Multi-Element Composition of Diatom Chaetoceros spp. from Natural Phytoplankton Assemblages of the Russian Arctic Seas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Environmental Setting
2.2. Field Studies
2.3. Analytical Methods
3. Results
4. Discussion
4.1. Biogenic Silica
4.2. Major Elements
4.3. Iron
4.4. Aluminum
4.5. Trace Elements
4.6. Rare Earth Elements
4.7. Global and Regional Levels of Chemical Element Contents in Phytoplankton: Issues of Estimating Background Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morel, F.M.M.; Milligan, A.J.; Saito, M.A. Marine Bioinorganic Chemistry: The Role of Trace Metals in the Oceanic Cycles of Major Nutrients. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 6, pp. 113–143. [Google Scholar]
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: London, UK, 1979; ISBN 0121204502. [Google Scholar]
- Twining, B.S.; Baines, S.B. The Trace Metal Composition of Marine Phytoplankton. Ann. Rev. Mar. Sci. 2013, 5, 191–215. [Google Scholar] [CrossRef] [Green Version]
- Lobus, N.V.; Arashkevich, E.G.; Flerova, E.A. Major, trace, and rare-earth elements in the zooplankton of the Laptev Sea in relation to community composition. Environ. Sci. Pollut. Res. 2019, 26, 23044–23060. [Google Scholar] [CrossRef]
- Martin, J.H.; Knauer, G.A. The elemental composition of plankton. Geochim. Cosmochim. Acta 1973, 37, 1639–1653. [Google Scholar] [CrossRef]
- Li, Y.-H. Distribution patterns of the elements in the ocean: A synthesis. Geochim. Cosmochim. Acta 1991, 55, 3223–3240. [Google Scholar] [CrossRef]
- Masuzawa, T.; Suzuki, T.; Seki, K.; Kosugi, T.; Hibi, Y.; Yamamoto, M.; Takada, J.; Matsushita, R.; Yanada, M. Multielement compositions of marine phytoplankton samples from coastal areas of japan by instrumental neutron activation analysis. Biol. Trace Elem. Res. 1999, 71–72, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.M.; Price, N.M. The Biogeochemical Cycles of Trace Metals in the Oceans. Science 2003, 300, 944–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobus, N.V.; Flint, M.V.; Flerova, E.A.; Shcheglova, Y.V. Biochemical Composition and Energy Content in the Zooplankton of the Kara Sea. Oceanology 2020, 60, 778–786. [Google Scholar] [CrossRef]
- Baar de, H.J.W.; La Roche, J. Trace Metals in the Oceans: Evolution, Biology and Global Change. In Marine Science Frontiers for Europe; Springer: Berlin/Heidelberg, Germany, 2003; pp. 79–105. ISBN 3-540-40168-7. [Google Scholar]
- Morel, F.M.M.; Lam, P.J.; Saito, M.A. Trace Metal Substitution in Marine Phytoplankton. Annu. Rev. Earth Planet. Sci. 2020, 48, 491–517. [Google Scholar] [CrossRef] [Green Version]
- Twining, B.S.; Antipova, O.; Chappell, P.D.; Cohen, N.R.; Jacquot, J.E.; Mann, E.L.; Marchetti, A.; Ohnemus, D.C.; Rauschenberg, S.; Tagliabue, A. Taxonomic and nutrient controls on phytoplankton iron quotas in the ocean. Limnol. Oceanogr. Lett. 2021, 6, 96–106. [Google Scholar] [CrossRef]
- Sunda, W.G.; Huntsman, S.A. Processes regulating cellular metal accumulation and physiological effects: Phytoplankton as model systems. Sci. Total Environ. 1998, 219, 165–181. [Google Scholar] [CrossRef]
- Ho, T.-Y.; Quigg, A.; Finkel, Z.V.; Milligan, A.J.; Wyman, K.; Falkowski, P.G.; Morel, F.M.M. The elemental composition of some marine phytoplankton. J. Phycol. 2003, 39, 1145–1159. [Google Scholar] [CrossRef]
- Morel, F.M.M.; Hudson, R.J.M.; Price, N.M. Limitation of productivity by trace metals in the sea. Limnol. Oceanogr. 1991, 36, 1742–1755. [Google Scholar] [CrossRef]
- Savenko, V.S. Elementary chemical composition of ocean plankton. Geokhimiya 1989, 8, 1084–1089. [Google Scholar]
- Leonova, G.A.; Bobrov, V.A. Geochemical Role of Plankton of Continental Water Bodies in Siberian in Concentration and Biosedimentation of Microelements; Geo: Novosibirsk, Russia, 2012.
- Anikeev, V.V.; Dudarev, O.V.; Kasatkina, A.P. Influence of terrigenic and biogenic factors on formation of sedimentary fluxes of chemical elements in the coastal zone of the Sea of Japan. Geokhimiya 1996, 59–72. [Google Scholar]
- Moreno, A.R.; Martiny, A.C. Ecological Stoichiometry of Ocean Plankton. Annu. Rev. Mar. Sci. 2018, 10, 43–69. [Google Scholar] [CrossRef]
- Conley, D.J.; Schelske, C.L. Biogenic Silica. In Environmental Change Using Lake Sediments; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 281–293. [Google Scholar]
