Optical Characterization of Fluorescent Chitosan-Based Carbon Dots Embedded in Aqueous Natural Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Spectroscopic and Optical Techniques
2.3. Thermal Lens (TL)
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, M.N.V.R. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.-G. Chitosan and Its Antimicrobial Potential—A Critical Literature Survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Yu, H.; Abdin, Z.; Chen, Y.; Chen, Q.; Zhou, W.; Zhang, H.; Chen, X. Recent Progress on Synthesis, Property and Application of Modified Chitosan: An Overview. Int. J. Biol. Macromol. 2016, 88, 333–344. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, H.; Xiong, Q.; Zhang, Y.; Zhao, H.; Wang, G. A Fluorescent Chitosan Hydrogel Detection Platform for the Sensitive and Selective Determination of Trace Mercury(II) in Water. J. Mater. Chem. A 2015, 3, 19455–19460. [Google Scholar] [CrossRef]
- Padilha, A.C.; Vivas, M.G.; Melo, M.d.S.F.; Campos, M.G.N. Fluorescent Chitosan Nanoparticles as a Carrier System for Trackable Drug Delivery. Polym. Plas. Technol. Mater. 2021, 60, 862–871. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y.; Yang, Q.; Liu, Y. One-Step Synthesis of Amino-Functionalized Fluorescent Carbon Nanoparticles by Hydrothermal Carbonization of Chitosan. Chem. Commun. 2012, 48, 380–382. [Google Scholar] [CrossRef]
- Basumallick, S.; Campos, M.G.N.; Richardson, D.; Gesquiere, A.; Santra, S. Hydrothermally Treated Chitosan Spontaneously Forms Water-Soluble Spherical Particles Stable at a Wide pH Range. Int. J. Polym. Mater. Polym. Biomater. 2016, 65, 751–758. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; de Castro Bessa, N.U.; do Nascimento, J.F.; de Paula Cavalcante, C.S.; dos Santos Fontenelle, R.O.; da Silva Abreu, F.O.M. Synthesis of Luminescent Chitosan-based Carbon Dots for Candida albicans bioimaging. Int. J. Biol. Macromol. 2023, 227, 805–814. [Google Scholar] [CrossRef]
- Mathew, S.A.; Praveena, P.; Dhanavel, S.; Manikandan, R.; Senthilkumar, S.; Stephen, A. Luminescent Chitosan/Carbon Dots as an Effective Nano-Drug Carrier for Neurodegenerative Diseases. RSC Adv. 2020, 10, 24386–24396. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z. Carbon Quantum Dots and their Applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Zhang, D.-W.; Papaioannou, N.; David, N.M.; Luo, H.; Gao, H.; Tanase, L.C.; Degousée, T.; Samori, P.; Sapelkin, A.; Fenwick, O.; et al. Photoelectrochemical Response of Carbon Dots (CDs) Derived from Chitosan and Their Use in Electrochemical Imaging. Mater. Horiz. 2018, 5, 423–428. [Google Scholar] [CrossRef]
