Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Conditions, Salinity Treatments and Experimental Design
2.3. Measurements
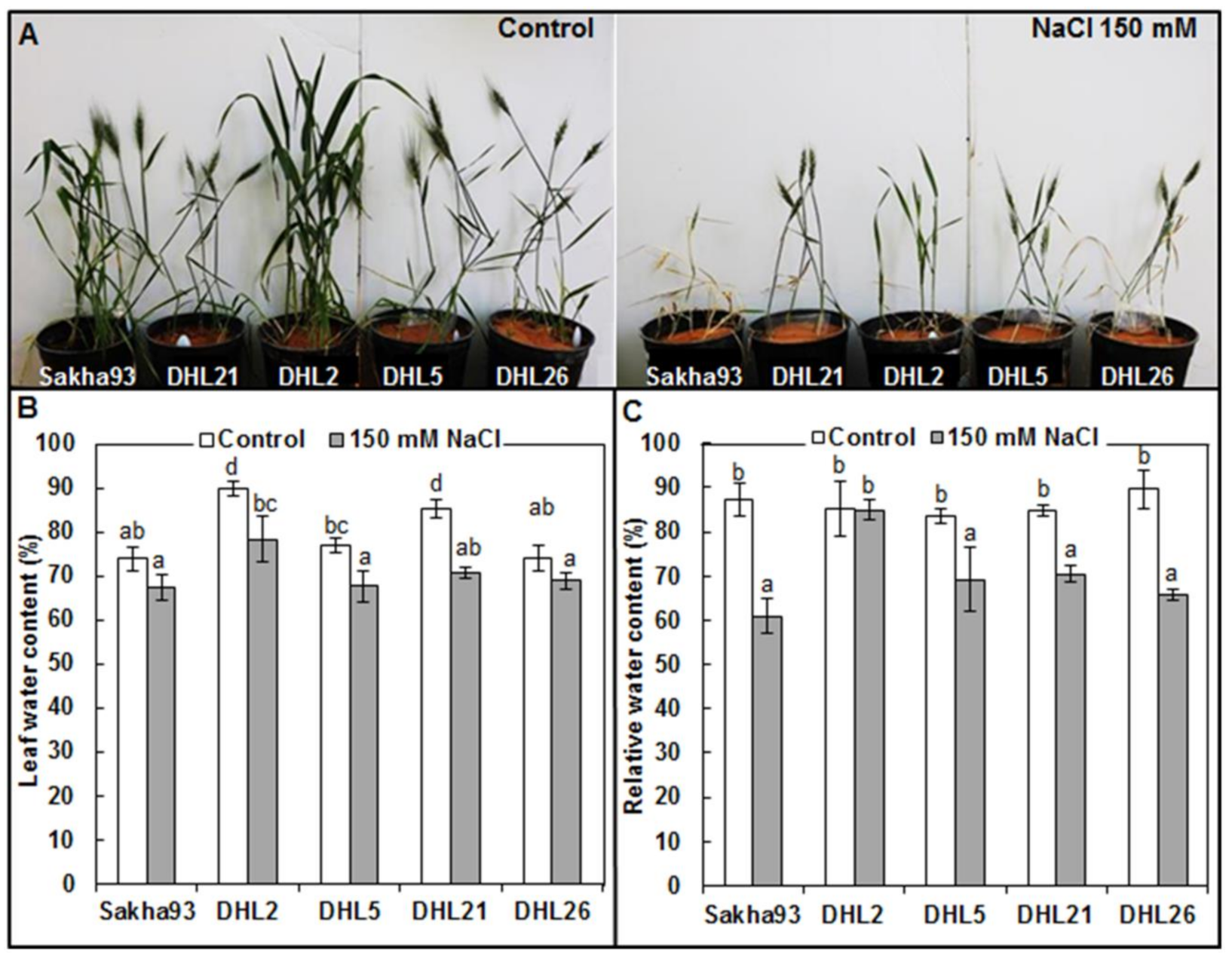
2.3.1. Leaf Water Status Parameters
2.3.2. Chlorophyll and Leaf Pigment Content Parameters
2.3.3. Flag Leaf Parameters
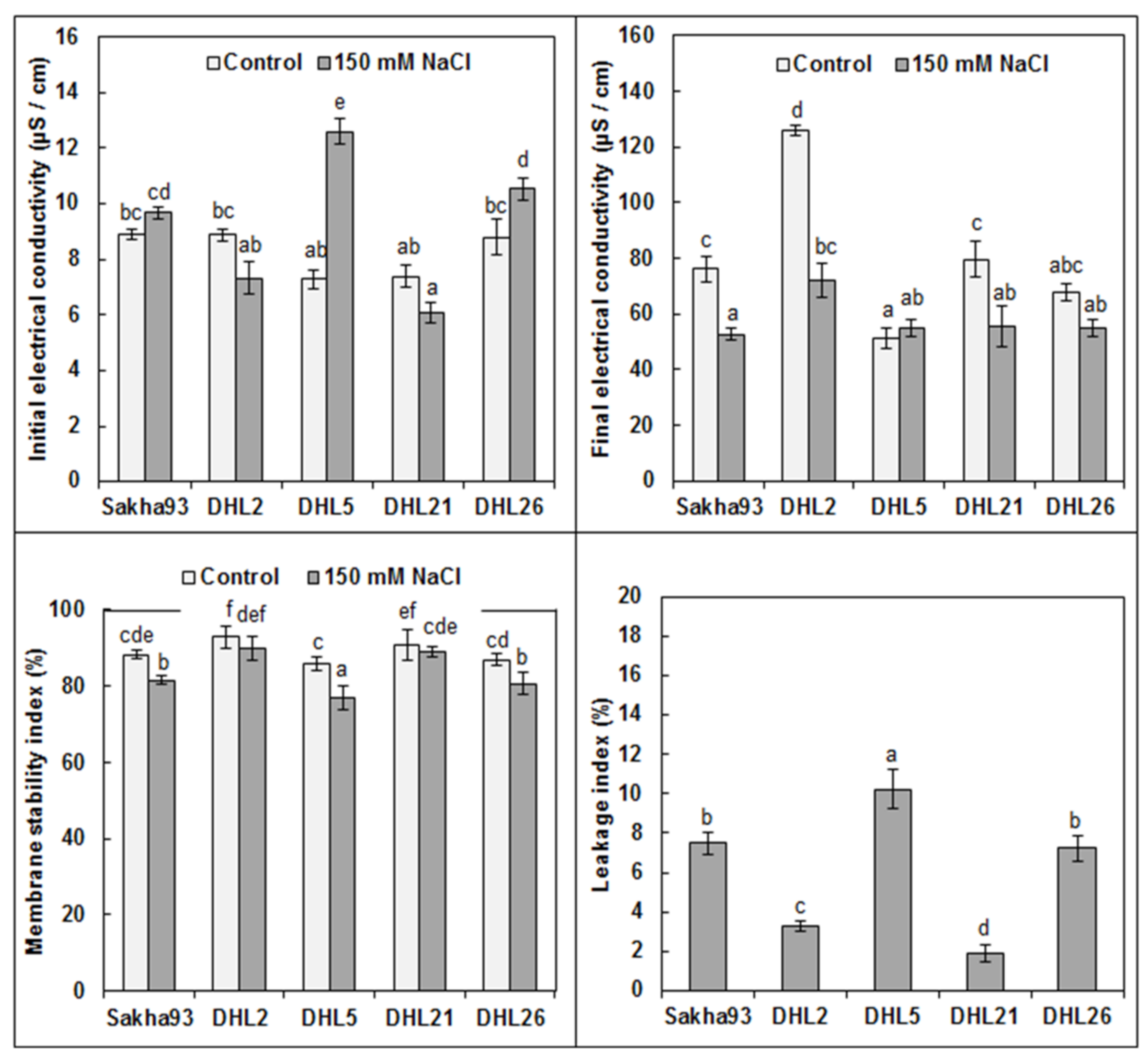
2.3.4. Membrane Injury Parameters
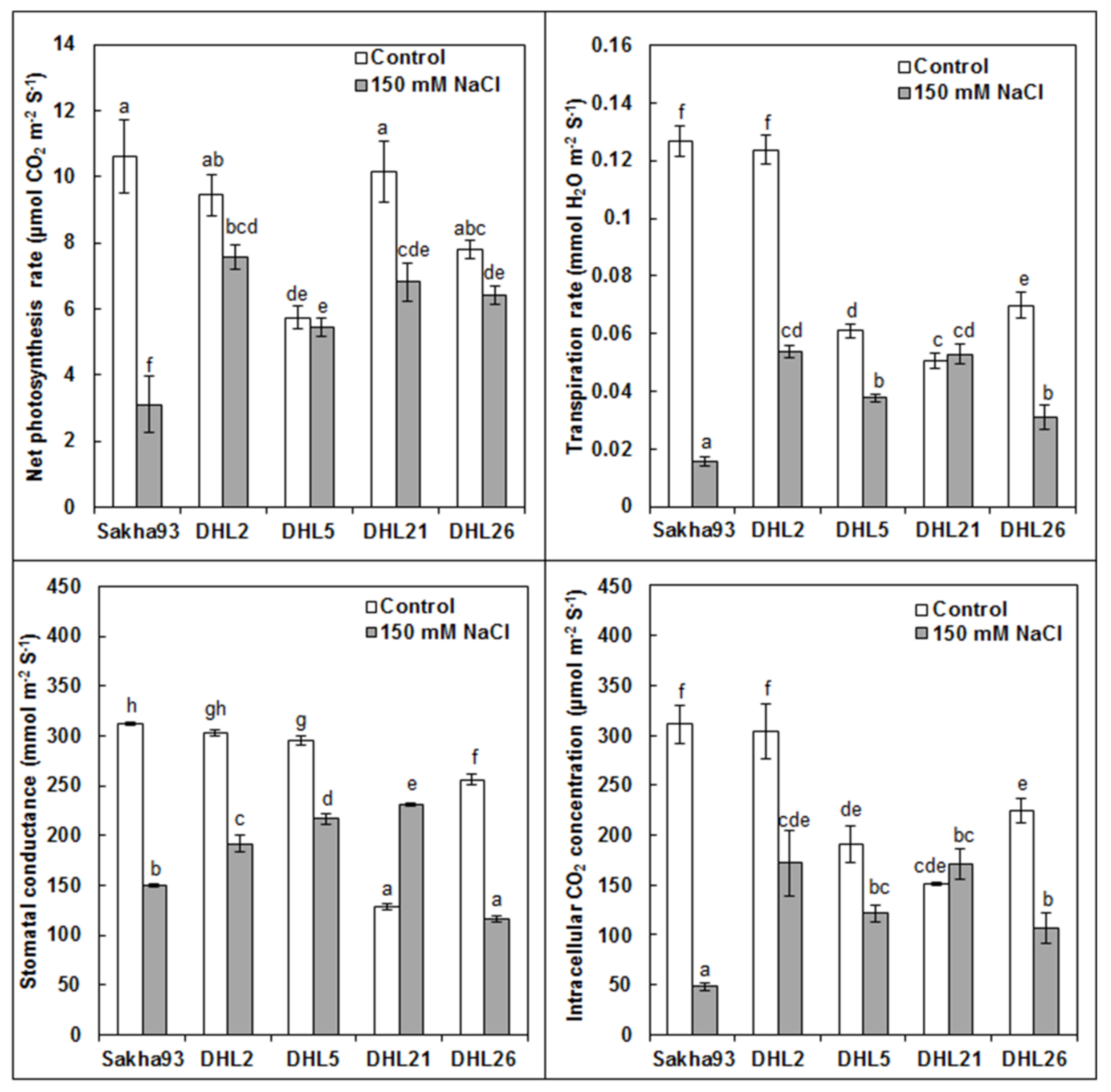
2.3.5. Photosynthetic Parameters
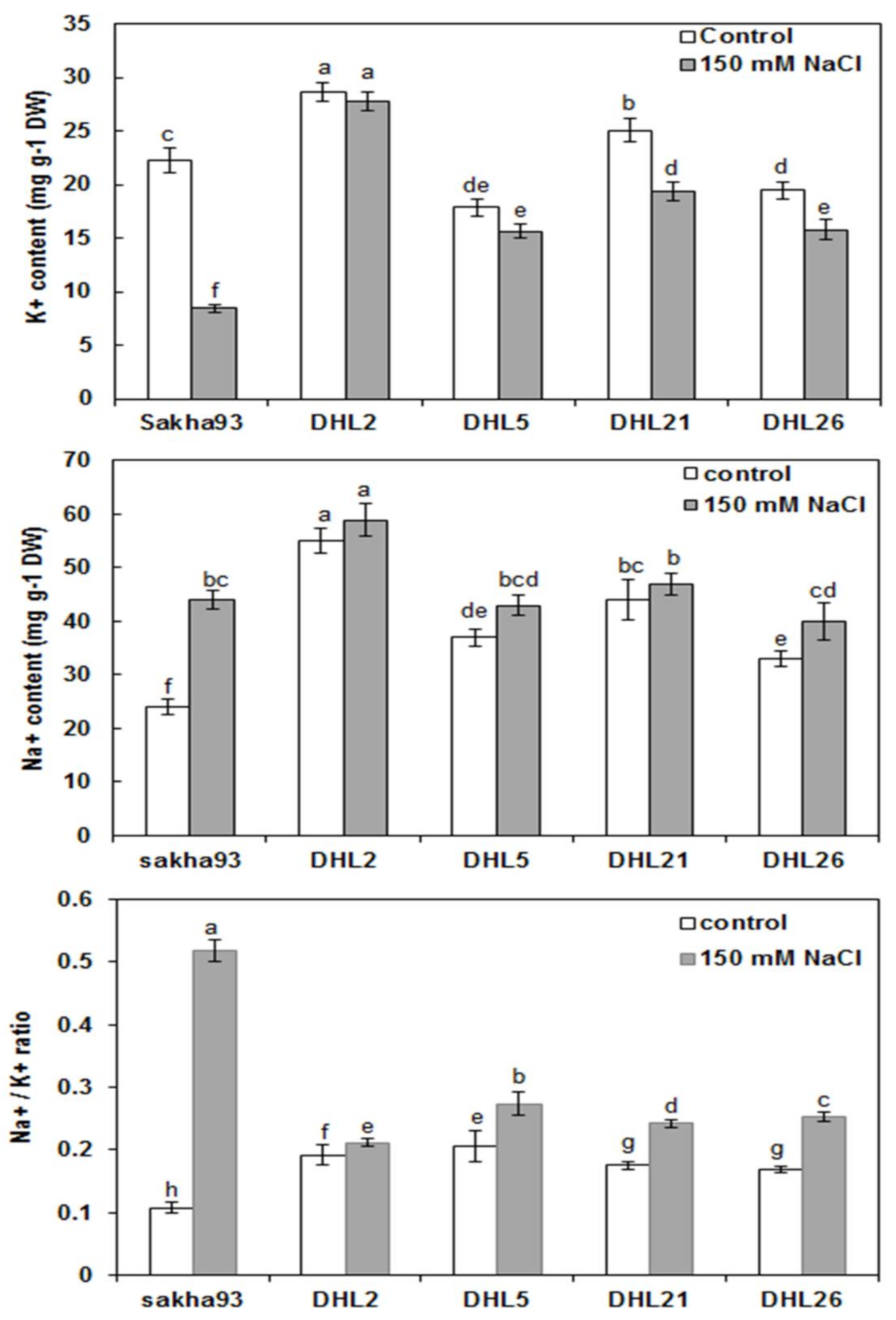
2.3.6. Ion Concentrations
2.3.7. Yield and Yield Components Parameters
2.4. Stress-Related Gene Expression Profiling
2.5. Statistical Analysis
3. Results
3.1. Comparative Performance of Agro-Physiologic Parameters
3.1.1. Leaf Water Status Parameters
3.1.2. Chlorophyll and Leaf Pigment Content Parameters
3.1.3. Membrane Injury Parameters
3.1.4. Photosynthetic Parameters
3.1.5. Flag Leaf Parameters
3.1.6. Shoot Ion Concentrations
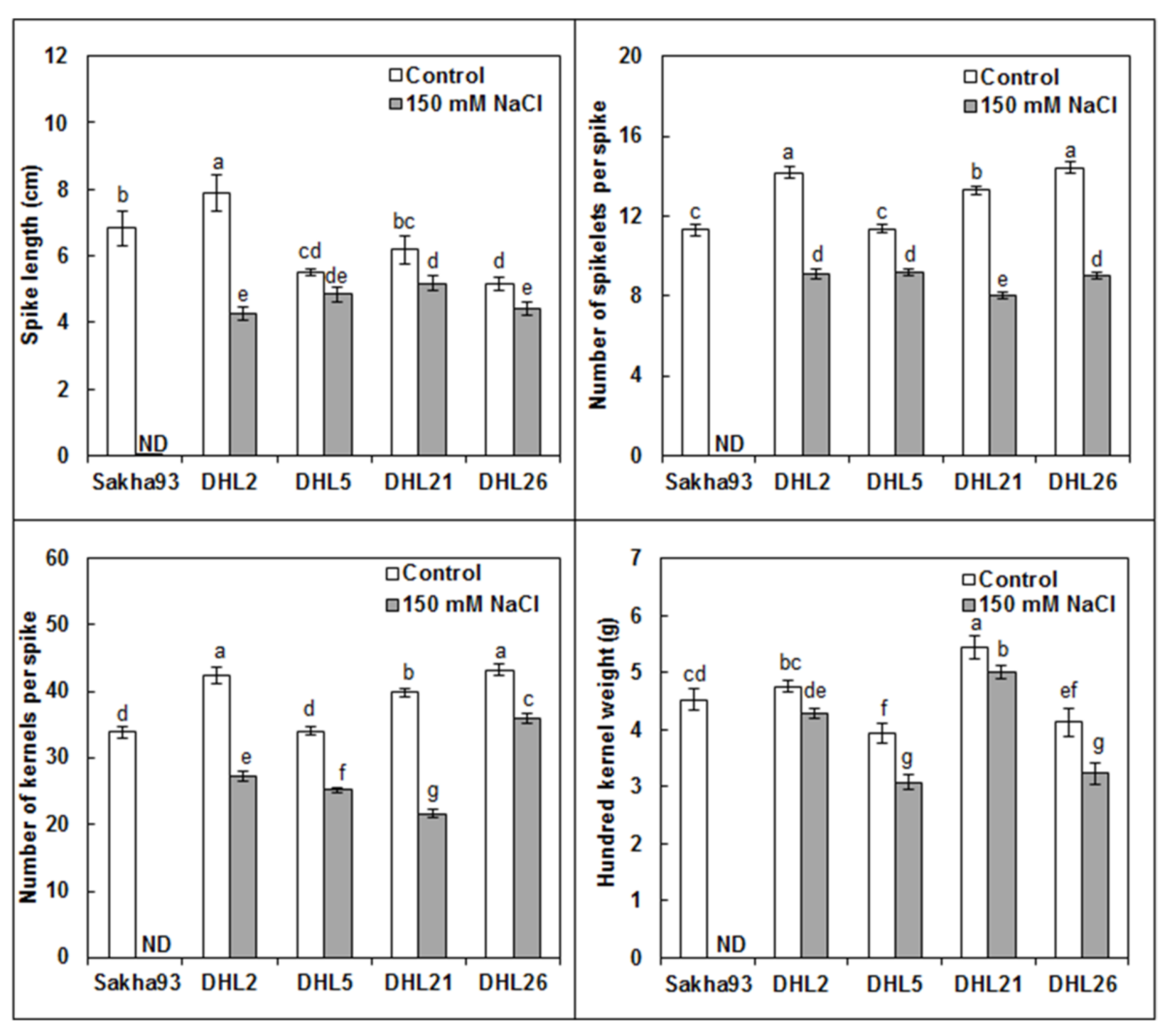
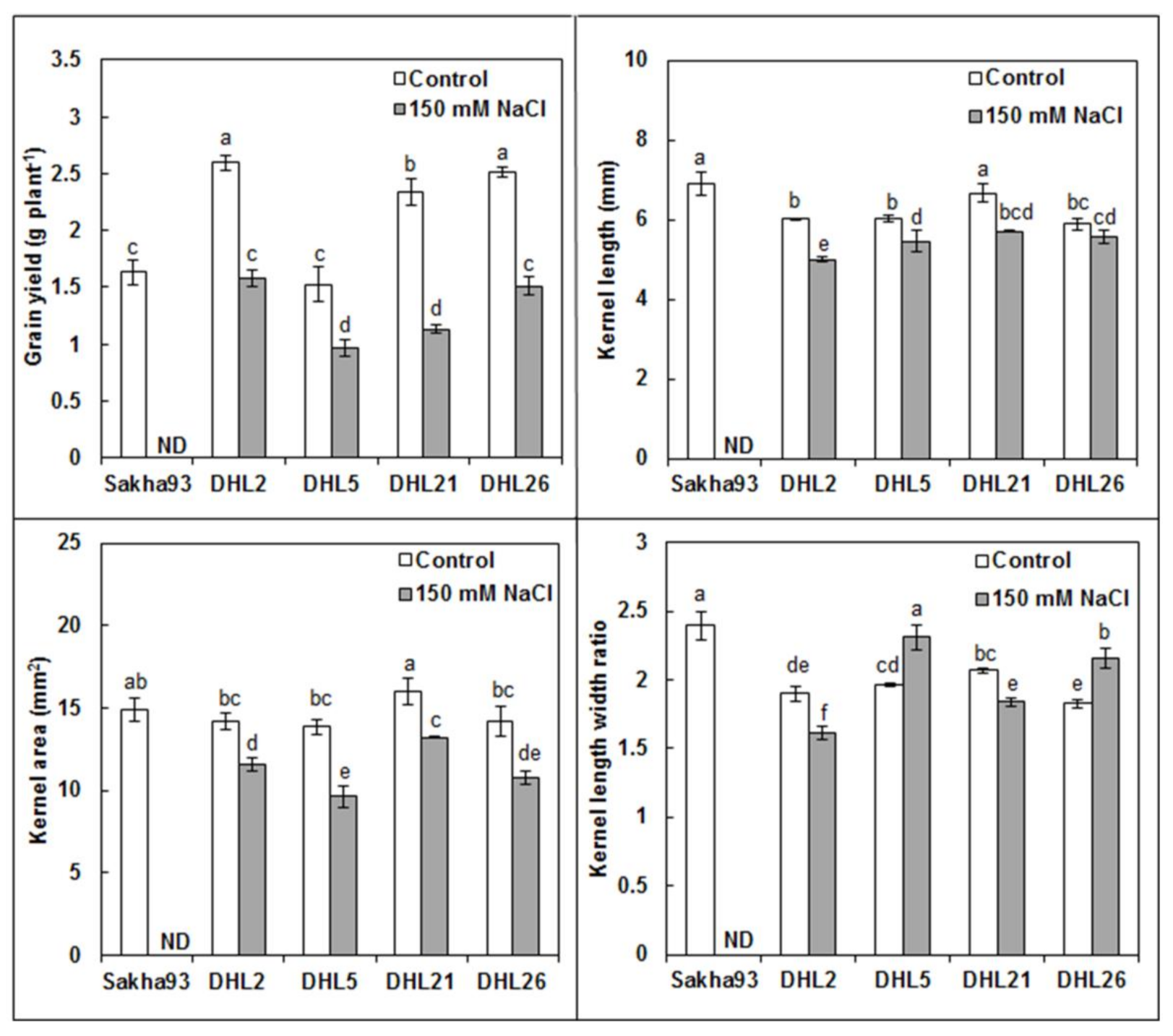
3.1.7. Yield and Yield Component Parameters
3.2. Expression Analysis of Related Genes under Salt Stress Conditions
3.3. Relationships between Measured Traits and Multivariate Analysis
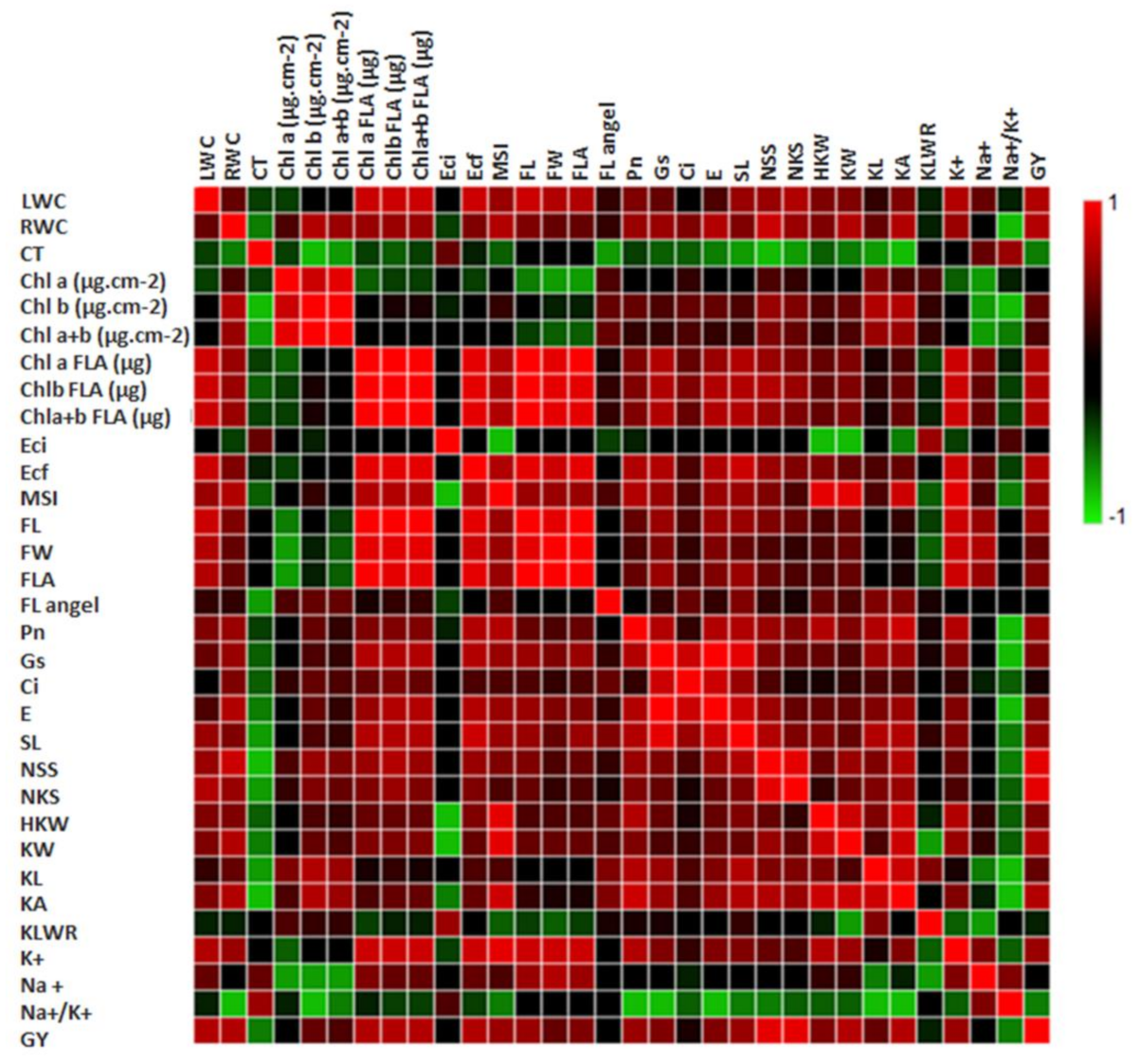
3.3.1. Relationships between Different Measured Traits
3.3.2. PCA of Different Measured Traits
3.3.3. Multiple Linear Regression and Path Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rybka, K.; Janaszek-Mańkowska, M.; Siedlarz, P.; Mańkowski, D.R. Machine learning in determination of water saturation deficit in wheat leaves on basis of Chl a fluorescence parameters. Photosynthetica 2019, 57, 226–230. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alotaibi, M.; Refay, Y.; Ghazy, A.; Zakri, A.; Al-Doss, A. Selection criteria for high-yielding and early-flowering bread wheat hybrids under heat stress. PLoS ONE 2020, 15, e0236351. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mantovani, E.E.; Simsek, S.; Jain, S.; Elias, E.M.; Mergoum, M. Genome wide genetic dissection of wheat quality and yield related traits and their relationship with grain shape and size traits in an elite × non-adapted bread wheat cross. PLoS ONE 2019, 14, e0221826. [Google Scholar] [CrossRef] [PubMed]
