Dyeing to Know: Harmonizing Nile Red Staining Protocols for Microplastic Identification
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection for Staining Experiments
2.2. Staining Dye Solution
2.3. Fenton Oxidation Pretreatment
2.4. NR Staining of MPs
3. Results
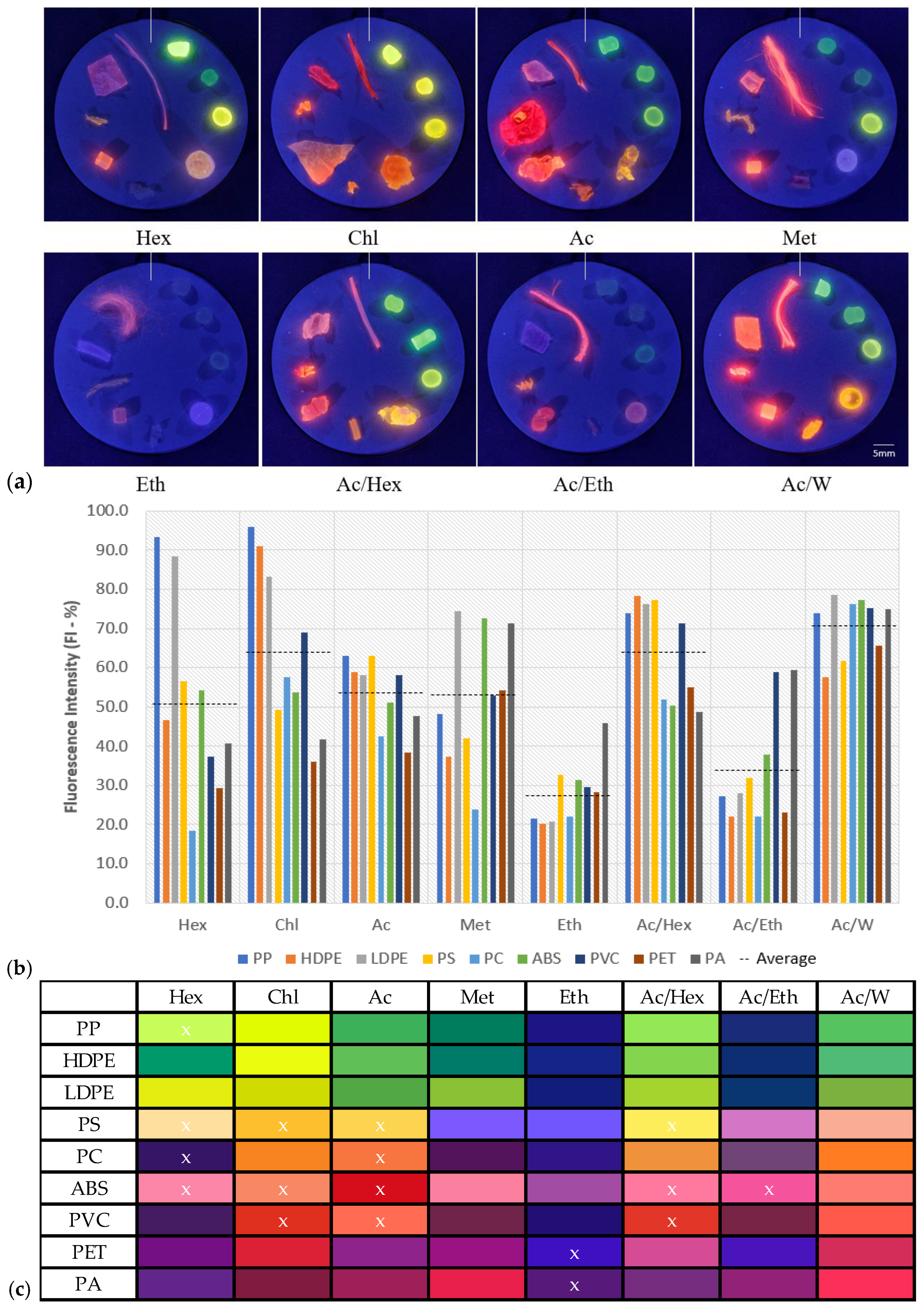
3.1. Effects of Solvent on Fluorescence Behavior
3.2. Solvent Mixtures
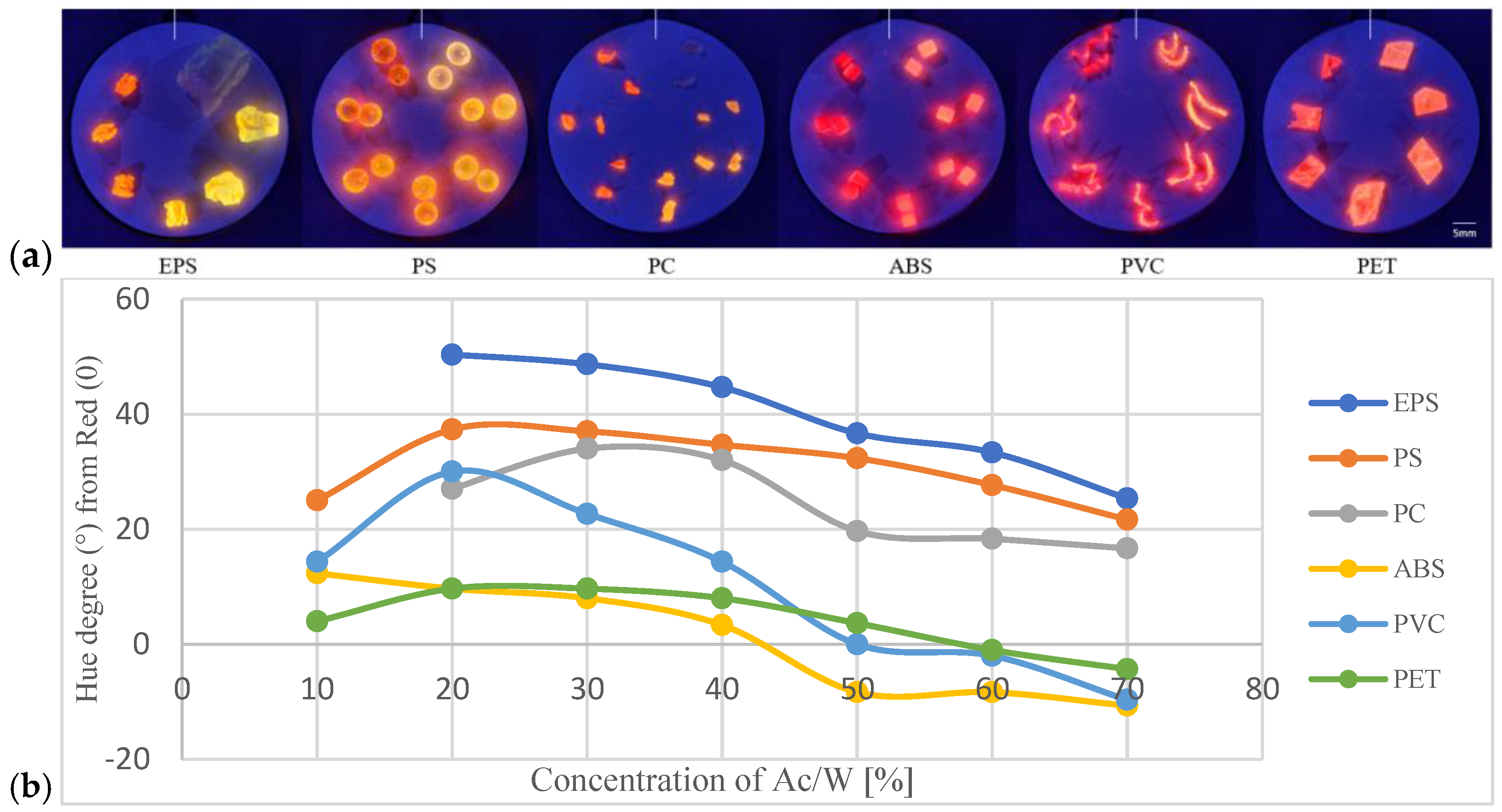
3.3. Optimizing Ac/W Mixtures
3.4. Classification Using Polarity-Induced Fluorescence
3.5. Evaluating the Classification Ability of MPs
3.6. Spectral Behavior of NR-Stained MPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| PP | Polypropylene |
| PS | Polystyrene |
| EPS | Expanded polystyrene |
| ABS | Acrylonitrile butadiene styrene |
| PC | Polycarbonate |
| PVC | Polyvinylchloride |
| PET | Polyethylene terephthalate |
| PA | Polyamide |
| Hex | n-Hexane |
| Chl | Chloroform |
| Ac | Acetone |
| Eth | Ethanol |
| Met | Methanol |
| Ac-Hex | Acetone with n-hexane |
| Ac-Eth | Acetone with ethanol |
| Ac-W | Acetone with water |
| NR | Nile Red |
| MPs | Microplastics |
| FI | Fluorescence intensity |
| NOM | Natural organic matter |
| ML | Machine Learning |
References
- Ritchie, H.; Samborska, V.; Roser, M. Plastic Pollution. Our World in Data, November 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 7 December 2023).
