Dopamine Modulates Drosophila Gut Physiology, Providing New Insights for Future Gastrointestinal Pharmacotherapy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly strains and Culture
2.2. Chemicals
2.3. Gut Contents
2.4. Area, Perimeter, Integrated Dye Intensity, and Number of Fecal Deposits
2.5. Fecal Pellets (pH) Hue Analysis
2.6. Gut Peristalsis
2.7. Activation of Phospholipase C Beta (PLC-β)
2.8. Expression Pattern of Dopaminergic Receptors in the Guts of Larvae and Adults
2.9. Statistical Analysis
3. Results
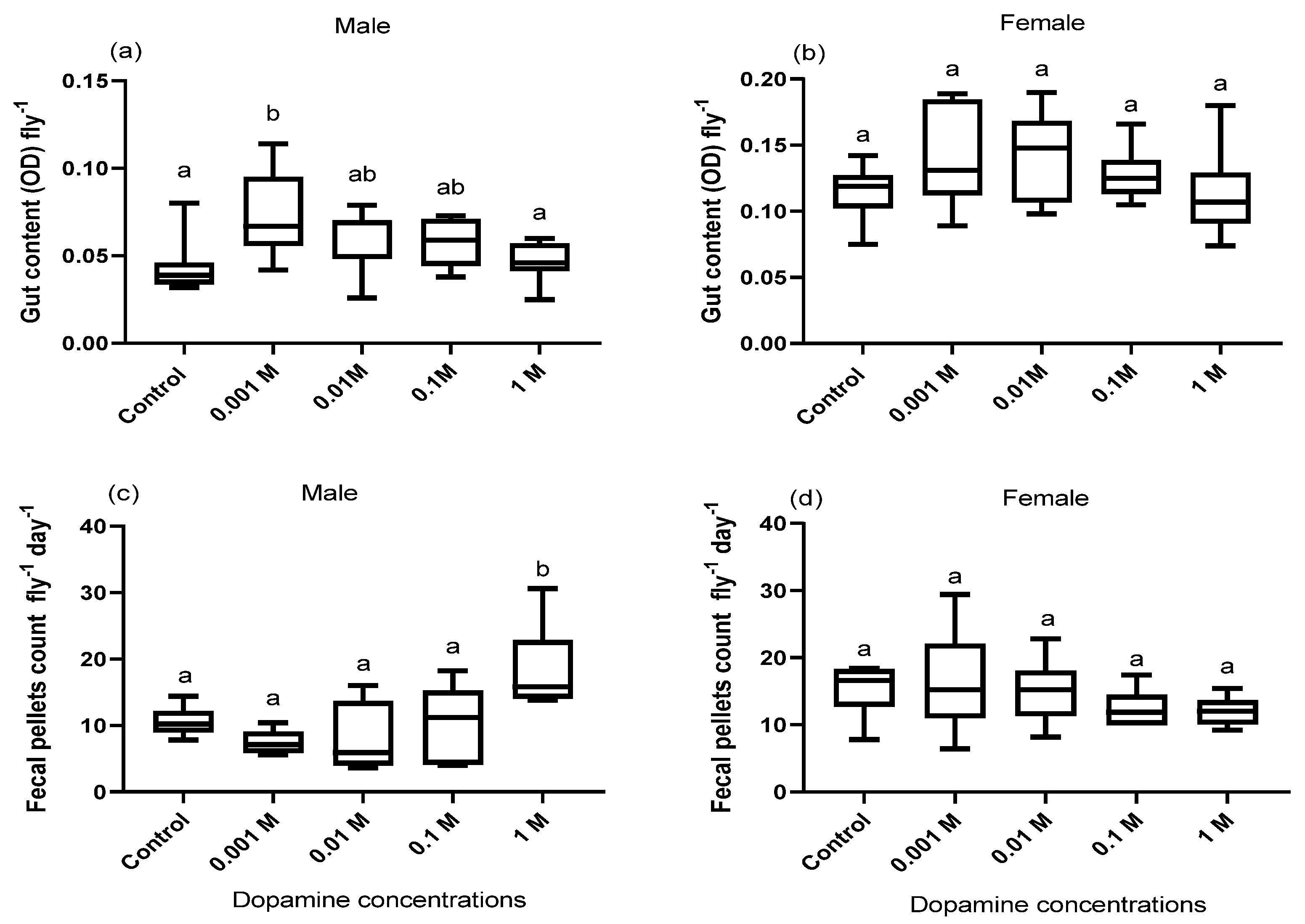
3.1. Effect of Dopamine on the Gut Contents
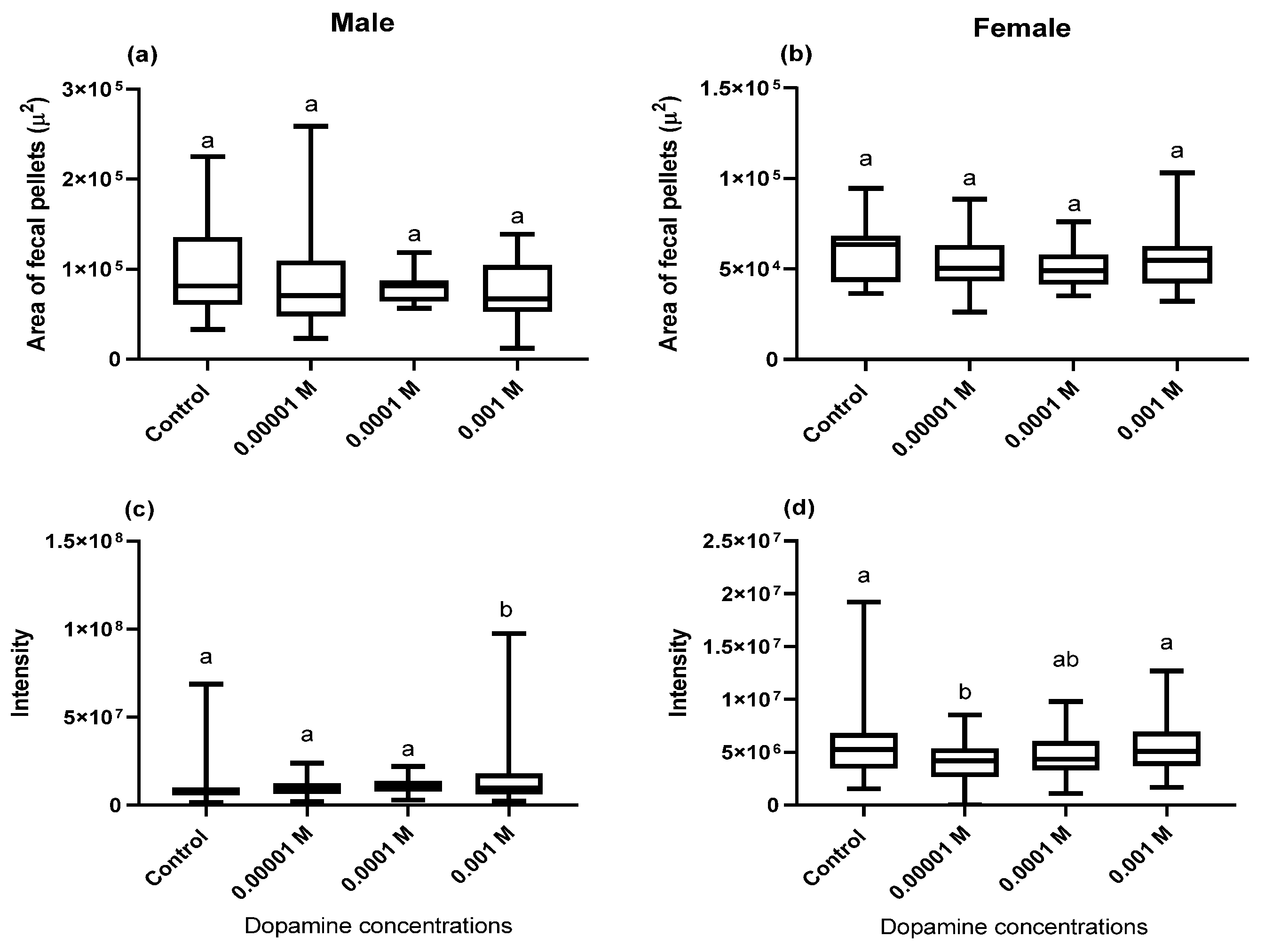
3.2. Effect of Dopamine on Defecation Rate
3.3. Fecal Pellets (pH) Hue Analysis
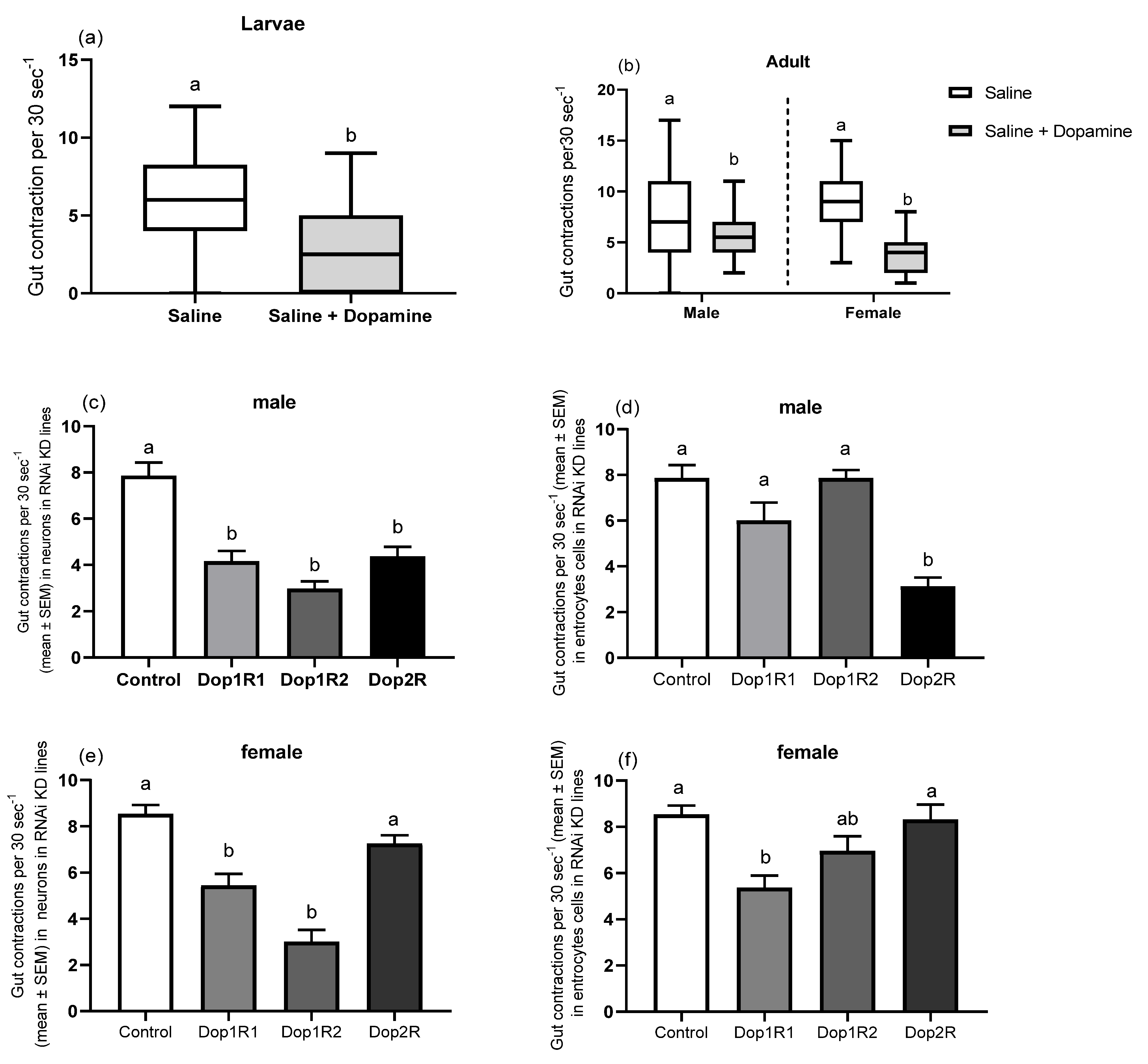
3.4. Gut Peristalsis
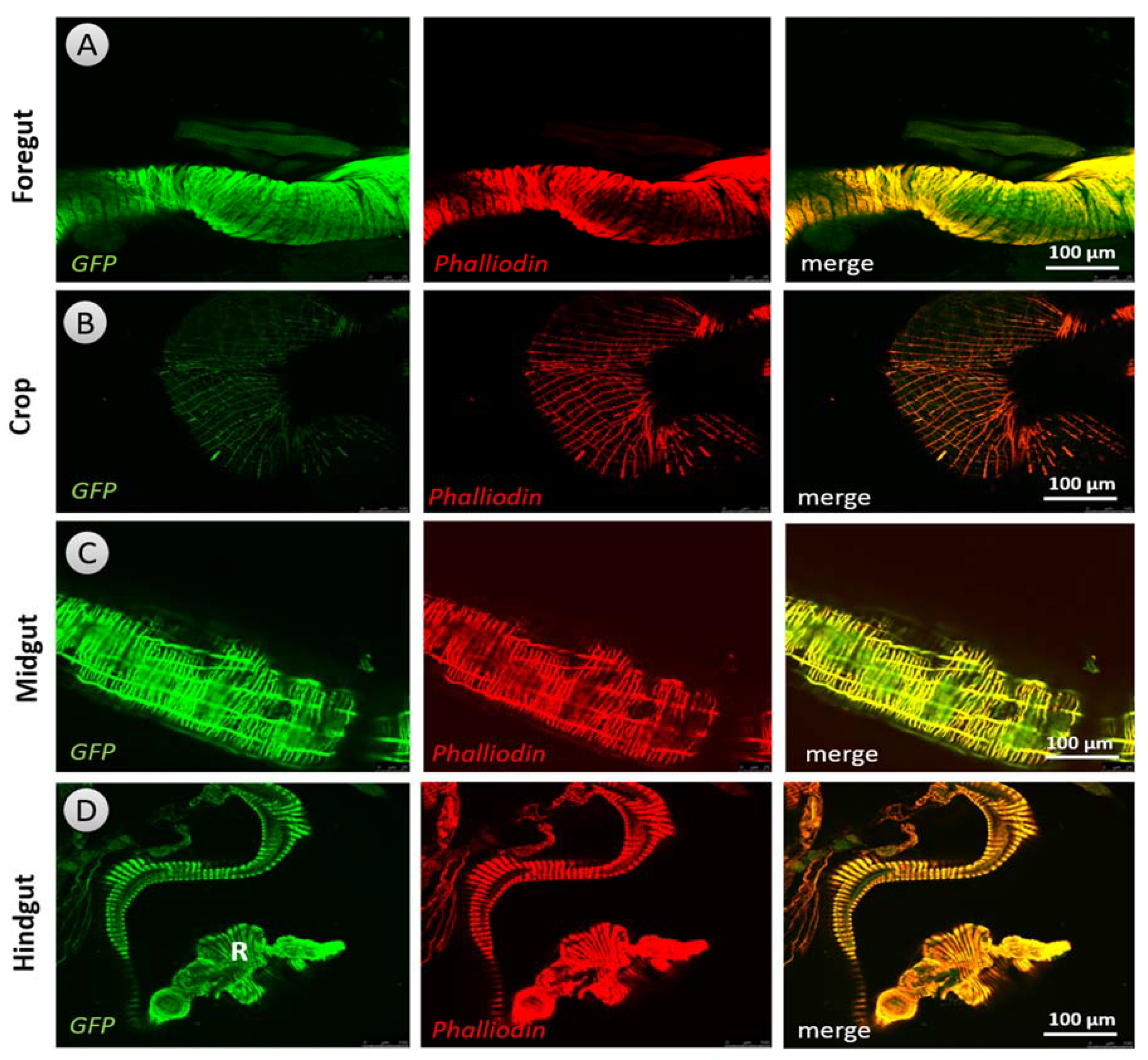
3.5. Expression Pattern of Dopaminergic Receptors
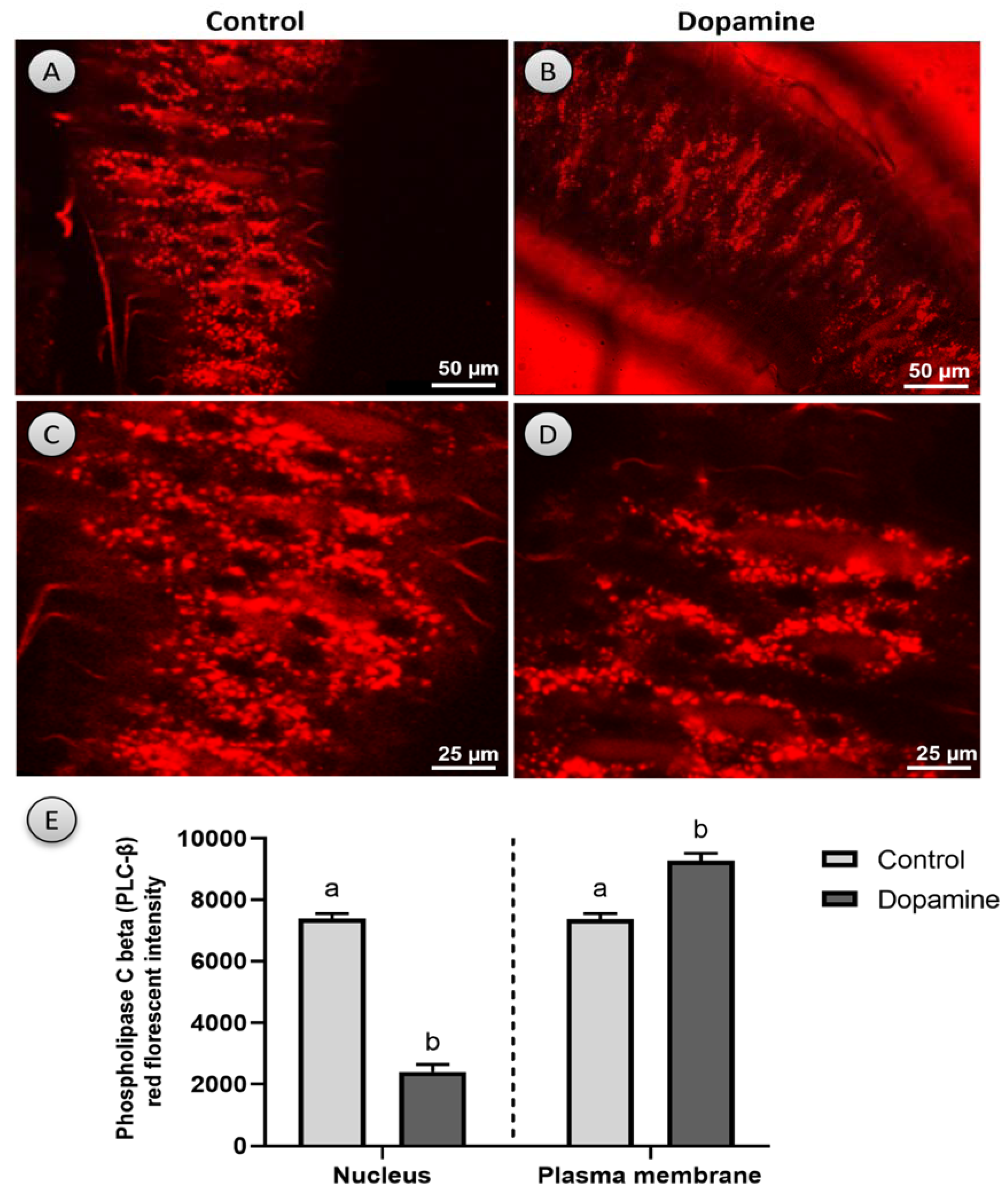
3.6. Phospholipase C Beta (PLC-β)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, T.N.; Taché, Y.; Debas, H.T. Opposing central and peripheral actions of brain-gut peptides: A basis for regulation of gastric function. Surgery 1985, 98, 183–190. [Google Scholar]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The Critical Modulators Regulating Gut-Brain Axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravati, S.; Rosani, A.; Warrington, S.J. Physiology, Catecholamines. