Investigation of Methylene Blue Removal from Aqueous Solutions Using Biochar Derived from Mango and Pitanga Pruning Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Preparation of Biochar
2.2. Physical–Chemical Characterization
2.3. Preparation of Biochar and MB Solution
2.4. Sorption Studies with Methylene Blue
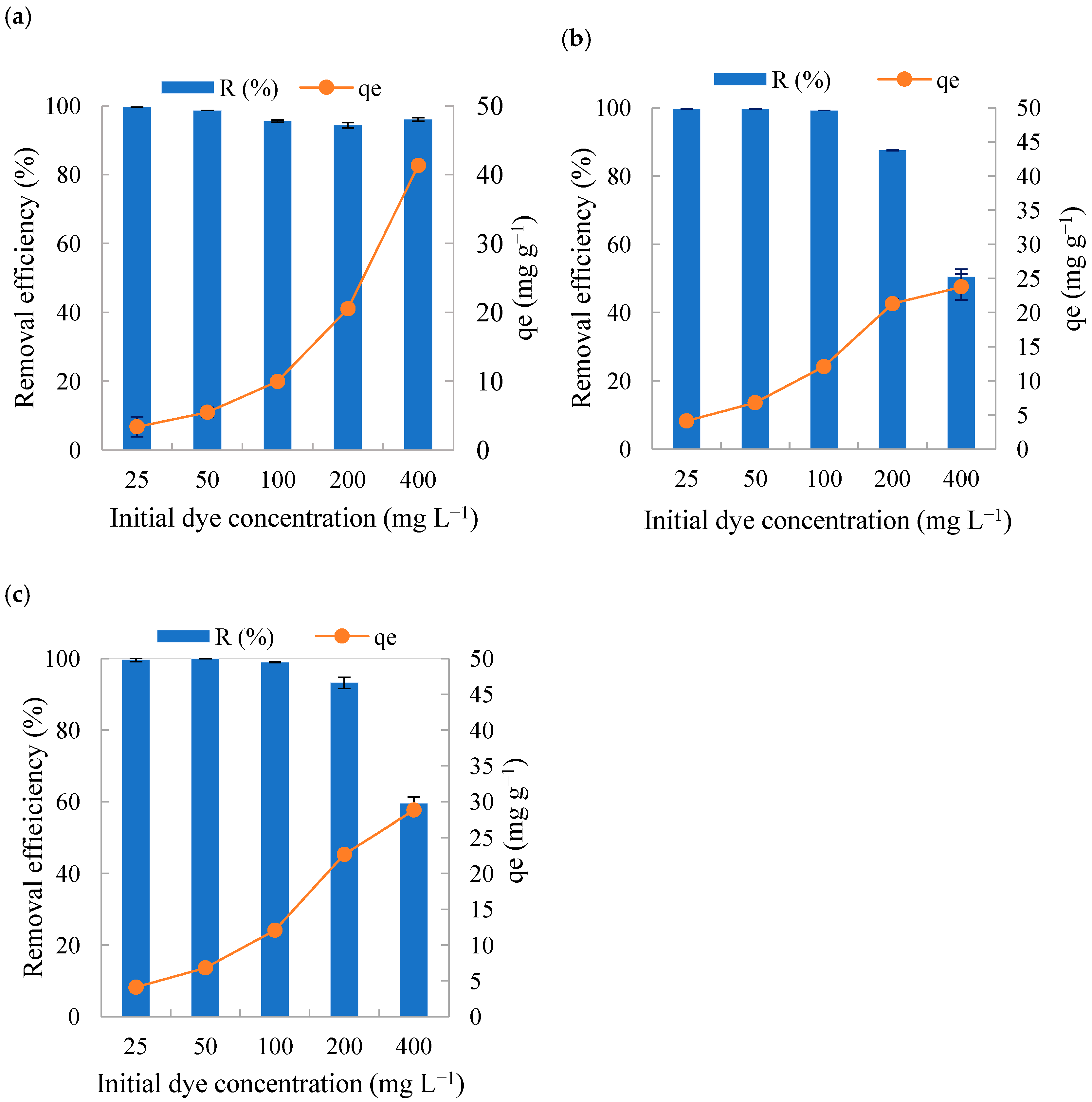
- Influence of initial dye concentration: To evaluate the influence of the initial MB concentration, the concentration of the dye solution was varied (25, 50, 100, 200 and 400 mg L−1), keeping the adsorbent mass (0.10 g) and the particle size fraction (<0.60 mm) fixed. This particle size was selected based on previous studies.
