1. Introduction
Direct thermal-printing technology is widely used for facsimiles, receipts and many kinds of tickets, because the printing systems involved are simple and do not require much maintenance [
1]. The key process in this technology is the production of colored images on thermo-sensitive paper by means of a thermal head. The paper consists of a paper substrate and a recording layer that includes a leuco dye and its developer. Fluoran dyes, known as “one-dye black”, are typically used as dye precursors. The black color is provided by strong absorption peaks around the yellow and blue regions, which are complementary in color [
2,
3]. The coloration reaction of fluoran dyes is shown in
Scheme 1. The color change in fluoran dyes occurs by means of a chemical reaction on the spiro-lacton ring. Ring cleavage results in π-conjugation of the chromophoric system, giving rise to an absorption band in the visible region. To carry out the ring cleavage reaction in this coloration system, acidic phenol derivatives, traditionally bisphenol A (BPA), are generally used as developers. However, recent reports have suggested that BPA may adversely affect human health, particularly through endocrine disruption [
4,
5,
6]. In addition, the low stability of the colored state poses a problem in the preservation of printed images over prolonged durations. Recently, bisphenol S and phenol-free substituents Perfumefast 201 and urea–urethane (UU) compounds have been used as alternatives to BPA [
7,
8,
9].
UU compounds, shown in
Figure 1, were synthesized as developers for high-performance thermo-sensitive paper [
10,
11]. UU compounds do not contain acidic protons, but they can develop the black color of fluoran dyes. They are also known to improve the speed of the printed images and have lower environmental loads than conventional phenolic developers. At present, these compounds are commercially available, but the mechanism governing color development and the reasons for the high stability of the resulting colored images are still unknown. The color development properties of UU compounds are influenced by their structural differences, which are the number of UU units, the position of the methyl substituent and the connecting group. Compounds
1 and
4 are known to provide excellent stability to printed images’ in-house performance test.
We analyzed the crystal structure of
5 and
6 and found that two types of intermolecular hydrogen bonds are formed between the urea groups and the urethane groups in these compounds [
12]. One is a bifurcated hydrogen bond between the two amino groups of the urea moiety and the carbonyl group of the urethane moiety. The other is a hydrogen bond between the amino group of the urethane moiety and the carbonyl group of the urea moiety. The ability of UU compounds to form multiple hydrogen bonds in the solid state was suggested to be advantageous for the color development of fluoran dyes and for stabilizing the colored state. In this study, we investigated the solid-state structure and thermal properties of black solids prepared from six UU compounds with a fluoran dye in order to interpret the mechanisms governing color development and stabilization. One of the best ways to understand structure–property relationships in solids is to analyze the crystal structure; in this case, however, our attempts to crystallize the black solids composed of the UU compounds and the fluoran dye only resulted in the formation of amorphous black solids. Hence, the two mechanisms were evaluated using infrared (IR) spectroscopy and differential scanning calorimetry (DSC) measurements on amorphous black solids, together with molecular orbital calculations for the UU compounds.
2. Materials and Methods
All UU compounds were supplied by Chemipro Kasei Kaisha, Ltd., and used without further purification. Their synthetic procedure and structural information are described in the patents [
10,
11]. In the patents, each compound is characterized by its IR spectrum. Characteristic peaks of each compound are transcribed below. The peaks of
1 and
2 appeared around 750 cm
−1, 840 cm
−1, 1020 cm
−1, 1500 cm
−1, 1600 cm
−1, 1720 cm
−1 and 3320 cm
−1. The peaks of
3 appeared around 550 cm
−1, 1030 cm
−1, 1110 cm
−1, 1150 cm
−1, 1590 cm
−1, 1700 cm
−1 and 3300 cm
−1. The peaks of
4 appeared around 990 cm
−1, 1110 cm
−1, 1320 cm
−1, 1590 cm
−1, 1700 cm
−1 and 3350 cm
−1. The peaks of
5 appeared around 880 cm
−1, 1000 cm
−1, 1040 cm
−1, 1440 cm
−1, 1720 cm
−1, 1720 cm
−1 and 3300 cm
−1. The peaks of
6 appeared around 1060 cm
−1, 1250 cm
−1, 1600 cm
−1, 1650 cm
−1, 1670 cm
−1, 1700 cm
−1 and 3300 cm
−1. Mass spectral data for
1 and
4 are also described in both patents; [M+H]
+ is detected at
m/
z 763 for
1 and
m/
z 785 for
4, respectively. We attempted to crystallize mixtures of the UU compounds and a common fluoran dye (2-anilino-6-dibutylamino-3-methylfluoran) with molar ratios of 2:1, 1:1 and 1:2 using liquid diffusion, vapor diffusion, melting and evaporation methods. Methyl ethyl ketone, acetone, tetrahydrofuran and 1,4-dioxane are good solvents for crystallization. For both the liquid and vapor diffusion methods, n-hexane, c-hexane and diethyl ether were used as poor solvents. X-ray diffraction data for the resulting solids were obtained by a Rigaku R-AXIS Rapid imaging-plate diffractometer using a graphite-monochromated Cu-
Kα radiation (50 kV, 40 mA). Powder samples were packed into a glass capillary tube (0.7 mm in diameter, 0.01 mm in thickness).
