Peptide Signatures for Prognostic Markers of Pancreatic Cancer by MALDI Mass Spectrometry Imaging
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Histopathological Assessment
2.2. Procedure of MALDI-Imaging
2.3. MALDI Imaging Analysis
2.4. Data Processing
2.5. Identification of Peptides by “Bottom-Up”-HPLC Mass Spectrometry
3. Results
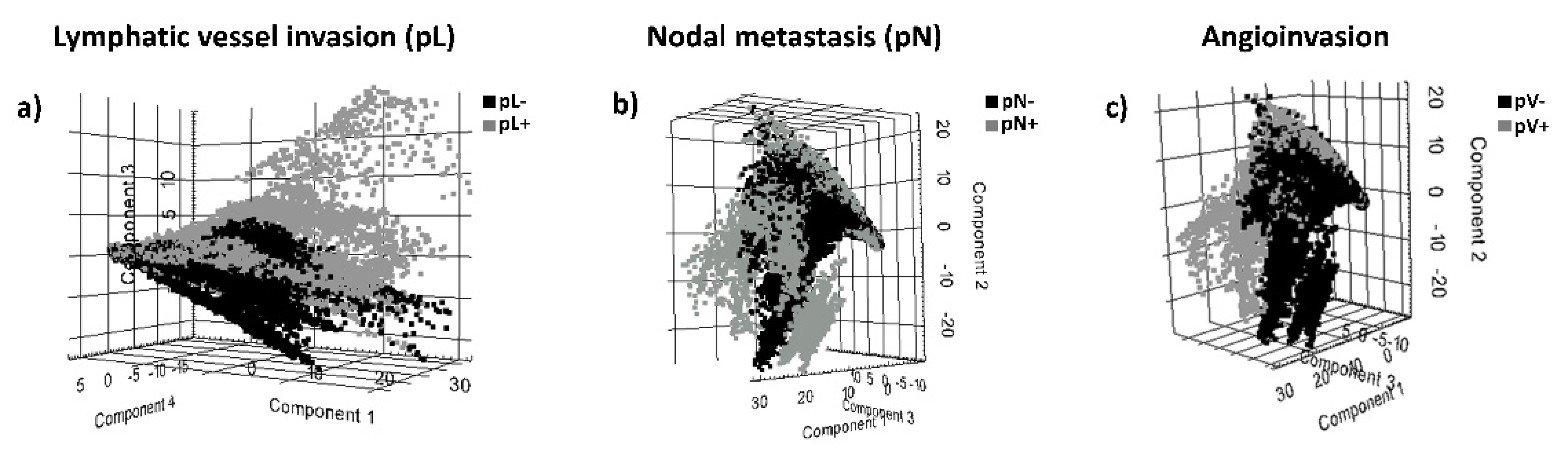
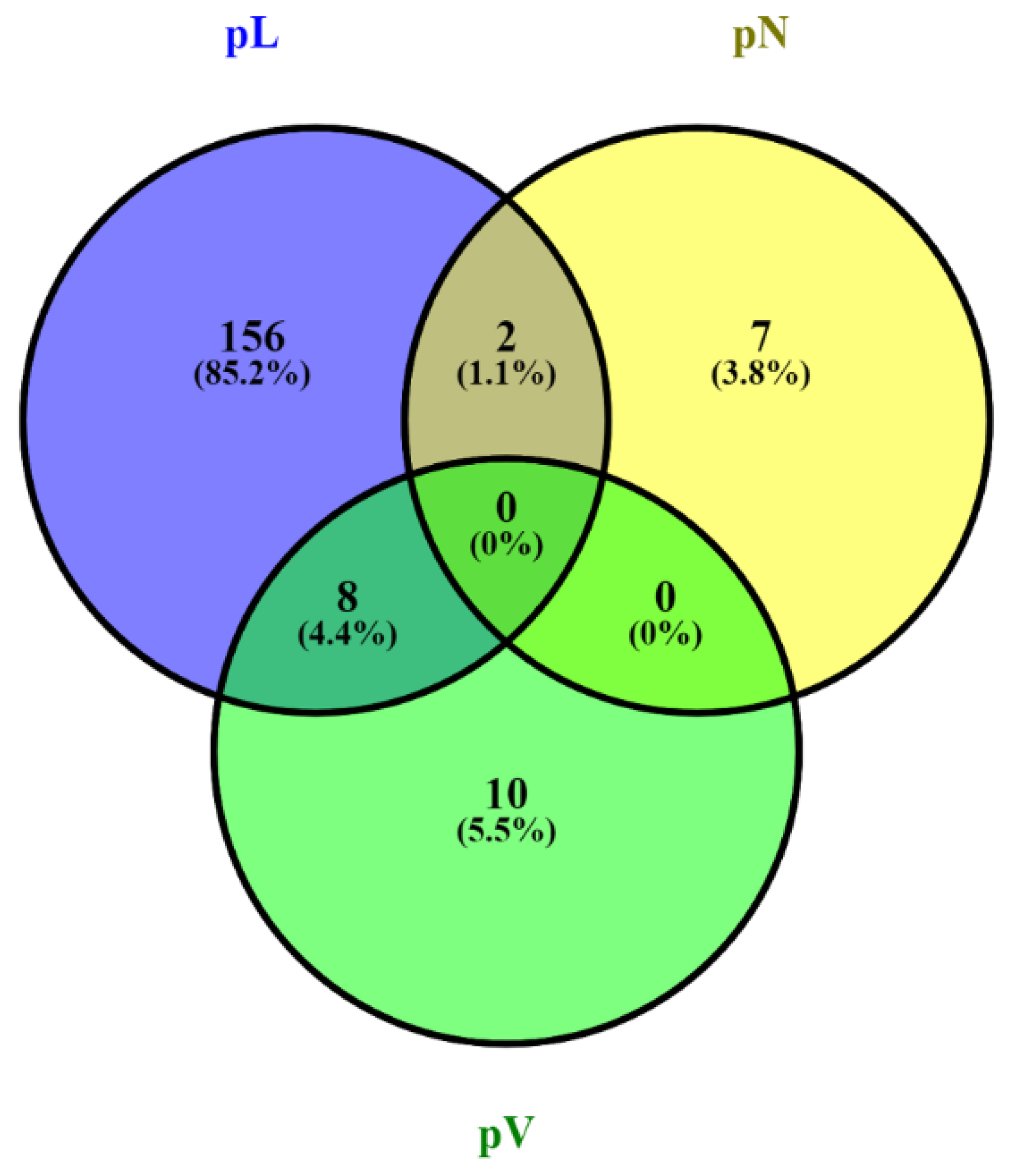
3.1. MALDI-MSI Data and Identification of Discriminative Peptide Signatures for Prognostic Histopathological Tumor Features
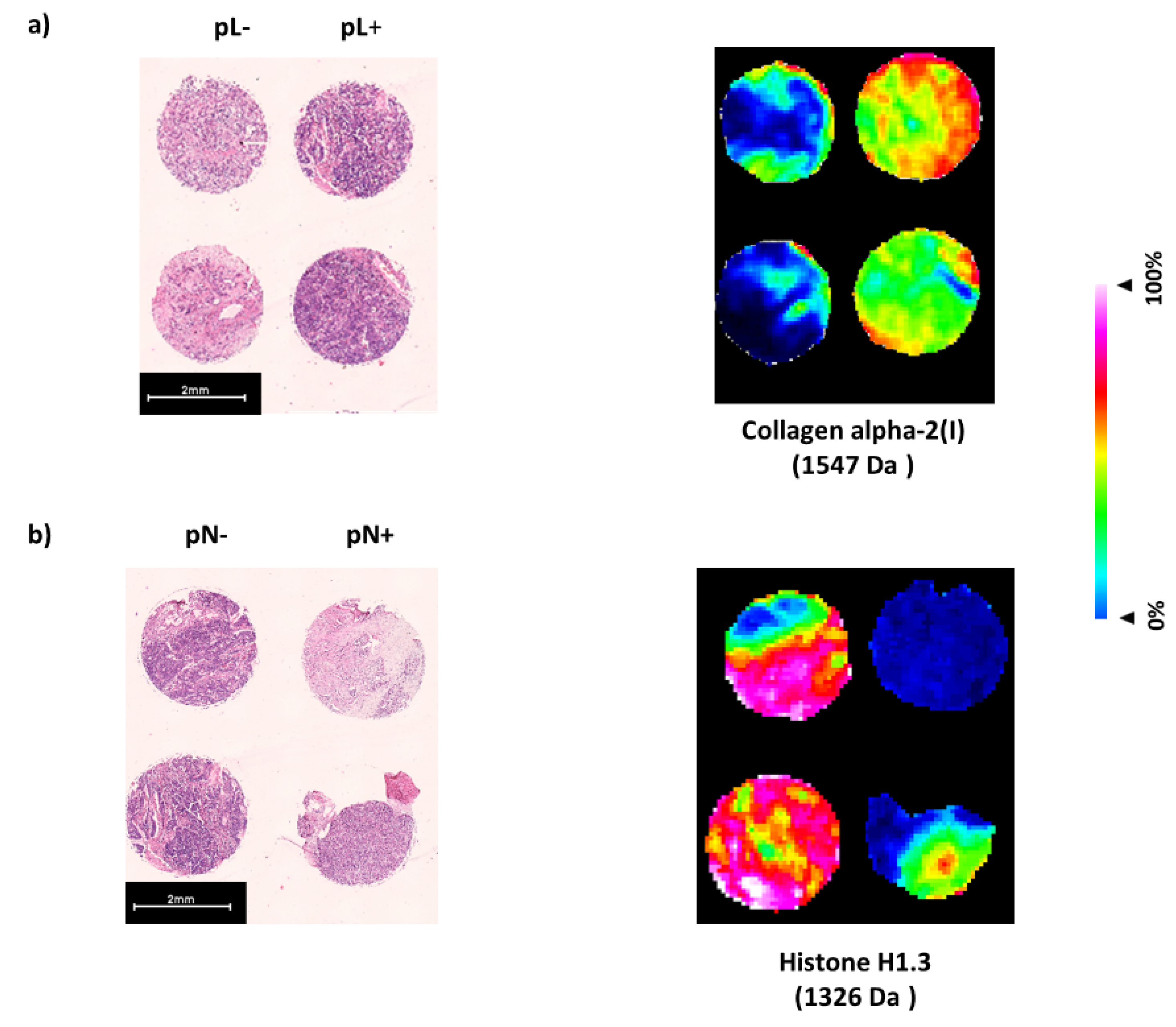
3.2. Identification of Proteins Linked to Discriminative Peptide Signatures from Pancreatic Cancer Tissue Sections
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Du, T.; Bill, K.A.; Ford, J.; Barawi, M.; Hayward, R.D.; Alame, A.; Berri, R.N. The diagnosis and staging of pancreatic cancer: A comparison of endoscopic ultrasound and computed tomography with pancreas protocol. Am. J. Surg. 2018, 215, 472–475. [Google Scholar] [CrossRef]
- Yeo, C.J.; Cameron, J.L.; Lillemoe, K.D.; Sitzmann, J.V.; Hruban, R.H.; Goodman, S.N.; Dooley, W.C.; Coleman, J.; Pitt, H.A. Pancreaticoduodenectomy for cancer of the head of the pancreas 201 patients. Ann. Surg. 1995, 221, 721–733. [Google Scholar] [CrossRef]