- Influence of adsorbent mass: to evaluate the influence of the adsorbent mass, the adsorbent mass was varied (0.01, 0.05, 0.10 and 0.20 g), keeping the initial concentration of MB (100 mg L−1) and the particle size fraction (<0.60 mm) fixed.
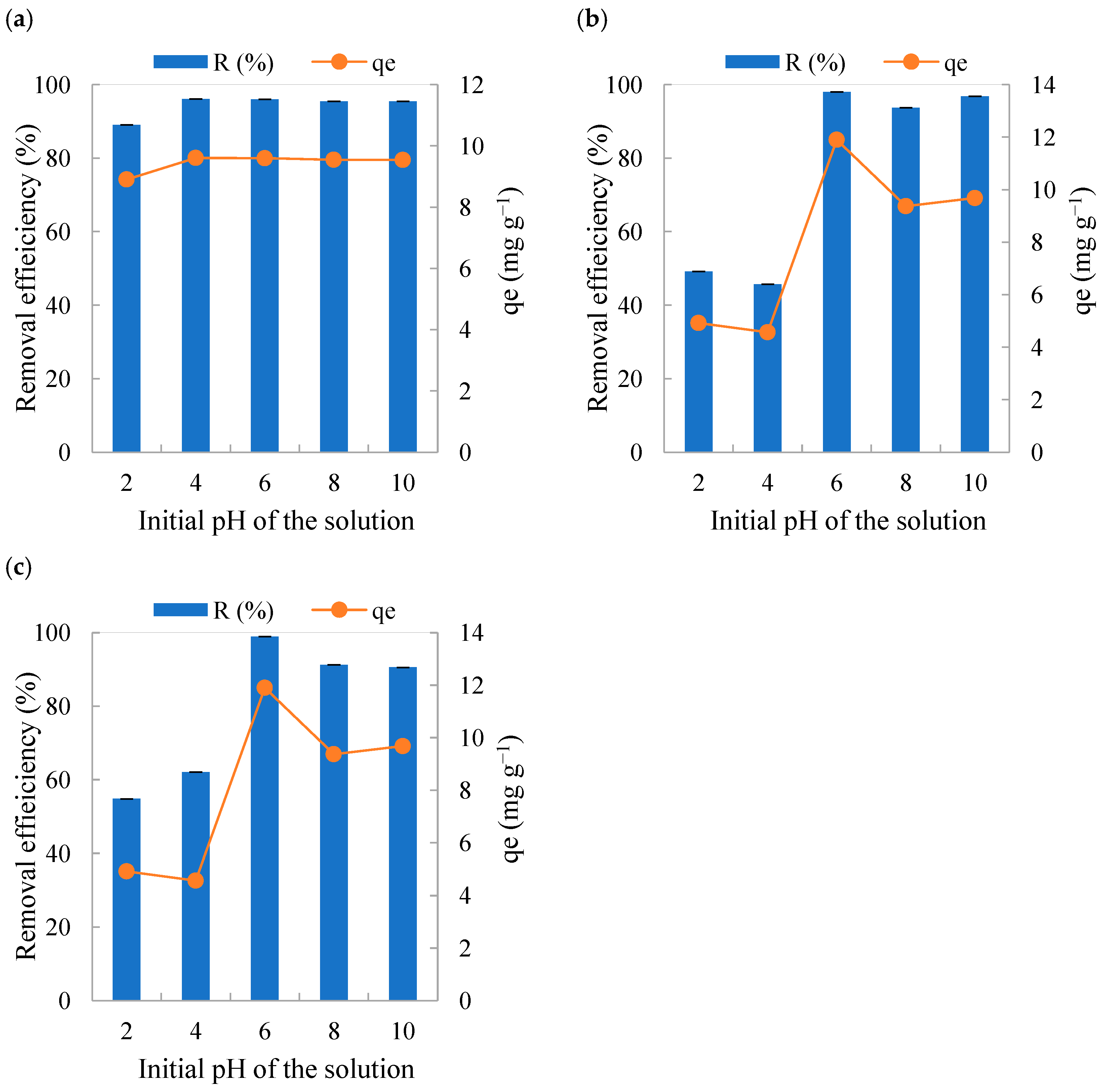
- Influence of initial pH: To evaluate the influence of the initial pH of the solution, the pH of the dye solution was varied using a 1 M NaOH and 1 M HCl solution to adjust pH to 2, 4, 6 (natural pH of the dye solution), 8 and 10. The adsorbent mass (0.10 g) and the particle size fraction (<0.60 mm) were kept constant.
