Physiology: An Important Tool to Assess the Welfare of Aquatic Animals
Simple Summary
Abstract
1. Introduction
1.1. Welfare
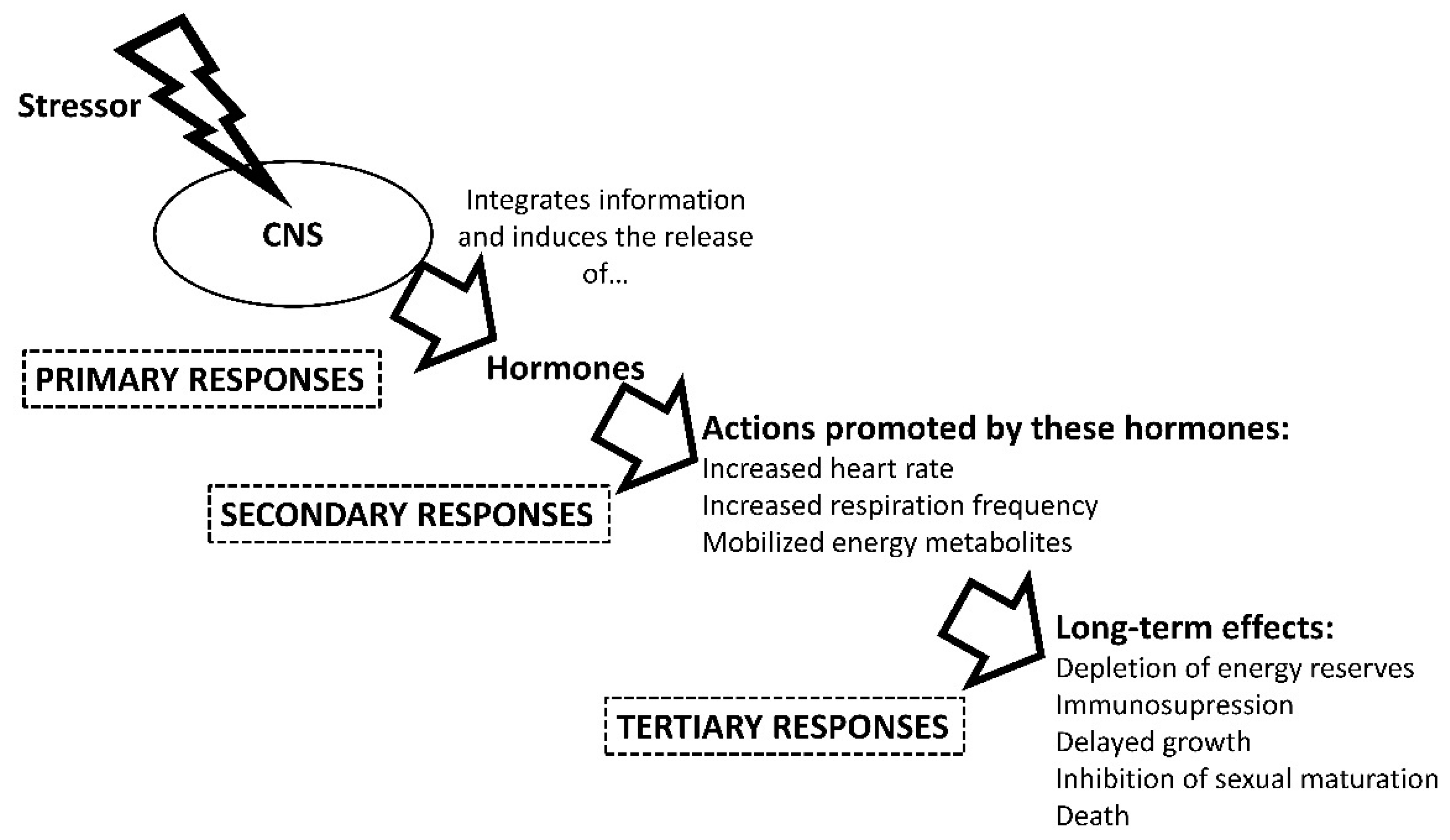
1.2. Stress Physiology
1.3. Areas of Interest
2. Taxonomic Differences
2.1. Crustaceans
2.2. Cephalopods
2.3. Elasmobranchs
2.4. Teleosts
2.5. Dipnoans
3. Future Approaches
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandoe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef]
- Kristiansen, T.S.; Bracke, M.B.M. A Brief Look into the Origins of Fish Welfare Science. In The Welfare of Fish. Animal Welfare; Kristiansen, T.S., Ferno, A., Pavlidis, M.A., van de Vis, H., Eds.; Springer: Cham, Switzerland, 2020; Volume 20. [Google Scholar]
- Webster, J. Animal Welfare Limping towards Eden: A Practical Approach to Redressing the Problem of Our Dominion over the Animals; Blackwell Publishing: Hoboken, NJ, USA, 2005; p. 283. [Google Scholar]
- EU. Treaty of Amsterdam Amending the Treaty on European Union, the Treaties Establishing the European Communities and Certain Related Acts. 1997. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:11997D/TXT&from=EN (accessed on 17 December 2020).