Amorphous black solids for the other experiments were prepared from mixtures of the UU compounds and the dye in molar ratios of 3:1, 2:1, 1:1, 1:2 and 1:3. These mixtures were dissolved in acetone, and the resulting colorless solutions were slowly evaporated at about 40 °C in a vial tube to yield the black solids.
IR spectra were recorded on a Perkin Elmer Spectrum BX FT-IR system for samples in KBr pellet form at room temperature. For each sample, the glass transition temperature (T
g) was determined using a Rigaku Thermo Plus 2 DSC8230 differential scanning calorimeter. About 3 mg of the black solids was placed in an aluminum pan and heated at a scan rate of 3.0 °C/min. Al
2O
3 was used as a reference. The optimized molecular structures of the UU compounds were calculated by the PM3 Hamiltonian [
13] using the CAChe 5.2 program package [
14].
3. Results and Discussion
Our attempts to obtain single crystals of black complexes of the UU compounds and the fluoran dye resulted in a mixture of small colorless crystals and powdery black solids. Powder X-ray diffraction of this mixture showed many peaks, but these peaks were assigned to the diffraction peaks of the crystals of individual components. Both components were thus found to crystallize separately under crystallization conditions. The measurement was also carried out for black solids isolated from the mixtures.
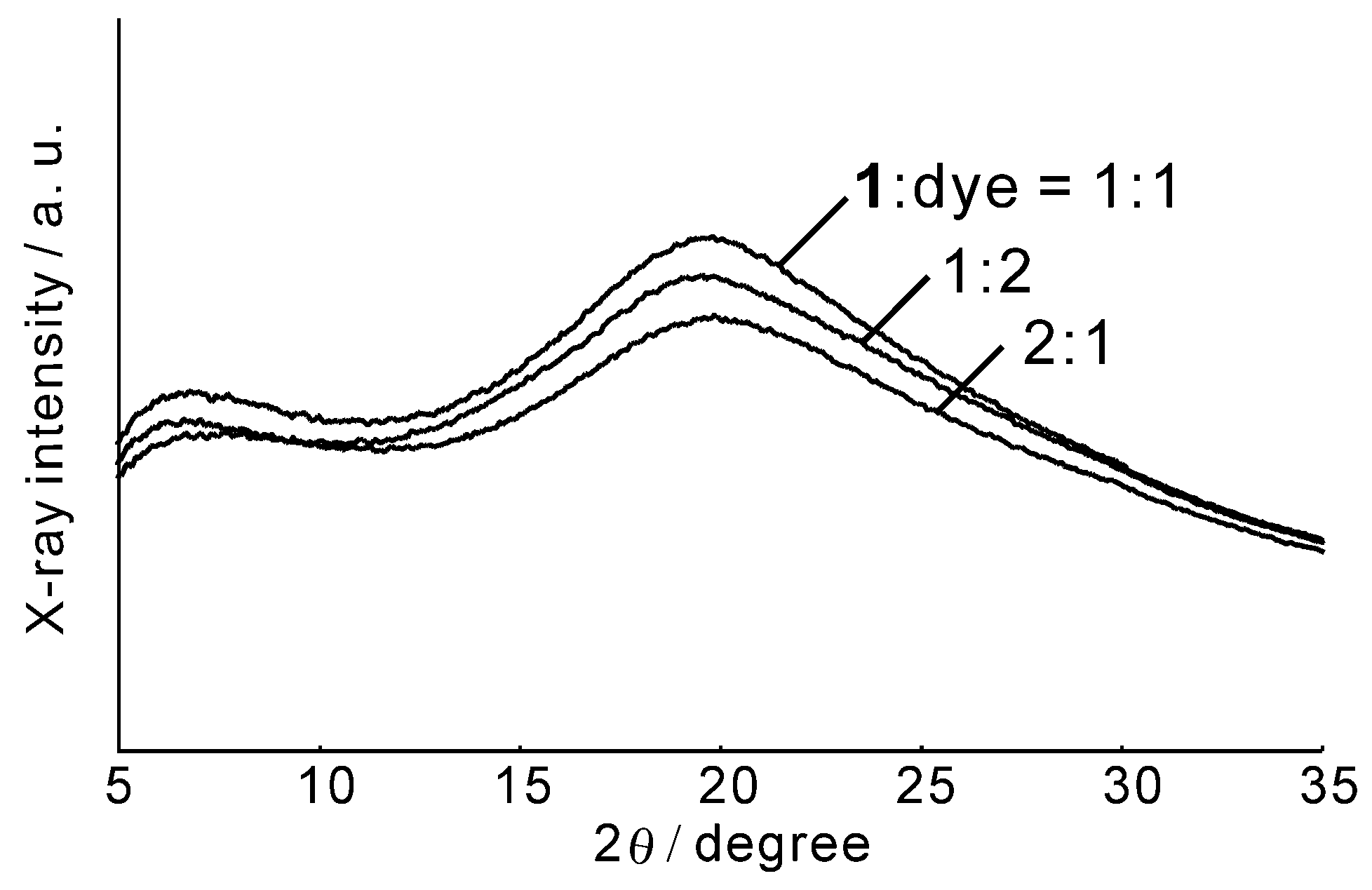