- Influence of the particle size: To evaluate the influence of particle size, the particle diameter of the adsorbent was varied (1.25–2.00, 0.60–1.25, 0.40–0.60, 0.25–0.40 and <0.25 mm), keeping the initial concentration of MB (100 mg L−1) fixed. The concentration was selected based on the results obtained previously, on the influence of initial dye concentration.
- Influence of the thermochemical process: to evaluate the influence of the thermochemical process, the sorption studies were conducted with three different types of BTP (BTP-R, BTP-300 and BTP-500).
2.5. Isotherm Studies
2.6. Desorption Studies
3. Results and Discussion
3.1. Characterization of Adsorbent Materials
3.2. Influence of the Initial Concentration of Methylene Blue on the Adsorption Studies
3.3. Influence of the Mass of the Adsorbent on Adsorption Studies
3.4. Influence of the pH of the Solution on Adsorption Studies
3.5. Influence of Adsorbent Particle Size on Adsorption Studies
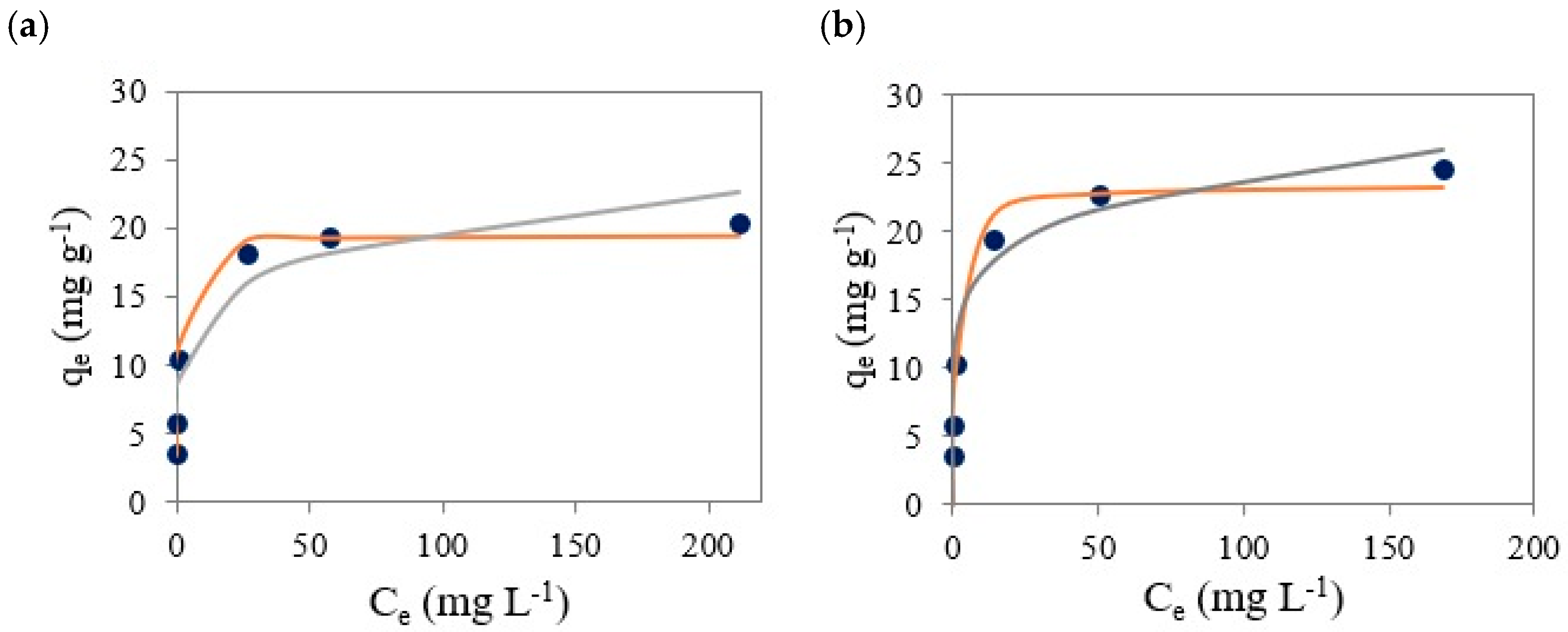
3.6. Isotherm Studies
3.7. Desorption Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTP | Biochar tree pruning |
| MB | Methylene blue |
| Adsorption capacity at equilibrium | |
| Adsorption efficiency at equilibrium |
References
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Advancing in wastewater treatment using sustainable electrosorbents. Curr. Opin. Electrochem. 2024, 44, 101450. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A review on recent advances of biochar from agricultural and forestry wastes: Preparation, modification and applications in wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Saleem, M. Sustainable production of activated carbon from indigenous Acacia etbaica tree branches employing microwave induced and low temperature activation. Heliyon 2024, 10, e24113. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Gurav, R.; Bhatia, S.K.; Choi, T.-R.; Choi, Y.-K.; Kim, H.J.; Song, H.-S.; Park, S.L.; Lee, H.S.; Lee, S.M.; Choi, K.-Y.; et al. Adsorptive removal of crude petroleum oil from water using floating pinewood biochar decorated with coconut oil-derived fatty acids. Sci. Total Environ. 2021, 781, 146636. [Google Scholar] [CrossRef]
