Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
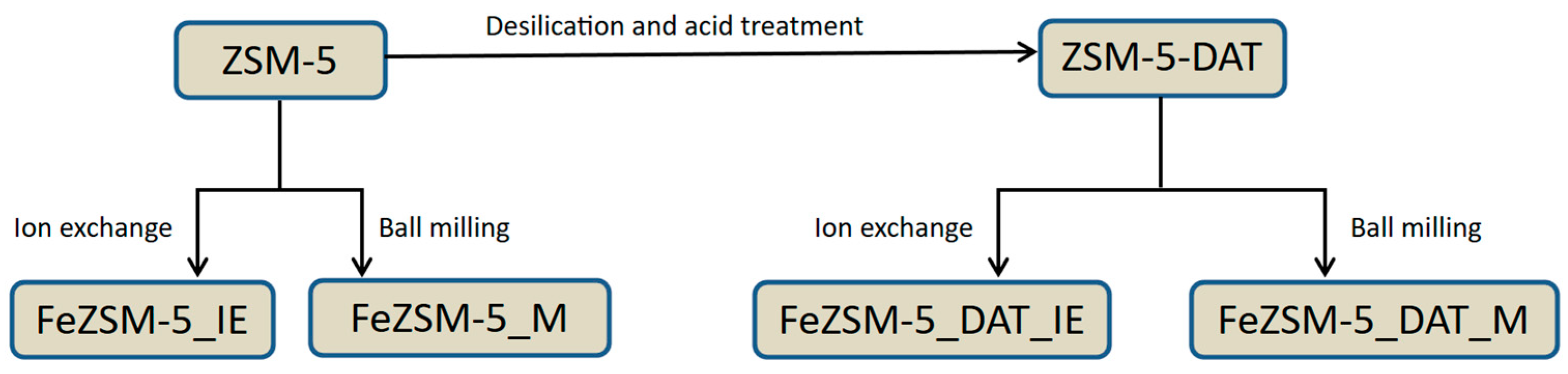
2.2. Zeolite Modifications
2.3. Metal Introduction
2.4. Catalyst Characterization
2.5. Catalytic Experiments
3. Results and Discussion
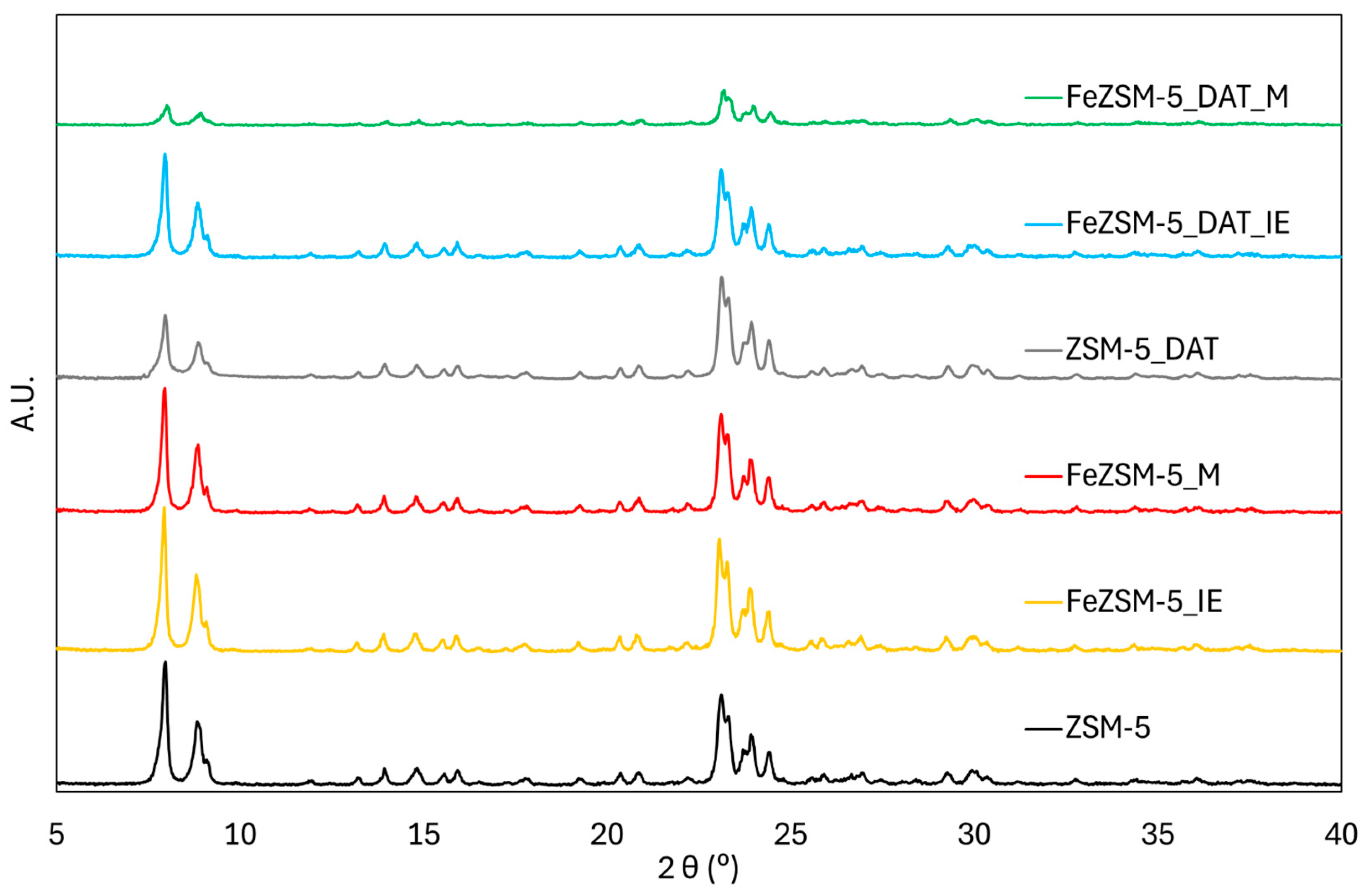
3.1. Zeolite Characterization
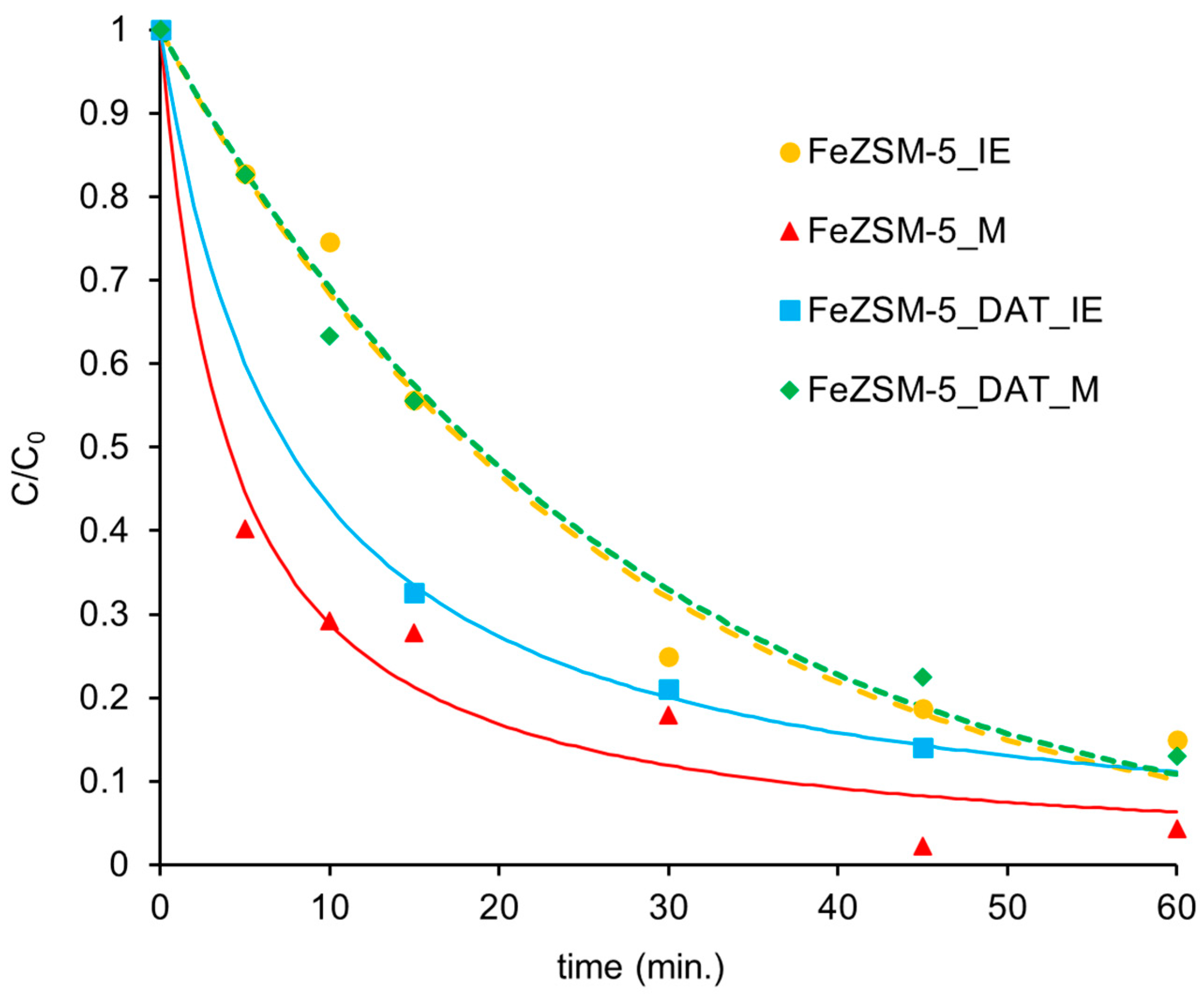
3.2. Heterogeneous Fenton Reaction
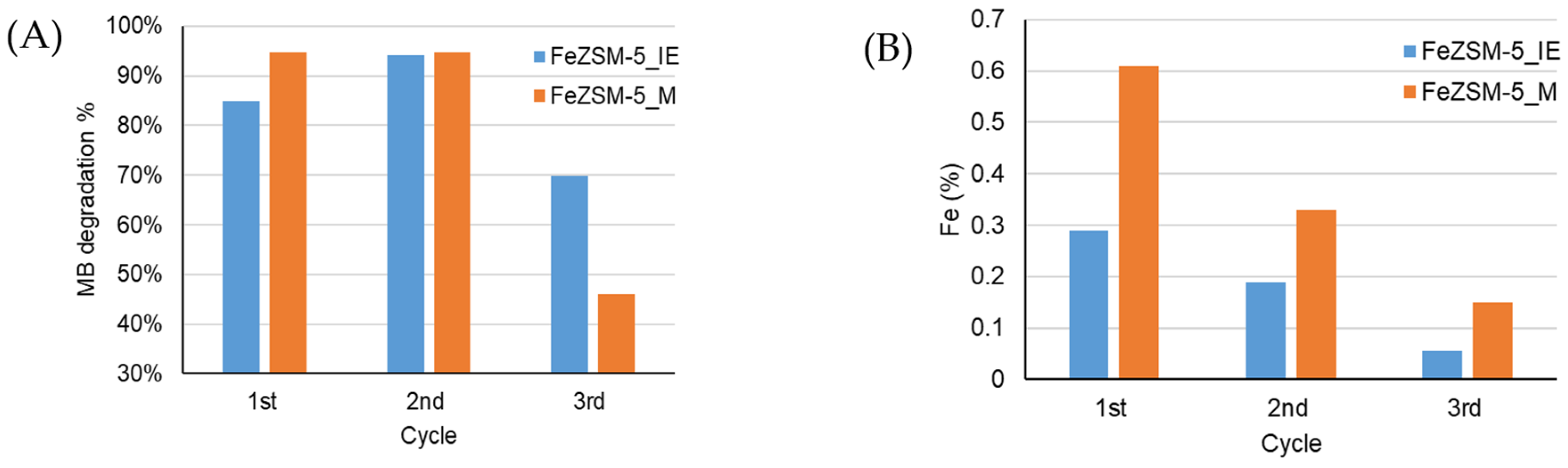
3.3. Regeneration Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- dos Santos, M.S.N.; Oro, C.E.D.; Wancura, J.H.C.; Dallago, R.M.; Tres, M.V. Fenton Process in Dye Removal. In Advanced Oxidation Processes in Dye-Containing Wastewater; Muthu, S.S., Khadir, A., Eds.; Springer Nature: Singapore, 2022; Volume 2, pp. 1–28. ISBN 978-981-19-0882-8. [Google Scholar]
- Fenton, H.J.H. LXXIII.—Oxidation of Tartaric Acid in Presence of Iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Goi, D. Catalytic Activity of Metals in Heterogeneous Fenton-like Oxidation of Wastewater Contaminants: A Review. Environ. Chem. Lett. 2021, 19, 2405–2424. [Google Scholar] [CrossRef]
- Pinto, I.S.X.; Pacheco, P.H.V.V.; Coelho, J.V.; Lorençon, E.; Ardisson, J.D.; Fabris, J.D.; de Souza, P.P.; Krambrock, K.W.H.; Oliveira, L.C.A.; Pereira, M.C. Nanostructured δ-FeOOH: An Efficient Fenton-like Catalyst for the Oxidation of Organics in Water. Appl. Catal. B Environ. 2012, 119–120, 175–182. [Google Scholar] [CrossRef]
