Cutaneous Manifestations in COVID-19: Report on 31 Cases from Five Countries
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, X.H.; Li, T.Y.; He, Z.C.; Ping, Y.F.; Liu, H.W.; Yu, S.C.; Mou, H.M.; Wang, L.H.; Zhang, H.R.; Fu, W.J.; et al. A pathological report of three COVID-19 cases by minimal invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020, 49, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Galván Casas, C.; Català, A.; Carretero Hernández, G.; Rodríguez-Jiménez, P.; Fernández-Nieto, D.; Rodríguez-Villa Lario, A.; Navarro Fernández, I.; Ruiz-Villaverde, R.; Falkenhain-López, D.; Llamas Velasco, M.; et al. Classification of the cutaneous manifestations of COVID-19: A rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020, 183, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, M.; Long, B. Dermatologic manifestations and complications of COVID-19. Am. J. Emerg. Med. 2020, 38, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Dianzani, C.; Agozzino, M.; Giuffrida, R.; Marangi, G.F.; Meo, N.D.; Morariu, S.H.; Persichetti, P.; Segreto, F.; Zalaudek, I.; et al. Cutaneous manifestations in confirmed COVID-19 patients: A systematic review. Biology 2020, 9, 449. [Google Scholar] [CrossRef]
- Stefaniak, A.A.; Białynicki-Birula, R.; Krajewski, P.K.; Matusiak, Ł.; Goldust, M.; Szepietowski, J.C. Itch in the era of COVID-19 pandemic: An unfolding scenario. Dermatol. Ther. 2020, 33, e13477. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Szepietowski, J.C.; Maj, J. Cutaneous hyperesthesia: A novel manifestation of COVID-19. Brain Behav. Immun. 2020, 87, 188. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Leerunyakul, K.; Kositkuljorn, C. Cutaneous manifestations in COVID-19: Lessons learned from current evidence. J. Am. Acad. Dermatol. 2020, 83, e57–e60. [Google Scholar] [CrossRef]