- Yang, G.; Jin, Q.; Xu, C.; Fan, S.; Wang, C.; Xie, P. Synthesis, Characterization and Antifungal Activity of Coumarin-Functionalized Chitosan Derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef]
- Kalaycioğlu, Z.; Torlak, E.; Akın-Evingür, G.; Ozen, I.; Erim, F.B. Antimicrobial and Physical Properties of Chitosan Films Incorporated with Turmeric Extract. Int. J. Biol. Macromol. 2017, 101, 882–888. [Google Scholar] [CrossRef]
- Bendokas, V.; Skemiene, K.; Trumbeckaite, S.; Stanys, V.; Passamonti, S.; Borutaite, V.; Liobikas, J. Anthocyanins: From Plant Pigments to Health Benefits at Mitochondrial Level. Crit. Rev. Food Sci. Nutr. 2020, 60, 3352–3365. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food colorans: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; de Freitas, V.; Mateus, N.; Calhau, C. Blueberry Anthocyanins and Pyruvic Acid Adducts: Anticancer Properties in Breast Cancer Cell Lines. Phytother. Res. 2010, 24, 1862–1869. [Google Scholar] [CrossRef]
- Blaszczyk, A.; Joachimiak-Lechman, K.; Sady, S.; Tański, T.; Szindler, M.; Drygata, A. Environmental Performance of Dye-Sensitized Solar Cells Based on Natural Dyes. Sol. Energy 2021, 215, 346–355. [Google Scholar] [CrossRef]
- De Lima, S.R.; Lourenço, L.R.; Thomaz, M.; Messias, D.N.; Andrade, A.A.; Pilla, V. Fluorescence Quantum Yields and Lifetimes of Aqueous Natural Dye Extracted from Tradescantia pallida purpurea at Different Hydrogen Potentials. Photochem 2023, 3, 1–14. [Google Scholar] [CrossRef]
- De Lima, S.R.; Felisbino, D.G.; Lima, M.R.S.; Chang, R.; Martins, M.M.; Goulart, L.R.; Andrade, A.A.; Messias, D.N.; Dos Santos, R.R.; Juliatti, F.C.; et al. Fluorescence Quantum Yield of Natural Dye Extracted from Tradescantia Pallida Purpurea as a Function of the Seasons: Preliminary Bioapplication as a Fungicide Probe for Necrotrophic Fungi. J. Photochem. Photobiol. B 2019, 200, 111631. [Google Scholar] [CrossRef]
- Lacombe, A.; Wu, V.C.H.; Tyler, S.; Edwards, K. Antimicrobial Action of the American Cranberry Constituents; Phenolics, Anthocyanins, and Organic Acids, Against Escherichia coli O157:H7. Int. J. Food Microbiol. 2010, 139, 102–107. [Google Scholar] [CrossRef]
