Methylene Blue as a Sensitizing Dye: Enhancement of the Photocatalytic Performance of a Peroxide-Functionalized Iron Molybdate by the Antenna Effect
Abstract
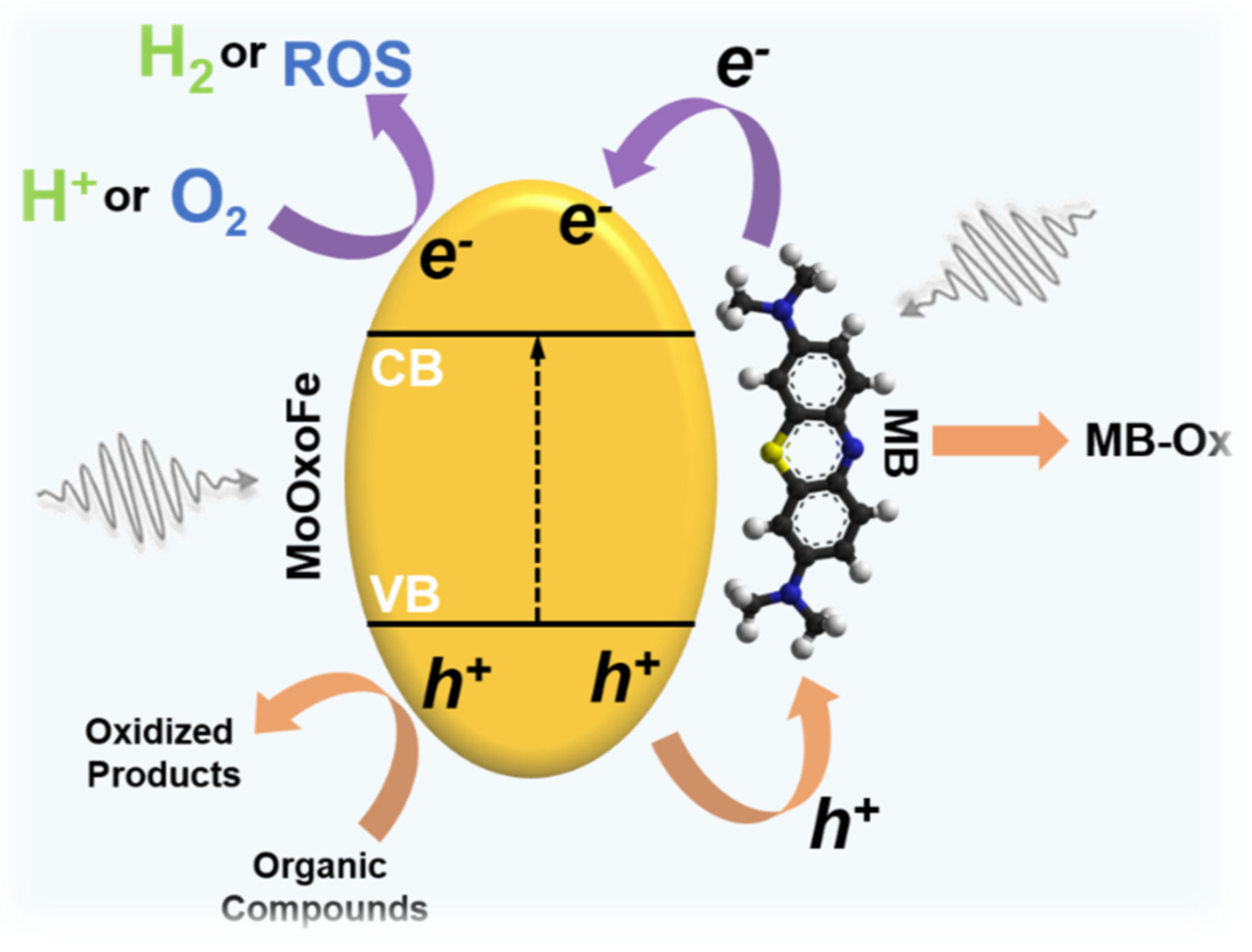
:1. Introduction
2. Experimental
2.1. Synthesis
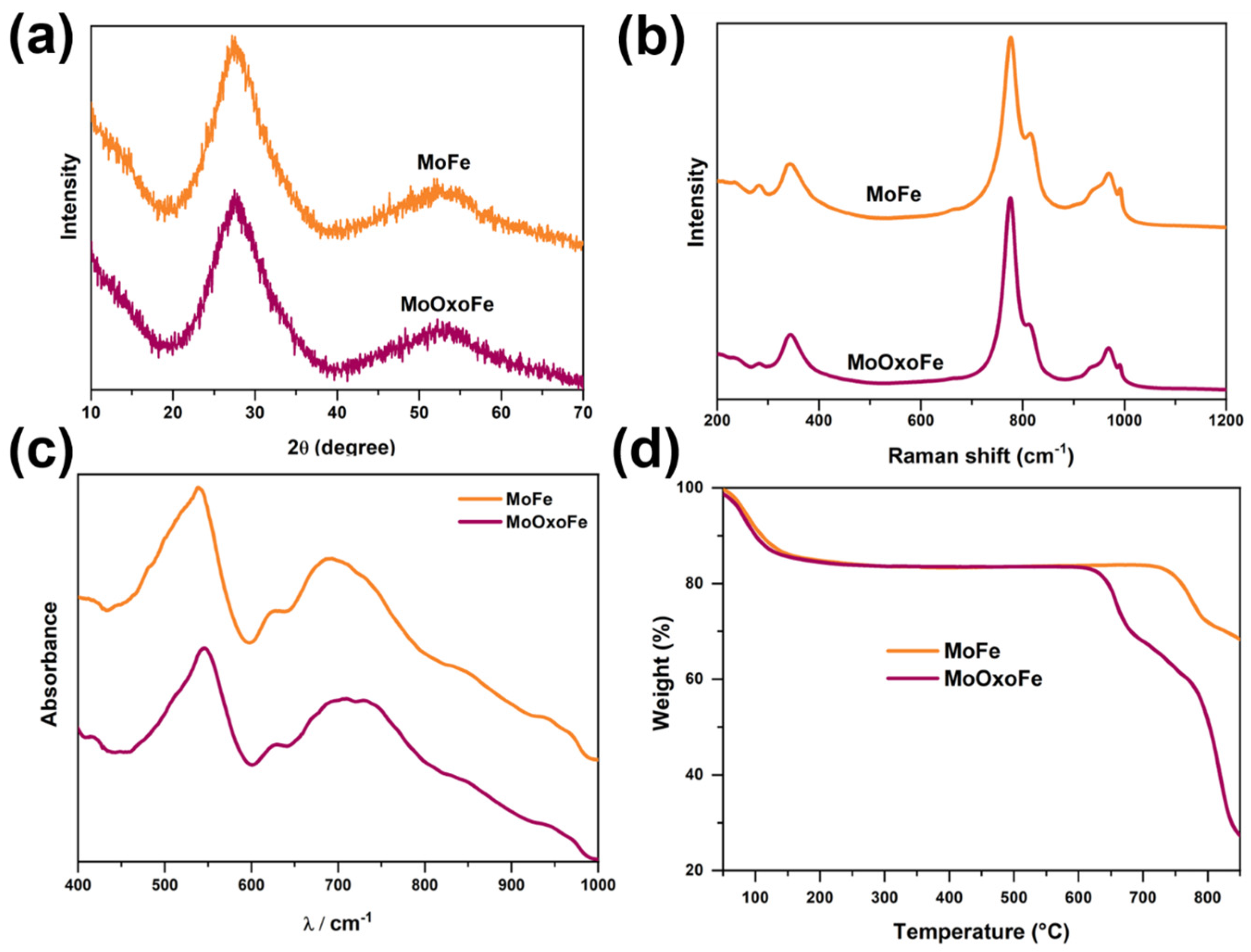
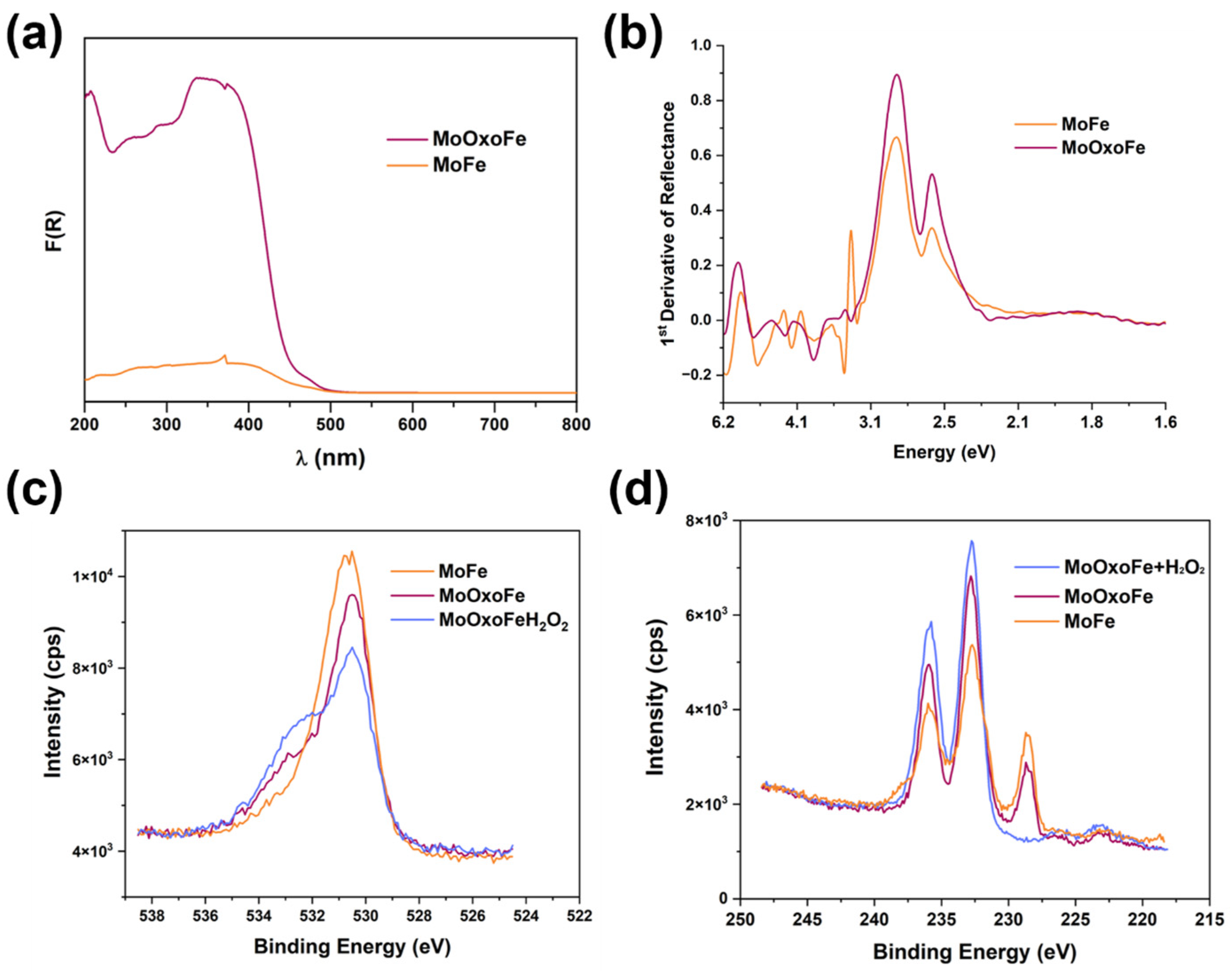
2.2. Characterizations
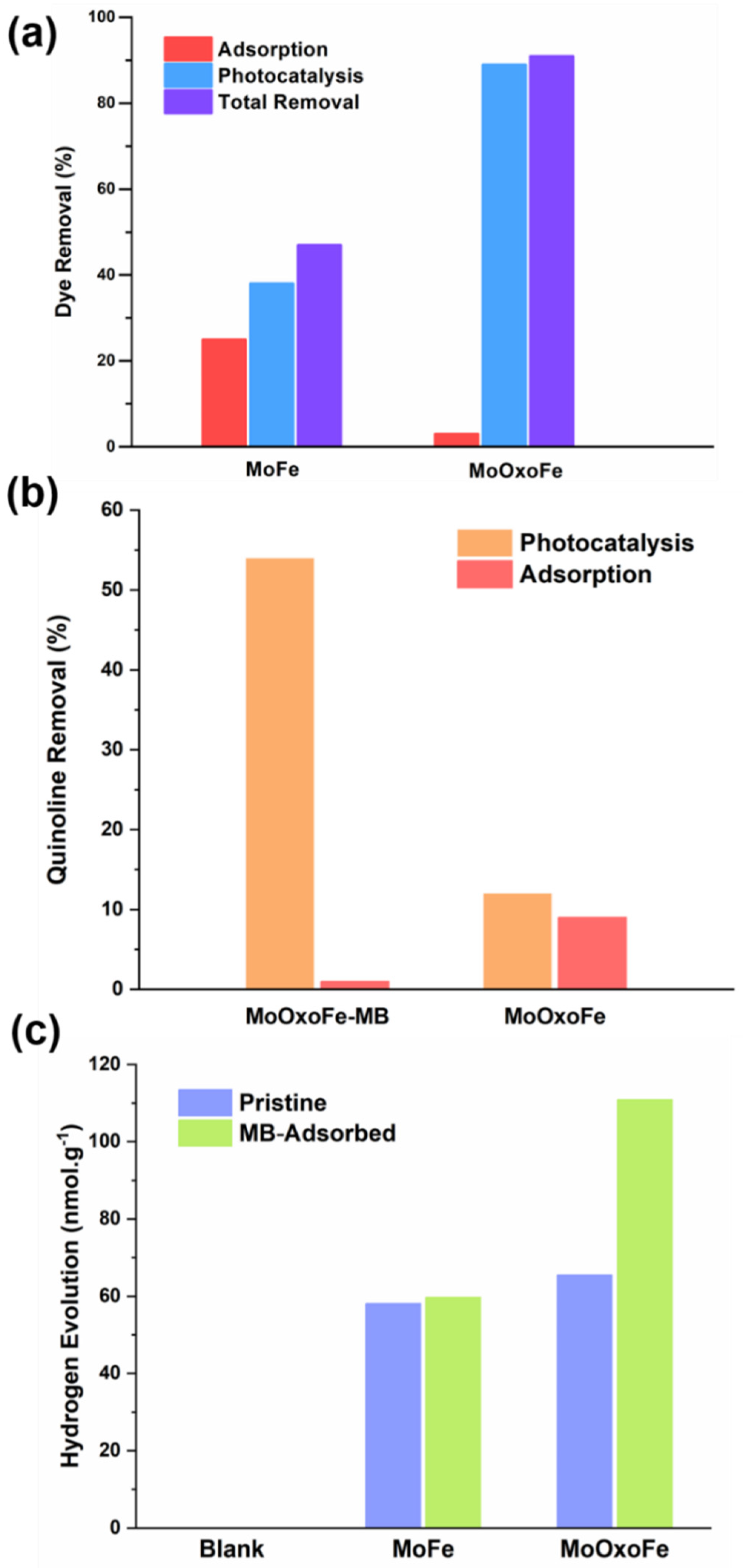
2.3. Photocatalysis
2.3.1. MB Test
2.3.2. Quinoline Test
2.3.3. Hydrogen Evolution Test
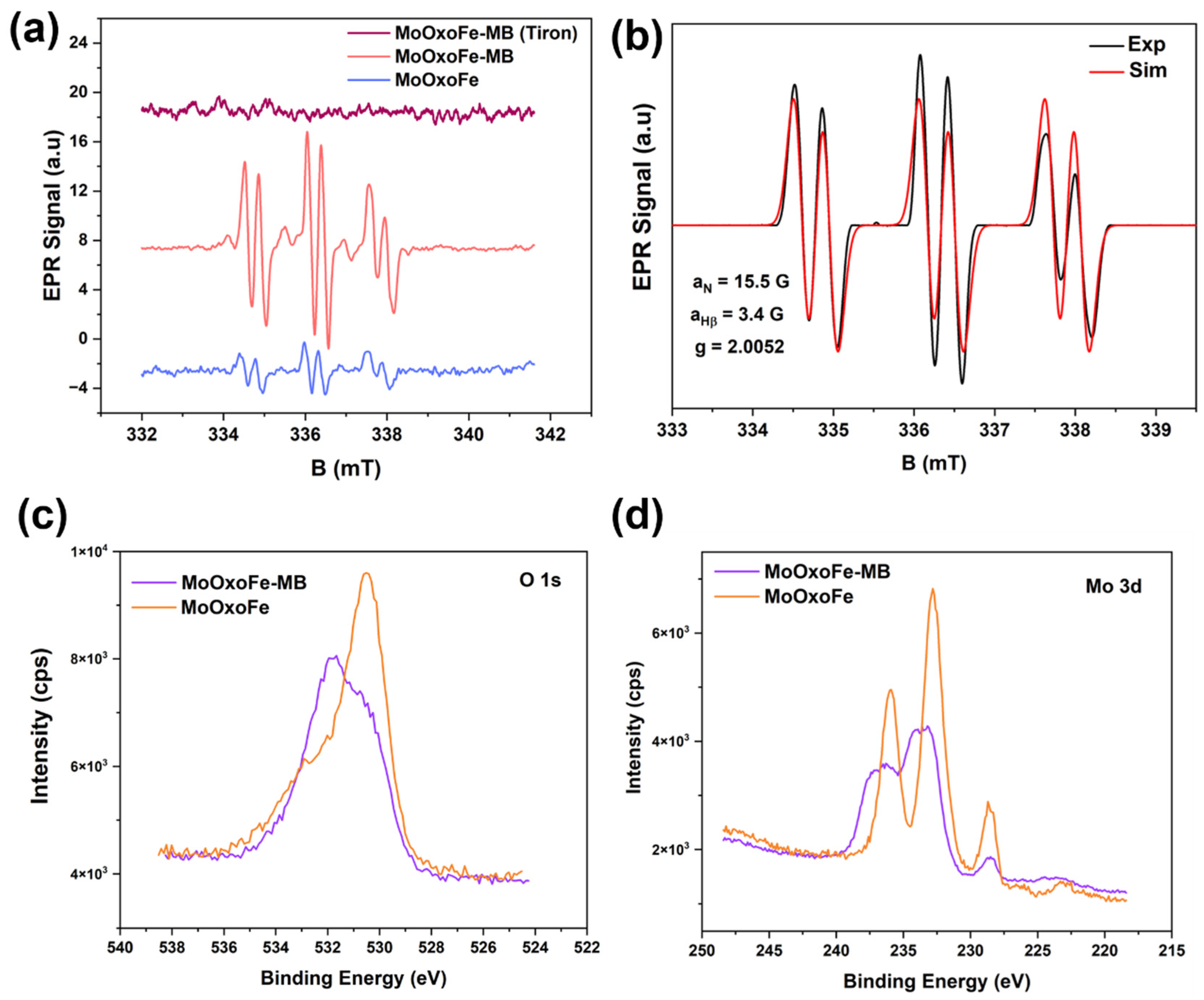
2.3.4. Reactive Species Test
3. Results and Discussion
Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaghoubi, S.; Mousavi, S.M.; Babapoor, A.; Binazadeh, M.; Lai, C.W.; Althomali, R.H.; Rahman, M.M.; Chiang, W.H. Photocatalysts for solar energy conversion: Recent advances and environmental applications. Renew. Sustain. Energy Rev. 2024, 200, 114538. [Google Scholar] [CrossRef]
- Dai, Y.; Xiong, Y. Control of selectivity in organic synthesis via heterogeneous photocatalysis under visible light. Nano Res. Energy 2022, 1, e9120006. [Google Scholar] [CrossRef]
- Gomes, G.H.M.; Gabriel, J.B.; Bruziquesi, C.G.O.; Victoria, H.V.; Krambrock, K.; Oliveira, L.C.A.; Mohallem, N.D.S. The role of oxygen vacancies in TT-Nb2O5 nanoparticles for the photoconversion of glycerol into solketal. Ceram. Int. 2023, 49, 14719–14732. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef] [PubMed]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef]
- Cao, G.M.; Hu, X.L.; Liao, L.L.; Yan, S.S.; Song, L.; Chruma, J.J.; Gong, L.; Yu, D.G. Visible-light photoredox-catalyzed umpolung carboxylation of carbonyl compounds with CO2. Nat. Commun. 2021, 12, 2–11. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. Defective dopant-free TiO2 as an efficient visible light-active photocatalyst. Catalysts 2021, 11, 978. [Google Scholar] [CrossRef]