- European Food Safety Authority (EFSA). General Approach to Fish Welfare and to the Concept of Sentience in Fish; EFSA: Parma, Italy, 2009. [Google Scholar]
- Browman, H.L.; Cooke, S.J.; Cowx, I.G.; Derbyshire, S.W.G.; Kasumyan, A.; Key, B.; Rose, J.D.; Schwab, A.; Skiftesvik, A.B.; Stevens, E.D.; et al. Welfare of aquatic animals: Where things are, where they are going, and what it means for research, aquaculture, recreational angling, and commercial fishing. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 2019, 76, 82–92. [Google Scholar] [CrossRef]
- OIE. Aquatic Animal Health Code; OIE: Paris, France, 2019. [Google Scholar]
- FAO. Welfare of Fishes in Aquaculture; FAO: Budapest, Hungary, 2019. [Google Scholar]
- EU. Council Directive 98/58/EC of 20 July 1998 Concerning the Protection of Animals Kept for Farming Purposes. 1998. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A31998L0058 (accessed on 17 December 2020).
- EU. Consolidated Text: Regulation (EC) No 882/2004 of the European Parliament and of the Council of 29 April 2004 on Official Controls Performed to Ensure the Verification of Compliance with Feed and Food Law, Animal Health and Animal Welfare Rules. 2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02017R0625-20191214 (accessed on 17 December 2020).
- EU. Council Regulation (EC) No 1/2005 of 22 December 2004 on the Protection of Animals during Transport and Related Operations and Amending Directives 64/432/EEC and 93/119/EC and Regulation (EC) No 1255/97. 2005. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32005R0001 (accessed on 17 December 2020).
- EU. Council Directive 2006/88/EC of 24 October 2006 on Animal Health Requirements for Aquaculture Animals and Products Thereof, and on the Prevention and Control of Certain Diseases in Aquatic Animals. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006L0088 (accessed on 17 December 2020).
- EU. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing (Text with EEA Relevance). 2009. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj (accessed on 17 December 2020).
- EU. Commission Regulation (EC) No 710/2009 of 5 August 2009 Amending Regulation (EC) No 889/2008 Laying down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007, as Regards Laying down Detailed Rules on Organic Aquaculture Animal and Seaweed Production. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32009R0710 (accessed on 17 December 2020).
- Diggles, B.K.; Cooke, S.J.; Rose, J.D.; Sawynok, W. Ecology and welfare of aquatic animals in wild capture fisheries. Rev. Fish Biol. Fish. 2011, 21, 739–765. [Google Scholar] [CrossRef]
- EU. Council Directive 1999/22/EC of 29 March 1999 Relating to the Keeping of Wild Animals in Zoos. 1999. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:31999L0022 (accessed on 17 December 2020).
- EU. EU Zoos Directive Good Practices Document; EU Commission: Luxembourg, 2015. [Google Scholar]
- EAZA. Standards for the Accommodation and Care of Animals in Zoos and Aquaria; EAZA: Amsterdam, The Netherlands, 2014. [Google Scholar]
- EU. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes (Text with EEA Relevance)Text with EEA Relevance. 2010. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 17 December 2020).
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the care and welfare of cephalopods in research -a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Tort, L.; Farrell, A.P.; Brauner, C. Biology of Stress in Fish; Academic Press: Cambridge, CA, USA, 2016; Volume 35, p. 602. [Google Scholar]
- Martins, C.I.; Galhardo, L.; Noble, C.; Damsgard, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2012, 38, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Broom, D.M. Animal welfare: Concepts and measurement. J. Anim. Sci. 1991, 69, 4167–4175. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Tresguerres, M.; Hamilton, T.J. Acid–base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef]
- Foster, C.; Amado, E.M.; Souza, M.M.; Freire, C.A. Do osmoregulators have lower capacity of muscle water regulation than osmoconformers? A study on decapod crustaceans. J. Exp. Zool. A 2010, 313, 80–94. [Google Scholar] [CrossRef]
- Freire, C.A.; Onken, H.; McNamara, J.C. A structure-function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. A 2008, 151, 272–304. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D. The Hormonal Control of Osmoregulation in Teleost Fish. In Encyclopedia of Fish Physiology: From Genome to Environment; Farrell, A.P., Ed.; Academic Press: San Diego, CA, USA, 2011; Volume 2, pp. 1466–1473. [Google Scholar]
- Wells, R.M.G. Blood-gas transport and hemoglobin function: Adaptations for functional and environmental hypoxia. In Fish Physiology: Hypoxia; Richards, J.G., Farrell, A.P., Brauner, C.J., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 255–299. [Google Scholar]