- Hartwig, W.; Hackert, T.; Hinz, U.; Gluth, A.; Bergmann, F.; Strobel, O.; Büchler, M.W.; Werner, J. Pancreatic cancer surgery in the new millennium: Better prediction of outcome. Ann. Surg. 2011, 254, 311–319. [Google Scholar] [CrossRef] [Green Version]
- Casadonte, R.; Caprioli, R.M. Proteomic analysis of formalin-fixed paraffin-embedded tissue by MALDI imaging mass spectrometry. Nat. Protoc. 2011, 6, 1695–1709. [Google Scholar] [CrossRef] [Green Version]
- Cornett, D.S.; Reyzer, M.L.; Chaurand, P.; Caprioli, R.M. MALDI imaging mass spectrometry: Molecular snapshots of biochemical systems. Nat. Methods 2007, 4, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Walch, A.; Rauser, S.; Deininger, S.O.; Höfler, H. MALDI imaging mass spectrometry for direct tissue analysis: A new frontier for molecular histology. Histochem. Cell Biol. 2008, 130, 421–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassuhn, W.; Klein, O.; Darb-Esfahani, S.; Lammert, H.; Handzik, S.; Taube, E.T.; Schmitt, W.D.; Keunecke, C.; Horst, D.; Dreher, F.; et al. Classification of Molecular Subtypes of High-Grade Serous Ovarian Cancer by MALDI-Imaging. Cancers 2021, 13, 1512. [Google Scholar] [CrossRef] [PubMed]
