Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
2.2. Bacterial Isolation and Identification
2.3. Bacterial Identification by MALDITOF-TOF
2.4. Inoculum Preparation and Temperature Experiment
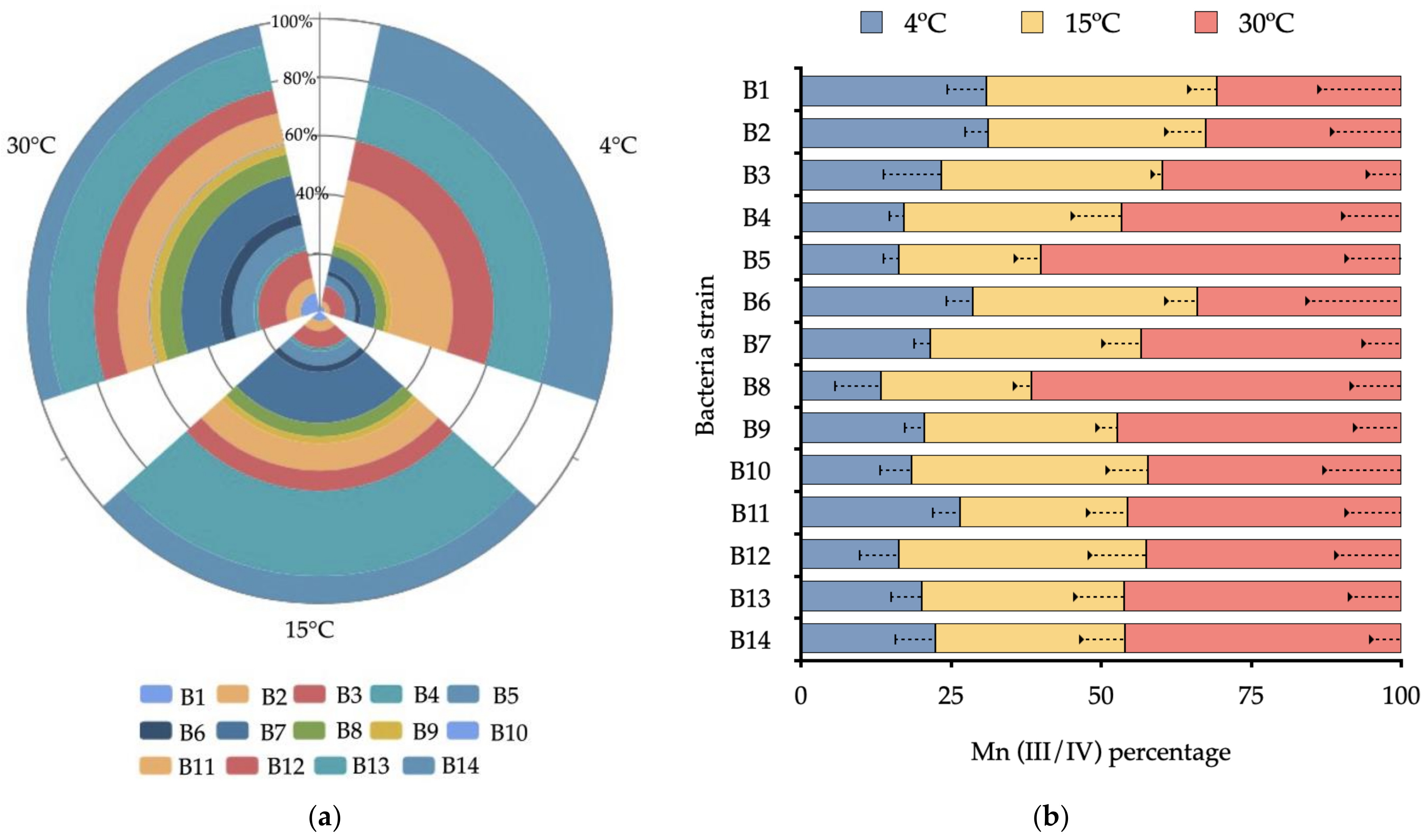
2.5. Quantification of Mn(III/IV) and Bacterial Growth Kinetics
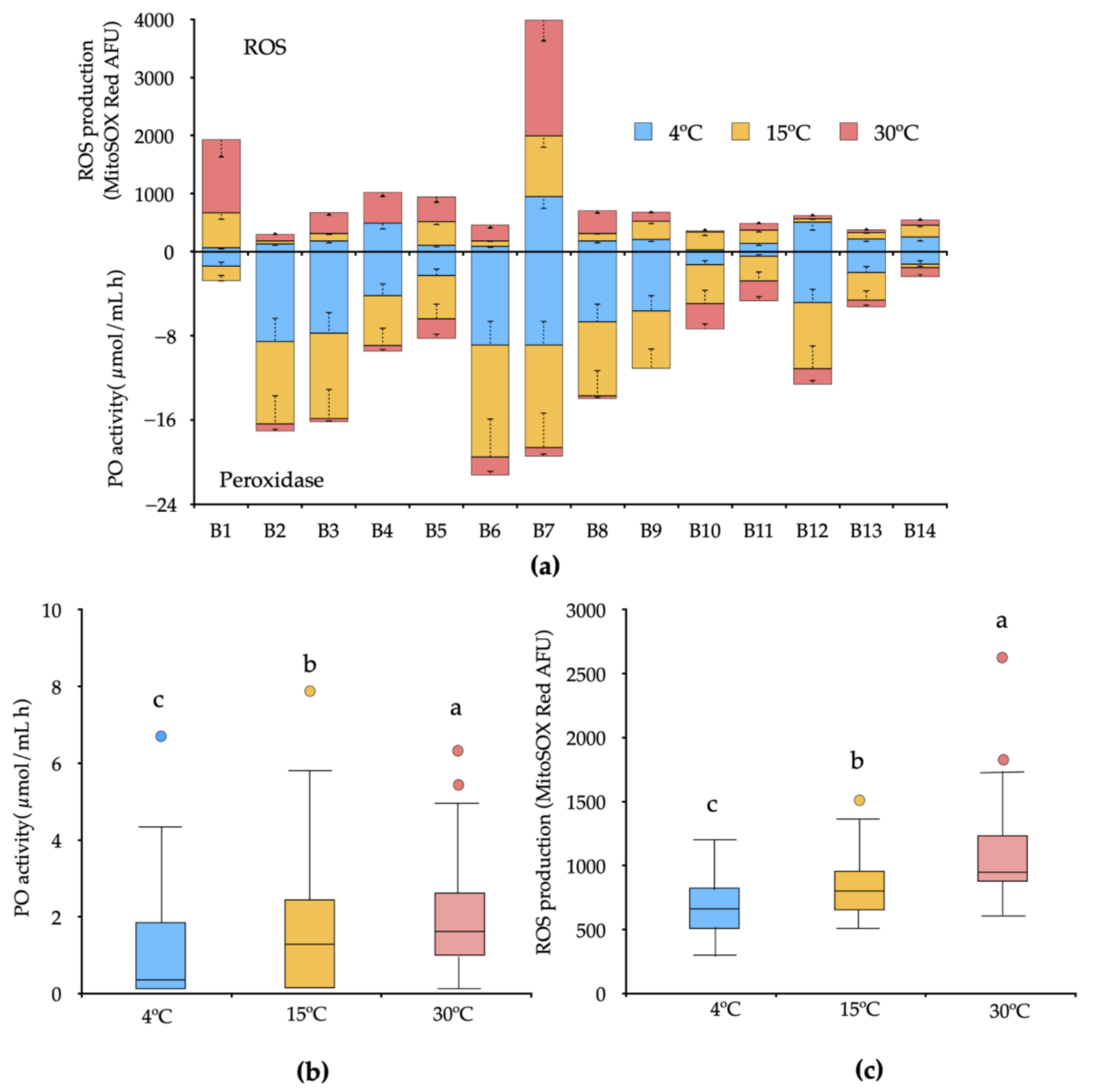
2.6. Peroxidase Activity and ROS Production
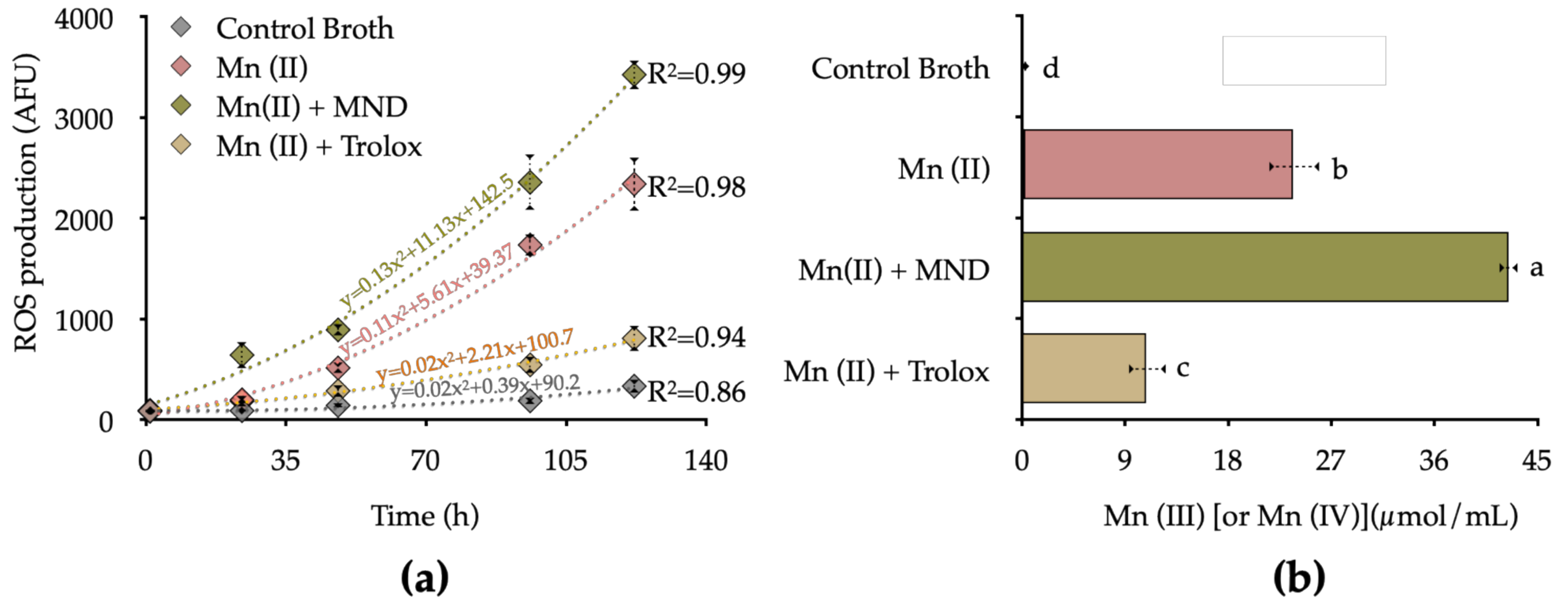
2.7. Contribution of ROS Production on Mn(II) Oxidation
2.8. Influence of Mn(II) on Membrane Potential, Viability, and Bacterial Morphology
2.9. Statistical Analysis
3. Results
3.1. Manganese Oxidizing Bacteria in Antarctic Soils
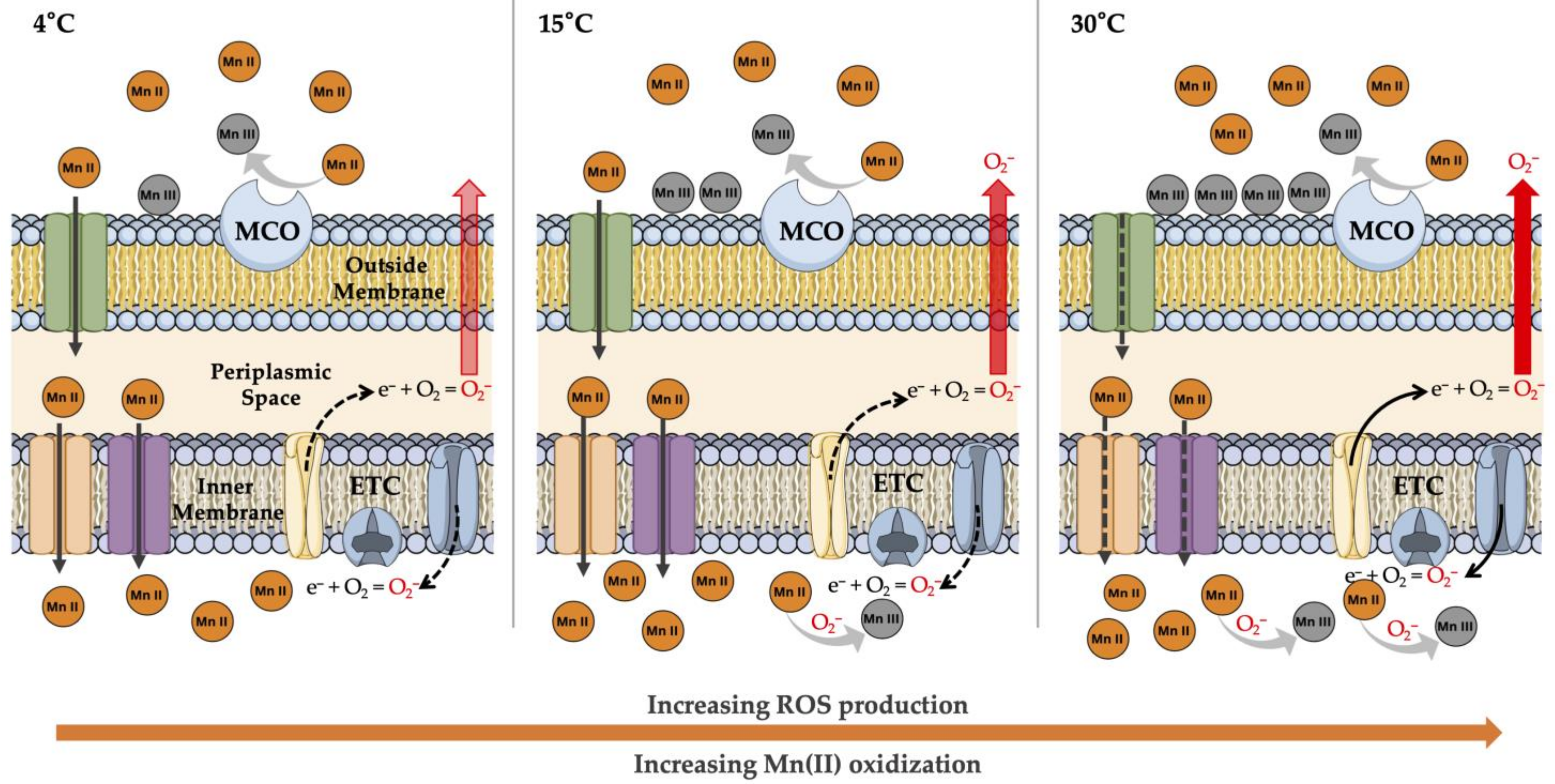
3.2. Effect of Temperature on Bacterial Growth and Mn(II) Oxidation
3.3. Peroxidase Activity and ROS Production under Increased Temperature
3.4. Influence of ROS Production on Mn Oxidation
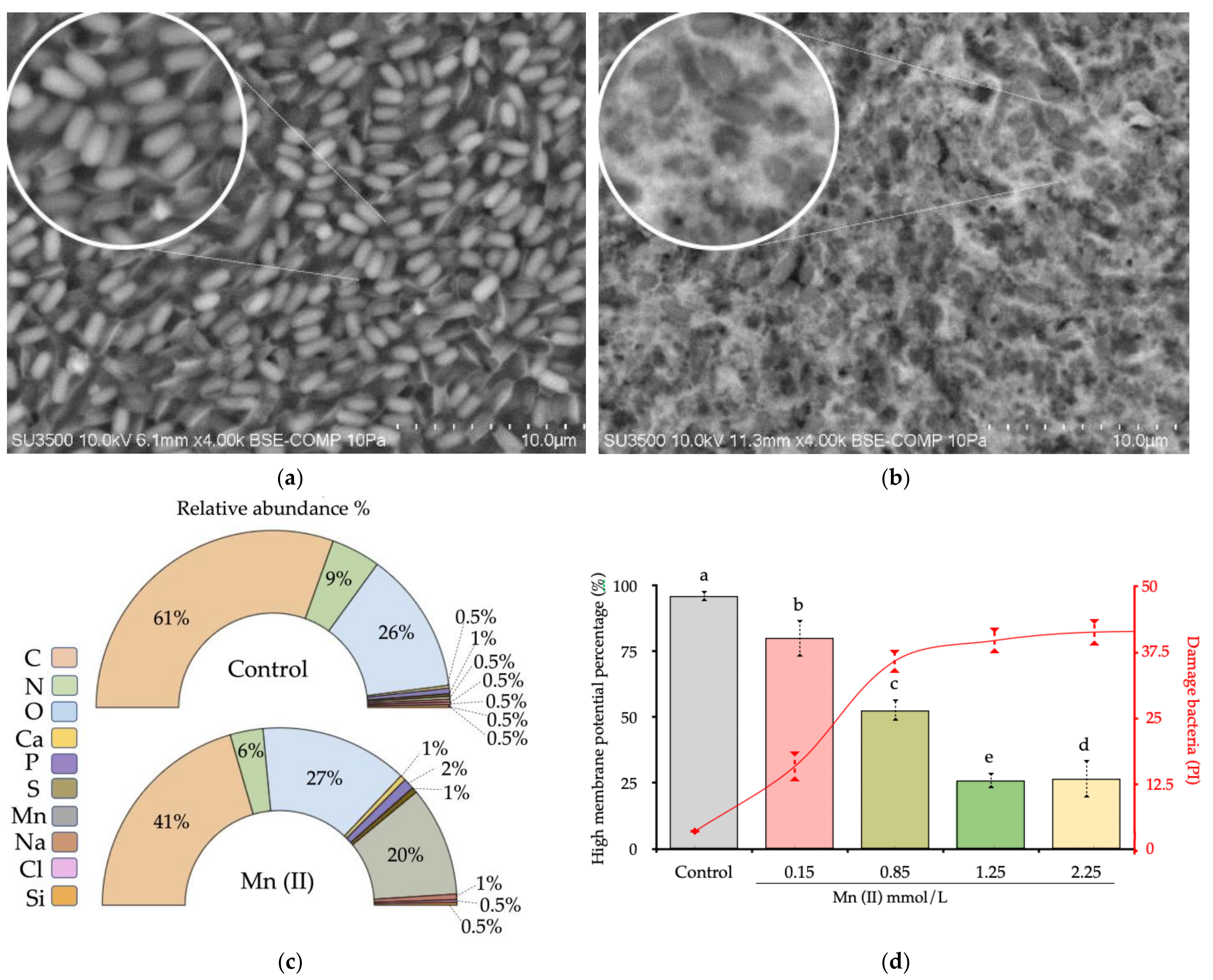
3.5. Scanning Electron Microscopy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vodyanitskii, Y.; Vasil’ev, A.A.; Lessovaia, S.; Sataev, E.F.; Sivtsov, A.V. Formation of manganese oxides in soils. Eurasian Soil Sci. 2004, 37, 572–584. [Google Scholar]
- Reid, A.S.J.; Miller, M.H. The manganese cycle in soils: Ii. forms of soil manganese in equilibrium with solution manganese. Can. J. Soil Sci. 1963, 43, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Spiro, T.G.; Bargar, J.R.; Sposito, G.; Tebo, B.M. Bacteriogenic Manganese Oxides. Acc. Chem. Res. 2010, 43, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Geszvain, K.; Butterfield, C.; Davis, R.; Madison, A.; Lee, S.-W.; Parker, D.; Soldatova, A.; Spiro, T.; Luther, G.; Tebo, B. The molecular biogeochemistry of manganese(II) oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef]
- Templeton, A.S.; Staudigel, H.; Tebo, B.M. Diverse Mn(II)-Oxidizing Bacteria Isolated from Submarine Basalts at Loihi Seamount. Geomicrobiol. J. 2005, 22, 127–139. [Google Scholar] [CrossRef]