- Barragán-Méndez, C.; Sobrino, I.; Marin-Rincon, A.; Fernandez-Boo, S.; Costas, B.; Mancera, J.M.; Ruiz-Jarabo, I. Acute-stress biomarkers in three octopodidae species after bottom trawling. Front. Physiol. 2019, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Jensen, F.B.; Fago, A.; Weber, R.E. Haemoglobin structure and function. In Fish Respiration; Perry, S.F., Tufts, B.L., Eds.; Academic Press: San Diego, CA, USA, 1998; Volume 17, pp. 1–40. [Google Scholar]
- Storey, K.B.; Storey, J.M. Carbohydrate metabolism in cephalopod molluscs. In Metabolic Biochemistry and Molecular Biomechanics; Hochachka, P.W., Wilbur, K.M., Eds.; Volume The Molusca; Academic Press: New York, NY, USA, 1983; Volume 1, pp. 91–136. [Google Scholar]
- Speers-Roesch, B.; Treberg, J.R. The unusual energy metabolism of elasmobranch fishes. Comp. Biochem. Physiol. A 2010, 155, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Soengas, J.L.; Sangiao-Alvarellos, S.; Laiz-Carrion, R.; Mancera, J.M. Energy metabolism and osmotic acclimation in teleost fish. In Fish Osmoregulation; Baldiserotto, B., Mancera, J.M., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 277–307. [Google Scholar]
- Decker, H.; Jaenicke, E. Recent findings on phenoloxidase activity and antimicrobial activity of hemocyanins. Dev. Comp. Immunol. 2004, 28, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Hirata, T.; Nishioka, T.; Sakaguchi, M. Hemocyte components in crustaceans convert hemocyanin into a phenoloxidase-like enzyme. Comp. Biochem. Physiol. B 2003, 134, 135–141. [Google Scholar] [CrossRef]
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef]
- Vizcaíno, R. Study of Immune Parameters in the Skin Mucus and Hemolymph of the Common Octopus, Octopus vulgaris, under Stressful Conditions: Selection of Potential Biomarkers of Welfare and Health. Ph.D. Thesis, Universidade de Vigo, Vigo, Spain, 2016. [Google Scholar]
- Gestal, C.; Castellanos-Martinez, S. Understanding the cephalopod immune system based on functional and molecular evidence. Fish Shellfish Immunol. 2015, 46, 120–130. [Google Scholar] [CrossRef]
- Loker, E.S.; Adema, C.M.; Zhang, S.M.; Kepler, T.B. Invertebrate immune systems--not homogeneous, not simple, not well understood. Immunol. Rev. 2004, 198, 10–24. [Google Scholar] [CrossRef]
- Vazquez, L.; Alpuche, J.; Maldonado, G.; Agundis, C.; Pereyra-Morales, A.; Zenteno, E. Review: Immunity mechanisms in crustaceans. Innate Immun. 2009, 15, 179–188. [Google Scholar] [CrossRef]
- Porte, C.; Janer, G.; Lorusso, L.C.; Ortiz-Zarragoitia, M.; Cajaraville, M.P.; Fossi, M.C.; Canesi, L. Endocrine disruptors in marine organisms: Approaches and perspectives. Comp. Biochem. Physiol. C 2006, 143, 303–315. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovas. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- van der Oost, R.; Beyer, J.; Vermeulen, N.P. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ. Toxicol. Pharmacol. 2003, 13, 57–149. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.D.; Bradshaw, D. Hormonal control of salt and water balance in vertebrates. Gen. Comp. Endocrinol. 2006, 147, 3–8. [Google Scholar] [CrossRef]
- McEwen, B.S.; Wingfield, J.C. The concept of allostasis in biology and biomedicine. Horm. Behav. 2003, 43, 2–15. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Malham, S.K.; Lacoste, A.; Gelebart, F.; Cueff, A.; Poulet, S.A. A first insight into stress-induced neuroendocrine and immune changes in the octopus Eledone cirrhosa. Aquat. Living Resour. 2002, 15, 187–192. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. C 1998, 120, 1–27. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Barragán-Méndez, C.; Jerez-Cepa, I.; Fernandez-Castro, M.; Sobrino, I.; Mancera, J.M.; Aerts, J. Plasma 1alpha-hydroxycorticosterone as biomarker for acute stress in catsharks (Scyliorhinus canicula). Front. Physiol. 2019, 10, 1217. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Taylor, A.C.; Geoffrey Moore, P. Physiological stress in decapod crustaceans (Munida rugosa and Liocarcinus depurator) discarded in the Clyde Nephrops fishery. J. Exp. Mar. Biol. Ecol. 2001, 259, 215–229. [Google Scholar] [CrossRef]
- Costas, B.; Conceicao, L.; Aragao, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.; Afonso, A. Physiological responses of Senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; González-Duarte, M.M.; Sobrino, I.; Vila, Y.; Mancera, J.M.; Ruiz-Jarabo, I. Physiological recovery after bottom trawling as a method to manage discards: The case study of Nephrops norvegicus and Squilla mantis. Mar. Policy 2020, 116, 103895. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Barton, B.A.; McLeay, D.J. Stress and acclimation. In Methods of Fish Biology; Schreck, C.B., Moyle, P.B., Eds.; American Fisheries Society: Bethesda MD, USA, 1990; pp. 451–489. [Google Scholar]