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ranjbar-Slamloo, Y.; Fazlali, Z. Dopamine and Noradrenaline in the Brain; Overlapping or Dissociate Functions? Front. Mol. Neurosci. 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Undieh, A.S. Pharmacology of signaling induced by dopamine D1-like receptor activation. Pharmacol. Ther. 2010, 128, 37–60. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, J.E. Dopamine as A Possible Neurotransmitter in Gastric Relaxation. Gastroenterology 1976, 71, 1019–1022. [Google Scholar] [CrossRef]
- Schuurkes, J.A.; Van Nueten, J.M. Is dopamine an inhibitory modulator of gastrointestinal motility? Scand. J. Gastroenterol. Suppl. 1981, 67, 33–36. [Google Scholar] [PubMed]
- N. King, J.; Gerring, E.L. Biphasic disruption of fasting equine gut motility by dopamine—A preliminary study. J. Vet. Pharmacol. Ther. 1988, 11, 354–361. [Google Scholar] [CrossRef]
- Poirier, A.-A.; Aubé, B.; Côté, M.; Morin, N.; Di Paolo, T.; Soulet, D. Gastrointestinal Dysfunctions in Parkinson’s Disease: Symptoms and Treatments. Parkinsons. Dis. 2016, 2016, 1–23. [Google Scholar] [CrossRef]
- Anderson, G.; Noorian, A.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2007, 207, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.-M.; Chen, X.; Luo, D.-Z.; Zhang, X.-H.; Xue, H.; Zheng, L.-F.; Yang, N.; Wang, X.-M.; Zhu, J.-X. Alteration of dopaminergic markers in gastrointestinal tract of different rodent models of Parkinson’s disease. Neuroscience 2008, 153, 634–644. [Google Scholar] [CrossRef]