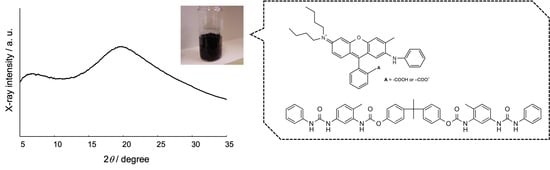
Figure 2 shows the X-ray diffraction diagram of the black solids prepared from
1 and the dye using three different ratios. A broad halo around 20° was observed for all samples. This result clearly indicates that the black solids are amorphous, and not crystalline. The other UU compounds provided the same crystallization result. Consequently, the optimal preparation conditions for obtaining amorphous black solids were determined and the resulting black solids were used for the other experiments.
IR measurements were then performed on the black solids to examine the structures of the related chemical species. Previous reports had suggested that the ring-opened colored structure of fluoran dyes is in the form of a carboxylic acid [
15] or a carboxylate [
16]. As summarized in
Table 1, in the former case, carbonyl C=O stretching absorption was found around 1760 cm
–1, while the carboxylate form provided two C=O stretching bands around 1550–1660 cm
–1 (anti-symmetric mode) and 1360–1400 cm
–1 (symmetric mode). The black solids prepared from the six UU compounds with the fluoran dye all showed a broad absorption band around 1750 cm
–1, and the anti-symmetric and symmetric bands were not observed. We therefore deduced that the dye exists as a carboxylic acid in the black solids.
C=O stretching bands were also recognized in the UU compounds.