- Klein, O.; Kanter, F.; Kulbe, H.; Jank, P.; Denkert, C.; Nebrich, G.; Schmitt, W.D.; Wu, Z.; Kunze, C.A.; Sehouli, J.; et al. MALDI-imaging for classification of epithelial ovarian cancer histotypes from a tissue microarray using machine learning methods. Proteom. Clin. Appl. 2019, 13, 1700181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.A.; Chakravarthy, A.B.; Rosenbluth, J.M.; Mi, D.; Seeley, E.H.; Granja-Ingram, N.D.M.; Olivares, M.G.; Kelley, M.C.; Mayer, I.A.; Meszoely, I.M.; et al. Identification of Markers of Taxane Sensitivity Using Proteomic and Genomic Analyses of Breast Tumors from Patients Receiving Neoadjuvant Paclitaxel and Radiation. Clin. Cancer Res. 2010, 16, 681–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulbe, H.; Klein, O.; Wu, Z.; Taube, E.T.; Kassuhn, W.; Horst, D.; Darb-Esfahani, S.; Jank, P.; Abobaker, S.; Ringel, F.; et al. Discovery of prognostic markers for early-stage high-grade serous ovarian cancer by MALDI-Imaging. Cancers 2020, 12, 2000. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hundsdoerfer, P.; Schulte, J.H.; Astrahantseff, K.; Boral, S.; Schmelz, K.; Eggert, A.; Klein, O. Discovery of Spatial Peptide Signatures for Neuroblastoma Risk Assessment by MALDI Mass Spectrometry Imaging. Cancers 2021, 13, 3184. [Google Scholar] [CrossRef]
- Grüner, B.M.; Hahne, H.; Mazur, P.K.; Trajkovic-Arsic, M.; Maier, S.; Esposito, I.; Kalideris, E.; Michalski, C.W.; Kleeff, J.; Rauser, S.; et al. MALDI imaging mass spectrometry for in situ proteomic analysis of preneoplastic lesions in pancreatic cancer. PLoS ONE 2012, 7, e39424. [Google Scholar] [CrossRef]
- Grüner, B.M.; Winkelmann, I.; Feuchtinger, A.; Sun, N.; Balluff, B.; Teichmann, N.; Herner, A.; Kalideris, E.; Steiger, K.; Braren, R.; et al. Modeling therapy response and spatial tissue distribution of erlotinib in pancreatic cancer. Mol. Cancer Ther. 2016, 15, 1145–1152. [Google Scholar] [CrossRef] [Green Version]
- National Comprehensive Cancer Network Website. NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma, Version 1.2015. Available online: www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 30 December 2014).