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic waste release caused by COVID-19 and its fate in the global ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef] [PubMed]
- UN. Environment. Plastic Pollution. UNEP—UN Environment Programme. Available online: http://www.unep.org/plastic-pollution (accessed on 7 December 2023).
- Cai, L.; Wang, J.; Peng, J.; Wu, Z.; Tan, X. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments. Sci. Total Environ. 2018, 628, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Paluselli, A.; Fauvelle, V.; Galgani, F.; Sempéré, R. Phthalate Release from Plastic Fragments and Degradation in Seawater. Environ. Sci. Technol. 2019, 53, 166–175. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Jang, M.; Han, G.M.; Jung, S.W.; Shim, W.J. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type. Environ. Sci. Technol. 2017, 51, 4368–4376. [Google Scholar] [CrossRef]
- Du, H.; Xie, Y.; Wang, J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mater. 2021, 418, 126377. [Google Scholar] [CrossRef]
- Peeken, I.; Primpke, S.; Beyer, B.; Gütermann, J.; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef]
- Hasenmueller, E.A.; Baraza, T.; Hernandez, N.F.; Finegan, C.R. Cave sediment sequesters anthropogenic microparticles (including microplastics and modified cellulose) in subsurface environments. Sci. Total Environ. 2023, 893, 164690. [Google Scholar] [CrossRef]
- Napper, I.E.; Davies, B.F.R.; Clifford, H.; Elvin, S.; Koldewey, H.J.; Mayewski, P.A.; Miner, K.R.; Potocki, M.; Elmore, A.C.; Gajurel, A.P.; et al. Reaching New Heights in Plastic Pollution—Preliminary Findings of Microplastics on Mount Everest. One Earth 2020, 3, 621–630. [Google Scholar] [CrossRef]
- Jamieson, A.J.; Brooks, L.S.R.; Reid, W.D.K.; Piertney, S.B.; Narayanaswamy, B.E.; Linley, T.D. Microplastics and synthetic particles ingested by deep-sea amphipods in six of the deepest marine ecosystems on Earth. R. Soc. Open Sci. 2019, 6, 180667. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Wan, Y.; Wu, C.; Xue, Q.; Hui, X. Effects of plastic contamination on water evaporation and desiccation cracking in soil. Sci. Total Environ. 2019, 654, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Menéndez-Pedriza, A.; Jaumot, J. Interaction of Environmental Pollutants with Microplastics: A Critical Review of Sorption Factors, Bioaccumulation and Ecotoxicological Effects. Toxics 2020, 8, 40. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, J.; Sohn, J.; Kim, C. Health Effects of Microplastic Exposures: Current Issues and Perspectives in South Korea. Yonsei Med. J. 2023, 64, 301–308. [Google Scholar] [CrossRef]
- Conti, G.O.; Ferrante, M.; Banni, M.; Favara, C.; Nicolosi, I.; Cristaldi, A.; Fiore, M.; Zuccarello, P. Micro-and nano-plastics in edible fruit and vegetables. The first diet risks assessment for the general population. Environ. Res. 2020, 187, 109677. [Google Scholar] [CrossRef]
- Dehaut, A.; Cassone, A.-L.; Frère, L.; Hermabessiere, L.; Himber, C.; Rinnert, E.; Rivière, G.; Lambert, C.; Soudant, P.; Huvet, A.; et al. Microplastics in seafood: Benchmark protocol for their extraction and characterization. Environ. Pollut. 2016, 215, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kunz, A.; Shim, W.J.; Walther, B.A. Microplastic contamination of table salts from Taiwan, including a global review. Sci. Rep. 2019, 9, 10145. [Google Scholar] [CrossRef]
- Scircle, A.; Cizdziel, J.V. Detecting and Quantifying Microplastics in Bottled Water using Fluorescence Microscopy: A New Experiment for Instrumental Analysis and Environmental Chemistry Courses. J. Chem. Educ. 2020, 97, 234–238. [Google Scholar] [CrossRef]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic contamination of packaged meat: Occurrence and associated risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Lebreton, L.; Egger, M.; Slat, B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci. Rep. 2019, 9, 12922. [Google Scholar] [CrossRef]