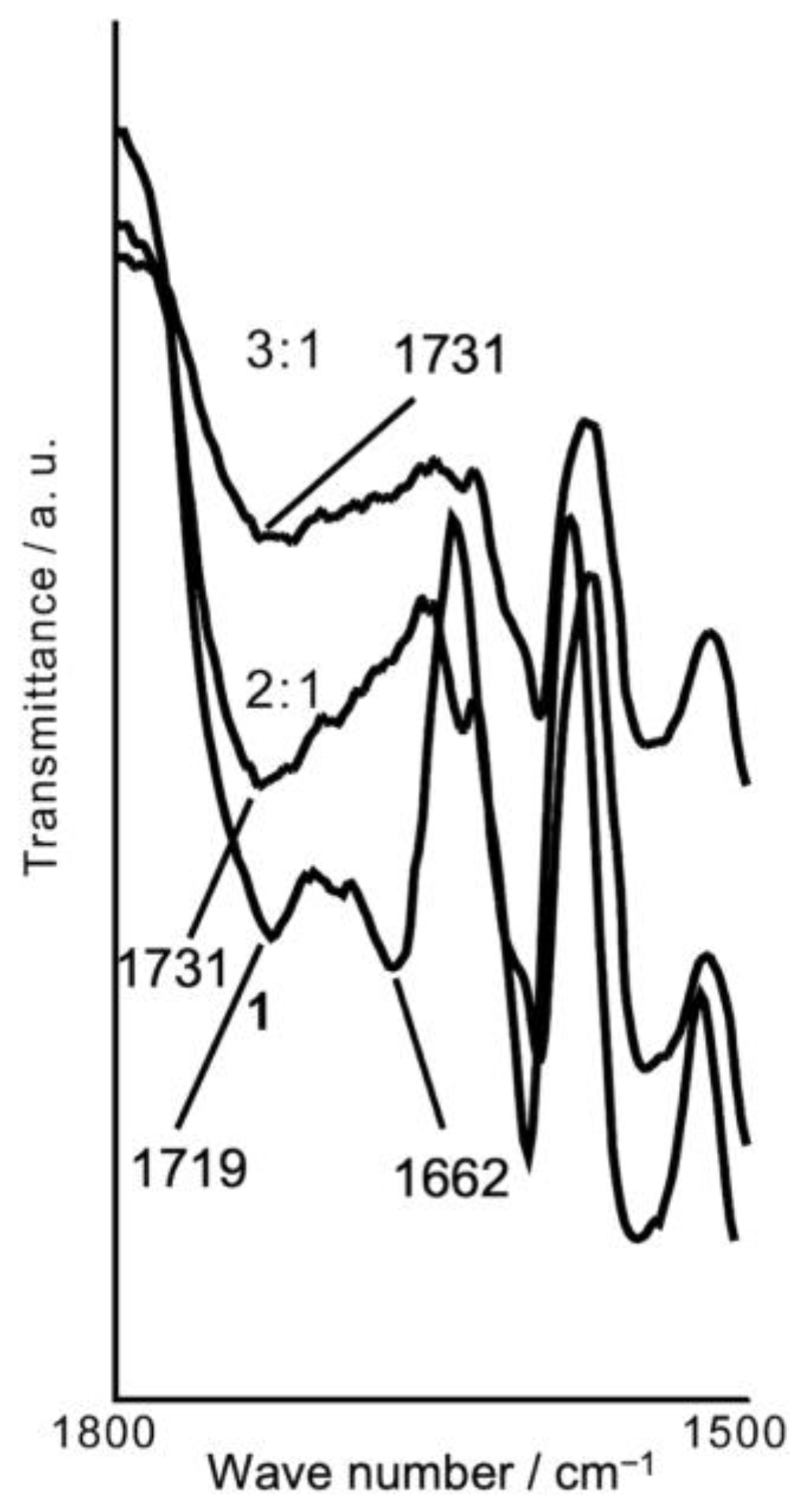
Figure 3 illustrates the IR spectra in the carboxyl C=O stretching region for
1 and the black solid of
1 and the dye with molar ratios (
1:dye) of 2:1 and 3:1. The strong bands of
1 around 1719 and 1662 cm
–1 were assigned to the urethane and urea groups, respectively [
18,
19,
20]. The other UU compounds also showed these two C=O bands with almost the same intensity. However, for the black solids, the urea C=O band around 1662 cm
–1 disappeared, as shown in
Figure 3. This result indicates that the urea group impinges on fluoran dye coloration. Amide groups can potentially work as proton donors due to amide resonance, as shown in
Scheme 2 [
21,
22]. However, the
pKa values of urea derivatives are usually very large, as shown in
Table 2, so it is difficult for urea derivatives to give rise to the coloration reaction.