- Rybka, K.; Nita, Z. Physiological requirements for wheat ideotypes in response to drought threat. Acta Physiol. Plant. 2015, 37, 1–13. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; El-Hendawy, S.; Al-Suhaibani, N.; El-Kafafi, S.; Seleiman, M.F. Detecting Salt Tolerance in Doubled Haploid Wheat Lines. Agronomy 2019, 9, 211. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Royo, C.; Serret, M.D. Breeding for Yield Potential and Stress Adaptation in Cereals. Crit. Rev. Plant Sci. 2008, 27, 377–412. [Google Scholar] [CrossRef]
- Medina, S.; Vicente, R.; Nieto-Taladriz, M.T.; Aparicio, N.; Chairi, F.; Vergara-Diaz, O.; Araus, J.L. The Plant-Transpiration Response to Vapor Pressure Deficit (VPD) in Durum Wheat Is Associated With Differential Yield Performance and Specific Expression of Genes Involved in Primary Metabolism and Water Transport. Front. Plant Sci. 2019, 9, 1994. [Google Scholar] [CrossRef]
- Al-Ashkar, I.; Alderfasi, A.; Ben Romdhane, W.; Seleiman, M.F.; El-Said, R.A.; Al-Doss, A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Maatougui, M.; Michael, M.; Slash, M.; Haghparast, R.; Rahmanian, M.; Taheri, A.; Al-Yassin, A.; Benbelkacem, A.; et al. Plant breeding and climate changes. J. Agric. Sci. 2010, 148, 627–637. [Google Scholar] [CrossRef]
- Ben Rejeb, I.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef]
- Zhu, J. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Arzani, A. Improving salinity tolerance in crop plants: A biotechnological view. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 373–383. [Google Scholar] [CrossRef]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shokat, S.; Großkinsky, D.K. Tackling Salinity in Sustainable Agriculture—What Developing Countries May Learn from Approaches of the Developed World. Sustainability 2019, 11, 4558. [Google Scholar] [CrossRef]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant Responses to Salt Stress: Adaptive Mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Almeida, P.; Katschnig, D.; De Boer, A.H. HKT Transporters—State of the Art. Int. J. Mol. Sci. 2013, 14, 20359–20385. [Google Scholar] [CrossRef]