- Villarruel-López, A.; Ascencio, F.; Nuño, K. Microalgae, a Potential Natural Functional Food Source—A Review. Polish J. Food Nutr. Sci. 2017, 67, 251–263. [Google Scholar] [CrossRef] [Green Version]
- Lobus, N.V.; Udalov, A.A. Chemical composition of brown algae Laminaria digitata (Hudson) J.V. Lamouroux, 1813 and Fucus distichus (Linnaeus, 1767) from the bays of the Novaya Zemlya Archipelago (the Kara Sea). Russ. J. Mar. Biol. 2021, 47, 407–412. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as healthy ingredients for functional food: A review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Bio/Technol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchamy, A.; Poulain, J.; Wincker, P.; Iudicone, D.; de Vargas, C.; Bittner, L.; et al. Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. USA 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [Green Version]
- Booth, B.; Larouche, P.; Bélanger, S.; Klein, B.; Amiel, D.; Mei, Z.-P. Dynamics of Chaetoceros socialis blooms in the North Water. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 5003–5025. [Google Scholar] [CrossRef]
- Schiffrine, N.; Tremblay, J.-É.; Babin, M. Growth and Elemental Stoichiometry of the Ecologically-Relevant Arctic Diatom Chaetoceros gelidus: A Mix of Polar and Temperate. Front. Mar. Sci. 2020, 6, 790. [Google Scholar] [CrossRef] [Green Version]
- Lovejoy, C.; Legendre, L.; Price, N.M. Prolonged diatom blooms and microbial food web dynamics: Experimental results from an Arctic polynya. Aquat. Microb. Ecol. 2002, 29, 267–278. [Google Scholar] [CrossRef]
- Gogorev, R.M.; Samsonov, N.I. The genus Chaetoceros (Bacillariophyta) in Arctic and Antarctic. Nov. Sist. Nizshikh Rastenii 2016, 50, 56–111. [Google Scholar] [CrossRef]
- Lebeau, T.; Robert, J.-M. Diatom cultivation and biotechnologically relevant products. Part I: Cultivation at various scales. Appl. Microbiol. Biotechnol. 2003, 60, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, G.E.; Ackman, R.G.; Ratnayake, W.M.N. Fatty Acid Composition of Three Cultured Algal Species (Isochvysis galbana, Chaetoceros gracilis and Chaetoceros calcitrans) Used as Food for Bivalve Larvae. J. World Aquac. Soc. 1990, 21, 122–130. [Google Scholar] [CrossRef]
- Whyte, J.N.C. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 1987, 60, 231–241. [Google Scholar] [CrossRef]
- Palanisamy, K.M.; Paramasivam, P.; Maniam, G.P.; Rahim, M.H.A.; Govindan, N.; Chisti, Y. Production of lipids by Chaetoceros affinis in media based on palm oil mill effluent. J. Biotechnol. 2021, 327, 86–96. [Google Scholar] [CrossRef]
- Bhattacharjya, R.; Kiran Marella, T.; Tiwari, A.; Saxena, A.; Kumar Singh, P.; Mishra, B. Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioresour. Technol. 2020, 318, 124073. [Google Scholar] [CrossRef]
- Drozdova, A.N.; Nedospasov, A.A.; Lobus, N.V.; Patsaeva, S.V.; Shchuka, S.A. CDOM Optical Properties and DOC Content in the Largest Mixing Zones of the Siberian Shelf Seas. Remote Sens. 2021, 13, 1145. [Google Scholar] [CrossRef]
- Drits, A.V.; Pasternak, A.F.; Kravchishina, M.D.; Arashkevich, E.G.; Sukhanova, I.N.; Flint, M.V. The Role of Plankton in the Vertical Flux in the East Siberian Sea Shelf. Oceanology 2019, 59, 669–677. [Google Scholar] [CrossRef]
- Sukhanova, I.N.; Flint, M.V.; Fedorov, A.V.; Sakharova, E.G.; Artemyev, V.A.; Makkaveev, P.N.; Nedospasov, A.A. Phytoplankton of the Khatanga Bay, Shelf and Continental Slope of the Western Laptev Sea. Oceanology 2019, 59, 648–657. [Google Scholar] [CrossRef]
- Portnova, D.A.; Garlitska, L.A.; Polukhin, A.A. The effect of estuarine system on the meiofauna and nematodes in the East Siberian Sea. Sci. Rep. 2021, 11, 19306. [Google Scholar] [CrossRef] [PubMed]
- Flint, M.V.; Poyarkov, S.G.; Rymsky-Korsakov, N.A. Ecosystems of the Siberian Arctic Seas-2017 (Cruise 69 of the R/V Akademik Mstislav Keldysh). Oceanology 2018, 58, 315–318. [Google Scholar] [CrossRef]
- Bezzubova, E.M.; Seliverstova, A.M.; Zamyatin, I.A.; Romanova, N.D. Heterotrophic Bacterioplankton of the Laptev and East Siberian Sea Shelf Affected by Freshwater Inflow Areas. Oceanology 2020, 60, 62–73. [Google Scholar] [CrossRef]
- Kopylov, A.I.; Kosolapov, D.B.; Zabotkina, E.A.; Romanenko, A.V.; Sazhin, A.F. Distribution and Relationship between Heterotrophic Organisms and Viruses on the East Siberian Sea Shelf. Oceanology 2021, 61, 220–232. [Google Scholar] [CrossRef]
- Sukhanova, I.N.; Flint, M.V.; Georgieva, E.J.; Lange, E.K.; Kravchishina, M.D.; Demidov, A.B.; Nedospasov, A.A.; Polukhin, A.A. The structure of phytoplankton communities in the eastern part of the Laptev Sea. Oceanology 2017, 57, 75–90. [Google Scholar] [CrossRef]