- Tonini, M.; Cipollina, L.; Poluzzi, E.; Crema, F.; Corazza, G.R.; De Ponti, F. Clinical implications of enteric and central D 2 receptor blockade by antidopaminergic gastrointestinal prokinetics. Aliment. Pharmacol. Ther. 2004, 19, 379–390. [Google Scholar] [CrossRef]
- Li, Z.S. Physiological Modulation of Intestinal Motility by Enteric Dopaminergic Neurons and the D2 Receptor: Analysis of Dopamine Receptor Expression, Location, Development, and Function in Wild-Type and Knock-Out Mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.; Micci, M.-A.; Pasricha, S.; Pasricha, P.J. The D2/D3 Agonist PD128907 (R-(+)-trans-3,4a,10b-Tetrahydro-4-Propyl-2H,5H-[1]Benzopyrano [4,3-b]-1,4-Oxazin-9-ol) Inhibits Stimulated Pyloric Relaxation and Spontaneous Gastric Emptying. Dig. Dis. Sci. 2009, 54, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Yoshida, N. The possible involvement of dopamine D3 receptors in the regulation of gastric emptying in rats. Life Sci. 2010, 87, 638–642. [Google Scholar] [CrossRef]
- Makhlouf, G.M.; Murthy, K.S. Signal Transduction in Gastrointestinal Smooth Muscle. Cell. Signal. 1997, 9, 269–276. [Google Scholar] [CrossRef]