- Dennis, P.G.; Newsham, K.K.; Rushton, S.P.; O’Donnell, A.G.; Hopkins, D.W. Soil bacterial diversity is positively associated with air temperature in the maritime Antarctic. Sci. Rep. 2019, 9, 2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsham, K.K.; Davey, M.L.; Hopkins, D.W.; Dennis, P.G. Regional Diversity of Maritime Antarctic Soil Fungi and Predicted Responses of Guilds and Growth Forms to Climate Change. Front. Microbiol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, C.A.; Newsham, K.K.; Cox, F.; Garnett, M.H.; Robinson, C.H.; Dungait, J.A.J. Predicting climate change impacts on maritime Antarctic soils: A space-for-time substitution study. Soil Biol. Biochem. 2020, 141, 107682. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Benhua, S.; Dennis, P.G.; Badalucco, L.; Rushton, S.P.; Newsham, K.K.; O’Donnell, A.G.; Hartley, I.P.; Hopkins, D.W. Responses to increases in temperature of heterotrophic micro-organisms in soils from the maritime Antarctic. Polar Biol. 2015, 38, 1153–1160. [Google Scholar] [CrossRef]
- Thamdrup, B.; Hansen, J.W.; Jørgensen, B.B. Temperature dependence of aerobic respiration in a coastal sediment. FEMS Microbiol. Ecol. 1998, 25, 189–200. [Google Scholar] [CrossRef]
- Piazza, A.; Ciancio Casalini, L.; Pacini, V.A.; Sanguinetti, G.; Ottado, J.; Gottig, N. Environmental Bacteria Involved in Manganese(II) Oxidation and Removal From Groundwater. Front Microbiol 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mols, M.; Ceragioli, M.; Abee, T. Heat stress leads to superoxide formation in Bacillus cereus detected using the fluorescent probe MitoSOX. Int. J. Food Microbiol. 2011, 151, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Mols, M.; Pier, I.; Zwietering, M.H.; Abee, T. The impact of oxygen availability on stress survival and radical formation of Bacillus cereus. Int. J. Food Microbiol. 2009, 135, 303–311. [Google Scholar] [CrossRef]
- Brynildsen, M.P.; Winkler, J.A.; Spina, C.S.; MacDonald, I.C.; Collins, J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotechnol. 2013, 31, 160–165. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bleier, L.; Dröse, S. Superoxide generation by complex III: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [Green Version]
