Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Material
2.3. Metabolic and Hormonal Profile in Blood Serum
2.4. Statistical Analysis
3. Results
3.1. Body Weight and General Metabolic Profile
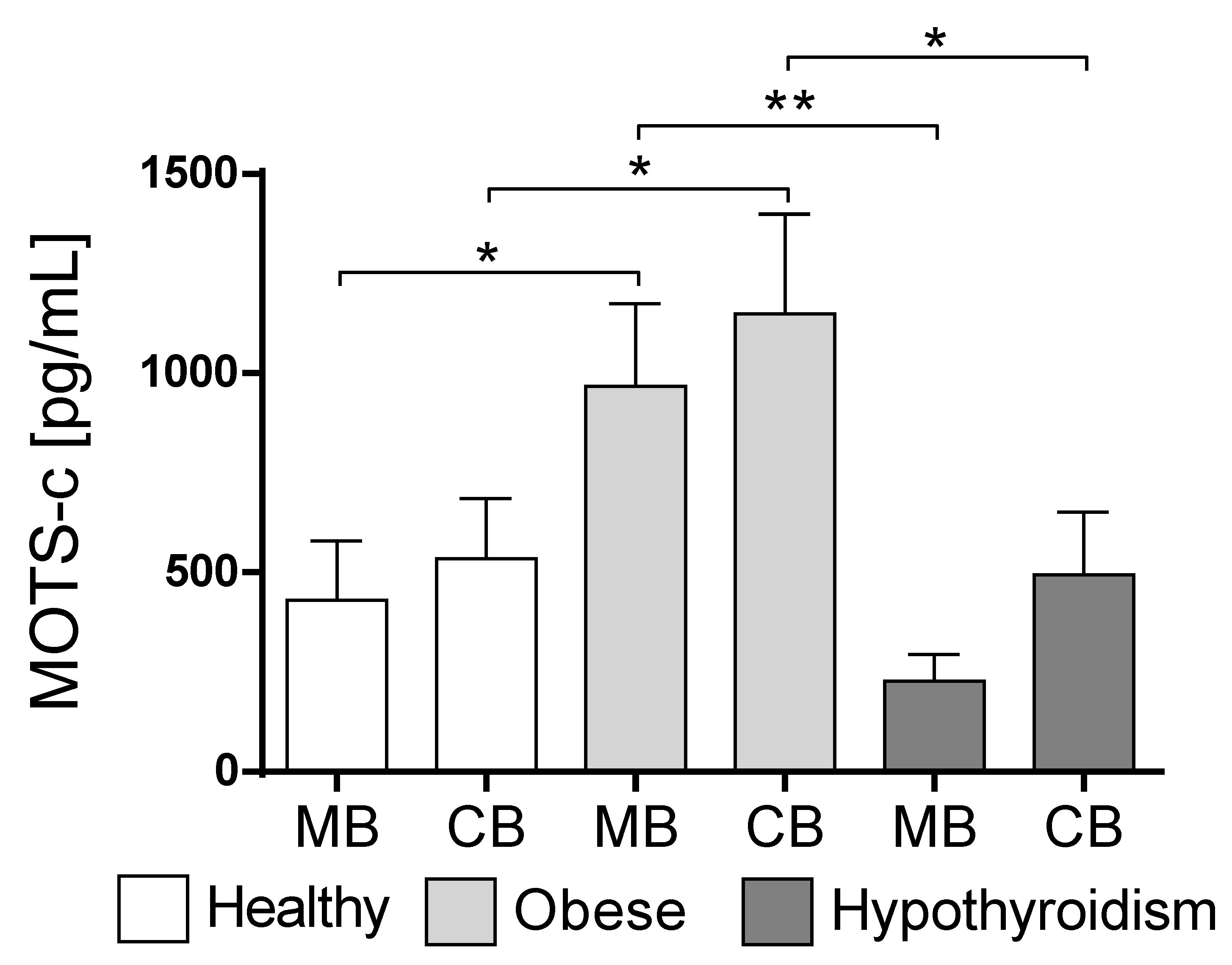
3.2. MOTS-c Concentration Changes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-, A.; Wan, J.; Kim, S.; Mehta, H.; Hevener, A.L.; De, R.; et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Niikura, T.; Tajima, H.; Yasukawa, T.; Sudo, H.; Ito, Y.; Kita, Y.; Kawasumi, M.; Kouyama, K.; Doyu, M.; et al. A Rescue Factor Abolishing Neuronal Cell Death by a Wide Spectrum of Familial Alzheimer’s Disease Genes and Aβ. Proc. Natl. Acad. Sci. USA 2001, 98, 6336–6341. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Zhai, D.; Cabezas, E.; Welsh, K.; Nouraini, S.; Satterthwait, A.C.; Reed, J.C. Humanin Peptide Suppresses Apoptosis by Interfering with Bax Activation. Nature 2003, 423, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, M.; Liu, B.; Hashimoto, Y.; Ma, L.; Lee, K.W.; Niikura, T.; Nishimoto, I.; Cohen, P. Interaction between the Alzheimer’s Survival Peptide Humanin and Insulin-like Growth Factor-Binding Protein 3 Regulates Cell Survival and Apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 13042–13047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Physiol. Behav. 2019, 176, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Kim, K.H.; Cohen, P.; Angeles, L.; States, U. MOTS-c: A Novel Mitochondrial-Derived Peptide Regulating Muscle and Fat Metabolism. Free. Radic. Biol. Med. 2016, 100, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Wolkowitz, O.M.; Srihari, V.H.; Reus, V.I.; Goetzl, L.; Kapogiannis, D.; Heninger, G.R.; Mellon, S.H. Abnormal Levels of Mitochondrial Proteins in Plasma Neuronal Extracellular Vesicles in Major Depressive Disorder. Mol. Psychiatry 2021, in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Jia, W.; Liu, G.; Zhang, J.; Wang, B.; Li, J.; Cui, P.; Li, X.; Lager, S.; et al. Hyperandrogenism and Insulin Resistance Modulate Gravid Uterine and Placental Ferroptosis in PCOS-like Rats. J. Endocrinol. 2020, 246, 247–263. [Google Scholar] [CrossRef]