- Tian, K.; Pan, J.; Liu, Y.; Wang, P.; Zhong, M.; Dong, Y.; Wang, M. Fe-ZSM-5 Zeolite Catalyst for Heterogeneous Fenton Oxidation of 1,4-Dioxane: Effect of Si/Al Ratios and Contributions of Reactive Oxygen Species. Environ. Sci. Pollut. Res. 2024, 31, 19738–19752. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, X.; Lv, G.; Zhai, Y.; Yang, Z.; Zhu, Y.; Li, H.; Wang, F. Influence of Zeolite Carriers on the Dyes Degradation for Framework Fe-Doped Zeolite Catalysts. J. Sol.-Gel. Sci. Technol. 2019, 91, 54–62. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.; Shang, X.; He, T. Fe-Cu Binary Oxide Loaded Zeolite as Heterogeneous Fenton Catalyst for Degradation of Carbamazepine at near-Neutral pH. Environ. Sci. Pollut. Res. 2022, 29, 73181–73190. [Google Scholar] [CrossRef] [PubMed]
- Ning, M.; Li, L.; Xu, X. Degradation of Methylene Blue Using Fe-ZSM-5 Zeolite as a Heterogeneous Fenton Catalyst. J. Phys. Conf. Ser. 2023, 2463, 012055. [Google Scholar] [CrossRef]
- Hammond, C. Sn-Substituted Zeolites as Heterogeneous Catalysts for Liquid-Phase Catalytic Technologies. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2017; Volume 177, pp. 567–611. ISBN 978-0-12-805090-3. [Google Scholar]