- Polyakova, Y.I.; Kryukova, I.M.; Martynov, F.M.; Novikhin, A.E.; Abramova, E.N.; Kassens, H.; Hölemann, J. Community structure and spatial distribution of phytoplankton in relation to hydrography in the Laptev Sea and the East Siberian Sea (autumn 2008). Polar Biol. 2021, 44, 1229–1250. [Google Scholar] [CrossRef]
- Sakshaug, E.; Slagstad, D. Light and productivity of phytoplankton in polar marine ecosystems: A physiological view. Polar Res. 1991, 10, 69–86. [Google Scholar] [CrossRef]
- Janout, M.A.; Hölemann, J.; Waite, A.M.; Krumpen, T.; Appen, W.; Martynov, F. Sea-ice retreat controls timing of summer plankton blooms in the Eastern Arctic Ocean. Geophys. Res. Lett. 2016, 43, 12493–12501. [Google Scholar] [CrossRef] [Green Version]
- Koukina, S.E.; Lobus, N.V.; Shatravin, A.V. Dataset on the abundance, enrichment and partitioning of chemical elements between the filtered, particulate and sedimentary phases in the Cai River estuary (South China Sea). Data Br. 2021, 107412. [Google Scholar] [CrossRef]
- Karandashev, V.K.; Turanov, A.N.; Orlova, T.A.; Lezhnev, A.E.; Nosenko, S.V.; Zolotareva, N.I.; Moskvitina, I.R. Use of the inductively coupled plasma mass spectrometry for element analysis of environmental objects. Inorg. Mater. 2008, 44, 1491–1500. [Google Scholar] [CrossRef]
- Karpov, Y.A.; Orlova, V.A. Modern methods of autoclave sample preparation in chemical analysis of substances and materials. Inorg. Mater. 2008, 44, 1501–1508. [Google Scholar] [CrossRef]
- Orlova, V.A. Analytical Autoclaves: Autoclave Preparation of Samples in Chemical Analysis; Central Scientific Research Institute of Agrochemical Service of Agriculture Moscow: Moscow, Russia, 2003; ISBN 5-9238-0025-X. [Google Scholar]
- Samczyński, Z.; Dybczyński, R.S.; Polkowska-Motrenko, H.; Chajduk, E.; Pyszynska, M.; Danko, B.; Czerska, E.; Kulisa, K.; Doner, K.; Kalbarczyk, P. Two New Reference Materials Based on Tobacco Leaves: Certification for over a Dozen of Toxic and Essential Elements. Sci. World J. 2012, 2012, 216380. [Google Scholar] [CrossRef] [Green Version]
- Tesson, B.; Genet, M.J.; Fernandez, V.; Degand, S.; Rouxhet, P.G.; Martin-Jézéquel, V. Surface chemical composition of diatoms. ChemBioChem 2009, 10, 2011–2024. [Google Scholar] [CrossRef]
- Paasche, E. Silicon content of five marine plankton diatom species measured with a rapid filter method. Limnol. Oceanogr. 1980, 25, 474–480. [Google Scholar] [CrossRef]
- Martin-Jézéquell, V.; Lopez, P.J. Silicon—A Central Metabolite for Diatom Growth and Morphogenesis. In Silicon Biomineralization. Progress in Molecular and Subcellular Biology; Müller, W.E.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 99–124. [Google Scholar]
- Sicko-Goad, L.M.; Schelske, C.L.; Stoermer, E.F. Estimation of intracellular carbon and silica content of diatoms from natural assemblages using morphometric techniques. Limnol. Oceanogr. 1984, 29, 1170–1178. [Google Scholar] [CrossRef]
- Paasche, E. Silicon. In The Physiological Ecology of Phytoplankton. Studies in Ecology; Morris, I., Ed.; University California Press: Berkeley, CA, USA, 1980; pp. 259–284. [Google Scholar]
- Brzezinski, M.A. The Si:C:N ratio of marine diatoms: Interspecific variability and the effect of some en-vironmental variables. J. Phycol. 1985, 21, 347–357. [Google Scholar] [CrossRef]
- Su, Y.; Lundholm, N.; Ellegaard, M. Effects of abiotic factors on the nanostructure of diatom frustules—Ranges and variability. Appl. Microbiol. Biotechnol. 2018, 102, 5889–5899. [Google Scholar] [CrossRef]
- Pančić, M.; Torres, R.R.; Almeda, R.; Kiørboe, T. Silicified cell walls as a defensive trait in diatoms. Proc. R. Soc. B Biol. Sci. 2019, 286, 20190184. [Google Scholar] [CrossRef] [Green Version]
- Javaheri, N.; Dries, R.; Burson, A.; Stal, L.J.; Sloot, P.M.A.; Kaandorp, J.A. Temperature affects the silicate morphology in a diatom. Sci. Rep. 2015, 5, 11652. [Google Scholar] [CrossRef]
- Panagiotopoulos, C.; Goutx, M.; Suroy, M.; Moriceau, B. Phosphorus limitation affects the molecular composition of Thalassiosira weissflogii leading to increased biogenic silica dissolution and high degradation rates of cellular carbohydrates. Org. Geochem. 2020, 148, 104068. [Google Scholar] [CrossRef]