- Guo, Q.; Chang, B.; Yu, Q.-L.; Xu, S.-T.; Yi, X.-J.; Cao, S.-C. Adiponectin Treatment Improves Insulin Resistance in Mice by Regulating the Expression of the Mitochondrial-Derived Peptide MOTS-c and Its Response to Exercise via APPL1–SIRT1–PGC-1α. Diabetologia 2020, 63, 2675–2688. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Bettahi, I.; Jerobin, J.; Chandra, P.; Khalil, C.A.; Skarulis, M.; Atkin, S.L.; Abou-Samra, A.B. Mitochondrial-Derived Peptides Are down Regulated in Diabetes Subjects. Front. Endocrinol. (Lausanne) 2019, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Fuku, N.; Pareja-Galeano, H.; Zempo, H.; Alis, R.; Arai, Y.; Lucia, A.; Hirose, N. The Mitochondrial-Derived Peptide MOTS-c: A Player in Exceptional Longevity? Aging Cell 2015, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Wojciechowicz, T.; Sassek, M.; Leciejewska, N.; Jasaszwili, M.; Billert, M.; Małek, E.; Szczepankiewicz, D.; Misiewicz-Mielnik, M.; et al. The Role of Peptide Hormones Discovered in the 21st Century in the Regulation of Adipose Tissue Functions. Genes (Basel) 2021, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, J.S.T.; Merry, T.L. Mitochondrial-Derived Peptides and Exercise. Biochim. Biophys. Acta-Gen. Subj. 2021, 1865, 130011. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c Interacts Synergistically with Exercise Intervention to Regulate PGC-1α Expression, Attenuate Insulin Resistance and Enhance Glucose Metabolism in Mice via AMPK Signaling Pathway. Biochim. Biophys. Acta-Mol. Basis Dis. 2021, 1867, 166126. [Google Scholar] [CrossRef] [PubMed]
- Kapur, A.; McIntyre, H.D.; Hod, M. Type 2 Diabetes in Pregnancy. Endocrinol. Metab. Clin. N. Am. 2019, 48, 511–531. [Google Scholar] [CrossRef] [PubMed]
- Dow, M.L.; Szymanski, L.M. Effects of Overweight and Obesity in Pregnancy on Health of the Offspring. Endocrinol. Metab. Clin. N. Am. 2020, 49, 251–263. [Google Scholar] [CrossRef]
- Casey, B.M.; Leveno, K.J. Thyroid Disease in Pregnancy. Obstet Gynecol. 2006, 108, 1283–1292. [Google Scholar] [CrossRef]
- Caballero, B. Humans against Obesity: Who Will Win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Warchoł, M.; Wojciechowska, M.; Kupsz, J.; Sot-Szewczyk, M.H.; Michalak, M.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Krauss, H. Association of Cord Blood Ghrelin, Leptin and Insulin Concentrations in Term Newborns with Anthropometric Parameters at Birth. J. Pediatr. Endocrinol. Metab. 2018, 31, 151–157. [Google Scholar] [CrossRef]