- Carvalho, A.P.; Nunes, N.; Martins, A. Hierarchical Zeolites: Preparation, Properties and Catalytic Applications. In Comprehensive Guide for Mesoporous Materials: Properties and Development; Aliofkhazraei, M., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; Volume 3, ISBN 978-1-63482-091-2. [Google Scholar]
- Verboekend, D.; Mitchell, S.; Milina, M.; Groen, J.C.; Pérez-Ramírez, J. Full Compositional Flexibility in the Preparation of Mesoporous MFI Zeolites by Desilication. J. Phys. Chem. C 2011, 115, 14193–14203. [Google Scholar] [CrossRef]
- Elvas-Leitão, R.; Martins, F.; Borbinha, L.; Marranita, C.; Martins, A.; Nunes, N. Probing Substrate/Catalyst Effects Using QSPR Analysis on Friedel-Crafts Acylation Reactions over Hierarchical BEA Zeolites. Molecules 2020, 25, 5682. [Google Scholar] [CrossRef]
- Nunes, N.; Carvalho, A.P.; Elvas-Leitão, R.; Martins, F.; Fernandes, A.; Rocha, J.; Martins, A. Exploring the Effect of Hierarchical Porosity in BEA Zeolite in Friedel-Crafts Acylation of Furan and Benzofuran. Catalysts 2022, 12, 1064. [Google Scholar] [CrossRef]
- Ma, S.; Jing, J.; Liu, P.; Li, Z.; Jin, W.; Xie, B.; Zhao, Y. High Selectivity and Effectiveness for Removal of Tetracycline and Its Related Drug Resistance in Food Wastewater through Schwertmannite/Graphene Oxide Catalyzed Photo-Fenton-like Oxidation. J. Hazard. Mater. 2020, 392, 122437. [Google Scholar] [CrossRef] [PubMed]