- Bakir, A.; McGoran, A.R.; Silburn, B.; Russell, J.; Nel, H.; Lusher, A.L.; Amos, R.; Shadrack, R.S.; Arnold, S.J.; Castillo, C.; et al. Creation of an international laboratory network towards global microplastics monitoring harmonisation. Sci. Rep. 2024, 14, 12714. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-L.; Thomas, K.V.; Luo, Z.; Gowen, A.A. FTIR and Raman imaging for microplastics analysis: State of the art, challenges and prospects. TrAC Trends Anal. Chem. 2019, 119, 115629. [Google Scholar] [CrossRef]
- Schwaferts, C.; Niessner, R.; Elsner, M.; Ivleva, N.P. Methods for the analysis of submicrometer- and nanoplastic particles in the environment. TrAC Trends Anal. Chem. 2019, 112, 52–65. [Google Scholar] [CrossRef]
- Fries, E.; Dekiff, J.H.; Willmeyer, J.; Nuelle, M.-T.; Ebert, M.; Remy, D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ. Sci. Process. Impacts 2013, 15, 1949. [Google Scholar] [CrossRef]
- Shim, W.J.; Hong, S.H.; Eo, S.E. Identification methods in microplastic analysis: A review. Anal. Methods 2017, 9, 1384–1391. [Google Scholar] [CrossRef]
- Ho, D.; Liu, S.; Wei, H. The glowing potential of Nile red for microplastics Identification: Science and mechanism of fluorescence staining. Microchem. J. 2023, 197, 109708. [Google Scholar] [CrossRef]
- Kalaronis, D.; Ainali, N.M.; Evgenidou, E.; Kyzas, G.Z.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Microscopic techniques as means for the determination of microplastics and nanoplastics in the aquatic environment: A concise review. Green Anal. Chem. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Advances in microplastics detection: A comprehensive review of methodologies and their effectiveness. TrAC Trends Anal. Chem. 2024, 170, 117440. [Google Scholar] [CrossRef]
- Silva, A.B.; Bastos, A.S.; Justino, C.I.; da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T.A. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19. [Google Scholar] [CrossRef]
- Rumin, J.; Bonnefond, H.; Saint-Jean, B.; Rouxel, C.; Sciandra, A.; Bernard, O.; Cadoret, J.-P.; Bougaran, G. The use of fluorescent Nile red and BODIPY for lipid measurement in microalgae. Biotechnol. Biofuels 2015, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Jiang, L.; Liu, R.; He, M.; Cui, X.; Wang, C. Comprehensive assessment of factors influencing Nile red staining: Eliciting solutions for efficient microplastics analysis. Mar. Pollut. Bull. 2021, 171, 112698. [Google Scholar] [CrossRef]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.T.; Horn, H.; Schuhen, K. The potential of fluorescent dyes—Comparative study of Nile red and three derivatives for the detection of microplastics. Anal. Bioanal. Chem. 2021, 413, 1059–1071. [Google Scholar] [CrossRef]
- Shim, W.J.; Song, Y.K.; Hong, S.H.; Jang, M. Identification and quantification of microplastics using Nile Red staining. Mar. Pollut. Bull. 2016, 113, 469–476. [Google Scholar] [CrossRef]
- Tamminga, M.; Hengstmann, E.; Fischer, E. Nile Red Staining as a Subsidiary Method for Microplastic Quantification: A Comparison of Three Solvents and Factors Influencing Application Reliability. J. Earth Sci. Environ. Stud. 2017, 2, 1–8. [Google Scholar]
- Erni-Cassola, G.; Gibson, M.I.; Thompson, R.C.; Christie-Oleza, J.A. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ. Sci. Technol. 2017, 51, 13641–13648. [Google Scholar] [CrossRef]
- Nel, H.A.; Chetwynd, A.J.; Kelleher, L.; Lynch, I.; Mansfield, I.; Margenat, H.; Onoja, S.; Goldberg Oppenheimer, P.; Sambrook Smith, G.H.; Krause, S. Detection limits are central to improve reporting standards when using Nile red for microplastic quantification. Chemosphere 2021, 263, 127953. [Google Scholar] [CrossRef]
- Campanale, C.; Savino, I.; Pojar, I.; Massarelli, C.; Uricchio, V.F. A Practical Overview of Methodologies for Sampling and Analysis of Microplastics in Riverine Environments. Sustainability 2020, 12, 6755. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division, Report; NOAA: Washington, DC, USA, 2015. [CrossRef]
- Sebők-Nagy, K.; Biczók, L. Effect of hydrogen-bonding on the fluorescent properties of Nile Red and N-(4-pyridyl)-1,2-naphthalimide. Magy. Kem. F. Kem. Kozlemenyek 2004, 109–110, 29–33. [Google Scholar]