- Song, C.; Xiao, L.; Chen, Y.; Yang, F.; Meng, H.; Zhang, W.; Zhang, Y.; Wu, Y. TiO2-Based Catalysts with Various Structures for Photocatalytic Application: A Review. Catalysts 2024, 14, 366. [Google Scholar] [CrossRef]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors. Int. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- Gonuguntla, S.; Kamesh, R.; Pal, U.; Chatterjee, D. Dye sensitization of TiO2 relevant to photocatalytic hydrogen generation: Current research trends and prospects. J. Photochem. Photobiol. C Photochem. Rev. 2023, 57, 100621. [Google Scholar] [CrossRef]
- Michalec, K.; Kusior, A. From adsorbent to photocatalyst: The sensitization effect of SnO2 surface towards dye photodecomposition. Molecules 2021, 26, 7123. [Google Scholar] [CrossRef]
- Castillo-Robles, J.A.; Rocha-Rangel, E.; Ramírez-De-león, J.A.; Caballero-Rico, F.C.; Armendáriz-Mireles, E.N. Advances on dye-sensitized solar cells (DSSCs) nanostructures and natural colorants: A review. J. Compos. Sci. 2021, 5, 288. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dye. Pigment. 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Sudarsan, S.; Venthan, S.M.; Kumar, P.S.; Anandkumar, M.; Uchaev, D.A.; Trofimov, E.A.; Rangasamy, G. Investigation of the surface and photocatalytic behaviour of copper–iron–molybdate Cu7.26Fe7.26Mo12O48 nanocomposites prepared from discarded printed circuit boards. New J. Chem. 2025, 49, 2685–2693. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, P.; Sharma, G.; Dhiman, P.; Shekh, M.; Sillanpää, M.; Stadler, F.J. Recent progress in advanced strategies to enhance the photocatalytic performance of metal molybdates for H2 production and CO2 reduction. J. Alloys Compd. 2024, 971, 172665. [Google Scholar] [CrossRef]
