [CrossRef]
- Fischer-Cripps, A.C. Nanoindentation, 2nd ed.; Springer: New York, NY, USA, 2004; pp. xxii, 263p. [Google Scholar]
- Mikhailov, K.E. Eggshell structure, parataxonomy and phylogenetic analysis: Some notes on articles published from 2002 to 2011. Hist. Biol. 2013, 26, 144–154. [Google Scholar] [CrossRef]
- Gill, F.B. Ornithology, 3rd ed.; W.H. Freeman: New York, NY, USA, 2007; pp. xxvi, 758p. [Google Scholar]
- Dennis, J.E.; Xiao, S.-Q.; Agarwal, M.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. Microstructure of matrix and mineral components of eggshells from white leghorn chickens (Gallus gallus). J. Morphol. 1996, 228, 287–306. [Google Scholar] [CrossRef]
- Fecheyr-Lippens, D.C.; Igic, B.; D’Alba, L.; Hanley, D.; Verdes, A.; Holford, M.; Waterhouse, G.I.N.; Grim, T.; Hauber, M.E.; Shawkey, M.D. The cuticle modulates ultraviolet reflectance of avian eggshells. Biol. Open 2015, 4, 753–759. [Google Scholar] [CrossRef]
- Tyler, C.; Simkiss, K. A study of egg shells of ratite birds. Proc. Zool. Soc. Lond. 1959, 133, 201–243. [Google Scholar] [CrossRef]
- Mikhailov, K.E. Eggshell structure in the Shoebill and pelecaniform birds: Comparison with Hamerkop, herons, ibises, and storks. Can. J. Zool. 1995, 73, 1754–1770. [Google Scholar] [CrossRef]
- Mikhailov, K.E. Avian Eggshells: An Atlas of Scanning Electron Micrographs; British Ornithologists’ Club: Tring, UK, 1997. [Google Scholar]
- Cristianne, M.M.C.; Maxwell, T.H. Recent patents on eggshell: shell and membrane applications. Recent Pat. Food Nutr. Agric. 2011, 3, 1–8. [Google Scholar] [CrossRef]
- Currey, J.D.; Miller, A.; Phillips, D.C.; Williams, R.J.P. Effects of differences in mineralization on the mechanical properties of bone. Philos. Trans. R. Soc. Lond. Ser. B-Biol. Sci. 1984, 304, 509–518. [Google Scholar] [CrossRef]
- Tanaka, C.B.; Zhou, Y.; Gludovatz, B.; Kruzic, J.J. Anisotropic fracture resistance of avian eggshell. J. Mech. Behav. Biomed. Mater. 2020, 110, 103888. [Google Scholar] [CrossRef] [PubMed]
- Busse, B.; Hahn, M.; Soltau, M.; Zustin, J.; Püschel, K.; Duda, G.N.; Amling, M. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: Mineralization, morphology and biomechanics of human single trabeculae. Bone 2009, 45, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Fodor, D.; Bondor, C.; Albu, A.; Simon, S.-P.; Craciun, A.; Muntean, L. The value of osteopontin in the assessment of bone mineral density status in postmenopausal women. J. Invest. Med. 2013, 61, 15. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.; Koppala, S.; Swamiappan, S. Bioactivity studies of calcium magnesium silicate prepared from eggshell waste by sol–gel combustion synthesis. J. Asian Ceram. Soc. 2015, 3, 173–177. [Google Scholar] [CrossRef]
- Hajji, S.; Mzoughi, N. Kinetic, equilibrium and thermodynamic studies for the removal of lead ions from aqueous solutions by using low cost adsorbents: A comparative study. IOSR J. Appl. Chem. 2018, 11, 12–24. [Google Scholar] [CrossRef]
- Igic, B.; Braganza, K.; Hyland, M.M.; Silyn-Roberts, H.; Cassey, P.; Grim, T.; Rutila, J.; Moskat, C.; Hauber, M.E. Alternative mechanisms of increased eggshell hardness of avian brood parasites relative to host species. J. R. Soc. Interface 2011, 8, 1654–1664. [Google Scholar] [CrossRef][Green Version]
- Arias, J.L.; Fink, D.J.; Xiao, S.Q.; Heuer, A.H.; Caplan, A.I. Biomineralization and eggshells: Cell-mediated acellular compartments of mineralized extracellular matrix. Int. Rev. Cytol. 1993, 145, 217–250. [Google Scholar] [CrossRef] [PubMed]
- Nys, Y.; Gautron, J.; Garcia-Ruiz, J.M.; Hincke, M.T. Avian eggshell mineralization: Biochemical and functional characterization of matrix proteins. C. R. Palevol 2004, 3, 549–562. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.; Kalin, O.; Nys, Y.; Garcia-Ruiz, J.M.M. Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poult. Sci. 2002, 43, 395–403. [Google Scholar] [CrossRef] [PubMed]