- Platten, J.D.; Cotsaftis, O.; Berthomieu, P.; Bohnert, H.; Davenport, R.J.; Fairbairn, D.J.; Horie, T.; Leigh, R.A.; Lin, H.-X.; Luan, S.; et al. Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci. 2006, 11, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Byrt, C.S.; Xu, B.; Krishnan, M.; Lightfoot, D.J.; Athman, A.; Jacobs, A.K.; Watson-Haigh, N.S.; Plett, D.; Munns, R.; Tester, M.; et al. The Na+ transporter, TaHKT1;5-D, limits shoot Na+accumulation in bread wheat. Plant J. 2014, 80, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhushan, B.; Gaikwad, K.; Yadav, O.P.; Kumar, S.; Rai, R.D. Induced defence responses of contrasting bread wheat genotypes under differential salt stress imposition. Indian J. Biochem. Biophys. 2015, 52, 75–85. [Google Scholar] [PubMed]
- Sathee, L.; Sairam, R.K.; Chinnusamy, V.; Jha, S.K. Differential transcript abundance of salt overly sensitive (SOS) pathway genes is a determinant of salinity stress tolerance of wheat. Acta Physiol. Plant. 2015, 37, 1–10. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Zwart, A.B.; Hare, R.A.; Rathjen, A.J.; Munns, R.E. Impact of ancestral wheat sodium exclusion genes Nax1 and Nax2 on grain yield of durum wheat on saline soils. Funct. Plant Biol. 2012, 39, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The Roles of Environmental Factors in Regulation of Oxidative Stress in Plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Dadshani, S.; Sharma, R.C.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Multi-dimensional evaluation of response to salt stress in wheat. PLoS ONE 2019, 14, e0222659. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Nahar, K.; Fujita, M. Exogenous Proline and Glycine Betaine Mediated Upregulation of Antioxidant Defense and Glyoxalase Systems Provides Better Protection against Salt-Induced Oxidative Stress in Two Rice (Oryza sativa L.) Varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M. Antioxidant Activity and Osmolyte Concentration of Sorghum (Sorghum bicolor) and Wheat (Triticum aestivum) Genotypes under Salinity Stress. Asian J. Plant Sci. 2009, 8, 240–244. [Google Scholar] [CrossRef]
- Barakat, N.A. Oxidative stress markers and antioxidant potential of wheat treated with phytohormones under salinity stress. J. Stress Physiol. Biochem. 2011, 7, 250–267. [Google Scholar]