- Yu, J.; Nong, Q.; Jiang, X.; Liu, X.; Wu, Y.; He, Y. Novel Fe2(MoO4)3/g-C3N4 heterojunction for efficient contaminant removal and hydrogen production under visible light irradiation. Sol. Energy 2016, 139, 355–364. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Hill, G.; Wilson, J.H. Raman spectroscopy of iron molybdate catalyst systems: Part I. Prep. Unsupported Catal. J. Mol. Catal. 1990, 63, 65–94. [Google Scholar]
- Xu, Q.; Jia, G.; Zhang, J.; Feng, Z.; Li, C. Surface Phase Composition of Iron Molybdate Catalysts Studied by UV Raman Spectroscopy. J. Phys. Chem. C 2008, 112, 9387–9393. [Google Scholar]
- House, M.P.; Carley, A.F.; Echeverria-valda, R.; Bowker, M. Effect of Varying the Cation Ratio within Iron Molybdate Catalysts for the Selective Oxidation of Methanol. J. Phys. Chem. C 2008, 112, 4333–4341. [Google Scholar] [CrossRef]
- Belhekar, A.A.; Ayyappan, S.; Ramaswamy, A.V. FT-IR studies on the evolution of different phases and their interaction in ferric molybdate—Molybdenum trioxide catalysts. J. Chem. Technol. Biotechnol. 1994, 59, 395–402. [Google Scholar] [CrossRef]
- Brookes, C.; Wells, P.P.; Cibin, G.; Dimitratos, N.; Jones, W.; Morgan, D.J.; Bowker, M. Molybdenum oxide on Fe2O3 core-shell catalysts: Probing the nature of the structural motifs responsible for methanol oxidation catalysis. ACS Catal. 2014, 4, 243–250. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ibrahim, A.A.; Rayan, D.A.; Sanad, M.M.S.; Helmy, I.M. Photo-Fenton-like degradation of Rhodamine B dye from waste water using iron molybdate catalyst under visible light irradiation, Environ. Nanotechnol. Monit. Manag. 2017, 8, 175–186. [Google Scholar] [CrossRef]