- Riahi, Z.; Khan, A.; Rhim, J.-W.; Shin, G.H.; Kim, J.T. Carrageenan-Based Active and Intelligent Packaging Films Integrated with Anthocyanin and TiO2-Doped Carbon Dots Derived from Sweet Potato Peels. Int. J. Biol. Macromol. 2024, 259, 129371. [Google Scholar] [CrossRef]
- Khan, A.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Carrageenan-Based Multifunctional Packaging Films Containing Zn-Carbon Dots/Anthocyanin Derived from Kohlrabi Peel for Monitoring Quality and Extending the Shelf Life of Shrimps. Food Chem. 2024, 432, 137215. [Google Scholar] [CrossRef]
- Wagh, R.V.; Riahi, Z.; Kim, J.T.; Rhim, J.-W. Carrageenan-Based Functional Films Hybridized with Carbon Dots and Anthocyanins from Rose Petals for Smart Food Packaging Applications. Int. J. Biol. Macromol. 2024, 272, 132817. [Google Scholar] [CrossRef]
- Koshy, R.R.; Koshy, J.T.; Mary, S.K.; Sadanandan, S.; Jisha, S.; Pothan, L.A. Preparation of pH Sensitive Film Based on Starch/Carbon Nano Dots Incorporating Anthocyanin for Monitoring Spoilage of Pork. Food Control. 2021, 126, 108039. [Google Scholar] [CrossRef]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Cellulose Nanofiber-Based Multifunctional Films Integrated with Carbon Dots and Anthocyanins from Brassica oleracea for Active and Intelligent Food Packaging Applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef]
- Shi, Z.; Daun, H.; Francis, F.J. Major Anthocyanin from Tradescantia pallida: Identification by LSI-MS and Chemical Analyses. J. Food Sci. 1993, 58, 1068–1069. [Google Scholar] [CrossRef]
- Shi, Z.; Lin, M.; Francis, F.J. Anthocyanins of Tradescantia pallida. Potential Food Colorants. J. Food Sci. 1992, 57, 761–765. [Google Scholar] [CrossRef]
- Santos, T.T.S.; Lourenço, L.R.; De Lima, S.R.; Goulart, L.R.; Messias, D.N.; Andrade, A.A.; Pilla, V. Fluorescence Quantum Yields and Lifetimes of Annatto Aqueous Solutions Dependent on Hydrogen Potential: Applications in Adulterated Milk. J. Photochem. Photobiol. 2021, 8, 100080. [Google Scholar] [CrossRef]
- Pilla, V.; Gonçalves, A.C.; Dos Santos, A.A.; Lodeiro, C. Lifetime and Fluorescence Quantum Yield of Two Fluorescein-Amino Acid-Based Compounds in Different Organic Solvents and Gold Colloidal Suspensions. Chemosensors 2018, 6, 26. [Google Scholar] [CrossRef]