- Medvedev, I.O.; Ramsey, A.J.; Masoud, S.T.; Bermejo, M.K.; Urs, N.; Sotnikova, T.D.; Beaulieu, J.-M.; Gainetdinov, R.R.; Salahpour, A. D1 Dopamine Receptor Coupling to PLC Regulates Forward Locomotion in Mice. J. Neurosci. 2013, 33, 18125–18133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitsouli, C.; Apidianakis, Y.; Perrimon, N. Homeostasis in Infected Epithelia: Stem Cells Take the Lead. Cell Host Microbe 2009, 6, 301–307. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.C. Intestinal morphogenesis. Curr. Opin. Gastroenterol. 2007, 23, 111–114. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- El Kholy, S.; Giesy, J.P.; Al Naggar, Y. Consequences of a short-term exposure to a sub lethal concentration of CdO nanoparticles on key life history traits in the fruit fly (Drosophila melanogaster). J. Hazard. Mater. 2021, 410, 124671. [Google Scholar] [CrossRef]
- El-Kholy, S.; Stephano, F.; Li, Y.; Bhandari, A.; Fink, C.; Roeder, T. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 2015, 361, 669–684. [Google Scholar] [CrossRef]
- Higuchi, C.; Kimura, M.T. Influence of photoperiod on low temperature acclimation for cold-hardiness in Drosophila auraria. Physiol. Entomol. 1985, 10, 303–308. [Google Scholar] [CrossRef]