- Louka, F.R.; Nguyen, L.T.; Albering, J.H.; Mautner, F.A.; Massoud, S.S. Unprecedented formation of a doubly bridged μ-peroxo-μ-pyrazolyldicarboxylato-dicobalt(III) complex. Inorg. Chem. Commun. 2012, 15, 269–271. [Google Scholar] [CrossRef]
- Sorrell, T.N.; Allen, W.E.; White, P.S. Sterically Hindered [Tris(imidazolyl)phosphine]copper Complexes: Formation and Reactivity of a Peroxo-Dicopper(II) Adduct and Structure of a Dinuclear Carbonate-Bridged Complex. Inorg. Chem. 1995, 34, 952–960. [Google Scholar] [CrossRef]
- Park, J.H.; Yoo, Y.B.; Lee, K.H.; Jang, W.S.; Oh, J.Y.; Chae, S.S.; Baik, H.K. Low-temperature, high-performance solution-processed thin-film transistors with peroxo-zirconium oxide dielectric. ACS Appl. Mater. Interfaces 2013, 5, 410–417. [Google Scholar] [CrossRef]
- Gütlich, P.; Schröder, C.; Schünemann, V. Mössbauer spectroscopy—An indispensable tool in solid state research. Spectrosc. Eur. 2012, 24, 21–32. [Google Scholar]
- Kuzmann, E.; Homonnay, Z.; Klencsár, Z.; Szalay, R. 57Fe mössbauer spectroscopy as a tool for study of spin states and magnetic interactions in inorganic chemistry. Molecules 2021, 26, 1062. [Google Scholar] [CrossRef]
- Oefner, N.; Heck, F.; Dürl, M.; Schumacher, L.; Siddiqui, H.K.; Kramm, U.I.; Hess, C.; Möller, A.; Albert, B.; Etzold, B.J.M. Activity, Selectivity and Initial Degradation of Iron Molybdate in the Oxidative Dehydrogenation of Ethanol. ChemCatChem 2022, 14, e202101219. [Google Scholar] [CrossRef]
- Castelão-Dias, M.; Costa BF, O.; Quinta-Ferreira, R.M. 57Fe Mössbauer Studies in Mo–Fe Supported Catalysts. Hyperfine Interact. 2001, 136, 9–16. [Google Scholar] [CrossRef]
- Manseri, K.; Hentit, H.; Elandaloussi, E.H.; Benaichouba, B.; Ouali, M.S. The role of cationic additions in molybdate multi-component catalysts. Hyperfine Interact. 2010, 198, 243–257. [Google Scholar] [CrossRef]