- Kołodziejski, P.A.; Pruszyńska-Oszmałek, E.; Korek, E.; Sassek, M.; Szczepankiewicz, D.; Kaczmarek, P.; Nogowski, L.; Maćkowiak, P.; Nowak, K.W.; Krauss, H.; et al. Serum Levels of Spexin and Kisspeptin Negatively Correlate with Obesity and Insulin Resistance in Women. Physiol. Res. 2018, 67, 45–56. [Google Scholar] [CrossRef]
- Baylan, F.A.; Karaküçük, S. Maternal Plasma Elabela Levels in Intrauterine Growth Restriction. Cukurova Med. J. 2021, 46, 1344–1350. [Google Scholar] [CrossRef]
- Zarse, K.; Ristow, M. A Mitochondrially Encoded Hormone Ameliorates Obesity and Insulin Resistance. Cell Metab. 2015, 21, 355–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, L.R.; Fernández-Verdejo, R.; Santos, J.L.; Galgani, J.E. Plasma MOTS-c Levels Are Associated with Insulin Sensitivity in Lean but Not in Obese Individuals. J. Investig. Med. 2018, 66, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, M.; Zhai, Y.; Li, Q.; Ye, Z.; Wang, L.; Luo, W.; Chen, J.; Lu, Z. MOTS-c Peptide Regulates Adipose Homeostasis to Prevent Ovariectomy-Induced Metabolic Dysfunction. J. Mol. Med. 2019, 97, 473–485. [Google Scholar] [CrossRef]
- Ramanjaneya, M.; Jerobin, J.; Bettahi, I.; Bensila, M.; Aye, M.; Siveen, K.S.; Sathyapalan, T.; Skarulis, M.; Abou-Samra, A.B.; Atkin, S.L. Lipids and Insulin Regulate Mitochondrial-Derived Peptide (MOTS-c) in PCOS and Healthy Subjects. Clin. Endocrinol. 2019, 91, 278–287. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Augustine, R.A.; Grattan, D.R. Hormone Interactions Regulating Energy Balance during Pregnancy. J. Neuroendocrinol. 2010, 22, 805–817. [Google Scholar] [CrossRef]
- Most, J.; Broskey, N.T.; Altazan, A.D.; Beyl, R.A.; St. Amant, M.; Hsia, D.S.; Ravussin, E.; Redman, L.M. Is Energy Balance in Pregnancy Involved in the Etiology of Gestational Diabetes in Women with Obesity? Cell Metab. 2019, 29, 231–233. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.D.; Kim, Y.J.; Cho, M.J.; Seok, J.; Kim, G.J.; Lee, C.H.; Ko, J.J.; Kim, Y.S.; Lee, J.H. The Mitochondrial-Derived Peptide MOTS-c Promotes Homeostasis in Aged Human Placenta-Derived Mesenchymal Stem Cells in Vitro. Mitochondrion 2021, 58, 135–146. [Google Scholar] [CrossRef]