- Cognigni, P.; Bailey, A.P.; Miguel-Aliaga, I. Enteric Neurons and Systemic Signals Couple Nutritional and Reproductive Status with Intestinal Homeostasis. Cell Metab. 2011, 13, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, G.C.; Tran, T.; Duttlinger, A.; Nichols, R. The drosulfakinin 0 (DSK 0) peptide encoded in the conserved Dsk gene affects adult Drosophila melanogaster crop contractions. J. Insect Physiol. 2007, 53, 1125–1133. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Wang, G.-J.; Baler, R.D. Reward, dopamine and the control of food intake: Implications for obesity. Trends Cogn. Sci. 2011, 15, 37–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmiter, R.D. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007, 30, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M. Dopamine and Food Addiction: Lexicon Badly Needed. Biol. Psychiatry 2013, 73, e15–e24. [Google Scholar] [CrossRef] [Green Version]
- Cersosimo, M.G.; Benarroch, E.E. Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 559–564. [Google Scholar] [CrossRef]
- Hayes, M.W.; Fung, V.S.; Kimber, T.E.; O’Sullivan, J.D. Current concepts in the management of Parkinson disease. Med. J. Aust. 2010, 192, 144–149. [Google Scholar] [CrossRef]
- Noack, C.; Schroeder, C.; Heusser, K.; Lipp, A. Cardiovascular effects of levodopa in Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 815–818. [Google Scholar] [CrossRef] [PubMed]