- Shen, J.; Lowe, R.D.; Snook, R.D. A Model for cw Laser Induced Mode- Mismatched Dual-Beam Thermal Lens Spectrometry. Chem. Phys. 1992, 165, 385–396. [Google Scholar] [CrossRef]
- Snook, R.D.; Lowe, R.D. Thermal Lens Spectrometry. A Review. Analyst 1995, 120, 2051–2068. [Google Scholar] [CrossRef]
- Pilla, V.; Munin, E.; Gesualdi, M.R.R. Measurement of the Thermo-Optic Coefficient in Liquids by Laser-Induced Conical Diffraction and Thermal Lens Techniques. J. Opt. A Pure Appl. Opt. 2009, 11, 105201. [Google Scholar] [CrossRef]
- Mendes, V.; De Lima, S.R.; Torres, J.O.B.; Antunes, A.; Messias, D.N.; Andrade, A.A.; Dantas, N.O.; Zilio, S.C.; Pilla, V. Preliminary Spectroscopic and Thermo-Optical Characterization of Anthocyanin Unpurified Crude Extracted from Tradescantia Pallida Purpurea. Dyes Pigm. 2016, 135, 57–63. [Google Scholar] [CrossRef]
- Baublis, A.; Spomer, A.; Berber-Jiménez, M.D. Anthocyanin Pigments: Comparison of Extract Stability. J. Food Sci. 1994, 59, 1219–1221. [Google Scholar] [CrossRef]
- Brouillard, R. Origin of the Exceptional Colour Stability of the Zebrina Anthocyanin. Phytochem 1981, 20, 143–145. [Google Scholar] [CrossRef]
- Shi, Z.; Lin, M.; Francis, F.J. Stability of Anthocyanins from Tradescantia pallida. J. Food Sci. 1992, 57, 758–760. [Google Scholar] [CrossRef]
- Puvvada, N.; Kumar, B.N.P.; Konar, S.; Kalita, H.; Mandal, M.; Pathak, A. Synthesis of Biocompatible Multicolor Luminescent Carbon Dots for Bioimaging Applications. Sci. Technol. Adv. Mater. 2012, 13, 045008. [Google Scholar] [CrossRef]
- Mewada, A.; Pandey, S.; Shinde, S.; Mishra, N.; Oza, G.; Thakur, M.; Sharon, M.; Sharon, M. Green Synthesis of Biocompatible Carbon Dots Using Aqueous Extract of Trapa bispinosa Peel. Mater. Sci. Eng. C 2013, 33, 2914–2917. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A Green and Facile Approach for the Synthesis of Water Soluble Fluorescent Carbon Dots from Banana Juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, W.; Pan, D.; Zhang, Z.; Fang, Y.; Wu, M. Controlled Synthesis of Green and Blue Luminescent Carbon Nanoparticles with High Yiels by the Carbonization of Sucrose. New J. Chem. 2010, 34, 591–593. [Google Scholar] [CrossRef]
- Munawaroh, H.; Fadillah, G.; Saputri, L.N.M.Z.; Hanif, Q.A.; Hidayat, R.; Wahyuningsih, S. The Co-Pigmentation of Anthocyanin Isolated from Mangosteen Pericarp (Garcinia mangostana L.) as Natural Dye for Dye-Sensitized Solar Cells (DSSC). IOP Conf. Ser. Mater. Sci. Eng. 2016, 107, 012061. [Google Scholar] [CrossRef]