- Arjona, F.J.; Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Gonçalves, O.; Pâscoa, I.; Martín del Río, M.P.; Mancera, J.M. Tertiary stress responses in Senegalese sole (Solea senegalensis Kaup, 1858) to osmotic challenge: Implications for osmoregulation, energy metabolism and growth. Aquaculture 2009, 287, 419–426. [Google Scholar] [CrossRef]
- Barragán-Méndez, C.; Ruiz-Jarabo, I.; Fuentes, J.; Mancera, J.M.; Sobrino, I. Survival rates and physiological recovery responses in the lesser-spotted catshark (Scyliorhinus canicula) after bottom-trawling. Comp. Biochem. Physiol. A 2019, 233, 1–9. [Google Scholar] [CrossRef]
- Sneddon, L.U.; Wolfenden, D.C.C.; Thomson, J.S. Stress management and welfare. Fish Physiol. 2016, 35, 463–539. [Google Scholar] [CrossRef]
- Toni, M.; Manciocco, A.; Angiulli, E.; Alleva, E.; Cioni, C.; Malavasi, S. Review: Assessing fish welfare in research and aquaculture, with a focus on European directives. Animal 2019, 13, 161–170. [Google Scholar] [CrossRef]
- Lieke, T.; Meinelt, T.; Hoseinifar, S.H.; Pan, B.; Straus, D.L.; Steinberg, C.E.W. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquac. 2019, 1–23. [Google Scholar] [CrossRef]
- European Commission. Welfare of Farmed Fish: Common Practices during Transport and at Slaughter; Directorate-General for Health and Food Safety: Luxembourg, 2017. [Google Scholar]
- Barragán-Méndez, C.; Sánchez-García, F.; Sobrino, I.; Mancera, J.M.; Ruiz-Jarabo, I. Air exposure in catshark (Scyliorhinus canicula) modify muscle texture properties: A pilot study. Fishes 2018, 3, 34. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Lyu, J.; Kong, C.; Song, S.; Luo, Y. The impact of stunning methods on stress conditions and quality of silver carp (Hypophthalmichthys molitrix) fillets stored at 4 degrees C during 72h postmortem. Food Chem. 2017, 216, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.V.; Reid, A.; Patterson, D.A.; Robinson, K.A.; Chapman, J.M.; Hinch, S.G.; Cooke, S.J. A synthesis to understand responses to capture stressors among fish discarded from commercial fisheries and options for mitigating their severity. Fish Fish. 2018, 1–19. [Google Scholar] [CrossRef]
- Ellis, J.R.; McCully Phillips, S.R.; Poisson, F. A review of capture and post-release mortality of elasmobranchs. J. Fish Biol. 2017, 90, 653–722. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Jarabo, I.; Amanajas, R.D.; Baldisserotto, B.; Mancera, J.M.; Val, A.L. Tambaqui (Colossoma macropomum) acclimated to different tropical waters from the Amazon basin shows specific acute-stress responses. Comp. Biochem. Physiol. A 2020, 110706. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Arjona, F.J.; Ruiz-Jarabo, I.; Garcia-Lopez, A.; Flik, G.; Mancera, J.M. Water temperature affects osmoregulatory responses in gilthead sea bream (Sparus aurata L.). J. Therm. Biol. 2020, 88, 102526. [Google Scholar] [CrossRef]
- Ruiz-Jarabo, I.; Tinoco, A.B.; Vargas-Chacoff, L.; Martos-Sitcha, J.A.; Rodríguez-Rua, A.; Cardenas, S.; Mancera, J.M. Environmental salinity affects growth and metabolism in fingerling meagre (Argyrosomus regius). Fishes 2019, 4, 6. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Oyarzún, R.; Paredes, R.; Saravia, R.; Morera, J.; Muñoz, J.L.; Ruiz-Jarabo, I.; Mancera, J.M. Stocking density affects the growth performance, intermediary metabolism, osmoregulation, and response to stress in Patagonian blennie Eleginops maclovinus. Aquaculture 2019, 515, 734565. [Google Scholar] [CrossRef]
- Olivotto, I.; Planas, M.; Simoes, N.; Holt, G.J.; Avella, M.A.; Calado, R. Advances in breeding and rearing marine ornamentals. J. World Aquac. Soc. 2011, 42, 135–166. [Google Scholar] [CrossRef]
- Diggles, B.K. Review of some scientific issues related to crustacean welfare. ICES (Int. Counc. Explor. Sea) J. Mar. Sci. 2019, 76, 66–81. [Google Scholar] [CrossRef]
- LeBlanc, G.A. Crustacean endocrine toxicology: A review. Ecotoxicology 2007, 16, 61–81. [Google Scholar] [CrossRef]
- Kallen, J.L.; Abrahamse, S.L.; Vanherp, F. Circadian rhythmicity of the crustacean hyperglycemic hormone (Chh) in the hemolymph of the crayfish. Biol. Bull. 1990, 179, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.L.; Eriksson, S.P.; Nordborg, M.; Styf, H.K. The effect of environmental stressors on the early development of the Norway lobster Nephrops norvegicus (L.). J. Exp. Mar. Biol. Ecol. 2015, 473, 35–42. [Google Scholar] [CrossRef]
- Webster, S.G. Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J. Exp. Biol. 1996, 199, 1579–1585. [Google Scholar]
- Raicevich, S.; Minute, F.; Finoia, M.G.; Caranfa, F.; Di Muro, P.; Scapolan, L.; Beltramini, M. Synergistic and antagonistic effects of thermal shock, air exposure, and fishing capture on the physiological stress of Squilla mantis (Stomatopoda). PLoS ONE 2014, 9, e105060. [Google Scholar] [CrossRef] [PubMed]