- Learman, D.R.; Voelker, B.M.; Madden, A.S.; Hansel, C.M. Constraints on superoxide mediated formation of manganese oxides. Front. Microbiol. 2013, 4, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Learman, D.R.; Voelker, B.M.; Vazquez-Rodriguez, A.I.; Hansel, C.M. Formation of manganese oxides by bacterially generated superoxide. Nat. Geosci. 2011, 4, 95–98. [Google Scholar] [CrossRef]
- Singh, R.; Beriault, R.; Middaugh, J.; Hamel, R.; Chenier, D.; Appanna, V.D.; Kalyuzhnyi, S. Aluminum-tolerant Pseudomonas fluorescens: ROS toxicity and enhanced NADPH production. Extrem. Life Under Extrem. Cond. 2005, 9, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, M.K.; Raghu, G.; Sharma, Y.V.R.K.; Biju, A.R.; Rajasekharan, M.V.; Shivaji, S. Increase in Oxidative Stress at Low Temperature in an Antarctic Bacterium. Curr. Microbiol. 2011, 62, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 20th ed.; Natural Resources Conservation Service, Deparment of Agriculture, United State.: Washington, DC, USA, 2014. [Google Scholar]
- Van Reeuwijk, L. Procedures for Soil Analysis; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- Stein, L.Y.; La Duc, M.T.; Grundl, T.J.; Nealson, K.H. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 2001, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, M.; Sugita, T.; Shimizu, M.; Ohode, Y.; Iwamoto, K.; de Vrind-de Jong, E.W.; de Vrind, J.P.; Corstjens, P.L. Partial purification and characterization of manganese-oxidizing factors of Pseudomonas fluorescens GB-1. Appl. Environ. Microbiol. 1997, 63, 4793–4799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingle, T.C.; Butler-Wu, S.M. MALDI-TOF mass spectrometry for microorganism identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef]
- Krumbein, W.; Altmann, H. A new method for the detection and enumeration of manganese oxidizing and reducing microorganisms. Helgoländer Wiss. Meeresunters 1973, 25, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Bach, C.E.; Warnock, D.D.; Van Horn, D.J.; Weintraub, M.N.; Sinsabaugh, R.L.; Allison, S.D.; German, D.P. Measuring phenol oxidase and peroxidase activities with pyrogallol, l-DOPA, and ABTS: Effect of assay conditions and soil type. Soil Biol. Biochem. 2013, 67, 183–191. [Google Scholar] [CrossRef] [Green Version]