- Fernandes, M.J.; Moreira, M.M.; Paíga, P.; Dias, D.; Bernardo, M.; Carvalho, M.; Lapa, N.; Fonseca, I.; Morais, S.; Figueiredo, S.; et al. Evaluation of the adsorption potential of biochars prepared from forest and agri-food wastes for the removal of fluoxetine. Bioresour. Technol. 2019, 292, 121973. [Google Scholar] [CrossRef]
- Fang, Z.; Gao, Y.; Zhang, F.; Zhu, K.; Shen, Z.; Liang, H.; Xie, Y.; Yu, C.; Bao, Y.; Feng, B.; et al. The adsorption mechanisms of oriental plane tree biochar toward bisphenol S: A combined thermodynamic evidence, spectroscopic analysis and theoretical calculations. Environ. Pollut. 2022, 310, 119819. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Alazba, A.A.; Amin, M.T. Eco-friendly nanocomposite of manganese-iron and plant waste derived biochar for optimizing Pb2+ adsorption: A response surface methodology approach. Desalination Water Treat. 2025, 322, 101091. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Costa, J.; Martins, A.; Fonseca, A.M.; Neves, I.C.; Nunes, N. Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation. Colorants 2025, 4, 10. [Google Scholar] [CrossRef]
- Lermen, A.M.; Fronza, C.S.; Diel, J.C.; Schein, D.; Clerici, N.J.; Guimarães, R.E.; Boligon, S.D.; Scher, A.C. A utilização de resíduos agroindustriais para adsorção do corante azul de metileno: Uma breve revisão / The use of agro-industrial waste for adsorption of the blue dye of methylene: A brief review. Braz. Appl. Sci. Rev. 2021, 5, 273–288. [Google Scholar] [CrossRef]
- Chahal, M.; Kumari, S.; Bhattacharya, A.; Garg, M.C. Evaluating sustainable agricultural waste biomass for methylene blue adsorption in wastewater treatment: A state-of-the-art review. Bioresour. Technol. Rep. 2024, 28, 101983. [Google Scholar] [CrossRef]
- Al-Asadi, S.T.; Mussa, Z.H.; Al-Qaim, F.F.; Kamyab, H.; Al-Saedi, H.F.S.; Deyab, I.F.; Kadhim, N.J. A comprehensive review of methylene blue dye adsorption on activated carbon from edible fruit seeds: A case study on kinetics and adsorption models. Carbon. Trends 2025, 20, 100507. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; Romão, E.L.; Bueno, T.; Papa, R. Adsorption of methylene blue on babassu coconut (Orbignya speciosa) mesocarp commercial biochar. Int. J. Environ. Sci. Technol. 2024, 21, 1671–1682. [Google Scholar] [CrossRef]