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e212–e213. [Google Scholar] [CrossRef]
- Estébanez, A.; Pérez-Santiago, L.; Silva, E.; Guillen-Climent, S.; García-Vázquez, A.; Ramón, M.D. Cutaneous manifestations in COVID-19: A new contribution. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e250–e251. [Google Scholar] [CrossRef]
- Mirza, F.N.; Malik, A.A.; Omer, S.B.; Sethi, A. Dermatologic manifestations of COVID-19: A comprehensive systematic review. Int. J. Dermatol. 2020. [Google Scholar] [CrossRef]
- Bandhala Rajan, M.; Kumar, M.P.; Bhardwaj, A. The trend of cutaneous lesions during COVID-19 pandemic: Lessons from a meta-analysis and systematic review. Int. J. Dermatol. 2020, 59, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, L.J.; Pereira, F.A. Eruption as a clinical manifestation of COVID-19: Photographs of a patient. Clin. Dermatol. 2020, 38, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhu, J.; Du, L. Neonatal management during coronavirus disease (COVID-19) outbreak: Chinese experiences. NeoReviews 2020, 21, e293–e297. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.; Wang, C.; Li, C.; Zhou, Z.; Liu, S.; Rong, Z. Clinical characteristics of COVID-19 in pregnancy: Analysis of nine cases. Chin. J. Perinat. Med. 2020, 23. [Google Scholar] [CrossRef]
- Colonna, C.; Monzani, N.A.; Rocchi, A.; Gianotti, R.; Boggio, F.; Gelmetti, C. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr. Dermatol. 2020, 37, 437–440. [Google Scholar] [CrossRef]
- Andina, D.; Noguera-Morel, L.; Bascuas-Arribas, M.; Gaitero-Tristán, J.; Alonso-Cadenas, J.A.; Escalada-Pellitero, S.; Hernández-Martín, Á.; de la Torre-Espi, M.; Colmenero, I.; Torrelo, A. Chilblains in children in the setting of COVID-19 pandemic. Pediatr. Dermatol. 2020, 37, 406–411. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; CDC COVID-19 Response Team. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020, 23, 334–336. [Google Scholar] [CrossRef]