- Claquin, P.; Martin-Jezequel, V.; Kromkamp, J.C.; Veldhuis, M.J.W.; Kraay, G.W. Uncoupling of silicon compared to carbon and nitrogen metabolism, and role of the cell cycle, in continuous cultures of Thalassiosira pseudonana (bacillariophyceae) under light, nitrogen and phosphorus control. J. Phycol. 2002, 38, 922–930. [Google Scholar] [CrossRef] [Green Version]
- Lynn, S.G.; Kilham, S.S.; Kreeger, D.A.; Interlandi, S.J. Effect of nutrient availability on the biochemical and elemental stoichiometry in the freshwater diatom Stephanodiscus minutulus (Bacillariophyceae). J. Phycol. 2000, 36, 510–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Rocha, C.; Hutchins, D.; Brzezinski, M.; Zhang, Y. Effects of iron and zinc deficiency on elemental composition and silica production by diatoms. Mar. Ecol. Prog. Ser. 2000, 195, 71–79. [Google Scholar] [CrossRef]
- Twining, B.S.; Baines, S.B.; Fisher, N.S. Element stoichiometries of individual plankton cells collected during the Southern Ocean Iron Experiment (SOFeX). Limnol. Oceanogr. 2004, 49, 2115–2128. [Google Scholar] [CrossRef]
- Basharina, T.N.; Danilovtseva, E.N.; Zelinskiy, S.N.; Klimenkov, I.V.; Likhoshway, Y.V.; Annenkov, V.V. The effect of titanium, zirconium and tin on the growth of diatom Synedra acus and morphology of its silica valves. Silicon 2012, 4, 239–249. [Google Scholar] [CrossRef]
- Rogato, A.; De Tommasi, E. Physical, chemical, and genetic techniques for diatom frustule modification: Applications in nanotechnology. Appl. Sci. 2020, 10, 8738. [Google Scholar] [CrossRef]
- Conley, D.J.; Kilham, S.S.; Theriot, E. Differences in silica content between marine and freshwater diatoms. Limnol. Oceanogr. 1989, 34, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Vrieling, E.; Poort, L.; Beelen, T.; Gieskes, W. Growth and silica content of the diatoms Thalassiosira weissflogii and Navicula salinarum at different salinities and enrichments with aluminium. Eur. J. Phycol. 1999, 34, 307–316. [Google Scholar] [CrossRef]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Global Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Rainbow, P.S. Trace Metal Accumulation in Marine Invertebrates: Marine Biology or Marine Chemistry? J. Mar. Biol. Assoc. UK 1997, 77, 195–210. [Google Scholar] [CrossRef]
- Giordano, M.; Norici, A.; Ratti, S.; Raven, J.A. Role of Sulfur for Algae: Acquisition, Metabolism, Ecology and Evolution. In Sulfur Metabolism in Phototrophic Organisms. Advances in Photosynthesis and Respiration; Hell, R., Dahl, C., Knaff, D., Leustek, T., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 397–415. [Google Scholar]
- Vollenweider, R.A. Elemental and biochemical composition of plankton biomass; some comments and explorations. Arch. Hydrobiol. 1985, 105, 11–29. [Google Scholar]
- Leonova, G.A.; Bobrov, V.A.; Bogush, A.A.; Bychinskii, V.A. Concentration of chemical elements by zooplankton of the White Sea. Oceanology 2013, 53, 54–70. [Google Scholar] [CrossRef]
- Baturin, G.N.; Emel’yanov, E.M.; Stryuk, V.L. Geochemistry of plankton and suspended matter in the Baltic Sea. Okeanologiya 1993, 33, 126–132. [Google Scholar]
- Greene, R.M.; Geider, R.J.; Falkowski, P.G. Effect of iron limitation on photosynthesis in a marine diatom. Limnol. Oceanogr. 1991, 36, 1772–1782. [Google Scholar] [CrossRef] [Green Version]
- Geider, R.J.; La Roche, J. The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosynth. Res. 1994, 39, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, N.; Planquette, H.; Dehairs, F.; van der Merwe, P.; Bowie, A.R.; Trull, T.W.; Laurenceau-Cornec, E.C.; Davies, D.; Bollinger, C.; Le Goff, M.; et al. Impact of the natural Fe-fertilization on the magnitude, stoichiometry and efficiency of particulate biogenic silica, nitrogen and iron export fluxes. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 117, 11–27. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Bruland, K.W. Iron-limited diatom growth and Si:N uptake ratios in a coastal upwelling regime. Nature 1998, 393, 561–564. [Google Scholar] [CrossRef]
- Bruland, K.W.; Middag, R.; Lohan, M.C. Controls of Trace Metals in Seawater. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Philadelphia, PA, USA, 2014; pp. 19–51. ISBN 9780080983004. [Google Scholar]
- Lasareva, E.V.; Parfenova, A.M.; Romankevich, E.A.; Lobus, N.V.; Drozdova, A.N. Organic Matter and Mineral Interactions Modulate Flocculation Across Arctic River Mixing Zones. J. Geophys. Res. Biogeosci. 2019, 124, 1651–1664. [Google Scholar] [CrossRef]