- Bayot, D.; Tinant, B.; Devillers, M. Water-soluble niobium peroxo complexes as precursors for the preparation of Nb-based oxide catalysts. Catal. Today 2003, 78, 439–447. [Google Scholar] [CrossRef]
- Würtele, C.; Sander, O.; Lutz, V.; Waitz, T.; Tuczek, F.; Schindler, S. Aliphatic C-H bond oxidation of toluene using copper peroxo complexes that are stable at room temperature. J. Am. Chem. Soc. 2009, 131, 7544–7545. [Google Scholar] [CrossRef] [PubMed]
- Pajić, D.N.; Djinović, P.; Dražić, G.; Grdadolnik, J.; Šket, P.; Cerkovnik, J.; Pintar, A. Structural stabilization and characterization of active peroxo species on TiO2-nanotube based materials in mild catalytic wet peroxide oxidation process. Appl. Catal. A Gen. 2018, 562, 276–283. [Google Scholar] [CrossRef]
- Bonino, F.; Damin, A.; Ricchiardi, G.; Ricci, M.; Spano, G.; Aloisio, R.D.; Zecchina, A.; Lamberti, C.; Prestipino, C.; Bordiga, S. Ti-Peroxo Species in the TS-1/H2O2/H2O System. J. Phys. Chem. B 2004, 108, 3573–3583. [Google Scholar]
- Nikolenko, M.V.; Kostynyuk, A.O.; Goutenoire, F.; Kalashnikov, Y.V. Chemical precipitation of iron(III) molybdate + molybdenum trioxide mixtures through continuous crystallization. Inorg. Mater. 2014, 50, 1140–1145. [Google Scholar] [CrossRef]
- Shaheen, W.M. Thermal behaviour of pure and binary Fe(NO3)3·9H2O and (NH4)6Mo7O24·4H2O systems. Mater. Sci. Eng. A 2007, 445, 113–121. [Google Scholar] [CrossRef]
- Brookes, C.; Wells, P.P.; Dimitratos, N.; Jones, W.; Gibson, E.K.; Morgan, D.J.; Cibin, G.; Nicklin, C.; Mora-Fonz, D.; Scanlon, D.O.; et al. The Nature of the Molybdenum Surface in Iron Molybdate the Active Phase in Selective Methanol Oxidation. J. Phys. Chem. C 2014, 118, 26155–26161. [Google Scholar] [CrossRef]