- Domingues, N.S.; Romão, E.L.; Alvim, D.S.; Marques, J.P.; Rodrigues, V.G.S.; Kasemodel, M.C. Use of Construction and Demolition Waste for the Treatment of Dye-Contaminated Water Toward Circular economy. Water Air Soil. Pollut. 2024, 235, 663. [Google Scholar] [CrossRef]
- Damahe, D.; Mayilswamy, N.; Kandasubramanian, B. Biochar/metal nanoparticles-based composites for Dye remediation: A review. Hybrid. Adv. 2024, 6, 100254. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- da Silva Antunes Martins, R.; da Silva, M.R.M.; Lourenço, M.A.D.S.; Kasemodel, M.C. Evaluation of the adsorption potential of iron mining tailing and its effect on raphanus sativus germination. Eng. Sanit. Ambient. 2024, 29, e20230150. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Lv, Z.; Wang, S. Effects of particle size on the adsorption behavior and antifouling performance of magnetic resins. Environ. Sci. Pollut. Res. 2023, 30, 11926–11935. [Google Scholar] [CrossRef]
- Kasemodel, M.C.; de Aguiar, L.G.; Rodrigues, V.G.S.; Romão, É.L. The Investigation of the Adsorption of Methylene Blue from Water by Torrefied Biomass. Colorants 2025, 4, 21. [Google Scholar] [CrossRef]
- Nhuchhen, D.; Basu, P.; Acharya, B. A Comprehensive Review on Biomass Torrefaction. Int. J. Renew. Energy Biofuels 2014, 2014, 506376. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Ghiasi, B.; Soelberg, N.R.; Sokhansanj, S. Biomass Torrefaction Process, Product Properties, Reactor Types, and Moving Bed Reactor Design Concepts. Front. Energy Res. 2021, 9, 728140. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Revista e Ampliada. Available online: https://www.embrapa.br (accessed on 11 September 2025).
- De Camargo, O.A.; Moniz, A.C.; Jorge, J.A.; Valadares, J.M.A.S. Métodos de Análise Química, Mineralógica e Física de Solos. Governo Do Estado de São Paulo Secretaria de Agricultura e Abastecimento Coordenadoria da Pesquisa Agropecuária Instituto Agronômico. 2009. Available online: https://www.iac.sp.gov.br (accessed on 11 September 2025).