- Sairam, R.K.; Rao, K.; Srivastava, G. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Mason, R.E.; Singh, R.P. Considerations When Deploying Canopy Temperature to Select High Yielding Wheat Breeding Lines under Drought and Heat Stress. Agronomy 2014, 4, 191–201. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; Rattey, A.R.; Farquhar, G.D.; Richards, R.A.; Condon, A.G. Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in wheat. Funct. Plant Biol. 2013, 40, 14–33. [Google Scholar] [CrossRef]
- Reynolds, M.; Pierre, C.S.; Saad, A.S.; Vargas, M.; Condon, A.G. Evaluating Potential Genetic Gains in Wheat Associated with Stress-Adaptive Trait Expression in Elite Genetic Resources under Drought and Heat Stress. Crop. Sci. 2007, 47, S-172. [Google Scholar] [CrossRef]
- Gautam, A.; Prasad, S.V.S.; Jajoo, A.; Ambati, D. Canopy Temperature as a Selection Parameter for Grain Yield and Its Components in Durum Wheat Under Terminal Heat Stress in Late Sown Conditions. Agric. Res. 2015, 4, 238–244. [Google Scholar] [CrossRef]
- Bahar, B.; Yildirim, M.; Barutcular, C.; Ibrahim, G. Effect of canopy temperature depression on grain yield and yield components in bread and durum wheat. Not. Bot. Horti Agrobot. Cluj-Napoca 2008, 36, 34–37. [Google Scholar]
- Ficklin, S.P.; Feltus, F.A. A Systems-Genetics Approach and Data Mining Tool to Assist in the Discovery of Genes Underlying Complex Traits in Oryza sativa. PLoS ONE 2013, 8, e68551. [Google Scholar] [CrossRef]
- Ball, S.T.; Konzak, C.F. Relationship between Grain Yield and Remotely-Sensed Data in Wheat Breeding Experiments. Plant Breed. 1993, 110, 277–282. [Google Scholar] [CrossRef]
- Urrea-Gómez, R.; Ceballos, H.; Pandey, S.; Filho, A.F.C.B.; León, L.A. A Greenhouse Screening Technique for Acid Soil Tolerance in Maize. Agron. J. 1996, 88, 806–812. [Google Scholar] [CrossRef]
- Grzesiak, S.; Hordyńska, N.; Szczyrek, P.; Grzesiak, M.T.; Noga, A.; Szechyńska-Hebda, M. Variation among wheat (Triticum easativum L.) genotypes in response to the drought stress: I—Selection approaches. J. Plant Interact. 2018, 14, 30–44. [Google Scholar] [CrossRef]
- Raja, S.; Shokat, S.; Azhar, F.M.; Azhar, M.T.; Khan, A.A. Screening of tomato (Solanum lycopersicum L.) genotypes at different salinity levels. J. Plant Breed. Crop Sci. 2012, 4, 94–100. [Google Scholar]