- Ren, J.-L.; Zhang, G.-L.; Zhang, J.; Shi, J.-H.; Liu, S.-M.; Li, F.-M.; Jin, J.; Liu, C.-G. Distribution of dissolved aluminum in the Southern Yellow Sea: Influences of a dust storm and the spring bloom. Mar. Chem. 2011, 125, 69–81. [Google Scholar] [CrossRef]
- Measures, C.I.; Vink, S. On the use of dissolved aluminum in surface waters to estimate dust deposition to the ocean. Glob. Biogeochem. Cycles 2000, 14, 317–327. [Google Scholar] [CrossRef]
- Lobus, N.V. Elemental composition of zooplankton in the Kara Sea and the bays on the eastern side of Novaya Zemlya. Oceanology 2016, 56, 809–818. [Google Scholar] [CrossRef]
- Lobus, N.V.; Drits, A.V.; Flint, M.V. Accumulation of Chemical Elements in the Dominant Species of Copepods in the Ob Estuary and the Adjacent Shelf of the Kara Sea. Oceanology 2018, 58, 405–415. [Google Scholar] [CrossRef]
- Gillmore, M.L.; Golding, L.A.; Angel, B.M.; Adams, M.S.; Jolley, D.F. Toxicity of dissolved and precipitated aluminium to marine diatoms. Aquat. Toxicol. 2016, 174, 82–91. [Google Scholar] [CrossRef]
- Golding, L.A.; Angel, B.M.; Batley, G.E.; Apte, S.C.; Krassoi, R.; Doyle, C.J. Derivation of a water quality guideline for aluminium in marine waters. Environ. Toxicol. Chem. 2015, 34, 141–151. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, L.; Liu, F.; Fortin, C.; Tan, Y.; Huang, L.; Campbell, P.G.C. Uptake and subcellular distribution of aluminum in a marine diatom. Ecotoxicol. Environ. Saf. 2019, 169, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Rutten, L.; Maddala, S.P.; Wu, H.; Eren, E.D.; Mezari, B.; Schreur-Piet, I.; Friedrich, H.; van Benthem, R.A.T.M. Modifying the thickness, pore size, and composition of diatom frustule in Pinnularia sp. with Al3+ ions. Sci. Rep. 2020, 10, 19498. [Google Scholar] [CrossRef] [PubMed]
- Machill, S.; Köhler, L.; Ueberlein, S.; Hedrich, R.; Kunaschk, M.; Paasch, S.; Schulze, R.; Brunner, E. Analytical studies on the incorporation of aluminium in the cell walls of the marine diatom Stephanopyxis turris. BioMetals 2013, 26, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Gehlen, M.; Beck, L.; Calas, G.; Flank, A.-M.; Van Bennekom, A.J.; Van Beusekom, J.E.E. Unraveling the atomic structure of biogenic silica: Evidence of the structural association of Al and Si in diatom frustules. Geochim. Cosmochim. Acta 2002, 66, 1601–1609. [Google Scholar] [CrossRef]
- Gehlen, M.; Heinze, C.; Maier-Reimer, E.; Measures, C.I. Coupled Al-Si geochemistry in an ocean general circulation model: A tool for the validation of oceanic dust deposition fields? Global Biogeochem. Cycles 2003, 17, 1028. [Google Scholar] [CrossRef] [Green Version]
- Van Bennekom, A.J.; Buma, A.G.J.; Nolting, R.F. Dissolved aluminium in the Weddell-Scotia Confluence and effect of Al on the dissolution kinetics of biogenic silica. Mar. Chem. 1991, 35, 423–434. [Google Scholar] [CrossRef]
- Van Beusekom, J.; Weber, A. Der Einfluß von Aluminium auf das Wachstum und die Entwicklung von Kieselalgen in der Nordsee. Dtsch. Hydrogr. Zeitschrift. Suppl. 1995, 5, 213–220. [Google Scholar]
- Koning, E.; Gehlen, M.; Flank, A.-M.; Calas, G.; Epping, E. Rapid post-mortem incorporation of aluminum in diatom frustules: Evidence from chemical and structural analyses. Mar. Chem. 2007, 106, 208–222. [Google Scholar] [CrossRef]
- Uitz, J.; Claustre, H.; Gentili, B.; Stramski, D. Phytoplankton class-specific primary production in the world’s oceans: Seasonal and interannual variability from satellite observations. Global Biogeochem. Cycles 2010, 24. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, Y.; Huang, L.; Fortin, C.; Campbell, P.G.C. Aluminum effects on marine phytoplankton: Implications for a revised Iron Hypothesis (Iron–Aluminum Hypothesis). Biogeochemistry 2018, 139, 123–137. [Google Scholar] [CrossRef] [Green Version]
- Gordeev, V.V.; Lisitzin, A.P. Geochemical interaction between the freshwater and marine hydrospheres. Russ. Geol. Geophys. 2014, 55, 562–581. [Google Scholar] [CrossRef]
- Riley, J.P.; Roth, I. The Distribution of Trace Elements in Some Species of Phytoplankton Grown in Culture. J. Mar. Biol. Assoc. UK 1971, 51, 63–72. [Google Scholar] [CrossRef]
- Martin, J.H. The possible transport of trace metals via moulted copepod exoskeletons. Limnol. Oceanogr. 1970, 15, 756–761. [Google Scholar] [CrossRef]