- Zhan, J.; Peng, R.; Wei, S.; Chen, J.; Peng, X.; Xiao, B. Ethanol-Precipitation-Assisted Highly Efficient Synthesis of Nitrogen-Doped Carbon Quantum Dots from Chitosan. ACS Omega 2019, 4, 22574–22580. [Google Scholar] [CrossRef]
- Dager, A.; Uchida, T.; Maekawa, T.; Tachibana, M. Synthesis and Characterization of Mono-disperse Carbon Quantum Dots from Fennel Seeds: Photoluminescence Analysis using Machine Learning. Sci. Rep. 2019, 9, 14004. [Google Scholar] [CrossRef]
- Pawar, S.; Togiti, U.K.; Bhattacharya, A.; Nag, A. Functionalized Chitosan–Carbon Dots: A Fluorescent Probe for Detecting Trace Amount of Water in Organic Solvents. ACS Omega 2019, 4, 11301–11311. [Google Scholar] [CrossRef]
- Da, X.; Han, Z.; Yang, Z.; Zhang, D.; Hong, R.; Tao, C.; Lin, H.; Huang, Y. Preparation of Multicolor Carbon Dots with High Fluorescence Quantum Yield and Application in White LED. Chem. Phys. Lett. 2022, 794, 139497. [Google Scholar] [CrossRef]
- Stachowska, J.D.; Murphy, A.; Mellor, C.; Fernandes, D.; Gibbons, E.N.; Krysmann, M.J.; Kelarakis, A.; Burgaz, E.; Moore, J.; Yeates, S.G. A Rich Gallery of Carbon Dots Based Photoluminescent Suspensions and Powders Derived by Citric Acid/Urea. Sci. Rep. 2021, 11, 10554. [Google Scholar] [CrossRef]
- Ma, C.; Yin, C.; Fan, Y.; Yang, X.; Zhou, X. Highly Efficient Synthesis of N-doped Carbon Dots with Excellent Stability Through Pyrolysis Method. J. Mater. Sci. 2019, 54, 9372–9384. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic Nitrogen-Doped Carbon Dots from Biowaste using Dwarf Banana Peel for Environmental and Biological Applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Baruah, U.; Gogoi, N.; Konwar, A.; Deka, M.J.; Chowdhury, D.; Majumdar, G. Carbon Dot Based Sensing of Dopamine and Ascorbic Acid. J. Nanopart. 2014, 2014, 178518. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; El-Bery, H.M.; Metwally, A.A.; Elshazly, M.; Hathout, R.M. Synthesis of CdS-Modified Chitosan Quantum Dots for the Drug Delivery of Sesamol. Carbohydr. Polym. 2019, 214, 90–99. [Google Scholar] [CrossRef]
- Zhai, H.; Zheng, B.; Yang, F.; Wang, M.; Xiao, D. Synthesis of Water-Soluble Fluorescent Carbon Dots from Setcreasea Purpurea Boom and Its Application for Br2 Detection. Anal. Methods 2018, 10, 151–157. [Google Scholar] [CrossRef]