- Sajjan, V.V.; Lunge, S.; Swamy, M.B.; Pandit, A.M. Livedo reticularis: A review of the literature. Indian Dermatol. Online J. 2015, 6, 315–321. [Google Scholar] [CrossRef]
- Teuwen, L.A.; Geldhof, V.; Pasut, A.; Carmeliet, P. COVID-19: The vasculature unleashed. Nat. Rev. Immunol. 2020, 20, 389–391. [Google Scholar] [CrossRef]
- Roca-Ginés, J.; Torres-Navarro, I.; Sánchez-Arráez, J.; Abril-Pérez, C.; Sabalza-Baztán, O.; Pardo-Granell, S.; Cózar, V.M.I.; Botella-Estrada, R.; Évole-Buselli, M. Assessment of acute acral lesions in a case series of children and adolescents during the covid-19 pandemic. JAMA Dermatol. 2020, e202340. [Google Scholar] [CrossRef]
- Hawkins, D. Differential occupational risk for COVID-19 and other infection exposure according to race and ethnicity. Am. J. Ind. Med. 2020, 63, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Campbell, M.; Sims, E.; Lawson, E.; Warren, F.; James, T.; Cox, S.; Howarth, A.; et al. Differential occupational risks to healthcare workers from SARS-CoV-2 observed during a prospective observational study. Elife 2020, 21, e60675. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Fernandez, A.P. Skin manifestations of COVID-19. Cleve Clin. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Henry, D.; Ackerman, M.; Sancelme, E.; Finon, A.; Esteve, E. Urticarial eruption in COVID-19 infection. J. Eur. Acad. Dermatol. Venereol. 2020, 4, e244–e245. [Google Scholar] [CrossRef] [PubMed]