This result agrees well with the fact that no coloration reaction was detected in solutions of the dye and the UU compounds. Strong acids with a low
pKa value, such as HCl and phenol derivatives [
23], are known to promote fluoran dye coloration in a solution. The UU compounds did not contribute to dye coloration in the solutions at room temperature and at a higher temperature. As solvent evaporates, black solids appeared from their colorless solutions. Specifically, the black solids appeared in the area where the solvent had evaporated. If the black solids dissolve in an appropriate solvent, the solution will be colorless. This means that the UU compounds can promote dye coloration only in the solid state. The coloration reaction is governed primarily by thermodynamic stabilization, which leads to a stable binary amorphous state. It is plausible that the deprotonation reaction of the urea NH group of the UU compounds occurs by direct contact between the UU compounds and the dye in the solid state. In fact, the coloration reaction occurs in a solvent-free system such as grinding or heating the mixture of the dye and the UU compounds. The conjugated base of the UU compounds can form a complex with the carboxylic acid of the colored dye. As mentioned above,
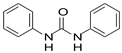
pKa is an important parameter in fluoran dye coloration. Our preliminary examination of dye coloration with several urea and urethane compounds in the solid state showed that urethane and N-phenyl urethane did not promote dye coloration. The same result was obtained when urea and thiourea were used. However, both N,N’-diphenylurea and N,N’-diphenylthiourea showed good developing properties on the dye.