- Yeo, B.R.; Pudge, G.J.F.; Bugler, K.G.; Rushby, A.V.; Kondrat, S.; Bartley, J.; Golunski, S.; Taylor, S.H.; Gibson, E.; Wells, P.P.; et al. The surface of iron molybdate catalysts used for the selective oxidation of methanol. Surf. Sci. 2016, 648, 163–169. [Google Scholar] [CrossRef]
- Nikolenko, N.V.; Kozhevnikov, I.V.; Kostyniuk, A.O.; Bayahia, H.; Kalashnykov, Y.V. Preparation of iron molybdate catalysts for methanol to formaldehyde oxidation based on ammonium molybdoferrate(II) precursor. J. Saudi Chem. Soc. 2018, 22, 372–379. [Google Scholar] [CrossRef]
- Douvas, A.M.; Vasilopoulou, M.; Georgiadou, D.G.; Soultati, A.; Davazoglou, D.; Vourdas, N.; Giannakopoulos, K.P.; Kontos, A.G.; Kennou, S.; Argitis, P. Sol-gel synthesized, low-temperature processed, reduced molybdenum peroxides for organic optoelectronics applications. J. Mater. Chem. C 2014, 2, 6290–6300. [Google Scholar] [CrossRef]
- Islam, T.; Roy, S.C.; Bayat, S.; Weret, M.A.; Hoffman, J.M.; Rao, K.R.; Sawicki, C.; Nie, J.; Alam, R.; Oketola, O.; et al. Mo3S13 Chalcogel: A High-Capacity Electrode for Conversion-Based Li-Ion Batteries. ChemSusChem 2024, 17, e202400084. [Google Scholar] [CrossRef]
- Wu, H.; Lian, K. The Development of Pseudocapacitive Molybdenum Oxynitride Electrodes for Supercapacitors. ECS Trans. 2014, 58, 67–75. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Ramalho, T.C.; Oliveira, L.C.A.; Carvalho, K.T.G.; Souza, E.F.; Da Cunha, E.F.F.; Nazzaro, M. Catalytic behavior of niobia species on oxidation reactions: Insights from experimental and theoretical models. J. Mater. Sci. 2008, 43, 5982–5988. [Google Scholar] [CrossRef]
- Molla, A.; Sahu, M.; Hussain, S. Under dark and visible light: Fast degradation of methylene blue in the presence of Ag-In-Ni-S nanocomposites. J. Mater. Chem. A 2015, 3, 15616–15625. [Google Scholar] [CrossRef]