- Gustafsson, O.J.; Briggs, M.T.; Condina, M.R.; Winderbaum, L.J.; Pelzing, M.; McColl, S.R.; Everest-Dass, A.V.; Packer, N.H.; Hoffmann, P. MALDI imaging mass spectrometry of N-linked glycans on formalin-fixed paraffin-embedded murine kidney. Anal. Bioanal. Chem. 2015, 407, 2127–2139. [Google Scholar] [CrossRef] [Green Version]
- Klein, O.; Strohschein, K.; Nebrich, G.; Oetjen, J.; Trede, D.; Thiele, H.; Alexandrov, T.; Giavalisco, P.; Duda, G.N.; von Roth, P.; et al. MALDI imaging mass spectrometry: Discrimination of pathophysiological regions in traumatized skeletal muscle by characteristic peptide signatures. Proteomics 2014, 14, 2249–2260. [Google Scholar] [CrossRef]
- Alexandrov, T.; Becker, M.; Guntinas-Lichius, O.; Ernst, G.; von Eggeling, F. MALDI-imaging segmentation is a powerful tool for spatial functional proteomic analysis of human larynx carcinoma. J. Cancer Res. Clin. Oncol. 2013, 139, 85–95. [Google Scholar] [CrossRef]
- Alexandrov, T.; Becker, M.; Deininger, S.O.; Ernst, G.; Wehder, L.; Grasmair, M.; von Eggeling, F.; Thiele, H.; Maass, P. Spatial segmentation of imaging mass spectrometry data with edge-preserving image denoising and clustering. J. Proteome Res. 2010, 9, 6535–6546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trede, D.; Schiffler, S.; Becker, M.; Wirtz, S.; Steinhorst, K.; Strehlow, J.; Aichler, M.; Kobarg, J.H.; Oetjen, J.; Dyatlov, A.; et al. Exploring three-dimensional matrix-assisted laser desorption/ionization imaging mass spectrometry data: Three-dimensional spatial segmentation of mouse kidney. Anal. Chem. 2012, 84, 6079–6087. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Cillero-Pastor, B.; Heeren, R.M. Matrix-Assisted Laser Desorption Ionization Mass Spectrometry Imaging for Peptide and Protein Analyses: A Critical Review of On-Tissue Digestion. J. Proteome Res. 2013, 13, 325–333. [Google Scholar] [CrossRef]
- Lim, K.H.; Chung, E.; Khan, A.; Cao, D.; Linehan, D.; Ben-Josef, E.; Wang-Gilliam, A. Neoadjuvant therapy of pancreatic cancer: The emerging paradigm? Oncologist 2012, 17, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Liu, S.; Wang, J.; Sun, M.Z.; Greenaway, F.T. ACTB in cancer. Clin. Chim. Acta 2013, 417, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Wei, X.; Yu, Q.; Niu, X.; Tang, S.; Song, L. A feature-based analysis identifies COL1A2 as a regulator in pancreatic cancer. J. Enzyme Inhib. Med. Chem. 2019, 34, 420–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svoronos, C.; Tsoulfas, G.; Souvatzi, M.; Chatzitheoklitos, E. Prognostic value of COL6A3 in pancreatic adenocarcinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, Y.; Ishihara, S.; Uchida, Y.; Tajima, K.; Mizutani, T.; Kawabata, K.; Haga, H. Filamin B enhances the invasiveness of cancer cells into 3D collagen matrices. Cell Struct. Funct. 2015, 40, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, S.; Tomita, Y.; Hoshida, Y.; Nagano, H.; Dono, K.; Umeshita, K.; Sakon, M.; Ishikawa, O.; Ohigashi, H.; Nakamori, S.; et al. Increased expression of valosin-containing protein (p97) is associated with lymph node metastasis and prognosis of pancreatic ductal adenocarcinoma. Ann. Surg. Oncol. 2004, 11, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bastola, P.; Neums, L.; Schoenen, F.J.; Chien, J. VCP inhibitors induce endoplasmic reticulum stress, cause cell cycle arrest, trigger caspase-mediated cell death and synergistically kill ovarian cancer cells in combination with Salubrinal. Mol. Oncol. 2016, 10, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Clauser, K.R.; Öhlund, D.; Rickelt, S.; Huang, Y.; Gupta, M.; Mani, D.R.; Carr, S.A.; Tuveson, D.A.; Hynes, R.O. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl. Acad. Sci. USA 2019, 116, 19609–19618. [Google Scholar] [CrossRef] [Green Version]