- Kong, B.S.; Min, S.H.; Lee, C.; Cho, Y.M. Mitochondrial-Encoded MOTS-c Prevents Pancreatic Islet Destruction in Autoimmune Diabetes. Cell Rep. 2021, 36, 109447. [Google Scholar] [CrossRef]
- Du, C.; Zhang, C.; Wu, W.; Liang, Y.; Wang, A.; Wu, S.; Zhao, Y.; Hou, L.; Ning, Q.; Luo, X. Circulating MOTS-c Levels Are Decreased in Obese Male Children and Adolescents and Associated with Insulin Resistance. Pediatr. Diabetes 2018, 19, 1058–1064. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Nikiforov, N.G.; Starodubova, A.V.; Popkova, T.V.; Orekhov, A.N. The Role of Mitochondria-Derived Peptides in Cardiovascular Diseases and Their Potential as Therapeutic Targets. Int. J. Mol. Sci. 2021, 22, 8770. [Google Scholar] [CrossRef] [PubMed]

| PARAMETER | Non-Obese (n-18) | Obese (n-22) | Hypothyroidism (n-17) |
|---|---|---|---|
| BEFORE PREGNANCY | |||
| Women’s age (years) | 30.09 ± 1.300 | 32.62 ± 1.243 | 29.75 ± 1.448 |
| Height (cm) | 168.3 ± 1.571 | 167.8 ± 1.245 | 166.8 ± 1.422 |
| Body weight (kg) | 63.88 ± 1.204 | 90.08 ± 3.536 ** | 67.25 ± 3.112 ## |
| BMI (kg/m2) | 22.54 ± 0.194 | 32.29 ± 1.107 ** | 24.32 ± 1.282 ## |
| ON THE DAY OF BIRTH | |||
| Body weight (kg) | 79.58 ± 1.903 | 98.32 ± 3.116 ** | 80.58 ± 2.861 # |
| PARAMETER | Non-Obese | Obese | Hypothyroidism |
|---|---|---|---|
| Child gender (M/F) | 9/9 | 10/12 | 10/7 |
| Body weight (kg) | 3.555 ± 0.146 | 3.544 ± 0.177 | 3.447 ± 0.090 |
| Head circumference (cm) | 36.11 ± 0.5536 | 36.3 ± 0.5287 | 36.06 ± 0.3147 |
| Chest circumference (cm) | 34.11 ± 0.5710 | 34.25 ± 0.5519 | 34.06 ± 0.3028 |
| Abdominal circumference (cm) | 34.33 ± 0.8205 | 35.35 ± 0.5679 | 34.65 ± 0.3202 |
| Thigh circumference (cm) | 12.39 ± 0.2574 | 12.5 ± 0.2565 | 12.24 ± 0.2016 |
| Arm circumference (cm) | 10.17 ± 0.2021 | 10.22 ± 0.2365 | 10 ± 0.1485 |
| Parameter | Non-Obese | Obese | Hypothyroidism | |||
|---|---|---|---|---|---|---|
| MB | CB | MB | CB | MB | CB | |
| Glucose (mg/dL) | 96.74 ± 4.39 | 88.70 ± 4.06 | 103.7 ± 2.62 | 97.32 ± 3.67 | 97.93 ± 2.72 | 90.01 ± 3.97 |
| NEFA (mmol/L) | 0.651 ± 0.055 | 0.408 ± 0.042 | 0.893 ± 0.094 | 0.418 ± 0.045 | 0.749 ± 0.081 | 0.542 ± 0.070 |
| Cholesterol (mg/dL) | 197.7 ± 9.46 | 185.1 ± 23.80 | 244.7 ± 8.79 | 172.6 ± 7.15 | 216.9 ± 11.66 | 206.4 ± 16.22 |