- Waldron, K.J.; Robinson, N.J. How do bacterial cells ensure that metalloproteins get the correct metal? Nat. Rev. Microbiol. 2009, 7, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sunda, W.G.; Huntsman, S.A. Effect of Zn, Mn, and Fe on Cd accumulation in phytoplankton: Implications for oceanic Cd cycling. Limnol. Oceanogr. 2000, 45, 1501–1516. [Google Scholar] [CrossRef]
- Jensen, E.L.; Clement, R.; Kosta, A.; Maberly, S.C.; Gontero, B. A new widespread subclass of carbonic anhydrase in marine phytoplankton. ISME J. 2019, 13, 2094–2106. [Google Scholar] [CrossRef] [Green Version]
- Gwenzi, W.; Mangori, L.; Danha, C.; Chaukura, N.; Dunjana, N.; Sanganyado, E. Sources, behaviour, and environmental and human health risks of high-technology rare earth elements as emerging contaminants. Sci. Total Environ. 2018, 636, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Kumar, P.; Singh, P.; Patil, S. (Eds.) Sensors in Water Pollutants Monitoring: Role of Material; Advanced Functional Materials and Sensors; Springer: Singapore, 2020; ISBN 978-981-15-0670-3. [Google Scholar]
- Adeel, M.; Lee, J.Y.; Zain, M.; Rizwan, M.; Nawab, A.; Ahmad, M.A.; Shafiq, M.; Yi, H.; Jilani, G.; Javed, R.; et al. Cryptic footprints of rare earth elements on natural resources and living organisms. Environ. Int. 2019, 127, 785–800. [Google Scholar] [CrossRef]
- MacMillan, G.A.; Chételat, J.; Heath, J.P.; Mickpegak, R.; Amyot, M. Rare earth elements in freshwater, marine, and terrestrial ecosystems in the eastern Canadian Arctic. Environ. Sci. Process. Impacts 2017, 19, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
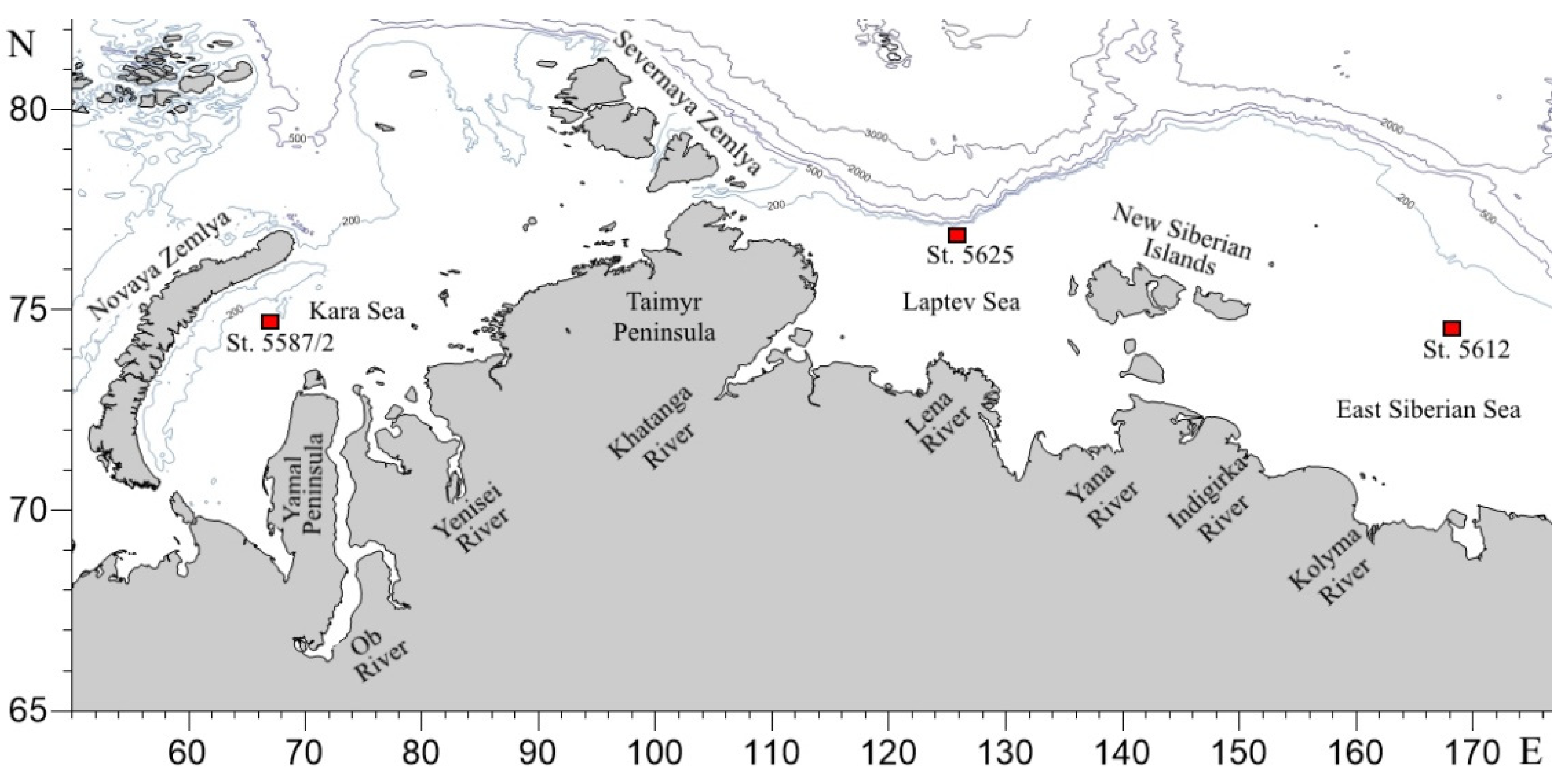

| Station | Location | Date | Latitude °N | Longitude °E | Depth, m | Salinity, PSU | Temperature, °C | ||
|---|---|---|---|---|---|---|---|---|---|
| Surface | 45-m | Surface | 45-m | ||||||
| 5587/2 | KS | 24.09.2017 | 74.7824 | 66.5917 | 189 | 25.22 | 33.99 | 3.46 | −1.74 |
| 5625 | LS | 16.09.2017 | 76.7729 | 125.7942 | 70 | 22.83 | 34.02 | 2.55 | −1.77 |
| 5612 | ESS | 08.09.2017 | 74.3833 | 168.1866 | 50 | 29.28 | 31.54 | 0.52 | −1.66 |
| Element | Detection Limit | Canadian Pondweed GSO 8921–2007 EK-1 | Oriental Basma Tobacco Leaves INCT–OBTL–5 | Polish Virginia Tobacco Leaves INCT–PVTL–6 | |||
|---|---|---|---|---|---|---|---|
| Measured Value | Certified Value * | Measured Values | Certified Value * | Measured Values | Certified Value * | ||
| Major Elements (% DW) | |||||||
| Na | 0.0006 | 0.71 | 0.69 ± 0.05 | 0.025 | 0.044 ** | 0.008 | 0.006 ** |
| Mg | 0.0003 | 0.33 | 0.32 ± 0.02 | 0.85 | 0.853 ± 0.034 | 0.23 | 0.241 ± 0.009 |
| P | 0.0002 | 0.25 | 0.24 ± 0.03 | 0.17 | 0.170 ± 0.012 | 0.24 | 0.242 ± 0.015 |
| S | 0.0012 | 0.33 | 0.34 ± 0.05 | 0.44 | 0.455 ± 0.091 | 0.35 | 0.378 ± 0.059 |
| K | 0.0006 | 3.28 | 3.22 ± 0.16 | 2.28 | 2.271 ± 0.076 | 2.42 | 2.640 ± 0.090 |
| Ca | 0.0044 | 2.88 | 2.80 ± 0.17 | 3.94 | 3.996 ± 0.142 | 2.28 | 2.297 ± 0.078 |
| Al | 0.0012 | 0.099 | 0.099 ± 0.012 | 0.18 | 0.198 ± 0.028 | 0.029 | 0.025 ± 0.005 |
| Fe | 0.0012 | 0.25 | 0.26 ± 0.01 | 0.14 | 0.149 ** | 0.024 | 0.026 ** |
| Trace Elements (µg g–1 DW) | |||||||
| Li | 0.04 | 1.50 | 1.44 ± 0.18 | 23.6 | 19.3 ** | 3.69 | 3.35 ± 0.67 |
| Be | 0.01 | 0.065 | 0.07 ** | 0.07 | 0.081 ** | 0.022 | – |
| B | 4 | 26.6 | 33 ± 10 | 34.4 | 33.6 ± 2.2 | 32.9 | 33.4 ± 1.9 |
| Ti | 2 | 49.4 | 77 ± 14 | 76.5 | 80.7 ** | 12.68 | 12.3 ** |
| V | 0.3 | 3.55 | 3.8 ± 0.4 | 4.0 | 4.12 ± 0.55 | 0.39 | 0.405 ± 0.06 |
| Cr | 0.4 | 4.73 | 5.1 ± 0.5 | 4.94 | 6.3 ** | 0.67 | 0.91 ** |
| Mn | 0.3 | 517 | 520 ± 30 | 179 | 180 ± 6 | 135 | 136 ± 5 |
| Co | 0.1 | 1.39 | 1.5 ± 0.1 | 0.93 | 0.98 ± 0.07 | 0.17 | 0.15 ± 0.01 |
| Ni | 0.5 | 3.58 | 3.7 ± 0.4 | 8.3 | 8.5 ± 0.49 | 1.47 | 1.49 ± 0.14 |
| Cu | 0.9 | 10.8 | 11.2 ± 0.4 | 9.74 | 10.1 ± 0.4 | 4.84 | 5.12 ± 0.2 |
| Zn | 0.3 | 19.1 | 20.6 ± 1.4 | 53.4 | 52.4 ± 1.8 | 44.49 | 43.6 ± 1.4 |
| Ga | 0.2 | 0.29 | 0.4 ** | 0.51 | – | 0.09 | – |
| As | 0.1 | 0.78 | 0.76 ± 0.02 | 0.78 | 0.67 ± 0.09 | 0.13 | 0.14 ± 0.01 |
| Se | 0.05 | 0.35 | 0.3 ** | 0.29 | – | 0.22 | – |
| Rb | 0.03 | 3.23 | 3.5 ± 0.3 | 22.9 | 19.1 ± 1 | 6.27 | 5.97 ± 0.28 |
| Sr | 0.3 | 170 | 174 ± 9 | 106.7 | 105 ± 5 | 135 | 133 ± 6 |
| Mo | 0.1 | 1.18 | 1.2 ** | 0.38 | 0.41 ± 0.06 | 0.42 | 0.4 ± 0.03 |
| Ag | 0.02 | 0.012 | 0.017 | 0.048 | 0.053 ± 0.011 | 0.018 | 0.019 ± 0.004 |
| Cd | 0.01 | 0.083 | 0.1 ± 0.02 | 2.67 | 2.64 ± 0.14 | 2.18 | 2.23 ± 0.12 |
| Sn | 0.1 | 0.15 | 0.12 ** | 0.13 | – | 0.05 | 0.031 ** |
| Sb | 0.02 | 0.072 | 0.08 ± 0.02 | 0.054 | 0.076 ± 0.013 | 0.035 | 0.037 ± 0.004 |
| Cs | 0.005 | 0.1 | 0.108 ± 0.008 | 0.29 | 0.288 ± 0.02 | 0.025 | 0.026 ** |