- Ting, D.T.; Wittner, B.S.; Ligorio, M.; Vincent, J.N.; Shah, A.M.; Miyamoto, D.T.; Aceto, N.; Bersani, F.; Brannigan, B.W.; Xega, K.; et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014, 8, 1905–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Zhao, L.; Budhu, A.; Forgues, M.; Jia, H.L.; Qin, L.X.; Yu, J.; Shi, X.; Tang, Z.; Wang, X.W. Let-7g targets collagen type I alpha2 and inhibits cell migration in hepatocellular carcinoma. J. Hepatol. 2010, 52, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009, 26, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, X.H.; Zhou, Y.F.; Lan, M.; Huang, S.H.; Liu, Z.L.; Shu, Y. Valosin-containing protein promotes metastasis of osteosarcoma through autophagy induction and anoikis inhibition via the ERK/NF-κβ/beclin-1 signaling pathway. Oncol. Lett. 2019, 18, 3823–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Li, G.; Wang, K.; Mu, Z.; Xie, Q.; Qu, H.; Lv, H.; Hu, B. Collagen Type VI Alpha 3 Chain Promotes Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Transforming Growth Factor β (TGF-β)/Smad Pathway. Med. Sci. Monit. 2018, 24, 5346–5354. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.H.; Chang, T.H.; Huang, Y.F.; Huang, H.D.; Chou, C.Y. COL1A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene 2014, 33, 3432–3440. [Google Scholar] [CrossRef]
- Aichler, M.; Walch, A. MALDI Imaging mass spectrometry: Current frontiers and perspectives in pathology research and practice. Lab. Investig. 2015, 95, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Winderbaum, L.; Koch, I.; Mittal, P.; Hoffmann, P. Classification of MALDI-MS imaging data of tissue microarrays using canonical correlation analysis-based variable selection. Proteomics 2016, 16, 1731–1735. [Google Scholar] [CrossRef] [Green Version]
- Boskamp, T.; Lachmund, D.; Oetjen, J.; Hernandez, Y.C.; Trede, D.; Maass, P.; Casadonte, R.; Kriegsmann, J.; Warth, A.; Dienemann, H.; et al. A new classification method for MALDI imaging mass spectrometry data acquired on formalin-fixed paraffin-embedded tissue samples. Biochim. Biophys. Acta 2017, 1865, 916–926. [Google Scholar] [CrossRef]
- Behrmann, J.; Etmann, C.; Boskamp, T.; Casadonte, R.; Kriegsmann, J.; Maass, P. Deep learning for tumor classification in imaging mass spectrometry. Bioinformatics 2017, 34, 1215–1223. [Google Scholar] [CrossRef]
- Ali, H.R.; Jackson, H.W.; Zanotelli, V.R.T.; Danenberg, E.; Fischer, J.R.; Bardwell, H.; Provenzano, E.; Rueda, O.M.; Chin, S.; Aparicio, S.; et al. Imaging mass cytometry and multiplatform genomics define the phenogenomic landscape of breast cancer. Nat. Cancer 2020, 1, 163–175. [Google Scholar] [CrossRef]
- Yagnik, G.; Liu, Z.; Rothschild, K.J.; Lim, M.J. Highly multiplexed immunohistochemical MALDI-MS imaging of biomarkers in tissues. J. Am. Soc. Mass Spectrom. 2021, 32, 977–988. [Google Scholar] [CrossRef] [PubMed]