We also investigated the mechanism underlying the stabilization of printed images on thermo-sensitive paper when the UU compounds are used; IR and DSC measurements were carried out on the black solids formed from the UU compounds with the fluoran dye.
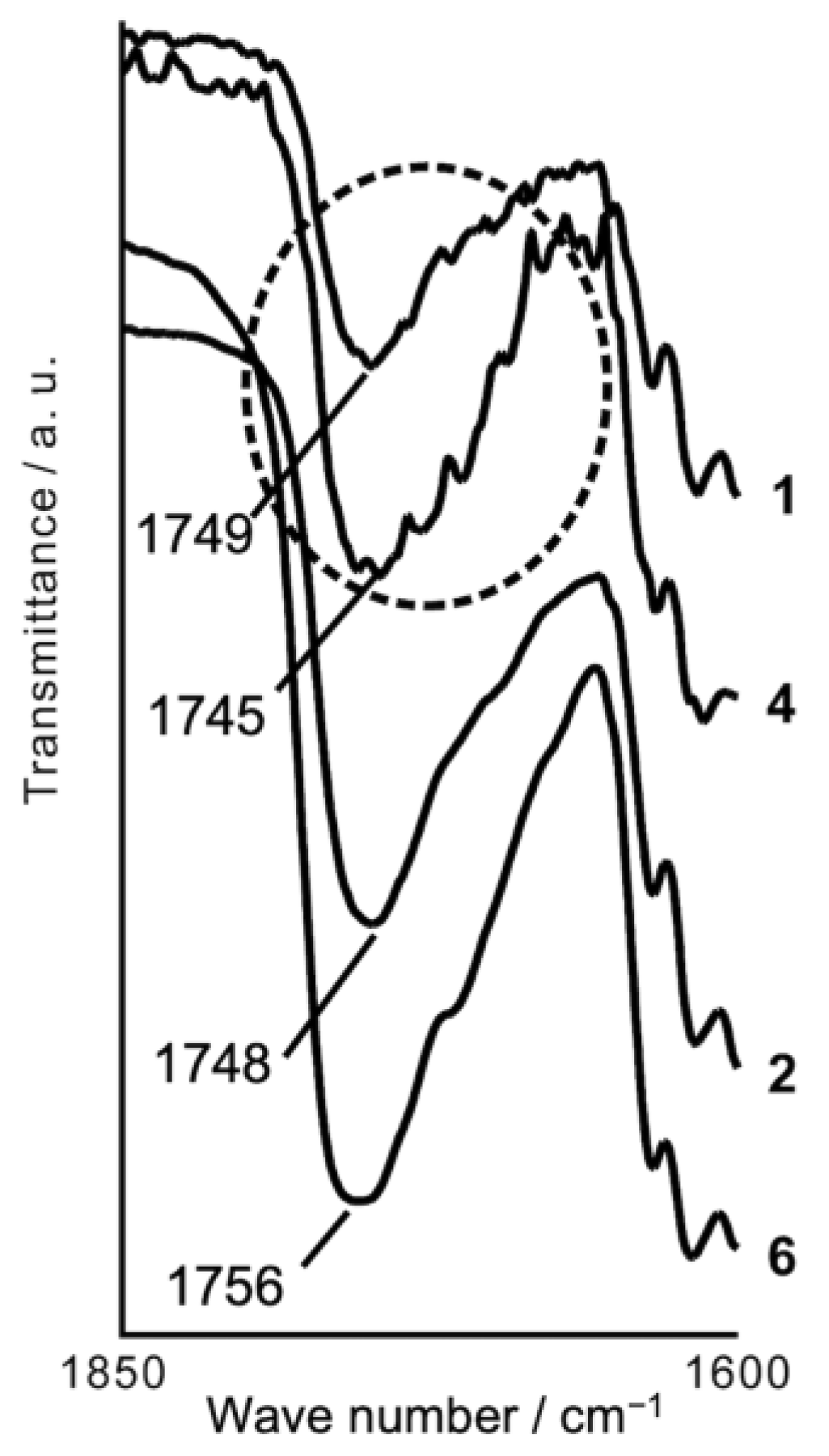
Figure 4 shows carboxyl C=O stretching bands of the colored dye with four UU compounds. Several small and discernible shoulder peaks were recognized for
1 and
4, whereas the black solids of
2 and
6 exhibited no shoulder peaks. This observation suggests that several colored structures of the dye exist in the black solids of
1 and
4. In the colored state, the ring-opened form of the dye is thought to form a complex with UU molecules via intermolecular hydrogen bonds. The structure of the complex could not be identified as a single form but was composed of various forms with different stoichiometries of UU and the dye. This is supported by the observations that UU compounds have the ability to form multiple hydrogen bonds and that black amorphous solids can be obtained by changing the mixture ratio of the dye and UU. The stability of the amorphous state of the black solids was examined by T
g measurements.
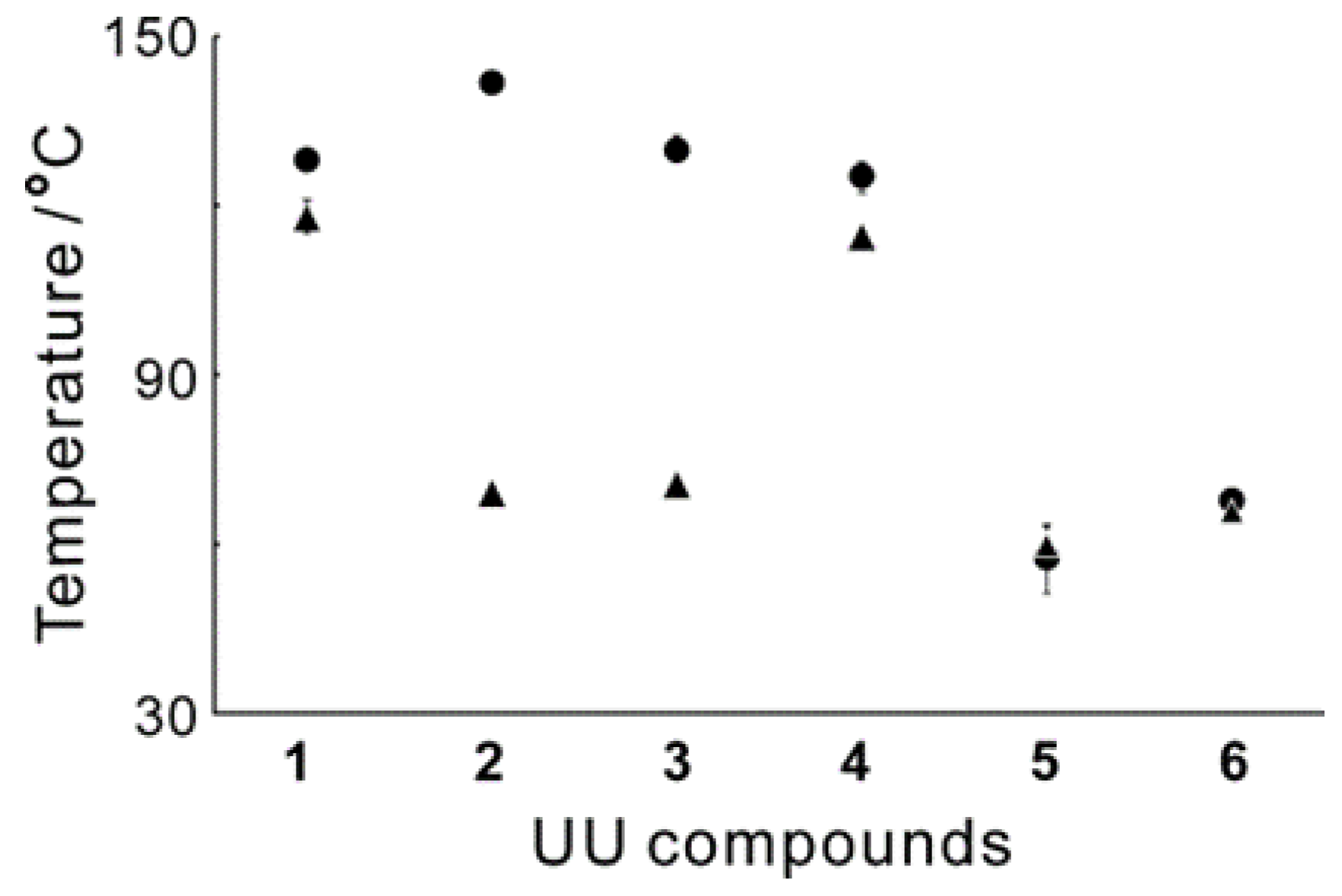
Figure 5 displays the averaged T
gs for the black solids of the six UU compounds with two different mixing ratios. Compounds
5 and