- McBee, M.E.; Chionh, Y.H.; Sharaf, M.L.; Ho, P.; Cai, M.W.L.; Dedon, P.C. Production of Superoxide in Bacteria Is Stress- and Cell State-Dependent: A Gating-Optimized Flow Cytometry Method that Minimizes ROS Measurement Artifacts with Fluorescent Dyes. Front. Microbiol. 2017, 8, 459. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.M.; Fridovich, I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 1979, 196, 385–395. [Google Scholar] [CrossRef]
- Iyanagi, T. On the mechanism of one-electron reduction of quinones by microsomal flavin enzymes: The kinetic analysis between cytochrome B5 and menadione. Free Radic. Res. Commun. 1990, 8, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Yang, Y.; Luo, T. Membrane potential based characterization by flow cytometry of physiological states in an aerobic anoxygenic phototrophic bacterium. Aquat. Microb. Ecol. 2004, 37, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Romanovaskaia, V.A.; Tashirev, A.B.; Gladka, G.B.; Tashireva, A.A. Temperature range for growth of the Antarctic microorganisms. Mikrobiolohichnyi Zhurnal 2012, 74, 13–19. [Google Scholar] [PubMed]
- Yu, H.; Leadbetter, J.R. Bacterial chemolithoautotrophy via manganese oxidation. Nature 2020, 583, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yang, M.; Zhang, S.; Zhao, D.; Duan, J.; Wang, W.; Yan, L. Iron and sulfur oxidation pathways of Acidithiobacillus ferrooxidans. World J. Microbiol. Biotechnol. 2019, 35, 60. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, C.N.; Soldatova, A.V.; Lee, S.-W.; Spiro, T.G.; Tebo, B.M. Mn(II,III) oxidation and MnO2 mineralization by an expressed bacterial multicopper oxidase. Proc. Natl. Acad. Sci. USA 2013, 110, 11731–11735. [Google Scholar] [CrossRef] [Green Version]