- Favaro, L.I.L.; Balcão, V.M.; Rocha, L.K.H.; Silva, E.C.; Oliveira, J.M.; Vila, M.M.D.C.; Tubino, M. Physicochemical Characterization of a Crude Anthocyanin Extract from the Fruits of Jussara (Euterpe edulis Martius): Potential for Food and Pharmaceutical Applications. J. Braz. Chem. Soc. 2018, 29, 2072–2088. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Wulandari, L.; Wartono, M.W.; Munawaroh, H.; Ramelan, A.H. The Effect of pH and Color Stability of Anthocyanin on Food Colorant. IOP Conf. Series: Mat. Sci. Eng. 2017, 193, 012047. [Google Scholar] [CrossRef]
- De Lima, S.R.; Pereira, G.J.; Messias, D.N.; Andrade, A.A.; Oliveira, E.; Lodeiro, C.; Zilio, S.C.; Pilla, V. Fluorescence Quantum Yield Determination of Molecules in Liquids by Thermally Driven Conical Diffraction. J. Lumin. 2018, 197, 175–179. [Google Scholar] [CrossRef]
- Domenegueti, J.F.M.; Andrade, A.A.; Pilla, V.; Zilio, S.C. Simultaneous Measurement of Thermo-Optic and Thermal Expansion Coefficients with a Single Arm Double Interferometer. Opt. Express 2017, 25, 313–319. [Google Scholar] [CrossRef]
- Pichardo-Molina, J.L.; Cardoso-Avila, P.E.; Flores-Villavicencio, L.L.; Gomez-Ortiz, N.M.; Rodriguez-Rivera, M.A. Fluorescent Carbon Nanoparticles Synthesized from Bovine Serum Albumin Nanoparticles. Int. J. Biol. Macromol. 2020, 142, 724–731. [Google Scholar] [CrossRef]
- Yu, J.; Song, N.; Zhang, Y.-K.; Zhong, S.-X.; Wang, A.-J.; Chen, J. Green Preparation of Carbon Dots by Jinhua Bergamot for Sensitive and Selective Fluorescent Detection of Hg2+ and Fe3+. Sens. Actuators B Chem. 2015, 214, 29–35. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef]
- Bano, D.; Kumar, V.; Singh, V.K.; Hasan, S.H. Green Synthesis of Fluorescent Carbon Quantum Dots for the Detection of Mercury(II) and Glutathione. New J. Chem. 2018, 42, 5814–5821. [Google Scholar] [CrossRef]
- Dong, Y.; Pang, H.; Yang, H.B.; Guo, C.; Shao, J.; Chi, Y.; Li, C.M.; Yu, T. Carbon-Based Dots Co-Doped with Nitrogen and Sulfur for High Quantum Yield and Excitation-Independent Emission. Angew. Chem. Int. Ed. 2013, 52, 7800–7804. [Google Scholar] [CrossRef]
