Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo
Abstract
:Simple Summary
Abstract
1. Introduction
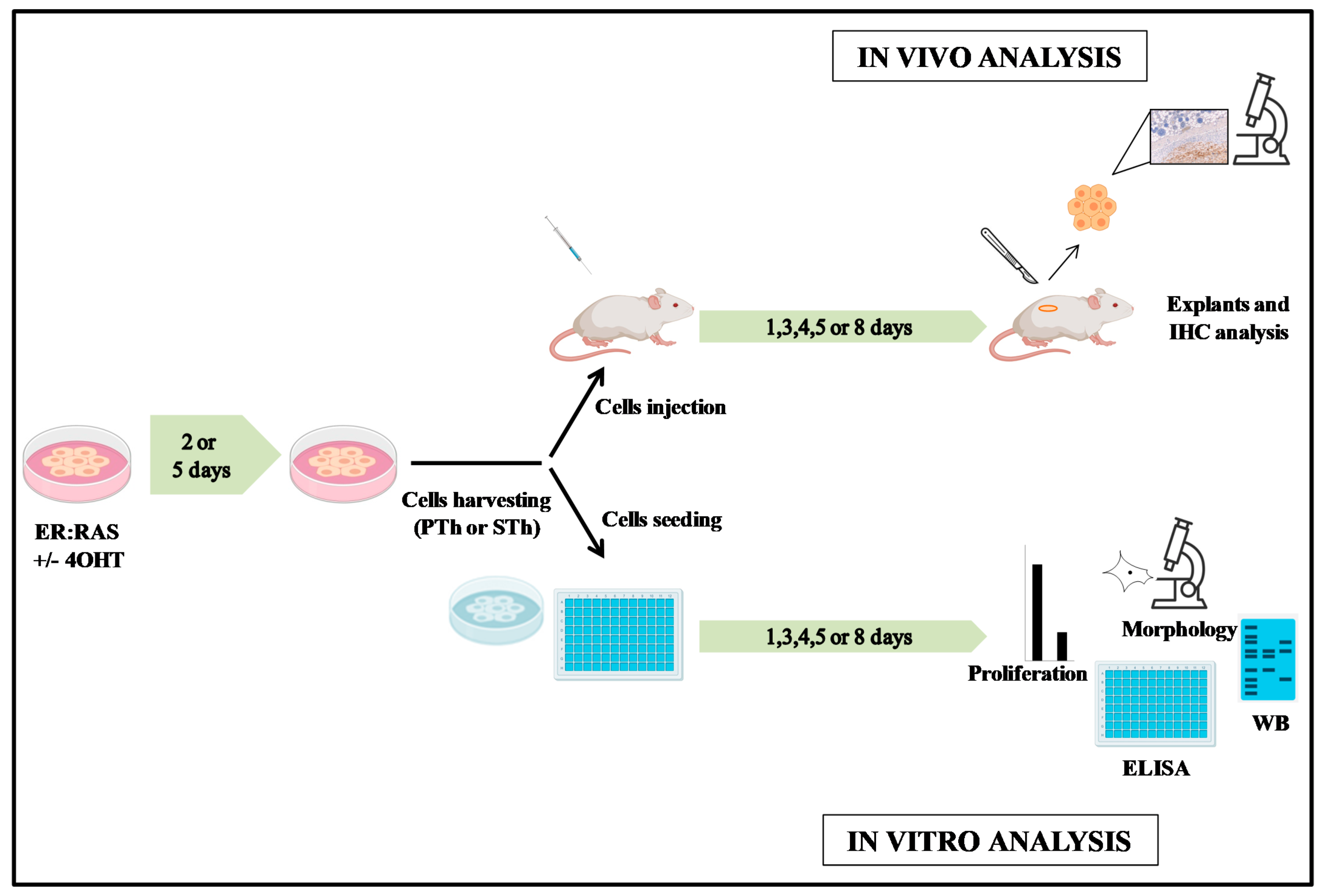
2. Materials and Methods
2.1. Thyroid Cell Cultures
2.2. In Vivo Studies
2.3. Histology and Immunohistochemistry
2.4. Western Blotting
2.5. Proliferation Assay
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
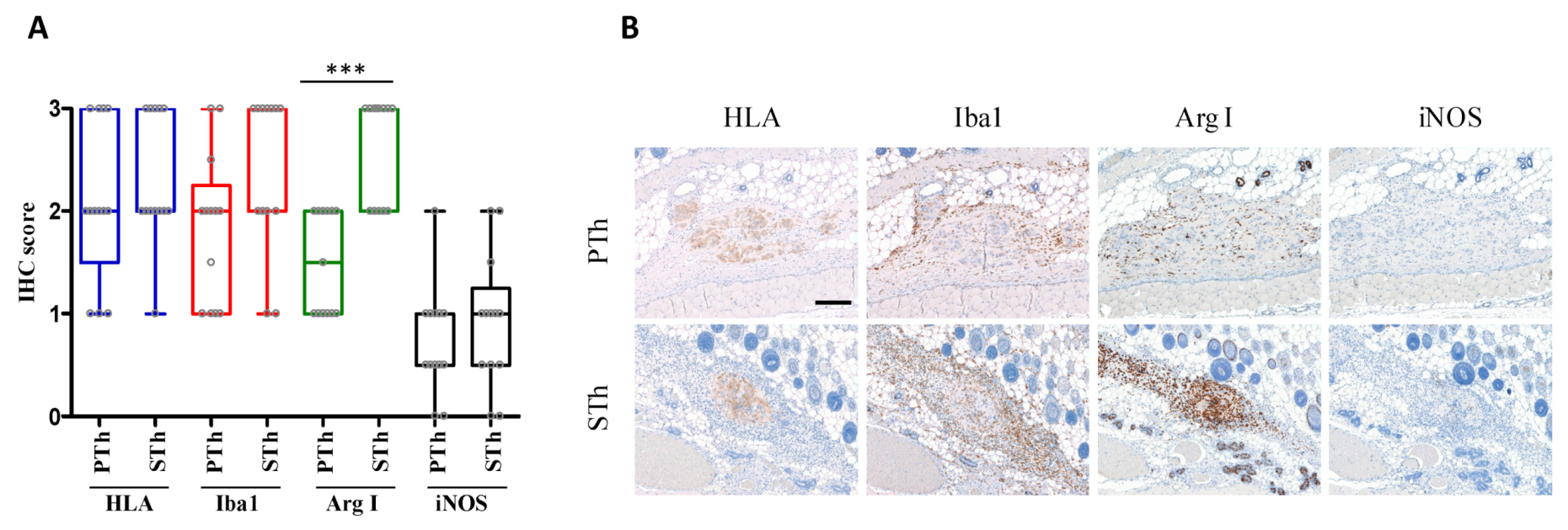
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Horn-Ross, P.L.; Lichtensztajn, D.Y.; Clarke, C.A.; Dosiou, C.; Oakley-Girvan, I.; Reynolds, P.; Gomez, S.L.; Nelson, D.O. Continued rapid increase in thyroid cancer incidence in california: Trends by patient, tumor, and neighborhood characteristics. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Vaccarella, S.; Dal, M.L.; Laversanne, M.; Bray, F.; Plummer, M.; Franceschi, S. The Impact of Diagnostic Changes on the Rise in Thyroid Cancer Incidence: A Population-Based Study in Selected High-Resource Countries. Thyroid 2015, 25, 1127–1136. [Google Scholar] [CrossRef]
- Cabanillas, M.E.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Miyauchi, A.; Oda, H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur. J. Surg. Oncol. 2018, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.B.; Lee, W.K.; Lee, S.G.; Ryu, H.; Lee, C.R.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Lee, E.J.; Chung, W.Y.; et al. Long-term oncologic outcomes of papillary thyroid microcarcinoma according to the presence of clinically apparent lymph node metastasis: A large retrospective analysis of 5,348 patients. Cancer Manag. Res. 2018, 10, 2883–2891. [Google Scholar] [CrossRef] [Green Version]
- Valerio, L.; Pieruzzi, L.; Giani, C.; Agate, L.; Bottici, V.; Lorusso, L.; Cappagli, V.; Puleo, L.; Matrone, A.; Viola, D.; et al. Targeted Therapy in Thyroid Cancer: State of the Art. Clin. Oncol. 2017, 29, 316–324. [Google Scholar] [CrossRef]
- Crusz, S.M.; Balkwill, F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015, 12, 584–596. [Google Scholar] [CrossRef]