- López, A.V.; Bolmaro, R.E.; Ávalos, M.; Gerschenson, L.N.; Reboreda, J.C.; Fiorini, V.D.; Tartalini, V.; Risso, P.; Hauber, M.E. How to build a puncture- and breakage-resistant eggshell? Mechanical and structural analyses of avian brood parasites and their hosts. J. Exp. Biol. 2021, 224, jeb243016. [Google Scholar] [CrossRef] [PubMed]
- Hearmon, R.F.S. The Elastic Constants of Anisotropic Materials. Rev. Mod. Phys. 1946, 18, 409–440. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, C.-C.; Liu, L.-G.; Sinogeikin, S.V.; Bass, J.D. Elasticity of single-crystal calcite and rhodochrosite by Brillouin spectroscopy. Am. Mineral. 2001, 86, 1525–1529. [Google Scholar] [CrossRef]

| Species | Common Name | N | wt. (%) | wt. S.D. | n | EFEM (GPa) | EFEM S.D. |
|---|---|---|---|---|---|---|---|
| Agapornis fischeri | Fischer’s lovebird | 4 | 84.40 | 0.94 | 4 | 24.60 | 1.90 |
| Agapornis personatus | Yellow-collared lovebird | 1 | 83.38 | 0.00 | 8 | 24.74 | 5.97 |
| Anas platyrhynchos | Mallard | 18 | 93.70 | 1.17 | 78 | 32.50 | 5.11 |
| Antigone antigone | Sarus crane | 9 | 94.71 | 0.45 | 2 | 25.03 | 2.35 |
| Ara ararauna | Blue and yellow macaw | 3 | 94.64 | 1.10 | 12 | 29.85 | 3.78 |
| Balearica regulorum | Gray-crowned crane | 8 | 94.53 | 0.31 | 14 | 30.39 | 6.53 |
| Columba livia domestica | Domestic pigeon | 6 | 94.60 | 0.29 | 10 | 26.39 | 3.30 |
| Dromaius novaehollandiae | Emu | 6 | 96.53 | 0.37 | 28 | 30.97 | 5.24 |
| Gallus gallus domesticus | Chicken | 4 | 95.08 | 0.61 | 13 | 30.77 | 4.35 |
| Merops philippinus | Blue-tailed bee-eater | 4 | 91.15 | 0.70 | 26 | 32.81 | 6.30 |
| Pavo cristatus | Indian peafowl | 10 | 94.71 | 1.14 | 29 | 34.81 | 4.39 |
| Phasianus colchicus | Ring-necked pheasant | 15 | 94.11 | 0.84 | 30 | 29.88 | 4.13 |
| Phoenicopterus chilensis | Chilean flamingo | 9 | 90.99 | 0.44 | 14 | 24.34 | 4.32 |
| Spheniscus demersus | African penguin | 3 | 92.71 | 0.38 | 22 | 25.64 | 4.23 |
| Struthio camelus | Ostrich | 9 | 96.46 | 0.22 | 7 | 47.76 | 4.03 |
| Taeniopygia guttata | Zebra finch | 3 | 83.07 | 0.56 | 26 | 23.28 | 11.78 |
| Species | Common Name | Texture | EFEM (GPa) | EFEM S.D. | n |
|---|---|---|---|---|---|
| Gallus gallus domesticus | Chicken | None | 30.77 | 4.35 | 13 |
| Alopochen aegyptiaca | Egyptian goose | Weak | 41.93 | 7.17 | 22 |
| Gallus sonneratii | Gray junglefowl | 36.23 | 1.77 | 3 | |
| Anas platyrhynchos | Mallard | Medium | 32.50 | 5.11 | 78 |
| Pavo cristatus | Indian peafowl | 34.81 | 4.39 | 29 | |
| Casuarius unappendiculatus | Northern cassowary | Strong | 34.60 | − | 2 |
| Dromaius novaehollandiae | Emu | 30.97 | 5.24 | 28 | |
| Struthio camelus | Ostrich | 47.76 | 4.03 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, P.-L.; Tseng, Y.-C.; Wu, H.-J.; Tsao, S.-H.; Wu, S.-P.; Wang, W.-C.; Hsieh, H.-I.; Juang, J.-Y. Elastic Moduli of Avian Eggshell. Biology 2021, 10, 989. https://doi.org/10.3390/biology10100989
Chiang P-L, Tseng Y-C, Wu H-J, Tsao S-H, Wu S-P, Wang W-C, Hsieh H-I, Juang J-Y. Elastic Moduli of Avian Eggshell. Biology. 2021; 10(10):989. https://doi.org/10.3390/biology10100989
Chicago/Turabian StyleChiang, Pei-Lin, Yu-Chien Tseng, Hsiao-Jou Wu, Shu-Han Tsao, Shang-Ping Wu, Wei-Cheng Wang, Hsin-I Hsieh, and Jia-Yang Juang. 2021. "Elastic Moduli of Avian Eggshell" Biology 10, no. 10: 989. https://doi.org/10.3390/biology10100989
APA StyleChiang, P.-L., Tseng, Y.-C., Wu, H.-J., Tsao, S.-H., Wu, S.-P., Wang, W.-C., Hsieh, H.-I., & Juang, J.-Y. (2021). Elastic Moduli of Avian Eggshell. Biology, 10(10), 989. https://doi.org/10.3390/biology10100989