- Chakraborty, K.; Mondal, S.; Ray, S.; Samal, P.; Pradhan, B.; Chattopadhyay, K.; Kar, M.K.; Swain, P.; Sarkar, R.K. Tissue Tolerance Coupled With Ionic Discrimination Can Potentially Minimize the Energy Cost of Salinity Tolerance in Rice. Front. Plant Sci. 2020, 11, 265. [Google Scholar] [CrossRef]
- Zafar, S.A.; Shokat, S.; Ahmed, H.G.M.-D.; Khan, A.; Ali, M.Z.; Atif, R.M. Assessment of salinity tolerance in rice using seedling based morpho-physiological indices. Adv. Life Sci. 2015, 2, 142–149. [Google Scholar]
- El-Hendawy, S.; Ruan, Y.; Hu, Y.; Schmidhalter, U. A Comparison of Screening Criteria for Salt Tolerance in Wheat under Field and Controlled Environmental Conditions. J. Agron. Crop. Sci. 2009, 195, 356–367. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Hassan, W.M.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative Performance of Multivariable Agro-Physiological Parameters for Detecting Salt Tolerance of Wheat Cultivars under Simulated Saline Field Growing Conditions. Front. Plant Sci. 2017, 8, 435. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Al-Ashkar, I.; Alotaibi, M.; Tahir, M.U.; Solieman, T.; Hassan, W.M. Combining Genetic Analysis and Multivariate Modeling to Evaluate Spectral Reflectance Indices as Indirect Selection Tools in Wheat Breeding under Water Deficit Stress Conditions. Remote. Sens. 2020, 12, 1480. [Google Scholar] [CrossRef]
- El-Hennawy, M.; Abdalla, A.; Shafey, S.; Al-Ashkar, I. Production of doubled haploid wheat lines (Triticum aestivum L.) using anther culture technique. Ann. Agric. Sci. 2011, 56, 63–72. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley and Sons, Inc.: New York, NY, USA, London, UK, Sydney, Australia, Toronto, ON, Canada, 1972. [Google Scholar]
- Clarke, J.M.; McCaig, T.N. Excised-Leaf Water Retention Capability as an Indicator of Drought Resistance of Triticum GenoTypes. Can. J. Plant Sci. 1982, 62, 571–578. [Google Scholar] [CrossRef]
- Barrs, H. Determination of water deficits in plant tissues. Water Deficit Plant Growth 1968, 1, 235–368. [Google Scholar]
- Armon, D. Copper enzymes in isolated chloroplast. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ben-Romdhane, W.; Ben-Saad, R.; Meynard, D.; Zouari, N.; Mahjoub, A.; Fki, L.; Guiderdoni, E.; Al-Doss, A.; Hassairi, A. Overexpression of AlTMP2 gene from the halophyte grass Aeluropus littoralis in transgenic tobacco enhances tolerance to different abiotic stresses by improving membrane stability and deregulating some stress-related genes. Protoplasma 2018, 255, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Toderich, K.; Shuyskaya, E.V.; Rakhmankulova, Z.F.; Bukarev, R.; Khujanazarov, T.; Zhapaev, R.; Ismail, S.; Gupta, S.K.; Yamanaka, N.; Boboev, F. Threshold Tolerance of New Genotypes of Pennisetum glaucum (L.) R. Br. to Salinity and Drought. Agronomy 2018, 8, 230. [Google Scholar] [CrossRef]
- Rubio, F.; Nieves-Cordones, M.; Horie, T.; Shabala, S. Doing ‘business as usual’comes with a cost: Evaluating energy cost of maintaining plant intracellular K+ homeostasis under saline conditions. New Phytol. 2020, 225, 1097–1104. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Steudle, E. Water uptake by plant roots: An integration of views. Plant Soil 2000, 226, 45–56. [Google Scholar] [CrossRef]
- Hussain, M.I.; Dakheel, A.J.A.; Reigosa, M.J. Genotypic differences in agro-physiological, biochemical and isotopic responses to salinity stress in quinoa (Chenopodium quinoa Willd.) plants: Prospects for salinity tolerance and yield stability. Plant Physiol. Biochem. 2018, 129, 411–420. [Google Scholar] [CrossRef]
- Reynolds, M.; Pask, A.; Mullan, D. Physiological Breeding I: Interdisciplinary Approaches to Improve Crop Adaptation; CIMMYT: Texcoco, Mexico, 2012. [Google Scholar]
- Reynolds, M.; Manes, Y.; Izanloo, A.; Langridge, P. Phenotyping approaches for physiological breeding and gene discovery in wheat. Ann. Appl. Biol. 2009, 155, 309–320. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hu, Y.; Yakout, G.M.; Awad, A.M.; Hafiz, S.E.; Schmidhalter, U. Evaluating salt tolerance of wheat genotypes using multiple parameters. Eur. J. Agron. 2005, 22, 243–253. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Sharma, R.C.; Shen, J.; Baum, M.; Ogbonnaya, F.C.; Léon, J.; Ballvora, A. Identification and Characterization of Salt Tolerance of Wheat Germplasm Using a Multivariable Screening Approach. J. Agron. Crop. Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Al-Ashkar, I.M.; Zaazaa, E.I.; Sabagh, A.E.; Barutcular, C. Physio-biochemical and molecular characterization for drought tolerance in rice genotypes at early seedling stage. J. Exp. Biol. Agric. Sci. 2016, 4, 675–687. [Google Scholar] [CrossRef]