- Mantovani, A.; Marchesi, F.; Jaillon, S.; Garlanda, C.; Allavena, P. Tumor-associated myeloid cells: Diversity and therapeutic targeting. Cell. Mol. Immunol. 2021, 18, 566–578. [Google Scholar] [CrossRef]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef]
- Borrello, M.G.; Alberti, L.; Fischer, A.; Degl’Innocenti, D.; Ferrario, C.; Gariboldi, M.; Marchesi, F.; Allavena, P.; Greco, A.; Collini, P.; et al. Induction of a proinflammatory programme in normal human thyrocytes by the RET/PTC1 oncogene. Proc. Natl. Acad. Sci. USA 2005, 102, 14825–14830. [Google Scholar] [CrossRef] [Green Version]
- Qing, W.; Fang, W.Y.; Ye, L.; Shen, L.Y.; Zhang, X.F.; Fei, X.C.; Chen, X.; Wang, W.Q.; Li, X.Y.; Xiao, J.C.; et al. Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid 2012, 22, 905–910. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Cho, S.W.; Min, H.S.; Kim, K.M.; Yeom, G.J.; Kim, E.Y.; Lee, K.E.; Yun, Y.G.; Park, D.J.; Park, Y.J. The expression of tumor-associated macrophages in papillary thyroid carcinoma. Endocrinol. Metab. 2013, 28, 192–198. [Google Scholar] [CrossRef]
- Ryder, M.; Ghossein, R.A.; Ricarte-Filho, J.C.; Knauf, J.A.; Fagin, J.A. Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocr. Relat. Cancer 2008, 15, 1069–1074. [Google Scholar] [CrossRef] [Green Version]
- Caillou, B.; Talbot, M.; Weyemi, U.; Pioche-Durieu, C.; Al, G.A.; Bidart, J.M.; Chouaib, S.; Schlumberger, M.; Dupuy, C. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS ONE 2011, 6, e22567. [Google Scholar] [CrossRef]
- Ryder, M.; Gild, M.; Hohl, T.M.; Pamer, E.; Knauf, J.; Ghossein, R.; Joyce, J.A.; Fagin, J.A. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS ONE 2013, 8, e54302. [Google Scholar] [CrossRef] [Green Version]
- Kuilman, T.; Peeper, D.S. Senescence-messaging secretome: SMS-ing cellular stress. Nat. Rev. Cancer 2009, 9, 81–94. [Google Scholar] [CrossRef]
- Collado, M.; Gil, J.; Efeyan, A.; Guerra, C.; Schuhmacher, A.J.; Barradas, M.; Benguria, A.; Zaballos, A.; Flores, J.M.; Barbacid, M.; et al. Tumour biology: Senescence in premalignant tumours. Nature 2005, 436, 642. [Google Scholar] [CrossRef]