- Baldereschi, M.; Di Carlo, A.; Rocca, W.A.; Vanni, P.; Maggi, S.; Perissinotto, E.; Grigoletto, F.; Amaducci, L.; Inzitari, D. Parkinson’s disease and parkinsonism in a longitudinal study: Two-fold higher incidence in men. Neurology 2000, 55, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Solla, P.; Cannas, A.; Ibba, F.C.; Loi, F.; Corona, M.; Orofino, G.; Marrosu, M.G.; Marrosu, F. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson’s disease. J. Neurol. Sci. 2012, 323, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinson’s Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef] [Green Version]
- Gillies, G.E.; Pienaar, I.S.; Vohra, S.; Qamhawi, Z. Sex differences in Parkinson’s disease. Front. Neuroendocrinol. 2014, 35, 370–384. [Google Scholar] [CrossRef] [Green Version]
- Auteri, M.; Zizzo, M.G.; Amato, A.; Serio, R. Dopamine induces inhibitory effects on the circular muscle contractility of mouse distal colon via D1- and D2-like receptors. J. Physiol. Biochem. 2016, 73, 395–404. [Google Scholar] [CrossRef]
- Kirschstein, T.; Dammann, F.; Klostermann, J.; Rehberg, M.; Tokay, T.; Schubert, R.; Köhling, R. Dopamine induces contraction in the proximal, but relaxation in the distal rat isolated small intestine. Neurosci. Lett. 2009, 465, 21–26. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2003, 2, 107–116. [Google Scholar] [CrossRef]