- Sharbatkhari, M.; Shobbar, Z.-S.; Galeshi, S.; Nakhoda, B. Wheat stem reserves and salinity tolerance: Molecular dissection of fructan biosynthesis and remobilization to grains. Planta 2016, 244, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Singh, P.; Mahajan, M.M.; Singh, N.K.; Kumar, D.; Kumar, K. Physiological and molecular response under salinity stress in bread wheat (Triticum aestivum L.). J. Plant Biochem. Biotechnol. 2019, 29, 125–133. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C.; Agarwal, S.; Meena, R.C. Differences in antioxidant activity in response to salinity stress in tolerant and susceptible wheat genotypes. Biol. Plant. 2005, 49, 85–91. [Google Scholar] [CrossRef]
- Bhutta, W. Antioxidant activity of enzymatic system of two different wheat (Triticum aestivum L.) cultivars growing under salt stress. Plant Soil Environ. 2011, 57, 101–107. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Activities of fructan- and sucrose-metabolizing enzymes in wheat stems subjected to water stress during grain filling. Planta 2004, 220, 331–343. [Google Scholar] [CrossRef]
- Maas, E.V.; Lesch, S.M.; Francois, L.E.; Grieve, C.M. Contribution of Individual Culms to Yield of Salt-Stressed Wheat. Crop. Sci. 1996, 36, 142–149. [Google Scholar] [CrossRef]
- Richard, C.; Munyinda, K.; Kinkese, T.; Osiru, D.S. Genotypic Variation in Seedling Tolerance to Aluminum Toxicity in Historical Maize Inbred Lines of Zambia. Agronomy 2015, 5, 200–219. [Google Scholar] [CrossRef]
- Barbosa-Neto, J.F.; Sorrells, M.E.; Cisar, G. Prediction of heterosis in wheat using coefficient of parentage and RFLP-based estimates of genetic relationship. Genome 1996, 39, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Cowen, N.M.; Frey, K.J. Relationship between genealogical distance and breeding behaviour in oats (Avena sativa L.). Euphytica 1987, 36, 413–424. [Google Scholar] [CrossRef]
- Cox, T.S.; Murphy, J.P. The effect of parental divergence on F2 heterosis in winter wheat crosses. Theor. Appl. Genet. 1990, 79, 241–250. [Google Scholar] [CrossRef]





| No. of Variables | Source | Without Deleting | After Deletion of NKS | After Deletion of NSS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Best Model | Stepwise | Forward | Best Model | Stepwise | Forward | Best Model | Stepwise | Forward | |||||||||||