- Najarian, D.J. Morbilliform exanthem associated with COVID-19. JAAD Case Rep. 2020, 6, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, A.R. COVID-19: An overview for dermatologists. Int. J. Dermatol. 2020, 59, 1437–1449. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, A.; Cuenca-Barrales, C.; Montero-Vilchez, T.; Molina-Leyva, A.; Arias-Santiago, S. Review of adverse cutaneous reactions of pharmacologic interventions for coronavirus disease 2019 (COVID-19): A guide for the dermatologist. J. Am. Acad Dermatol. 2020, 83, 1738–1748. [Google Scholar] [CrossRef]
| DERMATOLOGIC MANIFESTATIONS | |||||||
|---|---|---|---|---|---|---|---|
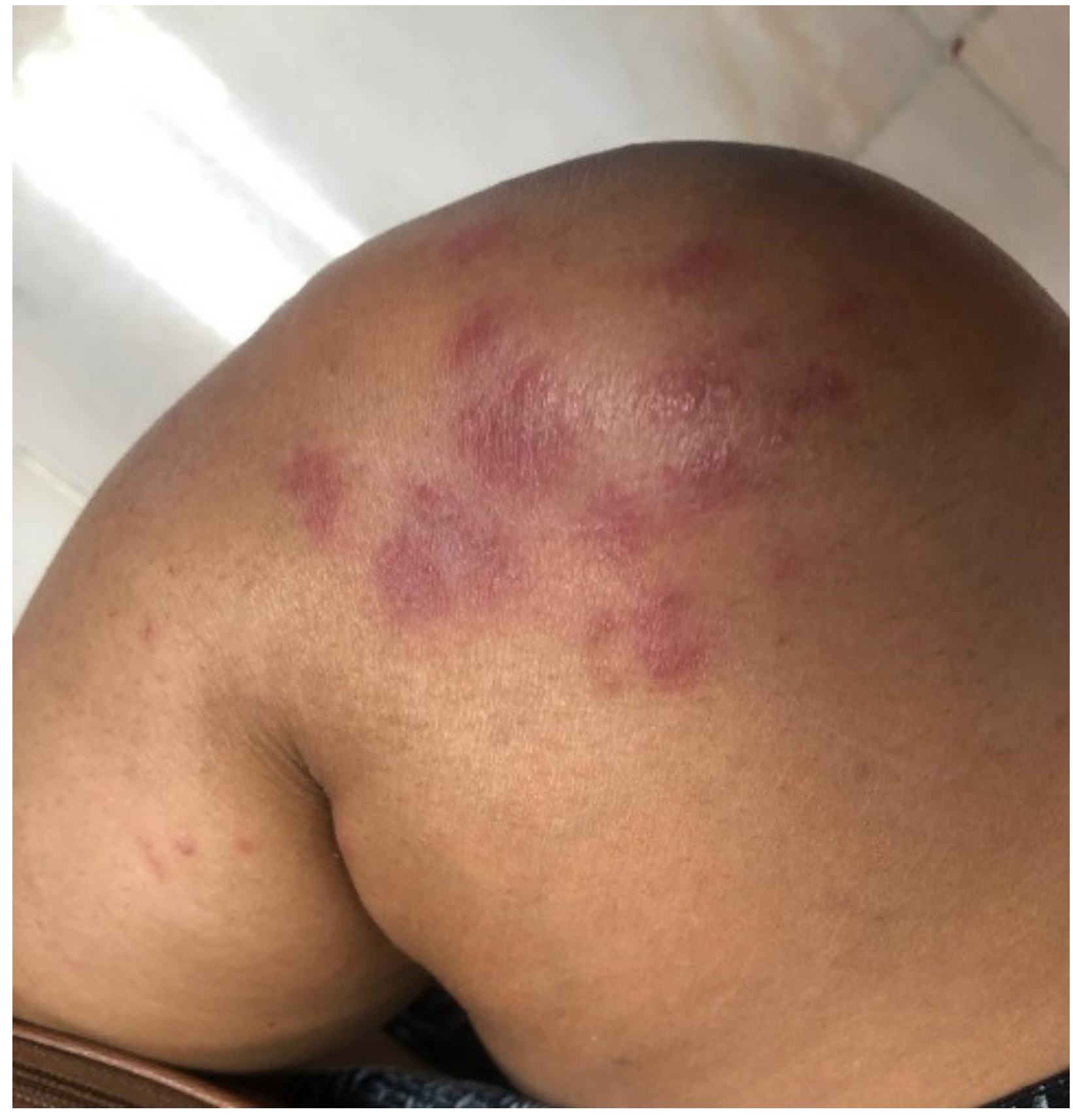
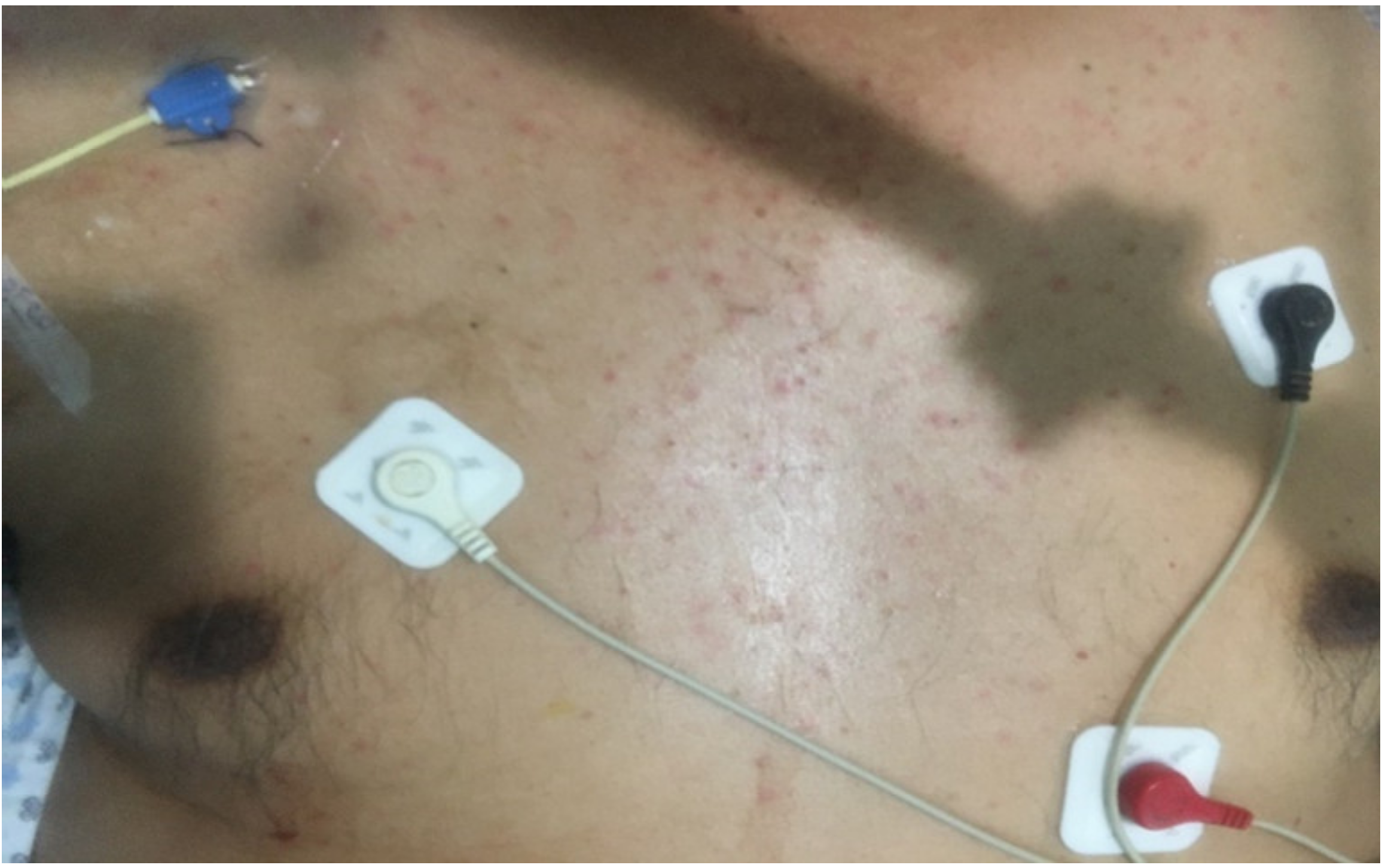
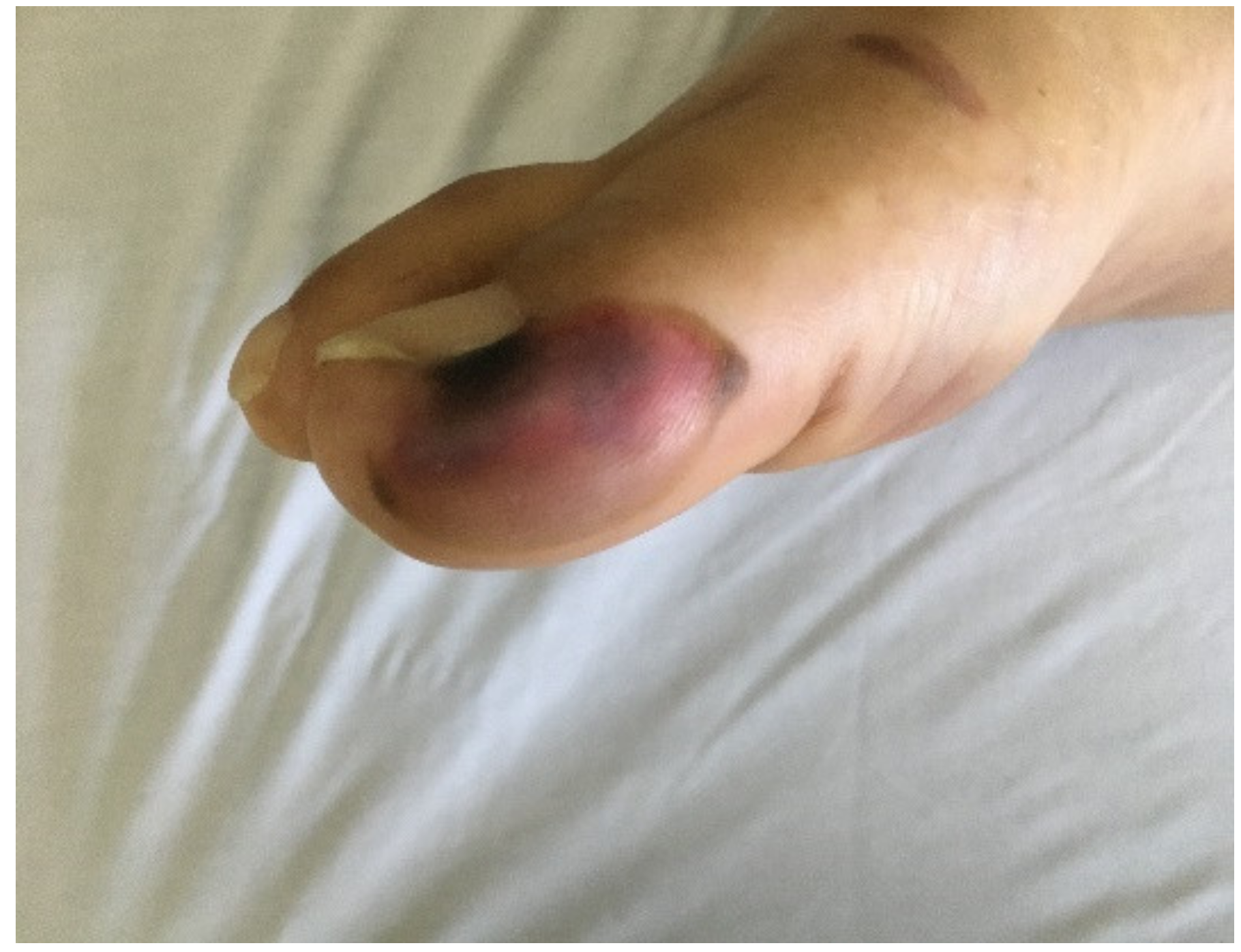
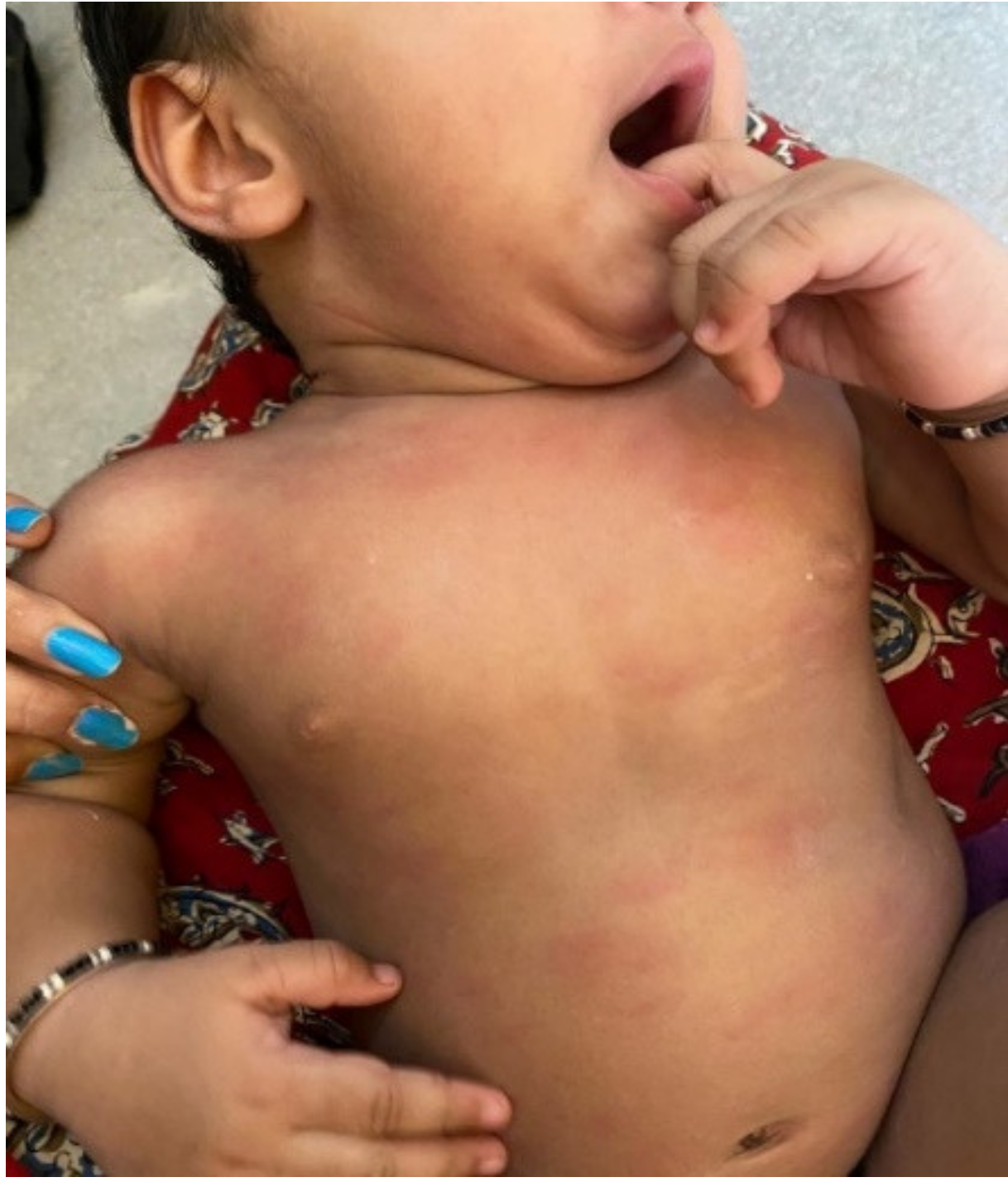
| Maculopapular Rash | Urticarial | Pseudochilblains | Petechiae/ Purpura | Distal Ischemia and Necrosis | Livedo Racemosa | Others | |
| COUNTRY | |||||||
| Argentina | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) |
| Dominican Republic | 1 (3.13) | 4 (12.50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| India | 0 (0) | 2 (6.25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mexico | 0 (0) | 1 (3.13) | 4 (12.50) | 2 (6.25) | 2 (6.25) | 4 (12.50) | 0 (0) |
| Spain | 3 (9.38) | 1 (3.13) | 3 (9.38) | 0 (0) | 0 (0) | 0 (0) | 3 (9.38) |
| SEX | |||||||
| Female | 3 (9.38) | 3 (9.38) | 2 (6.25) | 0 (0) | 0 (0) | 3 (9.38) | 2 (6.25) |
| Male | 2 (6.25) | 5 (15.63) | 5 (15.63) | 2 (6.25) | 2 (6.25) | 2 (6.25) | 1 (3.13) |
| CIVIL STATE | |||||||
| Married | 4 (12.50) | 8 (25) | 2 (6.25) | 1 (3.13) | 2 (6.25) | 2 (6.25) | 2 (6.25) |
| Single | 1 (3.13) | 0 (0) | 5 (15.63) | 0 (0) | 0 (0) | 2 (6.25) | 1 (3.13) |
| Widowhood | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 1 (3.13) | 0 (0) |
| AGE (years) | |||||||