- Davalos, A.R.; Coppe, J.P.; Campisi, J.; Desprez, P.Y. Senescent cells as a source of inflammatory factors for tumor progression. Cancer Metastasis Rev. 2010, 29, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Vizioli, M.G.; Possik, P.A.; Tarantino, E.; Meissl, K.; Borrello, M.G.; Miranda, C.; Anania, M.C.; Pagliardini, S.; Seregni, E.; Pierotti, M.A.; et al. Evidence of oncogene-induced senescence in thyroid carcinogenesis. Endocr. Relat. Cancer 2011, 18, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, M.G.; Santos, J.; Pilotti, S.; Mazzoni, M.; Anania, M.C.; Miranda, C.; Pagliardini, S.; Pierotti, M.A.; Gil, J.; Greco, A. Oncogenic RAS-induced senescence in human primary thyrocytes: Molecular effectors and inflammatory secretome involved. Oncotarget 2014, 5, 8270–8283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.H.; Choi, Y.W.; Lee, J.; Soh, E.Y.; Kim, J.H.; Park, T.J. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat. Commun. 2017, 8, 15208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minna, E.; Brich, S.; Todoerti, K.; Pilotti, S.; Collini, P.; Bonaldi, E.; Romeo, P.; De Cecco, L.; Dugo, M.; Perrone, F.; et al. Cancer Associated Fibroblasts and Senescent Thyroid Cells in the Invasive Front of Thyroid Carcinoma. Cancers 2020, 12, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzoni, M.; Mauro, G.; Erreni, M.; Romeo, P.; Minna, E.; Vizioli, M.G.; Belgiovine, C.; Rizzetti, M.G.; Pagliardini, S.; Avigni, R.; et al. Senescent thyrocytes and thyroid tumor cells induce M2-like macrophage polarization of human monocytes via a PGE2-dependent mechanism. J. Exp. Clin. Cancer. Res. 2019, 38, 208–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anania, M.C.; Sensi, M.; Radaelli, E.; Miranda, C.; Vizioli, M.G.; Pagliardini, S.; Favini, E.; Cleris, L.; Supino, R.; Formelli, F.; et al. TIMP3 regulates migration, invasion and in vivo tumorigenicity of thyroid tumor cells. Oncogene 2011, 30, 3011–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janis, K.; Hoeltke, J.; Nazareth, M.; Fanti, P.; Poppenberg, K.; Aronica, S.M. Estrogen decreases expression of chemokine receptors, and suppresses chemokine bioactivity in murine monocytes. Am. J. Reprod. Immunol. 2004, 51, 22–31. [Google Scholar] [CrossRef]