- Simunovic, F.; Yi, M.; Wang, Y.; Stephens, R.; Sonntag, K.C. Evidence for Gender-Specific Transcriptional Profiles of Nigral Dopamine Neurons in Parkinson Disease. PLoS ONE 2010, 5, e8856. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, I.; Keller-McGandy, C.; Bouzou, B.; Asteris, G.; Clark, T.W.; Frosch, M.P.; Standaert, D.G. Effects of gender on nigral gene expression and parkinson disease. Neurobiol. Dis. 2007, 26, 606–614. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Volkow, N.D.; Pan, Y.; Du, C. Chronic Cocaine Dampens Dopamine Signaling during Cocaine Intoxication and Unbalances D1 over D2 Receptor Signaling. J. Neurosci. 2013, 33, 15827–15836. [Google Scholar] [CrossRef] [Green Version]
- Chivero, E.T.; Ahmad, R.; Thangaraj, A.; Periyasamy, P.; Kumar, B.; Kroeger, E.; Feng, D.; Guo, M.-L.; Roy, S.; Dhawan, P.; et al. Cocaine Induces Inflammatory Gut Milieu by Compromising the Mucosal Barrier Integrity and Altering the Gut Microbiota Colonization. Sci. Rep. 2019, 9, 12187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suess, G. Cocaine Intake and the Gut Microbiota in Adolescent and Adult Male Rats: A Vicious Cycle? Georgia State University: Atlanta, GA, USA, 2020. [Google Scholar]
- Schuurkes, J.A.; Van Nueten, J.M. Domperidone improves myogenically transmitted antroduodenal coordination by blocking dopaminergic receptor sites. Scand. J. Gastroenterol. Suppl. 1984, 96, 101–110. [Google Scholar] [PubMed]