| 0 | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| 1 | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 15 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 17 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 25 | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 28 | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 29 | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 30 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) |
| 31 | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 32 | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 36 | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 39 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 43 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) |
| 47 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 49 | 0 (0) | 2 (6.25) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| 51 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) |
| 52 | 1 (3.13) | 1 (3.13) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 55 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 60 | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) |
| 61 | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) |
| 62 | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) | 1 (3.13) | 0 (0) | 1 (3.13) |
| 63 | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 67 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| 77 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| 81 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| ACADEMIC LEVEL | |||||||
| Primary School | 0 (0) | 5 (16.67) | 4 (13.33) | 2 (6.67) | 2 (6.67) | 3 (10) | 1 (3.33) |
| Secondary School | 1 (3.33) | 0 (0) | 2 (6.67) | 0 (0) | 0 (0) | 1 (3.33) | 0 (0) |
| University Degree | 3 (10) | 3 (10) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 2 (6.67) |
| OCCUPATION | |||||||
| Driver | 0 (0) | 0 (0) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 1 (3.33) |
| Handworker | 0 (0) | 3 (10) | 1 (3.33) | 1 (3.33) | 2 (6.67) | 2 (6.67) | 0 (0) |
| Healthcare worker | 0 (0) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.67) |
| Industrial | 3 (10) | 0 (0) | 1 (3.33) | 1 (3.33) | 0 (0) | 2 (6.67) | 0 (0) |
| Professor | 1 (3.33) | 1 (3.33) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Student | 0 (0) | 2 (6.67) | 2 (6.67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Unemployed | 0 (0) | 1 (3.33) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| COVID-19 STATUS | |||||||
| Confirmed RT-PCR | 2 (6.67) | 8 (26.67) | 6 (20) | 2 (6.67) | 2 (6.67) | 4 (13.33) | 2 (6.67) |
| Confirmed serological test | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.33) |
| Suspected | 1 (3.33) | 0 (0) | 1 (3.33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| COUGH | 3 (13.04) | 5 (21.74) | 4 (17.39) | 2 (8.70) | 2 (8.70) | 4 (17.39) | 3 (13.04) |
| DYSPNEA | 4 (16.67) | 6 (25) | 4 (16.67) | 2 (8.33) | 2 (8.33) | 4 (16.67) | 2 (8.33) |
| FEVER | 5 (17.24) | 7 (24.14) | 6 (20.69) | 2 (6.90) | 2 (6.90) | 4 (13.79) | 3 (10.34) |
| HEADACHE | 3 (11.54) | 7 (26.92) | 6 (23.08) | 1 (3.85) | 2 (7.69) | 4 (15.38) | 3 (11.54) |
| ASTHENIA | 4 (15.38) | 6 (23.08) | 5 (19.23) | 2 (7.69) | 2 (7.69) | 4 (15.38) | 3 (11.54) |
| NAUSEA, VOMITING, DIARRHOEA | 2 (50) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (50) |
| ANOSMIA/AGEUSIA | 1 (25) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 1 (25) | 1 (25) |
| PNEUMONIA | 1 (7.69) | 1 (7.69) | 3 (23.08) | 1 (7.69) | 2 (15.38) | 4 (30.77) | 1 (7.69) |
| SMOKING | 3 (30) | 3 (30) | 1 (10) | 0 (0) | 1 (10) | 1 (10) | 1 (10) |
| COPD | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 0 (0) |
| TYPE 2 DIABETES | 1 (11.11) | 1 (11.11) | 3 (33.33) | 1 (11.11) | 2 (22.22) | 1 (11.11) | 0 (0) |