- Hermosilla, D.; Han, C.; Nadagouda, M.N.; Machala, L.; Gascó, A.; Campo, P.; Dionysiou, D.D. Environmentally Friendly Synthesized and Magnetically Recoverable Designed Ferrite Photo-Catalysts for Wastewater Treatment Applications. J. Hazard. Mater. 2020, 381, 121200. [Google Scholar] [CrossRef] [PubMed]
- Quynh, H.G.; Thanh, H.V.; Phuong, N.T.T.; Duy, N.P.T.; Hung, L.H.; Dung, N.V.; Duong, N.T.H.; Long, N.Q. Rapid Removal of Methylene Blue by a Heterogeneous Photo-Fenton Process Using Economical and Simple-Synthesized Magnetite–Zeolite Composite. Environ. Technol. Innov. 2023, 31, 103155. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Dalto, F.; Kuźniarska-Biernacka, I.; Pereira, C.; Mesquita, E.; Soares, O.S.G.P.; Pereira, M.F.R.; Rosa, M.J.; Mestre, A.S.; Carvalho, A.P.; Freire, C. Solar Light-Induced Methylene Blue Removal over TiO2/AC Composites and Photocatalytic Regeneration. Nanomaterials 2021, 11, 3016. [Google Scholar] [CrossRef]
- Aleixo, R.; Elvas-Leitão, R.; Martins, F.; Carvalho, A.P.; Brigas, A.; Nunes, R.; Fernandes, A.; Rocha, J.; Martins, A.; Nunes, N. Zooming in with QSPR on Friedel-Crafts Acylation Reactions over Modified BEA Zeolites. Mol. Catal. 2019, 476, 110495. [Google Scholar] [CrossRef]
- Bencheqroun, Z.; Sahin, N.E.; Soares, O.S.G.P.; Pereira, M.F.R.; Zaitan, H.; Nawdali, M.; Rombi, E.; Fonseca, A.M.; Parpot, P.; Neves, I.C. Fe(III)-Exchanged Zeolites as Efficient Electrocatalysts for Fenton-like Oxidation of Dyes in Aqueous Phase. J. Environ. Chem. Eng. 2022, 10, 107891. [Google Scholar] [CrossRef]