| Triglycerides (mg/dL) | 233.9 ± 24.23 | 135.4 ± 20.40 | 339.3 ± 28.74 ** | 121.7 ± 22.65 | 267.1 ± 28.43 | 155.5 ± 24.43 |
| Adiponectin (µg/mL] | 9.95 ± 0.72 | 35.06 ± 2.62 | 7.84 ± 0.60 * | 35.22 ± 2.93 | 8.36 ± 0.87 | 32.04 ± 2.75 |
| Leptin (ng/mL) | 8.09 ± 0.45 | 7.59 ± 0.59 | 9.78 ± 0.28 ** | 6.84 ± 0.71 | 9.43 ± 0.22 * | 6.55 ± 0.87 |
| Insulin (ng/mL) | 11.42 ± 1.23 | 3.53 ± 0.69 | 16.71 ± 2.76 | 9.25 ± 4.33 | 10.88 ± 1.56 | 3.98 ± 1.03 |
| TSH (mIU/L) | 1.62 ± 0.18 | 8.95 ± 1.59 | 2.13 ± 0.47 | 9.75 ± 1.87 | 1.57 ± 0.17 | 11.85 ± 2.52 |
| T3 (ng/mL) | 1.48 ± 0.07 | 0.64 ± 0.07 | 1.44 ± 0.06 | 0.75 ± 0.15 | 1.15 ± 0.09 *# | 0.67 ± 0.05 |
| FT3 (pg/mL) | 2.451 ± 0.14 | 1.211 ± 0.13 | 2.235 ± 0.15 | 1.268 ± 0.20 | 1.917 ± 0.15 * | 1.235 ± 0.21 |
| T4 (µg/dL) | 8.76 ± 0.43 | 8.68 ± 0.26 | 8.58 ± 0.31 | 8.10 ± 0.28 | 8.22 ± 0.41 | 8.74 ± 0.25 |
| FT4 (ng/mL) | 1.13 ± 0.03 | 1.07 ± 0.03 | 1.12 ± 0.03 | 1.15 ± 0.02 | 1.05 ± 0.02 * | 1.10 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojciechowska, M.; Pruszyńska-Oszmałek, E.; Kołodziejski, P.A.; Krauss, H.; Leciejewska, N.; Szczepankiewicz, D.; Bień, J.; Skrzypski, M.; Wilczak, M.; Sassek, M. Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders. Biology 2021, 10, 1032. https://doi.org/10.3390/biology10101032
Wojciechowska M, Pruszyńska-Oszmałek E, Kołodziejski PA, Krauss H, Leciejewska N, Szczepankiewicz D, Bień J, Skrzypski M, Wilczak M, Sassek M. Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders. Biology. 2021; 10(10):1032. https://doi.org/10.3390/biology10101032
Chicago/Turabian StyleWojciechowska, Małgorzata, Ewa Pruszyńska-Oszmałek, Paweł A. Kołodziejski, Hanna Krauss, Natalia Leciejewska, Dawid Szczepankiewicz, Jakub Bień, Marek Skrzypski, Maciej Wilczak, and Maciej Sassek. 2021. "Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders" Biology 10, no. 10: 1032. https://doi.org/10.3390/biology10101032
APA StyleWojciechowska, M., Pruszyńska-Oszmałek, E., Kołodziejski, P. A., Krauss, H., Leciejewska, N., Szczepankiewicz, D., Bień, J., Skrzypski, M., Wilczak, M., & Sassek, M. (2021). Changes in MOTS-c Level in the Blood of Pregnant Women with Metabolic Disorders. Biology, 10(10), 1032. https://doi.org/10.3390/biology10101032