- Wolski, L.; Sobańska, K.; Walkowiak, A.; Akhmetova, K.; Gryboś, J.; Frankowski, M.; Ziolek, M.; Pietrzyk, P. Enhanced adsorption and degradation of methylene blue over mixed niobium-cerium oxide—Unraveling the synergy between Nb and Ce in advanced oxidation processes. J. Hazard. Mater. 2021, 415, 125665. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Almeida, L.D.; Victória, H.F.V.; Gomes, G.H.M.; Krambrock, K.; Robles-Azocar, P.A.; Pereira, M.C.; Oliveira, L.C.A. Niobium Oxides: The key role of hydroxylated surface on photocatalytic driven C–C reductive coupling of acetophenone. J. Catal. 2024, 436, 115580. [Google Scholar] [CrossRef]
- Buettner, G.R. Spin Trapping: Esr Parameters of Spin Adducts. Free Radic. Biol. Med. 1987, 3, 259–303. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Gomes, G.H.M.; Silva, I.F.; Rios, R.D.F.; Victória, H.F.V.; Krambrock, K.; Pereira, M.C.; Oliveira, L.C.A. Photocatalytic reduction of levulinic acid using thermally modified niobic acid. Chem. Eng. J. 2022, 450, 1385–1394. [Google Scholar] [CrossRef]
- Filho, J.B.G.; Silva, I.F.; Alafandi, M.; Rabeah, J. Aerobic Oxidation of 5-Hydroxymethylfurfural (HMF) in Aqueous Medium over Fe-Doped-Poly(heptazine imide) Photocatalysts: Unveiling the Bad Role of Hydroxyl Radical Generation on the Catalytic Performance. Molecules 2023, 28, 8077. [Google Scholar] [CrossRef]
- JFilho, B.G.; Rios, R.D.F.; Bruziquesi, C.G.O.; Ferreira, D.C.; Victória, H.F.V.; Krambrock, K.; Pereira, M.C.; Oliveira, L.C.A. A promising approach to transform levulinic acid into γ-valerolactone using niobic acid photocatalyst and the accumulated electron transfer technique. Appl. Catal. B Environ. 2021, 285, 119814. [Google Scholar] [CrossRef]