| ARTERIAL HYPERTENSION | 1 (16.67) | 0 (0) | 1 (16.67) | 1 (16.67) | 1 (16.67) | 2 (33.33) | 0 (0) |
| PRESENCE OF CUTANEOUS SYMPTOMS | |||||||
| Pain | 1 (2.13) | 1 (2.13) | 4 (8.51) | 2 (4.26) | 2 (4.26) | 2 (4.26) | 1 (2.13) |
| Burning | 2 (4.26) | 7 (14.89) | 6 (12.77) | 1 (2.13) | 1 (2.13) | 0 (0) | 2 (4.26) |
| Itch | 4 (8.51) | 7 (14.89) | 3 (6.38) | 1 (2.13) | 0 (0) | 0 (0) | 0 (0) |
| DURATION OF CUTANEOUS LESIONS (days) | |||||||
| 7 | 0 (0) | 2 (6.25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 8 | 0 (0) | 2 (6.25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 9 | 2 (6.25) | 3 (9.38) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 10 | 2 (6.25) | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) | 0 (0) |
| 11 | 1 (3.13) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (6.25) | 0 (0) |
| 12 | 0 (0) | 0 (0) | 3 (9.38) | 2 (6.25) | 0 (0) | 2 (6.25) | 0 (0) |
| 13 | 0 (0) | 0 (0) | 2 (6.25) | 0 (0) | 1 (3.13) | 0 (0) | 0 (0) |
| 14 | 0 (0) | 0 (0) | 2 (6.25) | 0 (0) | 1 (3.13) | 0 (0) | 1 (3.13) |
| 15 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) |
| 20 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3.13) |
| TREATMENTS | |||||||
| Paracetamol/ Acetominophen | 5 (6.02) | 5 (6.02) | 5 (6.02) | 2 (2.41) | 2 (2.41) | 6 (14.53) | 3(3.61) |
| Systemic corticosteroids | 1 (1.20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (2.41) | 1 (1.20) |
| Antihistamines | 3 (3.61) | 2 (2.41) | 0 (0) | 0 (0) | 0 (0) | 1 (1.20) | 1 (1.20) |
| Azithromycin | 2 (2.41) | 1 (1.20) | 2 (2.41) | 1 (1.20) | 1 (1.20) | 4 (4.82) | 1 (1.20) |
| NSAIDs | 0 (0) | 0 (0) | 4 (4.82) | 1 (1.20) | 1 (1.20) | 3 (3.61) | 0 (0) |
| Hydroxychloroquine | 1 (1.20) | 0 (0) | 2 (2.41) | 2 (2.41) | 1 (1.20) | 2 (2.41) | 1 (1.20) |
| Lopinavir, ritonavir | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.20) |