- Lund, H.S.; Wang, T.; Chang, E.S.; Pedersen, L.F.; Taylor, E.W.; Pedersen, P.B.; McKenzie, D.J. Recovery by the Norway lobster Nephrops norvegicus (L.) from the physiological stresses of trawling: Influence of season and live-storage position. J. Exp. Mar. Biol. Ecol. 2009, 373, 124–132. [Google Scholar] [CrossRef]
- Harris, R.R.; Andrews, M.B. Physiological changes in the Norway lobster Nephrops norvegicus (L.) escaping and discarded from commercial trawls on the West Coast of Scotland—II. Disturbances in haemolymph respiratory gases, tissue metabolites and swimming performance after capture and during recovery. J. Exp. Mar. Biol. Ecol. 2005, 320, 195–210. [Google Scholar] [CrossRef]
- Patterson, L.; Dick, J.T.A.; Elwood, R.W. Physiological stress responses in the edible crab, Cancer pagurus, to the fishery practice of de-clawing. Mar. Biol. 2007, 152, 265–272. [Google Scholar] [CrossRef]
- Fodor, I.; Urban, P.; Scott, A.P.; Pirger, Z. A critical evaluation of some of the recent so-called ‘evidence’ for the involvement of vertebrate-type sex steroids in the reproduction of mollusks. Mol. Cell. Endocrinol. 2020, 516, 110949. [Google Scholar] [CrossRef]
- Scott, A.P. Is there any value in measuring vertebrate steroids in invertebrates? Gen. Comp. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Demas, G.E.; Adamo, S.A.; French, S.S. Neuroendocrine-immune crosstalk in vertebrates and invertebrates: Implications for host defence. Funct. Ecol. 2011, 25, 29–39. [Google Scholar] [CrossRef]
- Lacoue-Labarthe, T.; Bustamante, P.; Horlin, E.; Luna-Acosta, A.; Bado-Nilles, A.; Thomas-Guyon, H. Phenoloxidase activation in the embryo of the common cuttlefish Sepia officinalis and responses to the Ag and Cu exposure. Fish Shellfish Immunol. 2009, 27, 516–521. [Google Scholar] [CrossRef]
- Malham, S.K.; Runham, N.W. A brief review of the immunology of Eledone cirrhosa. Afr. J. Aquat. Sci. 1998, 20, 385–391. [Google Scholar] [CrossRef]
- Malham, S.K.; Runham, N.W.; Secombes, C.J. Lysozyme and antiprotease activity in the lesser octopus Eledone cirrhosa (Lam.) (Cephalopoda). Dev. Comp. Immunol. 1998, 22, 27–37. [Google Scholar] [CrossRef]
- Oellermann, M.; Strugnell, J.M.; Lieb, B.; Mark, F.C. Positive selection in octopus haemocyanin indicates functional links to temperature adaptation. BMC Evol. Biol. 2015, 15, 133. [Google Scholar] [CrossRef]
- Oellermann, M.; Lieb, B.; Portner, H.O.; Semmens, J.M.; Mark, F.C. Blue blood on ice: Modulated blood oxygen transport facilitates cold compensation and eurythermy in an Antarctic octopod. Front. Zool. 2015, 12, 6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aguila, J.; Cuzon, G.; Pascual, C.; Domingues, P.M.; Gaxiola, G.; Sanchez, A.; Maldonado, T.; Rosas, C. The effects of fish hydrolysate (CPSP) level on Octopus maya (Voss and Solis) diet: Digestive enzyme activity, blood metabolites, and energy balance. Aquaculture 2007, 273, 641–655. [Google Scholar] [CrossRef]
- Sieiro, P.; Otero, J.; Aubourg, S.P. Biochemical composition and energy strategy along the reproductive cycle of female Octopus vulgaris in Galician waters (NW Spain). Front. Physiol. 2020, 11, 760. [Google Scholar] [CrossRef] [PubMed]
- Hochachka, P.W.; Fields, J.H.A. Arginine, glutamine, and proline as substrates for oxidation and or glycogenesis in cephalopod tissues. Pac. Sci. 1982, 36, 325–335. [Google Scholar]
- Fernández-Alacid, L.; Sanahuja, I.; Ordóñez-Grande, B.; Sánchez-Nuño, S.; Viscor, G.; Gisbert, E.; Herrera, M.; Ibarz, A. Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Sci. Total Environ. 2018, 644, 1323–1335. [Google Scholar] [CrossRef]
- Baker, M.E. Steroid receptors and vertebrate evolution. Mol. Cell. Endocrinol. 2019, 496, 110526. [Google Scholar] [CrossRef]
- Chapman, D.D.; Frisk, M.J.; Abercrombie, D.L.; Safina, C.; Gruber, S.H.; Babcock, E.A.; Feldheim, K.A.; Pikitch, E.K.; Ward-Paige, C.; Davis, B.; et al. Give Shark Sanctuaries a Chance. Science 2013, 339, 757. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Amaro, S.; Ordines, F.; Esteban, A.; García, C.; Guijarro, B.; Salmerón, F.; Terrasa, B.; Massutí, E. The diversity of recent trends for chondrichthyans in the Mediterranean reflects fishing exploitation and a potential evolutionary pressure towards early maturation. Sci. Rep. 2020, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Talwar, B.; Brooks, E.J.; Mandelman, J.W.; Grubbs, R.D. Stress, post-release mortality, and recovery of commonly discarded deep-sea sharks caught on longlines. Mar. Ecol. Progr. Ser. 2017, 582, 147–161. [Google Scholar] [CrossRef]
- Lambert, F.N.; Treberg, J.R.; Anderson, W.G.; Brandt, C.; Evans, A.N. The physiological stress response of the Atlantic stingray (Hypanus sabinus) to aerial exposure. Comp. Biochem. Physiol. A 2018, 219–220, 38–43. [Google Scholar] [CrossRef]