- Singh, R.; Goyal, A.; Sinha, S. Global insights into biochar: Production, sustainable applications, and market dynamics. Biomass Bioenergy 2025, 194, 107663. [Google Scholar] [CrossRef]
- Shahrun, M.S.; Rahman, M.H.A.; Baharom, N.A.; Jumat, F.; Saad, M.J.; Mail, M.F.; Zawawi, N.Z.; Suherman, F.H.S. Design of a pyrolysis system and the characterisation data of biochar produced from coconut shells, carambola pruning, and mango pruning using a low-temperature slow pyrolysis process. Data Brief 2024, 52, 109997. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Vaz, C.M.P.; Rodrigues, V.G.S. Characterization of mixtures of Brazilian Ultisol with urban pruning waste biochar at two different proportions. J. Soils Sediments 2024, 24, 3610–3625. [Google Scholar] [CrossRef]
- Azizian, S.; Eris, S. Adsorption isotherms and kinetics. Interface Sci. Technol. 2021, 33, 445–509. [Google Scholar] [CrossRef]
- Choudhury, P.; Manna, M.S.; Nag, S. A critical review on green synthesis and modification techniques of biochar: Comparison of efficacies towards adsorption capacities. Biomass Bioenergy 2025, 198, 107859. [Google Scholar] [CrossRef]
- Lin, S.L.; Zhang, H.; Chen, W.H.; Song, M.; Kwon, E.E. Low-temperature biochar production from torrefaction for wastewater treatment: A review. Bioresour. Technol. 2023, 387, 129588. [Google Scholar] [CrossRef]





| Parameter | Description | Reference |
|---|---|---|
| Physicochemical parameters (pH, ΔpH, Eh) | Tests performed by preparing a 1:2.5 solution (biomass/biochar:deionized water), homogenizing with a glass rod and reading on a digital pH meter (BEL Engineering). ΔpH was obtained from the difference between the pH measured in a 1 M potassium chloride (KCl) solution and the pH measured in water. | [22] |
| Electrical conductivity (EC) | Tests performed by preparing a 1:2 solution (biomass/biochar:deionized water), homogenizing with a glass rod and reading on a digital conductivity meter (BEL Engineering). | [23] |
| Parameters | BTP-R | BTP -300 | BTP-500 |
|---|---|---|---|
| pH (H2O) | 4.7 ± 0.0 | 7.3 ± 0.2 | 10.2 ± 0.1 |
| ΔpH | −0.2 ± 0.1 | −0.8 ± 0.3 | −0.6 ± 0.1 |
| Eh [mV] | 140.0 ± 2.8 | 10.0 ± 2.8 | −151.5 ± 2.1 |
| CE [µS cm−1] | 397.0 ± 0.0 | 1040.5 ± 37.5 | 1643.5 ± 65.8 |
| Isotherm | Parameter | BTP-300 | BTP-500 |
|---|---|---|---|
| Langmuir | qm (mg g−1) | 20.53 ± 5.47 | 23.40 ± 6.31 |
| KL (L mg−1) | 3.47 ± 2.91 | 4.47 ± 3.21 | |
| R2 | 0.9855 | 0.9691 | |
| Freundlich | n | 5.90 ± 0.22 | 6.55 ± 0.43 |
| KF (mg g−1) | 9.09 ± 0.35 | 11.88 ± 0.45 | |
| R2 | 0.9128 | 0.9570 | |
| Temkin | KT (mg g−1) | 5.37 × 107 | 5.37 × 107 |
| b (kJ mol−1) | 3.74 | 3.74 | |
| R2 | 0.6872 | 0.6404 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasemodel, M.C.; Rodrigues, V.G.S.; Farah Silva, J.M.R.; Vallim, B.S.C.; Romão, É.L. Investigation of Methylene Blue Removal from Aqueous Solutions Using Biochar Derived from Mango and Pitanga Pruning Waste. Colorants 2025, 4, 28. https://doi.org/10.3390/colorants4030028
Kasemodel MC, Rodrigues VGS, Farah Silva JMR, Vallim BSC, Romão ÉL. Investigation of Methylene Blue Removal from Aqueous Solutions Using Biochar Derived from Mango and Pitanga Pruning Waste. Colorants. 2025; 4(3):28. https://doi.org/10.3390/colorants4030028
Chicago/Turabian StyleKasemodel, Mariana Consiglio, Valéria Guimarães Silvestre Rodrigues, João Marcos Ribeiro Farah Silva, Bruna Soares Campelo Vallim, and Érica Leonor Romão. 2025. "Investigation of Methylene Blue Removal from Aqueous Solutions Using Biochar Derived from Mango and Pitanga Pruning Waste" Colorants 4, no. 3: 28. https://doi.org/10.3390/colorants4030028
APA StyleKasemodel, M. C., Rodrigues, V. G. S., Farah Silva, J. M. R., Vallim, B. S. C., & Romão, É. L. (2025). Investigation of Methylene Blue Removal from Aqueous Solutions Using Biochar Derived from Mango and Pitanga Pruning Waste. Colorants, 4(3), 28. https://doi.org/10.3390/colorants4030028