- Soldatova, A.V.; Romano, C.A.; Tao, L.; Stich, T.A.; Casey, W.H.; Britt, R.D.; Tebo, B.M.; Spiro, T.G. Mn(II) oxidation by the multicopper oxidase complex Mnx: A coordinated two-stage Mn(II)/(III) and Mn(III)/(IV) mechanism. J. Am. Chem. Soc. 2017, 139, 11381–11391. [Google Scholar] [CrossRef]
- Soldatova, A.V.; Tao, L.; Romano, C.A.; Stich, T.A.; Casey, W.H.; Britt, R.D.; Tebo, B.M.; Spiro, T.G. Mn(II) oxidation by the multicopper oxidase complex Mnx: A binuclear activation mechanism. J. Am. Chem. Soc. 2017, 139, 11369–11380. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, G.-J.; de Vrind, J.P.; Corstjens, P.L.; Cornelis, P.; Baysse, C.; de Vrind-de Jong, E.W. cumA, a gene encoding a multicopper oxidase, is involved in Mn2+ oxidation in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 1999, 65, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Geszvain, K.; McCarthy, J.K.; Tebo, B.M. Elimination of manganese (II, III) oxidation in Pseudomonas putida GB-1 by a double knockout of two putative multicopper oxidase genes. Appl. Environ. Microbiol. 2013, 79, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Corstjens, P.; De Vrind, J.; Goosen, T.; Jong, E.d.V.d. Identification and molecular analysis of the Leptothrix discophora SS-1 mofA gene, a gene putatively encoding a manganese-oxidizing protein with copper domains. Geomicrobiol. J. 1997, 14, 91–108. [Google Scholar] [CrossRef]
- Brouwers, G.; Corstjens, P.; De Vrind, J.; Verkamman, A.; De Kuyper, M.; De Vrind-De Jong, E. Stimulation of Mn2+ oxidation in Leptothrix discophora SS-1 by Cu2+ and sequence analysis of the region flanking the gene encoding putative multicopper oxidase MofA. Geomicrobiol. J. 2000, 17, 25–33. [Google Scholar]
- Nakama, K.; Medina, M.; Lien, A.; Ruggieri, J.; Collins, K.; Johnson, H.A. Heterologous expression and characterization of the manganese-oxidizing protein from Erythrobacter sp. strain SD21. Appl. Environ. Microbiol. 2014, 80, 6837–6842. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.; Johnson, H.; Caputo, N.; Davis, R.; Torpey, J.; Tebo, B.M. Mn(II) oxidation is catalyzed by heme peroxidases in “Aurantimonas manganoxydans” strain SI85-9A1 and Erythrobacter sp. strain SD-21. Appl. Environ. Microbiol. 2009, 75, 4130–4138. [Google Scholar] [CrossRef] [Green Version]