- Shadwick, R.E.; Farrell, A.P.; Brauner, C. Physiology of Elasmobranch Fishes: Internal Processes; Academic Press: London, UK, 2015; Volume 34B, p. 580. [Google Scholar]
- Manire, C.; Hueter, R. Serological changes associated with gill-net capture and restraint in three species of sharks. Trans. Am. Fish. Soc. 2001, 130, 1038–1048. [Google Scholar] [CrossRef]
- Hazon, N.; Henderson, I.W. Secretory dynamics of 1 alpha-hydroxycorticosterone in the elasmobranch fish, Scyliorhinus canicula. J. Endocrinol. 1984, 103, 205–211. [Google Scholar] [CrossRef]
- Ballantyne, J.S. Jaws: The inside story. The metabolism of elasmobranch fishes. Comp. Biochem. Physiol. B 1997, 118, 703–742. [Google Scholar] [CrossRef]
- Martins, C.L.; Walker, T.I.; Reina, R.D. Stress-related physiological changes and post-release survival of elephant fish (Callorhinchus milii) after longlining, gillnetting, angling and handling in a controlled setting. Fish. Res. 2018, 204, 116–124. [Google Scholar] [CrossRef]
- Gorissen, M.; Flik, G. The endocrinology of the stress reponse in fish: An adaptation-physiological view. Fish Physiol. 2016, 35, 75–111. [Google Scholar] [CrossRef]
- Faught, E.; Aluru, N.; Vijayan, M.M. The molecular stress repsonse. Fish Physiol. 2016, 35, 113–166. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Gorissen, M.; Mancera, J.M.; Ruiz-Jarabo, I. What can we learn from glucocorticoid administration in fish? Effects of cortisol and dexamethasone on intermediary metabolism of gilthead seabream (Sparus aurata L.). Comp. Biochem. Physiol. A 2019, 231, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Todgham, A.E.; Ackerman, P.A.; Bibeau, M.R.; Nakano, K.; Schulte, P.M.; Iwama, G.K. Heat shock protein genes and their functional significance in fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Skrzynska, A.K.; Maiorano, E.; Bastaroli, M.; Naderi, F.; Miguez, J.M.; Martinez-Rodriguez, G.; Mancera, J.M.; Martos-Sitcha, J.A. Impact of air exposure on vasotocinergic and isotocinergic systems in gilthead sea bream (Sparus aurata): New insights on fish stress response. Front. Physiol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Fernández-Alacid, L.; Sanahuja, I.; Ordoñez-Grande, B.; Sánchez-Nuño, S.; Herrera, M.; Ibarz, A. Skin mucus metabolites and cortisol in meagre fed acute stress- attenuating diets: Correlations between plasma and mucus. Aquaculture 2019, 499, 185–194. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, F.A.; Cuesta, A.; Esteban, M.A. Using skin mucus to evaluate stress in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2016, 59, 323–330. [Google Scholar] [CrossRef]
- Acerete, L.; Reig, L.; Alvarez, D.; Flos, R.; Tort, L. Comparison of two stunning/slaughtering methods on stress response and quality indicators of European sea bass (Dicentrarchus labrax). Aquaculture 2009, 287, 139–144. [Google Scholar] [CrossRef]
- López-Luna, J.; Vásquez, L.; Torrent, F.; Villarroel, M. Short-term fasting and welfare prior to slaughter in rainbow trout, Oncorhynchus mykiss. Aquaculture 2013, 400–401, 142–147. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Fernández-Castro, M.; Alameda-López, M.; González-Manzano, G.; Mancera, J.M.; Ruiz-Jarabo, I. Transport and recovery of gilthead seabream (Sparus aurata L.) sedated with AQUI-S and etomidate: Effects on intermediary metabolism and osmoregulation. Aquaculture 2021. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Marín-Rincón, A.; Martínez-Rodríguez, G.; Mancera, J.M.; Ruiz-Jarabo, I. A natural additive in the diet to improve growth and reduce energy expenditure of gilthead seabream (Sparus aurata L.): Attenuation of high stocking density stress responses. Aquaculture 2020, 524, 735263. [Google Scholar] [CrossRef]
- Boerrigter, J.G.; Manuel, R.; van den Bos, R.; Roques, J.A.; Spanings, T.; Flik, G.; van de Vis, H.W. Recovery from transportation by road of farmed European eel (Anguilla anguilla). Aquac. Res. 2013, 46, 1248–1260. [Google Scholar] [CrossRef]
- Manuel, R.; Boerrigter, J.; Roques, J.; van der Heul, J.; van den Bos, R.; Flik, G.; van de Vis, H. Stress in African catfish (Clarias gariepinus) following overland transportation. Fish Physiol. Biochem. 2014, 40, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.A.; Jerez-Cepa, I.; Cárcamo, C.B.; Toledo, P.; Flores, H.; Brokordt, K. Growth performance, physiological responses to hypoxia and flesh quality of Chilean croaker (Cilus gilberti) stocked at different densities. Aquaculture 2020, 525, 735316. [Google Scholar] [CrossRef]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Perelló, M.; Salmerón, C.; Riera-Codina, M.; Ibarz, A.; Fernández-Borrás, J.; Blasco, J.; Capilla, E.; et al. Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture 2017, 467, 28–40. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, L.; Liu, B.S.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Jiang, S.G.; Zhang, D.C. Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2020, 518, 734841. [Google Scholar] [CrossRef]