| Heparin | 0 (0) | 1 (1.20) | 3 (3.61) | 2 (2.41) | 2 (2.41) | 4 (4.82) | 1 (1.20) |
| INVASIVE MECHANICAL VENTILATION (injuries) | 1 (9.09) | 0 (0) | 3 (27.27) | 1 (9.09) | 2 (18.18) | 4 (36.36) | 0 (0) |
| NOREPINEPHRINE (detection of lesions while using amines) | 0 (0) | 0 (0) | 1 (20) | 1 (20) | 1 (20) | 2 (40) | 0 (0) |
| PATIENT SURVIVAL | 4 (20) | 7 (35) | 4 (20) | 1 (5) | 1 (5) | 0 (0) | 3 (15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Cerdeira, C.; Uribe-Camacho, B.I.; Silverio-Carrasco, L.; Méndez, W.; Mahesh, A.R.; Tejada, A.; Beirana, A.; Martinez-Herrera, E.; Alba, A.; Arenas, R.; et al. Cutaneous Manifestations in COVID-19: Report on 31 Cases from Five Countries. Biology 2021, 10, 54. https://doi.org/10.3390/biology10010054
Rodriguez-Cerdeira C, Uribe-Camacho BI, Silverio-Carrasco L, Méndez W, Mahesh AR, Tejada A, Beirana A, Martinez-Herrera E, Alba A, Arenas R, et al. Cutaneous Manifestations in COVID-19: Report on 31 Cases from Five Countries. Biology. 2021; 10(1):54. https://doi.org/10.3390/biology10010054
Chicago/Turabian StyleRodriguez-Cerdeira, Carmen, Brianda I. Uribe-Camacho, Lianet Silverio-Carrasco, Wennia Méndez, Ashwini R. Mahesh, Anakaren Tejada, Angelica Beirana, Erick Martinez-Herrera, Alfonso Alba, Roberto Arenas, and et al. 2021. "Cutaneous Manifestations in COVID-19: Report on 31 Cases from Five Countries" Biology 10, no. 1: 54. https://doi.org/10.3390/biology10010054
APA StyleRodriguez-Cerdeira, C., Uribe-Camacho, B. I., Silverio-Carrasco, L., Méndez, W., Mahesh, A. R., Tejada, A., Beirana, A., Martinez-Herrera, E., Alba, A., Arenas, R., & Szepietowski, J. C. (2021). Cutaneous Manifestations in COVID-19: Report on 31 Cases from Five Countries. Biology, 10(1), 54. https://doi.org/10.3390/biology10010054