- Ikeda, A.; Abe, C.; Matsuura, W.; Hasegawa, Y. Development of Methanol Permselective FAU-Type Zeolite Membranes and Their Permeation and Separation Performances. Membranes 2021, 11, 627. [Google Scholar] [CrossRef]
- Ghobadi, E.; Marquardt, A.; Zirdehi, E.M.; Neuking, K.; Varnik, F.; Eggeler, G.; Steeb, H. The Influence of Water and Solvent Uptake on Functional Properties of Shape-Memory Polymers. Int. J. Polym. Sci. 2018, 2018, e7819353. [Google Scholar] [CrossRef]
- Konde, S.; Ornik, J.; Prume, J.A.; Taiber, J.; Koch, M. Exploring the potential of photoluminescence spectroscopy in combination with Nile Red staining for microplastic detection. Mar. Pollut. Bull. 2020, 159, 111475. [Google Scholar] [CrossRef] [PubMed]
- Burke, J. Solubility Parameters: Theory and Application. Available online: https://cool.culturalheritage.org/coolaic/sg/bpg/annual/v03/bp03-04.html (accessed on 8 October 2022).
- Surface Free Energy Components by Polar/Dispersion and Acid—Base Analyses; and Hansen Solubility Parameters for Various Polymers. Available online: https://www.accudynetest.com/polytable_02.html (accessed on 1 December 2023).
- (PDF) Reverse Shape Selectivity of Hexane Isomer in Ligand Inserted MOF-74. ResearchGate. Available online: https://www.researchgate.net/publication/342173219_Reverse_shape_selectivity_of_hexane_isomer_in_ligand_inserted_MOF-74 (accessed on 11 April 2025).
- Critical Surface Tension and Contact Angle with Water for Various Polymers (Sort by Contact Angle). Available online: https://www.accudynetest.com/polytable_03.html?sortby=contact_angle (accessed on 1 December 2023).
- Zedníček, T. What Is a Dielectric Constant of Plastic Materials? Passive Components Blog. Available online: https://passive-components.eu/what-is-dielectric-constant-of-plastic-materials/ (accessed on 1 December 2023).
- Min, K.; Cuiffi, J.D.; Mathers, R.T. Ranking environmental degradation trends of plastic marine debris based on physical properties and molecular structure. Nat. Commun. 2020, 11, 727. [Google Scholar] [CrossRef]
- Farnand, S.P. Using ΔE metrics for measuring color difference in hard copy pictorial images. In Proceedings of the Color Imaging VIII: Processing, Hardcopy, and Applications, SPIE. Santa Clara, CA, USA, 21–24 January 2003; pp. 109–118. [Google Scholar]
- Delta E ≤ 2 Color Accuracy. Available online: https://www.viewsonic.com/colorpro/articles/detail/deltae2color-accuracy_3 (accessed on 5 December 2023).
- Hoekstra, S. How Do You Measure Color Accuracy? Nix Sensor Ltd. Available online: https://www.nixsensor.com/blog/measure-color-accuracy/ (accessed on 5 December 2023).
- Best, J. Colour Design: Theories and Applications; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Lin, J.; Yan, D.; Fu, J.; Chen, Y.; Ou, H. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020, 186, 116360. [Google Scholar] [CrossRef]
- Chaudhary, B.I.; Liotta, C.L.; Cogen, J.M.; Gilbert, M. Plasticized PVC. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Ahmad, Z.; Ahmad, Z. Polymer Dielectric Materials; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Delocalization of Electrons. Chemistry LibreTexts. Available online: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Chemical_Bonding/Valence_Bond_Theory/Delocalization_of_Electrons (accessed on 14 February 2024).
- Yasuda, T.; Okuno, T.; Yasuda, H. Contact Angle of Water on Polymer Surfaces. Langmuir 1994, 10, 2435–2439. [Google Scholar] [CrossRef]
- Van Melkebeke, M.; Janssen, C.; De Meester, S. Characteristics and Sinking Behavior of Typical Microplastics Including the Potential Effect of Biofouling: Implications for Remediation. Environ. Sci. Technol. 2020, 54, 8668–8680. [Google Scholar] [CrossRef]
- Rayssi, C.; El Kossi, S.; Dhahri, J.; Khirouni, K. Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1−xCo4x/3O3 (0 ≤ x ≤ 0.1). RSC Adv. 2018, 8, 17139–17150. [Google Scholar] [CrossRef]
- Dielectric Properties. Available online: https://polymerdatabase.com/polymer%20physics/Permitivity.html (accessed on 8 October 2022).