- Jia, R.; Liu, B.L.; Feng, W.R.; Han, C.; Huang, B.; Lei, J.L. Stress and immune responses in skin of turbot (Scophthalmus maximus) under different stocking densities. Fish Shellfish Immunol. 2016, 55, 131–139. [Google Scholar] [CrossRef]
- Calabrese, S.; Nilsen, T.O.; Kolarevic, J.; Ebbersson, L.O.E.; Pedrosa, C.; Fivelstad, S.; Hosfeld, C.; Stefansson, S.O.; Terjesen, B.F.; Takle, H.; et al. Stocking density limits for post-smolt Atlantic salmon (Salmo salar L.) with emphasis on production performance and welfare. Aquaculture 2017, 468, 363–370. [Google Scholar] [CrossRef]
- Wilson, S.M.; Raby, G.D.; Burnett, N.J.; Hinch, S.G.; Cooke, S.J. Looking beyond the mortality of bycatch: Sublethal effects of incidental capture on marine animals. Biol. Conserv. 2014, 171, 61–72. [Google Scholar] [CrossRef]
- Arkert, N.K.; Childs, A.R.; Duncan, M.I.; Farthing, M.; Potts, W.M. Physiological stress response and recovery of an important estuarine fishery species, dusky kob Argyrosomus japonicus, after a simulated catch-and-release event. Afr. J. Mar. Sci. 2020, 42, 339–345. [Google Scholar] [CrossRef]
- Tracey, S.R.; Hartmann, K.; Leef, M.; McAllister, J. Capture-induced physiological stress and postrelease mortality for Southern bluefin tuna (Thunnus maccoyii) from a recreational fishery. Can. J. Fish. Aquat. Sci. 2016, 73, 1547–1556. [Google Scholar] [CrossRef]
- Schlenker, L.S.; Latour, R.J.; Brill, R.W.; Graves, J.E. Physiological stress and post-release mortality of white marlin (Kajikia albida) caught in the United States recreational fishery. Conserv. Physiol. 2016, 4, cov066. [Google Scholar] [CrossRef] [PubMed]
- Anders, N.; Eide, I.; Lerfall, J.; Roth, B.; Breen, M. Physiological and flesh quality consequences of pre-mortem crowding stress in Atlantic mackerel (Scomber scombrus). PLoS ONE 2020, 15, e0228454. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.H.; Tobiassen, T.; Akse, L.; Evensen, T.H.; Migling, K.O. Capture induced stress and live storage of Atlantic cod (Gadus morhua) caught by trawl: Consequences for the flesh quality. Fish. Res. 2013, 147, 446–453. [Google Scholar] [CrossRef]
- Anderson, P.A.; Berzins, I.K.; Fogarty, F.; Hamlin, H.J.; Guillette, L.J. Sound, stress, and seahorses: The consequences of a noisy environment to animal health. Aquaculture 2011, 311, 129–138. [Google Scholar] [CrossRef]
- Sopinka, N.M.; Donaldson, M.R.; O´Connor, C.M.; Suski, C.D.; Cooke, S.J. Stress indicators in fish. Fish Biol. 2016, 35, 405–462. [Google Scholar] [CrossRef]
- Hoem, K.S.; Tveten, A.K. Current approaches in decoding the molecular mechanisms of long-term stress in adult farmed Atlantic salmon (Salmo salar). Rev. Aquac. 2019, 12, 1708–1720. [Google Scholar] [CrossRef]
- Magalhaes, C.S.; Cerqueira, M.A.; Schrama, D.; Moreira, M.J.; Boonanuntanasarn, S.; Rodrigues, P.M. A Proteomics and other Omics approach in the context of farmed fish welfare and biomarker discovery. Rev. Aquac. 2018, 12, 122–144. [Google Scholar] [CrossRef]
- Brinkmann, H.; Denk, A.; Zitzler, J.; Joss, J.J.; Meyer, A. Complete mitochondrial genome sequences of the South american and the Australian lungfish: Testing of the phylogenetic performance of mitochondrial data sets for phylogenetic problems in tetrapod relationships. J. Mol. Evol. 2004, 59, 834–848. [Google Scholar] [CrossRef]
- Idler, D.R.; Sangalang, G.B.; Truscott, B. Corticosteroids in the South American lungfish. Gen. Comp. Endocrinol. 1972, 3, 238–244. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Morgan, T.P.; Galvez, F.; Smith, R.W.; Kajimura, M.; Ip, Y.K.; Wood, C.M. The African lungfish (Protopterus dolloi): Ionoregulation and osmoregulation in a fish out of water. Physiol. Biochem. Zool. 2007, 80, 99–112. [Google Scholar] [CrossRef] [PubMed]
- da Silva, G.S.F.; Giusti, H.; Sanchez, A.P.; Carmo, J.M.; Glass, M.L. Aestivation in the South American lungfish, Lepidosiren paradoxa: Effects on cardiovascular function, blood gases, osmolality and leptin levels. Respir. Physiol. Neurobiol. 2008, 164, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Konno, N.; Shibuya, S.; Nogami, S. Cloning and expression of the epithelial sodium channel and its role in osmoregulation of aquatic and estivating African lungfish Protopterus annectens. Comp. Biochem. Physiol. B 2015, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Frick, N.T.; Bystriansky, J.S.; Ip, Y.K.; Chew, S.F.; Ballantyne, J.S. Lipid, ketone body and oxidative metabolism in the African lungfish, Protopterus dolloi following 60 days of fasting and aestivation. Comp. Biochem. Physiol. A 2008, 151, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Frick, N.T.; Bystriansky, J.S.; Ip, Y.K.; Chew, S.F.; Ballantyne, J.S. Carbohydrate and amino acid metabolism in fasting and aestivating African lungfish (Protopterus dolloi). Comp. Biochem. Physiol. A 2008, 151, 85–92. [Google Scholar] [CrossRef]