| Ba | 0.3 | 77.9 | 78 ± 7 | 62.6 | 67.4 ± 3.8 | 42.9 | 41.6 ± 1.9 |
| Hg | 0.04 | 0.017 | 0.03 ** | 0.018 | 0.021 ± 0.001 | 0.022 | 0.023 ± 0.002 |
| Tl | 0.001 | 0.016 | 0.02 ** | 0.052 | 0.051 ** | 0.025 | 0.023 ** |
| Pb | 0.2 | 1.12 | 1.1 ± 0.1 | 1.93 | 2.0 ± 0.3 | 0.82 | 0.97 ± 0.15 |
| Bi | 0.003 | 0.018 | 0.023 ** | 0.09 | – | 0.145 | 0.14 ** |
| Th | 0.02 | 0.38 | 0.4 ** | 0.48 | 0.5 ± 0.04 | 0.085 | 0.089 ± 0.007 |
| U | 0.004 | 1.42 | 1.4 ± 0.1 | 0.095 | 0.113 ** | 0.02 | 0.022 ** |
| Rare-Earth Elements (µg g–1 DW) | |||||||
| Sc | 0.01 | 0.35 | 0.38 ± 0.02 | 0.6 | 0.64 ± 0.027 | 0.19 | 0.06 ± 0.003 |
| Y | 0.01 | 1.18 | 1.3 ** | 0.98 | 0.963 ** | 0.22 | 0.218 ** |
| La | 0.003 | 2.03 | 2.1 ± 0.1 | 1.58 | 1.7 ± 0.1 | 0.50 | 0.54 ± 0.027 |
| Ce | 0.008 | 3.58 | 3.4 ± 0.3 | 2.84 | 3.0 ± 0.2 | 0.69 | 0.743 ± 0.051 |
| Pr | 0.001 | 0.41 | 0.42 ** | 0.33 | 0.321 ** | 0.08 | 0.083 ** |
| Nd | 0.006 | 1.56 | 1.6 ± 0.2 | 1.28 | 1.3 ± 0.1 | 0.32 | 0.32 ± 0.02 |
| Sm | 0.001 | 0.29 | 0.31 ± 0.03 | 0.25 | 0.26 ± 0.01 | 0.055 | 0.058 ± 0.004 |
| Eu | 0.006 | 0.04 | 0.047 ± 0.008 | 0.05 | 0.06 ± 0.004 | 0.011 | 0.014 ± 0.003 |
| Gd | 0.007 | 0.265 | 0.35 ± 0.08 | 0.23 | 0.243 ** | 0.052 | – |
| Tb | 0.005 | 0.038 | 0.041 ± 0.005 | 0.034 | 0.035 ± 0.002 | 0.007 | 0.008 ± 0.001 |
| Dy | 0.006 | 0.21 | 0.36 ± 0.13 | 0.185 | 0.184 ** | 0.037 | – |
| Ho | 0.001 | 0.04 | 0.47 ± 0.008 | 0.035 | 0.035 ** | 0.007 | – |
| Er | 0.001 | 0.12 | 0.13 ± 0.02 | 0.1 | 0.1 ± 0.01 | 0.0187 | 0.019 ± 0.003 |
| Tm | 0.002 | 0.015 | 0.021 ± 0.007 | 0.013 | 0.014 ** | 0.0024 | – |
| Yb | 0.007 | 0.1 | 0.074 ± 0.006 | 0.087 | 0.115 ± 0.02 | 0.015 | 0.028 |
| Lu | 0.003 | 0.015 | 0.019 ± 0.003 | 0.012 | 0.017 ** | 0.0024 | – |
| Element | The Kara Sea | The Laptev Sea | The East-Siberian Sea | Mean ± SE |
|---|---|---|---|---|
| Major Elements (% DW) | ||||
| Na | 0.09 | 0.28 | 0.78 | 0.38 ± 0.21 |
| Mg | 0.11 | 0.19 | 0.17 | 0.16 ± 0.03 |
| P | 0.27 | 0.39 | 0.50 | 0.39 ± 0.07 |
| S | 0.37 | 0.53 | 0.50 | 0.46 ± 0.05 |
| K | 0.02 | 0.12 | 0.32 | 0.15 ± 0.09 |
| Ca | 0.30 | 0.20 | 0.18 | 0.22 ± 0.04 |
| Si | 18.11 | 20.12 | 19.05 | 19.10 ± 0.58 |
| Al | 0.03 | 0.18 | 0.13 | 0.11 ± 0.04 |
| Fe | 0.07 | 0.35 | 0.14 | 0.19 ± 0.08 |
| Sum | 19.37 | 22.36 | 21.77 | 21.17 ± 1.0 |
| Trace Elements (μg g−1 DW) | ||||
| Li | 3.46 | 1.37 | 1.53 | 2.12 ± 0.67 |
| Be | 0.01 | 0.07 | 0.04 | 0.04 ± 0.01 |
| B | 45.6 | 37.0 | 37.6 | 40.1 ± 2.76 |
| Ti | 104 | 123 | 112 | 113 ± 5.42 |
| V | 3.5 | 6.2 | 2.9 | 4.19 ± 1.04 |
| Cr | 3.4 | 8.7 | 3.6 | 5.24 ± 1.73 |
| Mn | 17.5 | 108 | 129 | 84.7 ± 34.1 |
| Co | 0.24 | 1.08 | 0.84 | 0.72 ± 0.25 |
| Ni | 2.0 | 6.2 | 3.6 | 3.95 ± 1.22 |
| Cu | 14.6 | 18.0 | 12.0 | 14.88 ± 1.76 |
| Zn | 139 | 342 | 273 | 251.67 ± 59.48 |
| Ga | <d/l | <d/l | <d/l | - |
| As | 2.2 | 3.3 | 3.3 | 2.89 ± 0.33 |
| Se | 0.75 | <d/l | 2.0 | 1.38 |
| Rb | 0.50 | 3.3 | 3.3 | 2.38 ± 0.94 |
| Sr | 24.9 | 66.6 | 25.5 | 39.0 ± 13.8 |
| Mo | 1.7 | 1.2 | 0.51 | 1.15 ± 0.35 |
| Ag | 0.044 | 0.041 | 0.046 | 0.044 ± 0.002 |
| Cd | 0.3 | 0.57 | 1.31 | 0.73 ± 0.3 |
| Sn | 1.1 | 1.6 | 1.5 | 1.41 ± 0.14 |
| Sb | 0.12 | 0.49 | 0.18 | 0.26 ± 0.11 |
| Cs | 0.026 | 0.17 | 0.14 | 0.11 ± 0.04 |
| Ba | 45.9 | 84.6 | 95.5 | 75.33 ± 15.04 |
| Hg | 0.11 | 0.09 | 0.09 | 0.09 ± 0.006 |
| Tl | 0.21 | 0.02 | 0.012 | 0.082 ± 0.07 |
| Pb | 1.8 | 7.8 | 3.6 | 4.4 ± 1.77 |
| Bi | 0.025 | 0.043 | 0.019 | 0.03 ± 0.007 |
| Th | 0.051 | 0.28 | 0.15 | 0.16 ± 0.07 |
| U | 10.02 | 4.11 | 1.08 | 5.08 ± 2.68 |
| Sum | 423.07 | 825.83 | 714.35 | 654.42 ± 120.07 |
| Rare Earth Elements (μg g−1 DW) | ||||
| Sc | 0.45 | 0.92 | 0.91 | 0.76 ± 0.16 |
| Y | 0.12 | 0.56 | 0.35 | 0.34 ± 0.13 |
| La | 0.22 | 0.98 | 0.68 | 0.63 ± 0.22 |
| Ce | 0.52 | 2.1 | 1.2 | 1.25 ± 0.44 |
| Pr | 0.05 | 0.21 | 0.12 | 0.13 ± 0.05 |
| Nd | 0.25 | 0.97 | 0.60 | 0.61 ± 0.21 |
| Sm | 0.034 | 0.17 | 0.096 | 0.1 ± 0.04 |
| Eu | 0.009 | 0.11 | 0.025 | 0.047 ± 0.03 |
| Gd | 0.034 | 0.16 | 0.095 | 0.095 ± 0.04 |
| Tb | <d/l | 0.012 | <d/l | - |
| Dy | 0.028 | 0.12 | 0.073 | 0.074 ± 0.03 |
| Ho | 0.005 | 0.023 | 0.014 | 0.014 ± 0.005 |
| Er | <d/l | 0.056 | 0.021 | 0.038 |
| Tm | <d/l | 0.009 | <d/l | - |
| Yb | 0.010 | 0.062 | 0.036 | 0.036 ± 0.02 |
| Lu | <d/l | 0.007 | <d/l | - |
| Sum | 1.73 | 6.47 | 4.22 | 4.14 ± 1.37 |
| Element | Chaetoceros spp. a | Total Phytoplankton | Total Plankton | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coastal Areas b | Marine c | Ocean e | The White Sea f | The Baltic Sea g | The Sea of Japan h | Ocean | |||