- Taiwo, F.A. Mechanism of tiron as scavenger of superoxide ions and free electrons. Spectroscopy 2008, 22, 491–498. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Alharthi, A.I.; Qahtan, T.F.; Alotibi, S.; Ali, I.; Bakht, M.A. Green synthesis of xanthene derivatives through visible light-driven photocatalysis using blackberry dye-sensitized TiO2. J. Alloys Compd. 2024, 978, 173388. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filho, J.B.G.; Vieira, C.G.; Jesus, D.B.d.; Victória, H.F.V.; Soares, E.A.; Krambrock, K.; Pereira, M.C.; Oliveira, L.C.A. Methylene Blue as a Sensitizing Dye: Enhancement of the Photocatalytic Performance of a Peroxide-Functionalized Iron Molybdate by the Antenna Effect. Colorants 2025, 4, 14. https://doi.org/10.3390/colorants4020014
Filho JBG, Vieira CG, Jesus DBd, Victória HFV, Soares EA, Krambrock K, Pereira MC, Oliveira LCA. Methylene Blue as a Sensitizing Dye: Enhancement of the Photocatalytic Performance of a Peroxide-Functionalized Iron Molybdate by the Antenna Effect. Colorants. 2025; 4(2):14. https://doi.org/10.3390/colorants4020014
Chicago/Turabian StyleFilho, José Balena G., Clóvis G. Vieira, Daniel B. de Jesus, Henrique F. V. Victória, Edmar A. Soares, Klaus Krambrock, Márcio César Pereira, and Luiz Carlos A. Oliveira. 2025. "Methylene Blue as a Sensitizing Dye: Enhancement of the Photocatalytic Performance of a Peroxide-Functionalized Iron Molybdate by the Antenna Effect" Colorants 4, no. 2: 14. https://doi.org/10.3390/colorants4020014
APA StyleFilho, J. B. G., Vieira, C. G., Jesus, D. B. d., Victória, H. F. V., Soares, E. A., Krambrock, K., Pereira, M. C., & Oliveira, L. C. A. (2025). Methylene Blue as a Sensitizing Dye: Enhancement of the Photocatalytic Performance of a Peroxide-Functionalized Iron Molybdate by the Antenna Effect. Colorants, 4(2), 14. https://doi.org/10.3390/colorants4020014