- Aleksić, M.; Kušić, H.; Koprivanac, N.; Leszczynska, D.; Božić, A.L. Heterogeneous Fenton Type Processes for the Degradation of Organic Dye Pollutant in Water—The Application of Zeolite Assisted AOPs. Desalination 2010, 257, 22–29. [Google Scholar] [CrossRef]
- ASTM D5758-01; Standard Test Method for Determination of Relative Crystallinity of Zeolite ZSM-5 by X-Ray Diffraction. ASTM international: West Conshohocken, PA, USA, 2021. [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area, & Porosity, 2nd ed.; Academic Press: London, UK; New York, NY, USA, 1982; ISBN 978-0-12-300956-2. [Google Scholar]
- Phan, T.T.N.; Nikoloski, A.N.; Bahri, P.A.; Li, D. Adsorption and Photo-Fenton Catalytic Degradation of Organic Dyes over Crystalline LaFeO3-Doped Porous Silica. RSC Adv. 2018, 8, 36181–36190. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, Y.; Guan, X.; Liu, Y.; Nie, J.; Li, C. A Rapid Fenton Treatment of Bio-Treated Dyeing and Finishing Wastewater at Second-Scale Intervals: Kinetics by Stopped-Flow Technique and Application in a Full-Scale Plant. Sci. Rep. 2019, 9, 9689. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.C.; Parpot, P.; Soares, O.S.G.P.; Pereira, M.F.R.; Rombi, E.; Fonseca, A.M.; Correia Neves, I. Fenton-Type Bimetallic Catalysts for Degradation of Dyes in Aqueous Solutions. Catalysts 2020, 11, 32. [Google Scholar] [CrossRef]