- Dielectric Constant: Definition, Units, Formula, Plastic Values &Material List. Available online: https://omnexus.specialchem.com/polymer-properties/properties/dielectric-constant (accessed on 25 November 2022).
- La Mantia, F.P.; Schifani, R. Natural weathering of low density polyethylene: Part 2—Dielectric properties. Polym. Degrad. Stab. 1985, 10, 67–78. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Topics in Fluorescence Spectroscopy: Principles; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Fotopoulou, K.N.; Karapanagioti, H.K. Degradation of Various Plastics in the Environment. In Hazardous Chemicals Associated with Plastics in the Marine Environment; Takada, H., Karapanagioti, H.K., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–92. [Google Scholar] [CrossRef]
- Meyers, N.; Catarino, A.I.; Declercq, A.M.; Brenan, A.; Devriese, L.; Vandegehuchte, M.; De Witte, B.; Janssen, C.; Everaert, G. Microplastic detection and identification by Nile red staining: Towards a semi-automated, cost- and time-effective technique. Sci. Total Environ. 2022, 823, 153441. [Google Scholar] [CrossRef]
- Prasad, S.; Bennett, A.; Triantafyllou, M. Characterization of Nile Red-Stained Microplastics through Fluorescence Spectroscopy. J. Mar. Sci. Eng. 2024, 12, 1403. [Google Scholar] [CrossRef]
- Fang, C.; Luo, Y.; Naidu, R. Microplastics and nanoplastics analysis: Options, imaging, advancements and challenges. TrAC Trends Anal. Chem. 2023, 166, 117158. [Google Scholar] [CrossRef]



| WCA [50] | DE [51] | log Kow [52] |
|---|---|---|
| PP (102.1°) | PP (2.3) | PP (23.7) |
| PE (96.0°) | PE (2.3) | PE (23.6) |
| PS (87.4°) | PS (2.55) | PS (22.9) |
| PVC (85.6°) | ABS (2.95) | - |
| PC (82.0°) | PC (3.3) | PC (21.3) |
| ABS (80. 9°) | PVC (3.55) | PVC (16.2) |
| PET (72.5°) | PET (3.5) | PET (8.1) |
| PA (68.3°) | PA (4.5) | PA (4.5) |
| ∆E of MPs | PP | HDPE | LDPE | EPS | PS | PC | ABS | PVC | PET | PA |
|---|---|---|---|---|---|---|---|---|---|---|
| PP | 17.3 | 37.7 | 57.6 | 61.2 | 74.2 | 90.3 | 90.1 | 88.1 | 91.8 | |
| HDPE | 29.5 | 46.6 | 51.7 | 63.8 | 80.4 | 80.7 | 74.9 | 82.4 | ||
| LDPE | 20.5 | 24.2 | 37.1 | 55.5 | 56.5 | 55.2 | 59.2 | |||
| EPS | 10.1 | 18.8 | 39.7 | 42.1 | 38.3 | 45.5 | ||||
| PS | 13.3 | 32.2 | 34.0 | 35.5 | 37.5 | |||||
| PC | 22.0 | 25.4 | 26.6 | 29.5 | ||||||
| ABS | 5.4 | 22.1 | 9.5 | |||||||
| PVC | 24.3 | 4.7 | ||||||||
| PET | 24.7 | |||||||||
| PA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, D.; Masura, J. Dyeing to Know: Harmonizing Nile Red Staining Protocols for Microplastic Identification. Colorants 2025, 4, 20. https://doi.org/10.3390/colorants4020020
Ho D, Masura J. Dyeing to Know: Harmonizing Nile Red Staining Protocols for Microplastic Identification. Colorants. 2025; 4(2):20. https://doi.org/10.3390/colorants4020020
Chicago/Turabian StyleHo, Derek, and Julie Masura. 2025. "Dyeing to Know: Harmonizing Nile Red Staining Protocols for Microplastic Identification" Colorants 4, no. 2: 20. https://doi.org/10.3390/colorants4020020
APA StyleHo, D., & Masura, J. (2025). Dyeing to Know: Harmonizing Nile Red Staining Protocols for Microplastic Identification. Colorants, 4(2), 20. https://doi.org/10.3390/colorants4020020