- Xu, M.; Bradley, E.W.; Weivoda, M.M.; Hwang, S.M.; Pirtskhalava, T.; Decklever, T.; Curran, G.L.; Ogrodnik, M.; Jurk, D.; Johnson, K.O.; et al. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J. Gerontol. Biol. Sci. Med. Sci. 2017, 72, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Behmoaras, J.; Gil, J. Similarities and interplay between senescent cells and macrophages. J. Cell Biol. 2021, 220, e202010162. [Google Scholar] [CrossRef]
- Passaro, C.; Borriello, F.; Vastolo, V.; Di Somma, S.; Scamardella, E.; Gigantino, V.; Franco, R.; Marone, G.; Portella, G. The oncolytic virus dl922-947 reduces IL-8/CXCL8 and MCP-1/CCL2 expression and impairs angiogenesis and macrophage infiltration in anaplastic thyroid carcinoma. Oncotarget 2016, 7, 1500–1515. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzoni, M.; Mauro, G.; Minoli, L.; Cleris, L.; Anania, M.C.; Di Marco, T.; Minna, E.; Pagliardini, S.; Rizzetti, M.G.; Manenti, G.; et al. Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo. Biology 2021, 10, 985. https://doi.org/10.3390/biology10100985
Mazzoni M, Mauro G, Minoli L, Cleris L, Anania MC, Di Marco T, Minna E, Pagliardini S, Rizzetti MG, Manenti G, et al. Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo. Biology. 2021; 10(10):985. https://doi.org/10.3390/biology10100985
Chicago/Turabian StyleMazzoni, Mara, Giuseppe Mauro, Lucia Minoli, Loredana Cleris, Maria Chiara Anania, Tiziana Di Marco, Emanuela Minna, Sonia Pagliardini, Maria Grazia Rizzetti, Giacomo Manenti, and et al. 2021. "Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo" Biology 10, no. 10: 985. https://doi.org/10.3390/biology10100985
APA StyleMazzoni, M., Mauro, G., Minoli, L., Cleris, L., Anania, M. C., Di Marco, T., Minna, E., Pagliardini, S., Rizzetti, M. G., Manenti, G., Borrello, M. G., Scanziani, E., & Greco, A. (2021). Senescent Thyrocytes, Similarly to Thyroid Tumor Cells, Elicit M2-like Macrophage Polarization In Vivo. Biology, 10(10), 985. https://doi.org/10.3390/biology10100985