- Ribeiro, J.A.S.; Alves, J.F.; Salgado, B.C.B.; Oliveira, A.C.; Araújo, R.S.; Rodríguez-Castellón, E. Heterogeneous Photo-Fenton Degradation of Azo Dyes over a Magnetite-Based Catalyst: Kinetic and Thermodynamic Studies. Catalysts 2024, 14, 591. [Google Scholar] [CrossRef]
- Ibrahim, F.A.; Atran, A.A.; Hamdy, M.S. Kinetics, Isotherms, and Thermodynamics Studies on Adsorption of Methyl Green Dye onto Different Zeolites. J. Inorg. Organomet. Polym. 2024, 34, 3443–3456. [Google Scholar] [CrossRef]


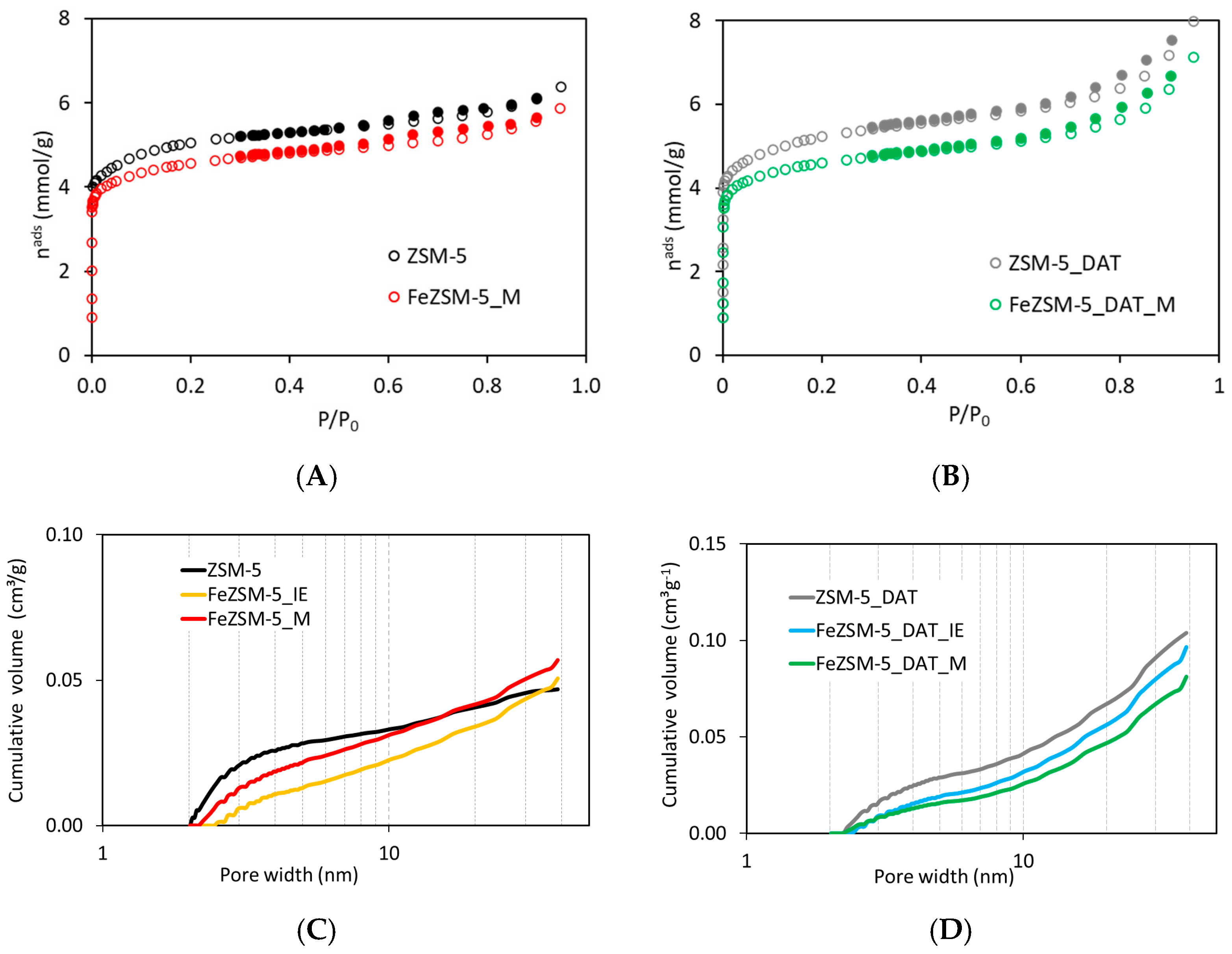
| Sample | CXRD 1 (%) | Vsuper (cm3 g−1) | Vultra (cm3 g−1) | Vmicro 2 (cm3 g−1) | Vmeso 3 (cm3 g−1) | Aext (m2 g−1) | Fe (wt.%) |
|---|---|---|---|---|---|---|---|
| ZSM-5 | 100 | 0.05 | 0.10 | 0.15 | 0.07 | 41 | - |
| FeZSM-5_IE | 100 | 0.04 | 0.10 | 0.14 | 0.07 | 43 | 0.29 |
| FeZSM-5_M | 100 | 0.04 | 0.10 | 0.14 | 0.07 | 41 | 0.61 |
| ZSM-5_DAT | 100 | 0.05 | 0.10 | 0.15 | 0.14 | 88 | - |
| FeZSM-5_DAT_IE | 94 | 0.05 | 0.10 | 0.15 | 0.13 | 36 | 0.33 |
| FeZSM-5_DAT_M | 36 | 0.03 | 0.10 | 0.13 | 0.12 | 62 | 0.65 |
| Samples | Pseudo-First-Order Model | ||||
|---|---|---|---|---|---|
| kap1 (min−1) | R2 | sFit | F | N | |
| FeZSM-5_IE | 0.038 ± 0.002 | 0.984 | 0.043 | 366 | 7 |
| FeZSM-5_M | 0.120 ± 0.020 | 0.909 | 0.099 | 60 | 7 |