- Sakakibara, R. Gastrointestinal dysfunction in movement disorders. Neurol. Sci. 2021, 42, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Cocco, L.; Follo, M.Y.; Manzoli, L.; Suh, P.-G. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 2015, 56, 1853–1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadamur, G.; Ross, E.M. Mammalian Phospholipase C. Annu. Rev. Physiol. 2013, 75, 127–154. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Kholy, S.; Wang, K.; El-Seedi, H.R.; Al Naggar, Y. Dopamine Modulates Drosophila Gut Physiology, Providing New Insights for Future Gastrointestinal Pharmacotherapy. Biology 2021, 10, 983. https://doi.org/10.3390/biology10100983
El Kholy S, Wang K, El-Seedi HR, Al Naggar Y. Dopamine Modulates Drosophila Gut Physiology, Providing New Insights for Future Gastrointestinal Pharmacotherapy. Biology. 2021; 10(10):983. https://doi.org/10.3390/biology10100983
Chicago/Turabian StyleEl Kholy, Samar, Kai Wang, Hesham R. El-Seedi, and Yahya Al Naggar. 2021. "Dopamine Modulates Drosophila Gut Physiology, Providing New Insights for Future Gastrointestinal Pharmacotherapy" Biology 10, no. 10: 983. https://doi.org/10.3390/biology10100983
APA StyleEl Kholy, S., Wang, K., El-Seedi, H. R., & Al Naggar, Y. (2021). Dopamine Modulates Drosophila Gut Physiology, Providing New Insights for Future Gastrointestinal Pharmacotherapy. Biology, 10(10), 983. https://doi.org/10.3390/biology10100983