| e | i | ||||||||
| in % DW | |||||||||
| Na | 0.38 | 0.05–5.72 | 8.85–13.83 | 3.0 | 5.3 | 3.9 | 3 | 3.5 | 3.3 |
| Mg | 0.16 | - | 1.1–1.64 | 0.8 | - | 0.67 | - | 1.1 | 9.4 |
| P | 0.39 | - | - | 1.0 | - | 0.25 | - | 0.8 | 0.28 |
| S | 0.46 | - | - | 0.5 | - | - | - | 0.55 | 0.83 |
| K | 0.15 | - | 1.1–1.33 | 1.2 | 1.2 | 1.01 | - | 0.9 | 5.2 |
| Ca | 0.22 | - | 0.53–0.65 | 0.45 | 1.1 | 1.21 | 1.5 | 1.9 | 1.4 |
| Si | 19.10 | - | 4.68–7.01 | 8.0 | - | - | - | 6.0 | 0.15 |
| Al | 0.11 | 0.23–1.75 | 0.004–0.04 | 0.01 | - | 0.27 | - | 0.01 | 0.0062 |
| Fe | 0.19 | 0.06–0.75 | 0.02–0.15 | 0.09 | 0.16 | 0.36 | 0.3 | 0.08 | 0.016 |
| in µg g−1 DW | |||||||||
| Li | 2.12 | - | - | 50 | - | 5.9 | - | 40 | 5 |
| Be | 0.04 | - | - | 0.6 | - | - | - | 0.4 | 0.003 |
| B | 40.1 | - | - | 30 | - | - | - | 50 | 120 |
| Sc | 0.76 | 0.4–2.43 | - | - | 0.28 | 0.76 | 0.19 | 0.2 | 0.07 |
| Ti | 113 | - | 27 | 100 | - | 350 | - | 50 | 11.0 |
| V | 4.19 | 13.4–38.5 | 3–5 d | 4 | 3.8 | 8.5 | - | 4 | 3.5 |
| Cr | 5.24 | 17.2–51.9 | 3.9 | 10 | 218.3 | 27.3 | 54.7 | 10 | 1.8 |
| Mn | 84.7 | 17–216 | 6.1–13.3 | 10 | 62.5 | 600 | - | 10 | 20 |
| Co | 0.72 | 0.24–1.83 | 38 d | 1.5 | 0.86 | 18 | 0.23 | 1.5 | 0.43 |
| Ni | 3.95 | - | 1.9–7.8 | 10 | 4.1 | 35 | 16.2 | 10 | 1.4 |
| Cu | 14.88 | - | 3.2–14.8 | 60 | 75.3 | 21 | 7 | 40 | 12 |
| Zn | 251.7 | 12–362 | 19–122 | 300 | 360 | 140 | 8.7 | 300 | 39 |
| Ga | < d/l | - | - | 0.2 | 1 | - | - | 0.2 | 0.5 |
| As | 2.89 | 3.3–9.6 | 12–36 d | 14 | 12.3 | 4.2 | 1.16 | 10 | 15 |
| Se | 1.38 | 1.3–4.32 | 3.5 d | 4 | 0.45 | 1.8 | - | 4 | 0.06 |
| Rb | 2.37 | - | - | 3 | 5.75 | 6 | - | 3 | 1.8 |
| Sr | 39.0 | 75–13,100 | 119–697 | 390 | 110 | 190 | - | 300 | 1100 |
| Y | 0.34 | - | - | - | 0.1 | - | - | 4 | - |
| Mo | 1.15 | - | - | 0.7 | 0.18 | 10 | 13.1 | 1 | 0.39 |
| Ag | 0.044 | - | 0.2–0.6 | 0.2 | - | 2.7 | - | 0.4 | 0.22 |
| Cd | 0.73 | - | 1.5–3.9 | 3 | 2.4 | 2 | 6.7 | 3 | 0.72 |
| Sn | 1.41 | - | - | 10 | - | - | - | 8 | 0.29 |
| Sb | 0.26 | 0.95–2.44 | - | 0.1 | 1.5 | 0.5 | - | 0.1 | 0.16 |
| Cs | 0.11 | 0.18–1.26 | 0.11 d | 0.03 | 0.25 | 0.32 | 1.06 | 0.04 | 0.072 |
| Ba | 75.33 | 666–1756 | 19–287 | 80 | 22 | 800 | - | 100 | 19 |
| La | 0.63 | 1.05–8.27 | - | - | 0.73 | 4.2 | 5.6 | 0.8 | 0.14 |
| Ce | 1.25 | 1.07–8.27 | - | - | 1.46 | 10 | 10.3 | 1.2 | 0.23 |
| Pr | 0.13 | - | - | - | - | - | - | 0.15 | - |
| Nd | 0.61 | - | - | - | - | 4.4 | - | 0.7 | - |
| Sm | 0.1 | 0.15–0.88 | - | - | 0.1 | 0.61 | 0.53 | 0.07 | - |
| Eu | 0.047 | 0.034–0.207 | - | - | 0.021 | 0.14 | 0.15 | 0.02 | - |
| Gd | 0.095 | - | - | - | - | - | - | 0.2 | - |
| Tb | 0.012 | - | - | - | 0.014 | 0.13 | 0.9 | 0.3 | - |
| Dy | 0.074 | - | - | - | - | - | - | 0.15 | - |
| Ho | 0.014 | - | - | - | - | - | - | 0.03 | - |
| Er | 0.038 | - | - | - | - | - | - | 0.09 | - |
| Tm | 0.009 | - | - | - | - | - | - | 0.015 | - |
| Yb | 0.036 | - | - | - | 0.058 | 0.29 | - | 0.07 | - |
| Lu | 0.007 | - | - | - | 0.008 | 0.04 | - | 0.015 | - |
| Hg | 0.09 | - | 0.16–0.19 | 0.1 | 0.034 | 0.24 | - | 0.2 | 0.03 |
| Tl | 0.082 | - | - | - | - | - | - | - | - |
| Pb | 4.4 | - | 7.2–9.2 | 20 | 16.6 | 25 | 10.2 | 20 | 8.7 |
| Bi | 0.03 | - | - | - | - | - | - | - | - |
| Th | 0.16 | 0.25–1.06 | 0.42 d | - | 0.21 | 0.87 | 0.44 | 0.1 | 0.1 |
| U | 5.08 | 1.4–9.4 | 0.7 d | 0.7 | - | 0.41 | - | 0.6 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobus, N.V.; Kulikovskiy, M.S.; Maltsev, Y.I. Multi-Element Composition of Diatom Chaetoceros spp. from Natural Phytoplankton Assemblages of the Russian Arctic Seas. Biology 2021, 10, 1009. https://doi.org/10.3390/biology10101009
Lobus NV, Kulikovskiy MS, Maltsev YI. Multi-Element Composition of Diatom Chaetoceros spp. from Natural Phytoplankton Assemblages of the Russian Arctic Seas. Biology. 2021; 10(10):1009. https://doi.org/10.3390/biology10101009
Chicago/Turabian StyleLobus, Nikolay V., Maxim S. Kulikovskiy, and Yevhen I. Maltsev. 2021. "Multi-Element Composition of Diatom Chaetoceros spp. from Natural Phytoplankton Assemblages of the Russian Arctic Seas" Biology 10, no. 10: 1009. https://doi.org/10.3390/biology10101009
APA StyleLobus, N. V., Kulikovskiy, M. S., & Maltsev, Y. I. (2021). Multi-Element Composition of Diatom Chaetoceros spp. from Natural Phytoplankton Assemblages of the Russian Arctic Seas. Biology, 10(10), 1009. https://doi.org/10.3390/biology10101009