- Geszvain, K.; Smesrud, L.; Tebo, B.M. Identification of a third Mn(II) oxidase enzyme in Pseudomonas putida GB-1. Appl. Environ. Microbiol. 2016, 82, 3774–3782. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, G.; Shahzad, T.; Andanson, L.; Bahn, M.; Wallenstein, M.D.; Fontaine, S. Catalytic power of enzymes decreases with temperature: New insights for understanding soil C cycling and microbial ecology under warming. Glob. Chang. Biol. 2018, 24, 4238–4250. [Google Scholar] [CrossRef] [PubMed]
- Barnese, K.; Gralla, E.B.; Valentine, J.S.; Cabelli, D.E. Biologically relevant mechanism for catalytic superoxide removal by simple manganese compounds. Proc. Natl. Acad. Sci. USA 2012, 109, 6892–6897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz, J.M.; Hansel, C.M.; Voelker, B.M.; Mendes, C.M.; Andeer, P.F.; Zhang, T. Widespread Production of Extracellular Superoxide by Heterotrophic Bacteria. Science 2013, 340, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Miteva, V. Diversity and ecology of psychrophilic microorganisms. Res. Microbiol. 2011, 162, 346–361. [Google Scholar] [CrossRef]
- Galeano, L.A.; Guerrero, M.; Sánchez, C.; Gil, A.; Vicente, M.A. Disinfection by Chemical Oxidation Methods. In Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment. The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2017; Volume 67. [Google Scholar]
- Pischedda, A.; Ramasamy, K.P.; Mangiagalli, M.; Chiappori, F.; Milanesi, L.; Miceli, C.; Pucciarelli, S.; Lotti, M. Antarctic marine ciliates under stress: Superoxide dismutases from the psychrophilic Euplotes focardii are cold-active yet heat tolerant enzymes. Sci. Rep. 2018, 8, 14721. [Google Scholar] [CrossRef] [PubMed]
- Lesser, M.P. Oxidative stress in marine environments: Biochemistry and physiological ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [Green Version]