6, both of which have one UU unit, exhibited low T
gs for both mixing ratios. Compounds
2 and
3 showed high T
gs when equal molar amounts of the UU compound and the dye were used, but their T
gs decreased when the molar ratio was 1:2. Compounds
1 and
4 showed high T
gs in both mixing ratios.
It is known that the thermal stability of amorphous molecular solids improves with an increase in their molecular weight and the number of potential conformations; the thermal stability also depends on the mode of molecular packing [
24]. The thermal stability of the black solids is influenced by the number of UU units. When equal molar amounts of the UU compound and the dye were used, the black solids of
1–
4 with two UU units exhibited a higher T
gs than the black solids of
5 and
6 with a single UU unit. This result can be attributed to the effect of the molecular weight described above. However, among the four UU compounds, the T
g for both
2 and
3 decreased by about 50% when the dye ratio of the mixture was increased. It is difficult to explain this result in definite terms, but a plausible explanation is that the number of possible colored structures in the black solids is correlated with the thermal stability of the resulting amorphous solids, because the number of molecular conformations is an important parameter in the design of stable amorphous molecular solids [
24]. Several clear shoulder peaks were found around the carboxyl C=O stretching band of the dye in the IR measurements for
1 and
4, whereas no clear shoulder peaks were recognized for
2 and
3.