- Aklakur, M. Natural antioxidants from sea: A potential industrial perspective in aquafeed formulation. Rev. Aquac. 2016, 10, 385–399. [Google Scholar] [CrossRef]
- Herrera, M.; Mancera, J.M.; Costas, B. The Use of Dietary Additives in Fish Stress Mitigation: Comparative Endocrine and Physiological Responses. Front. Endocrinol. 2019, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential Oils as Stress-Reducing Agents for Fish Aquaculture: A Review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Fernández-Castro, M.; del Santo, T.J.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Mancera, J.M.; Ruiz-Jarabo, I. Transport and recovery of gilthead seabream (Sparus aurata L.) sedated with clove oil and MS-222: Effects on stress axis regulation and intermediary metabolism. Front. Physiol. 2019, 10, 612. [Google Scholar] [CrossRef]
- Teles, M.; Oliveira, M.; Jerez-Cepa, I.; Franco-Martinez, L.; Tvarijonaviciute, A.; Tort, L.; Mancera, J.M. Transport and Recovery of Gilthead Sea Bream (Sparus aurata L.) Sedated With Clove Oil and MS222: Effects on Oxidative Stress Status. Front. Physiol. 2019, 10, 523. [Google Scholar] [CrossRef]
| System | Parameters | References 1 |
|---|---|---|
| Acid-base balance | H+, OH−, HCO3−, PO42−, SO42− | [24,25] |
| Hydric-ionic balance | H2O, osmolality, Na+, Cl−, K+, Ca2+, Mg2+, others | [26,27,28,29] |
| O2 (CO2) transport | Hemoglobin/hemocyanin, hematocrit | [30,31,32] |
| Energy management | Glucose, lactate, amino acids, triglycerides, free fatty acids, etc. | [33,34,35] |
| Immune system (Innate) | Physical barriers, cell-mediated defense (phagocytosis), humoral defense (antimicrobial enzymes, non-specific proteins, complement system), inflammation | [36,37,38,39,40,41,42] |
| Immune system (Adaptive) 2 | Cell-mediated defense (B- and T-lymphocytes) | [38] |
| Free radicals balance | Oxidative stress system | [43,44,45,46] |
| Others | Hormones, temperature, etc. | [43,47] |
| Taxonomic Group | Parameters | References 1 |
|---|---|---|
| Crustaceans | Crustacean hyperglycemic hormone (CHH). | [75,76,78] |
| Hemolymph pH, hemocyanin, glucose, lactate. | [55,57,79,80,81] | |
| Innate immune parameters (granulocytes, proPO, peroxidase or lysozyme activities). | [42,57] | |
| Cephalopods | Neuroendocrine factors (noradrenaline, dopamine). | [51] |
| Innate immune parameters (PO-like, proteases, antiproteases, peroxidase or lysozyme activities). | [31,86,87,88] | |
| Hemolymph pH, hemocyanin. | [31,89,90,91] | |
| Glucose, glycogen, amino acids, NOT lactate. | [31,33,92,93] | |
| Dermal mucus parameters (glucose, lactate, pH). | [69,94] | |
| Elasmobranchs | Catecholamines (adrenaline and noradrenaline). | [100] |
| Corticosteroids (1α-hydroxycorticosterone). | [53] | |
| Plasma pH, osmolality, ions, energy metabolites. | [53,60,101,102] | |
| Muscle amino acids and carbohydrates (glycogen). | [103] | |
| Teleosts | Neuroendocrine factors (CRH, TRH, POMCs, etc.). | [114,121] |
| Plasma catecholamines and cortisol. | [21,50,61,107,109] | |
| Cortisol in gills, scales or feces. | [111] | |
| Acid-Base balance. | [66,115] | |
| Hydro-mineral imbalances. | [128] | |
| Plasma hematocrit and energy metabolites. | [110,126,127] | |
| Plasma oxidative stress (CAT, SOD, GR, GST, etc.). | [122] | |
| Cellular parameters (Hsp70) in brain, liver and kidney. | [108,122] | |
| Mucus cortisol and energy metabolites. | [110] | |
| Plasma and skin mucus innate immune parameters. | [112,113,122,123] | |
| Intermediary metabolism in liver and muscle. | [116,117,118,119] | |
| Growth rate, condition index, hepatosomatic index. | [106,117,120] | |
| Dipnoans | Glucocorticoids (cortisol, corticosterone, cortisone, 11-deoxycortisol, 11-deoxycorticosterone and 11-dehydrocorticosterone) 2. | [136] |
| Mineralocorticoids (aldosterone) 2. | [136,139] | |
| Ions, carbohydrates and amino acids. | [139,140,141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerez-Cepa, I.; Ruiz-Jarabo, I. Physiology: An Important Tool to Assess the Welfare of Aquatic Animals. Biology 2021, 10, 61. https://doi.org/10.3390/biology10010061
Jerez-Cepa I, Ruiz-Jarabo I. Physiology: An Important Tool to Assess the Welfare of Aquatic Animals. Biology. 2021; 10(1):61. https://doi.org/10.3390/biology10010061
Chicago/Turabian StyleJerez-Cepa, Ismael, and Ignacio Ruiz-Jarabo. 2021. "Physiology: An Important Tool to Assess the Welfare of Aquatic Animals" Biology 10, no. 1: 61. https://doi.org/10.3390/biology10010061
APA StyleJerez-Cepa, I., & Ruiz-Jarabo, I. (2021). Physiology: An Important Tool to Assess the Welfare of Aquatic Animals. Biology, 10(1), 61. https://doi.org/10.3390/biology10010061