| Independent Variables | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | R2 | Direct Effect | |
| 2 | MSI/NKS | 0.94 | 0.75 | 0.94 | 0.75 | 0.94 | 0.75 | 0.94 | 0.75 | ||||||||||
| 2 | Chl a (0.38 cm−2)/NSS | 0.93 | 0.78 | 0.93 | 0.78 | 0.93 | 0.75 | 0.93 | 0.78 | 0.93 | 0.78 | ||||||||
| 3 | NKS/HKW/KL | 0.97 | 0.97 | ||||||||||||||||
| 3 | Chl a (0.38 cm−2)/FLA/NSS | 0.97 | |||||||||||||||||
| 4 | Chl a (0.38 cm−2)/Chl a + b(FLA)/NSS/KW | 0.99 | 0.99 | ||||||||||||||||
| 4 | FLA/E/NKS/Na+ | 0.99 | |||||||||||||||||
| 5 | RWC/Chl a (0.38 cm−2)/Chl b(FLA)/NSS/KW | 1.00 | 1.00 | ||||||||||||||||
| 5 | LWC/FLA/Gs/NKS/Na+ | 1.00 | |||||||||||||||||
| 1 | Chl a (0.38 cm−2) | 0.08 | 0.09 | 0.08 | 0.09 | 0.09 | 0.08 | 0.09 | 0.08 | 0.09 | |||||||||
| 1 | NKS | 0.62 | 0.62 | 0.82 | 0.62 | 0.82 | 0.62 | ||||||||||||
| 1 | NSS | 0.85 | 0.69 | 0.85 | 0.69 | 0.66 | 0.85 | 0.69 | 0.85 | 0.69 | |||||||||
| 1 | MSI | 0.13 | 0.13 | 0.12 | 0.13 | 0.12 | 0.13 | ||||||||||||
| Total indirect effect | 0.19 | 0.15 | 0.15 | 0.18 | 0.15 | 0.15 | 0.19 | 0.19 | 0.19 | ||||||||||
| Total R2 | 0.94 | 0.93 | 0.93 | 0.93 | 0.93 | 0.93 | 0.94 | 0.94 | 0.94 | ||||||||||
| Residual | 0.24 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.24 | 0.24 | 0.24 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ashkar, I.; Romdhane, W.B.; El-Said, R.A.; Ghazy, A.; Attia, K.; Al-Doss, A. Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions. Biology 2021, 10, 56. https://doi.org/10.3390/biology10010056
Al-Ashkar I, Romdhane WB, El-Said RA, Ghazy A, Attia K, Al-Doss A. Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions. Biology. 2021; 10(1):56. https://doi.org/10.3390/biology10010056
Chicago/Turabian StyleAl-Ashkar, Ibrahim, Walid Ben Romdhane, Rania A. El-Said, Abdelhalim Ghazy, Kotb Attia, and Abdullah Al-Doss. 2021. "Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions" Biology 10, no. 1: 56. https://doi.org/10.3390/biology10010056
APA StyleAl-Ashkar, I., Romdhane, W. B., El-Said, R. A., Ghazy, A., Attia, K., & Al-Doss, A. (2021). Agro-Physiologic Responses and Stress-Related Gene Expression of Four Doubled Haploid Wheat Lines under Salinity Stress Conditions. Biology, 10(1), 56. https://doi.org/10.3390/biology10010056