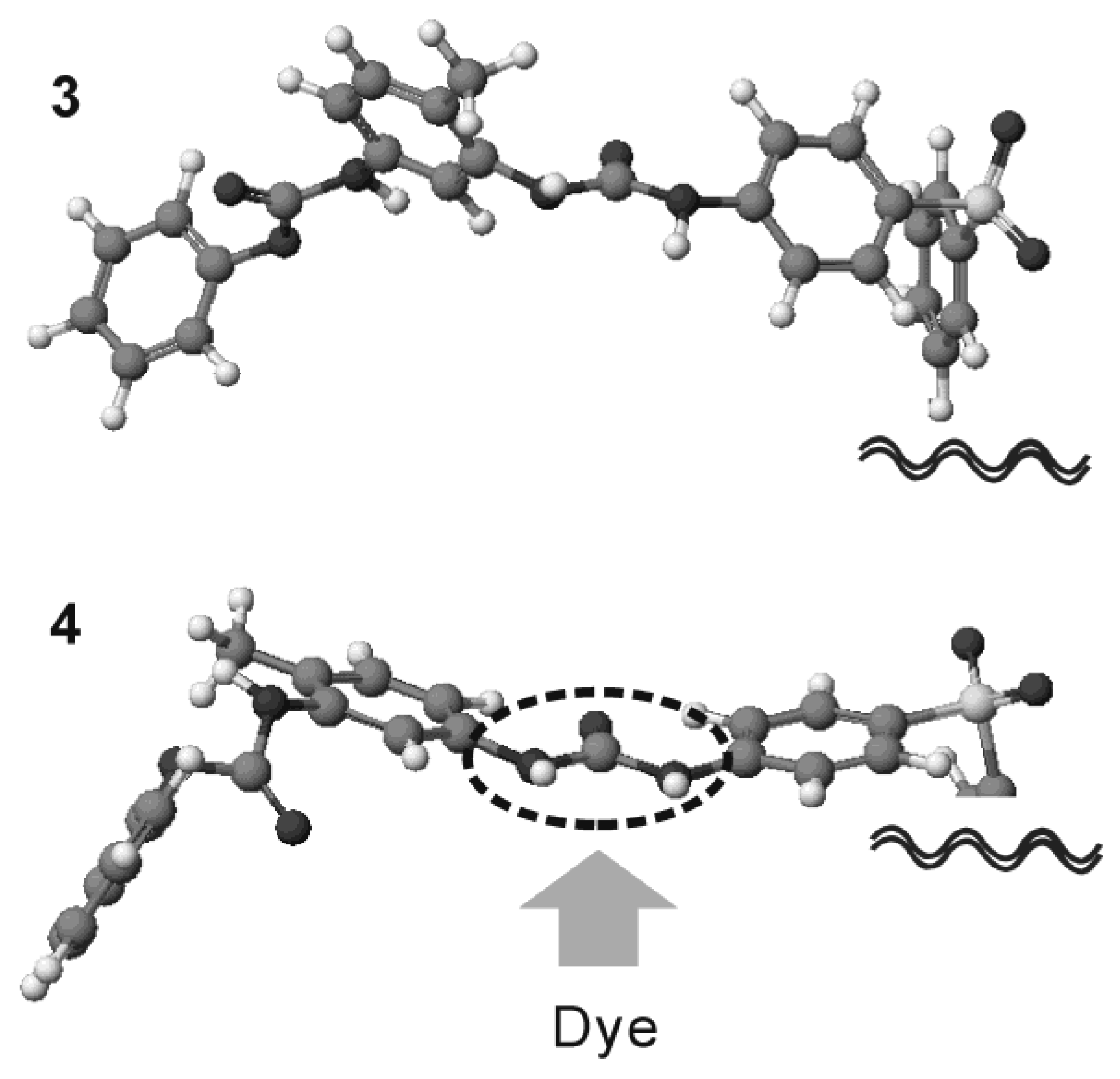
The molecular structures of the UU compounds were evaluated using molecular orbital calculations to interpret these observations in terms of the molecular structure. The optimized molecular structures of
3 and
4 are shown in
Figure 6. The difference in their chemical structure is found at the substitution position of the methyl group on the phenyl ring with both urea and urethane units. The geometrical features of the urea unit were found to be quite different depending on the position of the methyl group. In
3, the urea unit has a non-planar geometry due to steric hindrance between the carbonyl moiety and the methyl substituent. The same structural feature was recognized for
2. However, the methyl groups are located on the
p-position to the urea unit, thus providing a planar urea conformation in
4. Compound
1 has a similar molecular geometry. This difference in molecular conformation with respect to the urea unit has a significant effect on the compound’s hydrogen-bonding ability. Planar urea conformation plays an important role in forming strong hydrogen bonds based on bifurcated bond formation [
12]. Multiple hydrogen bonds are known to have a strong effect on the thermal stabilization of amorphous molecular solids, which leads to an increase in T
g [
25]. Deformation of the urea geometry therefore causes the T
g to decrease in the black solids prepared from
2 and
3. The same structural feature was observed for
5 and
6 with a single UU unit. This structural difference is reflected in the T
g of the black solids. When using this urea–urethane developer on high-performance thermo-sensitive paper, the important structural parameters to be considered for the stability of the colored state are the number of UU units and the planarity of the urea moiety in relation to the substituents. The acidity of the urea moiety is also an important property for the coloration reaction in the solid state.