| Patients | n = 18 |
|---|---|
| Age | |
| median age (years) | 67 |
| age range (years) | 36–77 |
| Sex | |
| Female | 8 (44%) |
| Male | 10 (56%) |
| Location of main tumor mass | |
| Pancreatic head | 14 (77%) |
| Pancreatic body | 1 (6%) |
| Pancreatic tail | 3 (17%) |
| Histopathological characteristics | |
| pT1 | 1 (6%) |
| pT2 | 1 (6%) |
| pT3 | 16 (88%) |
| pN+ | 12 (67%) |
| pN− | 6 (33%) |
| G1 | 1 (6%) |
| G2 | 11 (61%) |
| G3 | 5 (27%) |
| G4 | 1 (6%) |
| PN+ | 11 (61%) |
| pL+ | 8 (44%) |
| pL− | 10 (56%) |
| pV+ | 5 (27%) |
| pV− | 13 (73%) |
| Adenocarcinoma | 17 (94%) |
| Acinar cell carcinoma | 1 (6%) |
| MSI Mr [m/z] [Da] | Lymphatic Vessel Invasion (pL+) vs. None (pL−) (AUC) | LC-MS Mr [Da] | Deviation Δ [Da] | Protein |
|---|---|---|---|---|
| 1198.839 | 0.6005 | 1198.7052 | 0.1338 | Actin, cytoplasmic 1 |
| 1790.828 | 0.6128 | 1790.8874 | −0.0594 | |
| 1547.791 | 0.6213 | 1547.7901 | 0.0009 | Collagen alpha-2(I) chain |
| 1562.794 | 0.6343 | 1562.7900 | 0.0040 | |
| 805.481 | 0.6001 | 805.4568 | 0.0242 | Collagen alpha-3(VI) chain |
| 1467.68 | 0.6005 | 1467.7243 | −0.0443 | |
| 1628.804 | 0.6099 | 1628.8466 | −0.0426 | Filamin-B |
| 1766.824 | 0.6078 | 1766.9417 | −0.1177 | |
| 1326.808 | 0.6125 | 1326.7631 | 0.0449 | Histone H1.3 |
| 2059.968 | 0.6056 | 2060.1222 | −0.1542 | |
| 958.504 | 0.6056 | 958.5309 | −0.0269 | Spectrin beta chain, non-erythrocytic 1 |
| 2059.068 | 0.6165 | 2059.1005 | −0.0325 | |
| 1690.913 | 0.6006 | 1690.8475 | 0.0655 | Valosin-containing protein (VCP) |
| 1777.926 | 0.6156 | 1777.9513 | −0.0253 | |
| 1269.65 | 0.6235 | 1269.6794 | −0.0294 | Vinculin |
| 1428.674 | 0.6260 | 1428.7041 | −0.0301 | |
| 831.585 | 0,3786 | 831.4925 | 0.0925 | Histone H1.3 |
| 1326.808 | 0,3985 | 1326.7631 | 0.0449 | |
| 1562.794 | 0.60311 | 1562.7900 | 0.0040 | Collagen alpha-2(I) chain |
| 2026.963 | 0.6018 | 2027.0120 | −0.0490 | |
| 2056.067 | 0.6331 | 2056.0459 | 0.0211 | Myosin-11 |
| 2706.264 | 0.6078 | 2706.2320 | 0.0320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loch, F.N.; Klein, O.; Beyer, K.; Klauschen, F.; Schineis, C.; Lauscher, J.C.; Margonis, G.A.; Degro, C.E.; Rayya, W.; Kamphues, C. Peptide Signatures for Prognostic Markers of Pancreatic Cancer by MALDI Mass Spectrometry Imaging. Biology 2021, 10, 1033. https://doi.org/10.3390/biology10101033
Loch FN, Klein O, Beyer K, Klauschen F, Schineis C, Lauscher JC, Margonis GA, Degro CE, Rayya W, Kamphues C. Peptide Signatures for Prognostic Markers of Pancreatic Cancer by MALDI Mass Spectrometry Imaging. Biology. 2021; 10(10):1033. https://doi.org/10.3390/biology10101033
Chicago/Turabian StyleLoch, Florian N., Oliver Klein, Katharina Beyer, Frederick Klauschen, Christian Schineis, Johannes C. Lauscher, Georgios A. Margonis, Claudius E. Degro, Wael Rayya, and Carsten Kamphues. 2021. "Peptide Signatures for Prognostic Markers of Pancreatic Cancer by MALDI Mass Spectrometry Imaging" Biology 10, no. 10: 1033. https://doi.org/10.3390/biology10101033
APA StyleLoch, F. N., Klein, O., Beyer, K., Klauschen, F., Schineis, C., Lauscher, J. C., Margonis, G. A., Degro, C. E., Rayya, W., & Kamphues, C. (2021). Peptide Signatures for Prognostic Markers of Pancreatic Cancer by MALDI Mass Spectrometry Imaging. Biology, 10(10), 1033. https://doi.org/10.3390/biology10101033