- Niewerth, H.; Schuldes, J.; Parschat, K.; Kiefer, P.; Vorholt, J.A.; Daniel, R.; Fetzner, S. Complete genome sequence and metabolic potential of the quinaldine-degrading bacterium Arthrobacter sp. Rue61a. BMC Genom. 2012, 13, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shvinka, J.; Toma, M.; Galinina, N.; Skards, I.; Viesturs, U. Production of Superoxide Radicals during Bacterial Respiration. J. Gen. Microbiol. 1979, 113, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C.; Jamier, V.; Ba, L.A. Redox active secondary metabolites. Curr. Opin. Chem. Biol. 2011, 15, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Lanciano, P.; Khalfaoui-Hassani, B.; Selamoglu, N.; Ghelli, A.; Rugolo, M.; Daldal, F. Molecular mechanisms of superoxide production by complex III: A bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta 2013, 1827, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loor, G.; Kondapalli, J.; Schriewer, J.M.; Chandel, N.S.; Vanden Hoek, T.L.; Schumacker, P.T. Menadione triggers cell death through ROS-dependent mechanisms involving PARP activation without requiring apoptosis. Free Radic. Biol. Med. 2010, 49, 1925–1936. [Google Scholar] [CrossRef] [Green Version]
- Hohle, T.H.; O’Brian, M.R. Magnesium-dependent processes are targets of bacterial manganese toxicity. Mol. Microbiol. 2014, 93, 736–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant-Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef] [Green Version]
- Sträuber, H.; Müller, S. Viability states of bacteria--specific mechanisms of selected probes. Cytom. Part A J. Int. Soc. Anal. Cytol. 2010, 77, 623–634. [Google Scholar] [CrossRef]
- Zeinert, R.; Martinez, E.; Schmitz, J.; Senn, K.; Usman, B.; Anantharaman, V.; Aravind, L.; Waters, L.S. Structure–function analysis of manganese exporter proteins across bacteria. J. Biol. Chem. 2018, 293, 5715–5730. [Google Scholar] [CrossRef] [Green Version]

| SITE | APB 1 | MnOxb 2 | Strain Code | Closest Related Species | Class | log (Score) 3 |
|---|---|---|---|---|---|---|
| S1 | 5 × 105 ± 0.48 | 2 × 102 ± 0.73 | B1 | Microbacterium esteraromaticum | Actinobacteria | 2.34 |
| S2 | 2.1 × 105 ± 0.35 | 2.3 × 102 ± 0.89 | B2 | Pseudomonas extremorientalis | Gammaproteobacteria | 2.17 |