| FeZSM-5_DAT_IE | 0.061 ± 0.008 | 0.970 | 0.068 | 97 | 4 |
| FeZSM-5_DAT_M | 0.037 ± 0.002 | 0.990 | 0.032 | 529 | 6 |
| Samples | Pseudo-Second-Order Model | ||||
| kap2 (ppm−1 min−1) | R2 | sFit | F | N | |
| FeZSM-5_IE | 0.019 ± 0.003 | 0.940 | 0.0830 | 93 | 7 |
| FeZSM-5_M | 0.052 ± 0.006 | 0.978 | 0.0480 | 276 | 7 |
| FeZSM-5_DAT_IE | 0.100 ± 0.002 | 0.999 | 0.0007 | 8971 | 4 |
| FeZSM-5_DAT_M | 0.023 ± 0.003 | 0.975 | 0.0530 | 198 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.P.; Costa, J.; Martins, A.; Fonseca, A.M.; Neves, I.C.; Nunes, N. Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation. Colorants 2025, 4, 10. https://doi.org/10.3390/colorants4010010
Carvalho AP, Costa J, Martins A, Fonseca AM, Neves IC, Nunes N. Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation. Colorants. 2025; 4(1):10. https://doi.org/10.3390/colorants4010010
Chicago/Turabian StyleCarvalho, Ana P., José Costa, Angela Martins, António M. Fonseca, Isabel C. Neves, and Nelson Nunes. 2025. "Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation" Colorants 4, no. 1: 10. https://doi.org/10.3390/colorants4010010
APA StyleCarvalho, A. P., Costa, J., Martins, A., Fonseca, A. M., Neves, I. C., & Nunes, N. (2025). Zeolite Modification for Optimizing Fenton Reaction in Methylene Blue Dye Degradation. Colorants, 4(1), 10. https://doi.org/10.3390/colorants4010010