| S2 | 2.1 × 105 ± 0.35 | 2.3 × 102 ± 0.89 | B3 | Variovorax paradoxus | Betaproteobacteria | 2.15 |
| S3 | 4.4 × 105 ± 1.18 | 2.2 × 102 ± 0.27 | B4 | Arthrobacter psychrolactophilus | Actinobacteria | 2.09 |
| S3 | 4.4 × 105 ± 1.18 | 2.2 × 102 ± 0.27 | B5 | Chryseobacterium indoltheticum | Flavobacteria | 2.36 |
| S4 | 3.7 × 105 ± 0.20 | 2.7 × 102 ± 0.52 | B6 | Chryseobacterium chaponense | Flavobacteria | 2.13 |
| S5 | 6.7 × 106 ± 0.46 | 6.2 × 103 ± 0.72 | B7 | Arthrobacter oxydans | Actinobacteria | 2.16 |
| S6 | 1.5 × 106 ± 0.31 | 9.4 × 103 ± 0.33 | B8 | Rhodococcus erythropolis | Actinobacteria | 2.14 |
| S7 | 1.7 × 106 ± 0.26 | 2.3 × 102 ± 0.64 | B9 | Arthrobacter arylaitensis | Actinobacteria | 2.23 |
| S8 | 6.8 × 106 ± 0.46 | 6.5 × 103 ± 0.24 | B10 | Bacillus megaterium | Bacilli | 2.11 |
| S9 | 8.7 × 106 ± 0.55 | 2.4 × 103 ± 0.62 | B11 | Lactobacillus plantarum | Bacilli | 2.17 |
| S10 | 6.7 × 106 ± 0.46 | 6.2 × 103 ± 0.72 | B12 | Bacillus weihenstephanensis | Bacilli | 2.18 |
| S10 | 9.2 × 106 ± 0.31 | 7.8 × 102 ± 0.38 | B13 | Rhodococcus fascians | Actinobacteria | 2.16 |
| S10 | 6.7 × 106 ± 0.46 | 6.2 × 103 ± 0.72 | B14 | Sphingomonas echinoides | Alphaproteobacteria | 2.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jofré, I.; Matus, F.; Mendoza, D.; Nájera, F.; Merino, C. Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity. Biology 2021, 10, 1004. https://doi.org/10.3390/biology10101004
Jofré I, Matus F, Mendoza D, Nájera F, Merino C. Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity. Biology. 2021; 10(10):1004. https://doi.org/10.3390/biology10101004
Chicago/Turabian StyleJofré, Ignacio, Francisco Matus, Daniela Mendoza, Francisco Nájera, and Carolina Merino. 2021. "Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity" Biology 10, no. 10: 1004. https://doi.org/10.3390/biology10101004
APA StyleJofré, I., Matus, F., Mendoza, D., Nájera, F., & Merino, C. (2021). Manganese-Oxidizing Antarctic Bacteria (Mn-Oxb) Release Reactive Oxygen Species (ROS) as Secondary Mn(II) Oxidation Mechanisms to Avoid Toxicity. Biology, 10(10), 1004. https://doi.org/10.3390/biology10101004