- Ateia, E.E.; Rabie, O.; Mohamed, A.T. Assessment of the Correlation Between Optical Properties and CQD Preparation Approaches. Eur. Phys. J. Plus 2024, 139, 24. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, S.; Sun, L.; Wu, S.; Hu, G.; Pang, X.; Smith, A.T.; Hu, C.; Zeng, S.; Wang, W.; et al. Ultralong Lifetime and Efficient Room Temperature Phosphorescent Carbon Dots Through Multi-Confinement Structure Design. Nat. Commun. 2020, 11, 5591. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence Lifetime Measurements and Biological Imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef]
- Peng, J.; Gao, W.; Gupta, B.K.; Liu, Z.; Romero-Aburto, R.; Ge, L.; Song, L.; Alemany, L.B.; Zhan, X.; Gao, G.; et al. Graphene Quantum Dots Derived from Carbon Fibers. Nano Lett. 2012, 12, 844–849. [Google Scholar] [CrossRef]
- Felipe, D.G.; Serna, T.B.; Andrade, A.A.; Pilla, V. Synthesis of Fluorescent Carbon Dots from Raw Materials: An Overview of Textile Applications. Trends Text. Eng. Fashion. Technol. 2024, 10, 1155–1159. [Google Scholar] [CrossRef]
- Varman, G.A.; Kalanidhi, K.; Nagaraaj, P. Green Synthesis of Fluorescent Carbon Dots from Canon Ball Fruit for Sensitive Detection of Fe3+ and Catalytic Reduction of Textile Dyes. Dyes Pigm. 2022, 199, 110101. [Google Scholar] [CrossRef]
- Mogharbel, A.T.; Pashameah, R.A.; Alluhaybi, A.A.; Almahri, A.; Abumelha, H.M.; Habeebullah, T.M.; El-Metwaly, N.M. Development of a “Turn-off” Fluorescent Sensor for Acetone from Rice Straw-Derived Carbon Dots Immobilized onto Textile Cotton Mask. J. Mol. Liq. 2022, 362, 119666. [Google Scholar] [CrossRef]
- Thirumalaivasan, N.; Kanagaraj, K.; Logesh, K.; Chandrasekaran, S.; Kumar, S.; Subramanian, R.; Senthilkumar, N.; Kumar, A.; Angadi, V.J.; Al-Kahtani, A.A. Exploring Luminescent Carbon Dots Derived from Syrup Bottle Waste and Curcumin for Potential Antimicrobial and Bioimaging Applications. Chemosphere 2024, 354, 141592. [Google Scholar] [CrossRef]
- Vadia, F.Y.; Mehta, V.N.; ·Jha, S.; ·Park, T.J.; ·Malek, N.I.; Kailasa, S.K. Development of Simple Fluorescence Analytical Strategy for the Detection of Triazophos Using Greenish-Yellow Emissive Carbon Dots Derived from Curcuma longa. J. Fluoresc. 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.; Hu, Y. Blue/Yellow Emissive Carbon Dots Coupled with Curcumin: A Hybrid Sensor Toward Fluorescence Turn-On Detection of Fluoride Ion. J. Hazard. Mater. 2021, 411, 125184. [Google Scholar] [CrossRef]
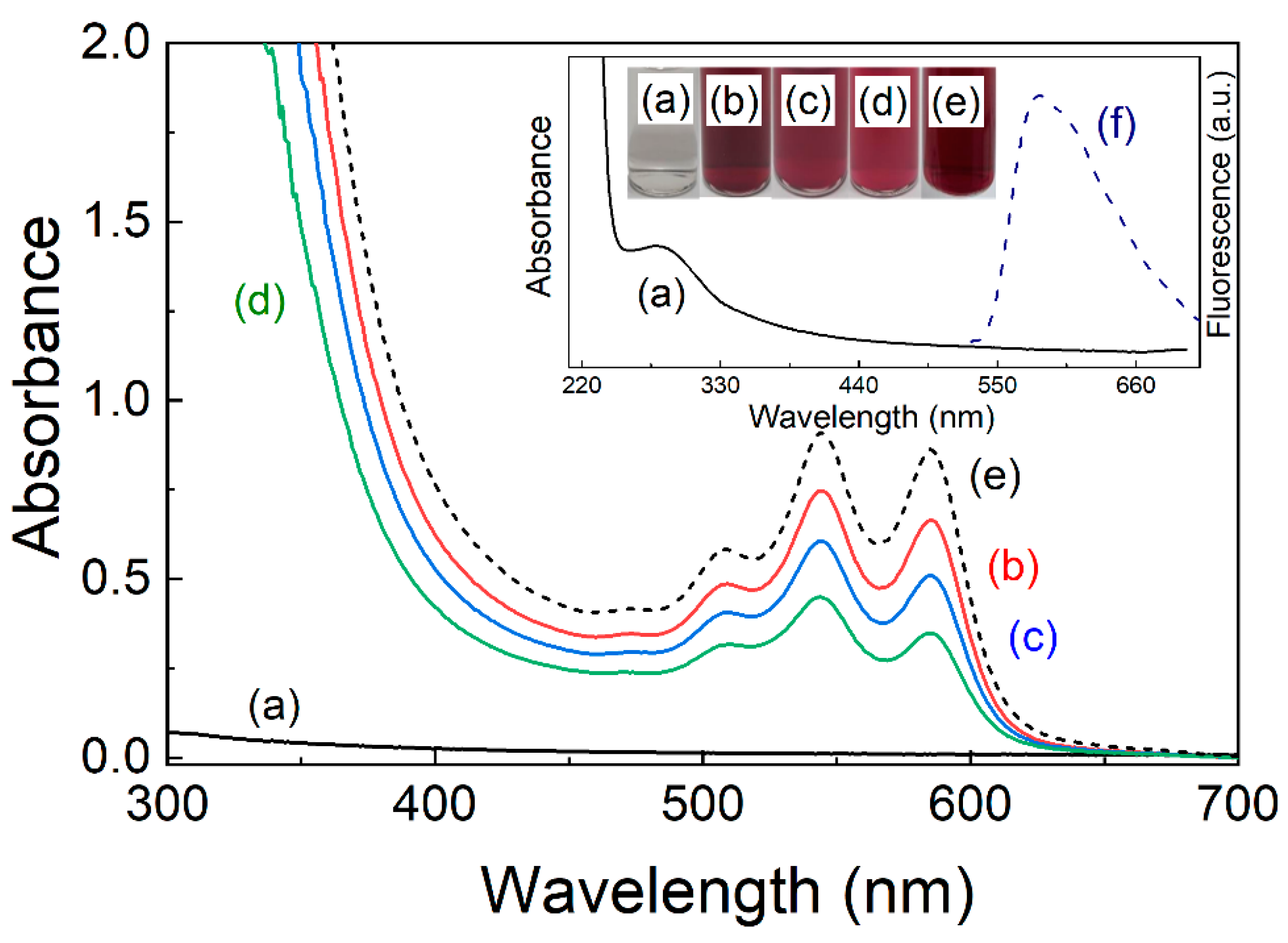
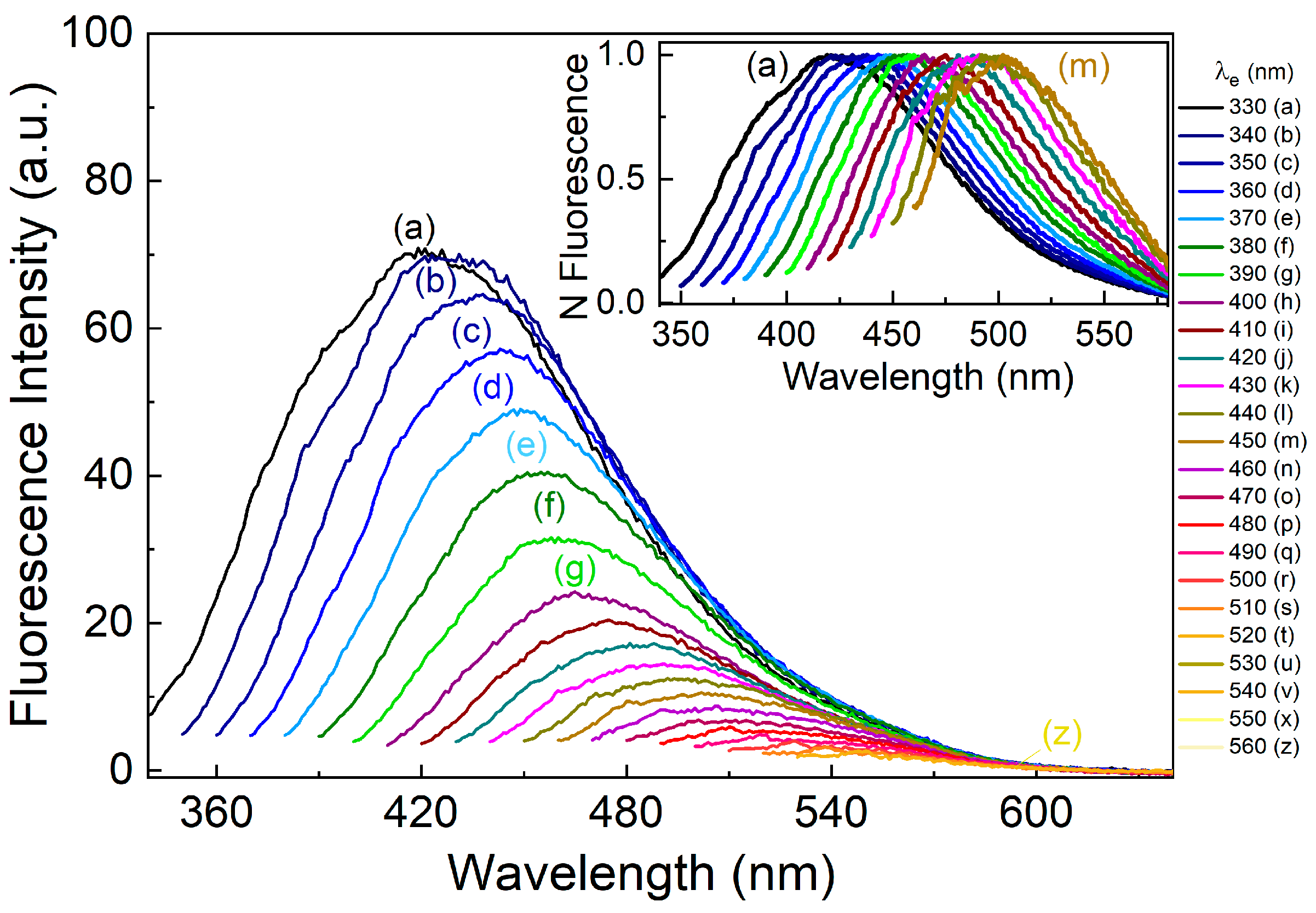
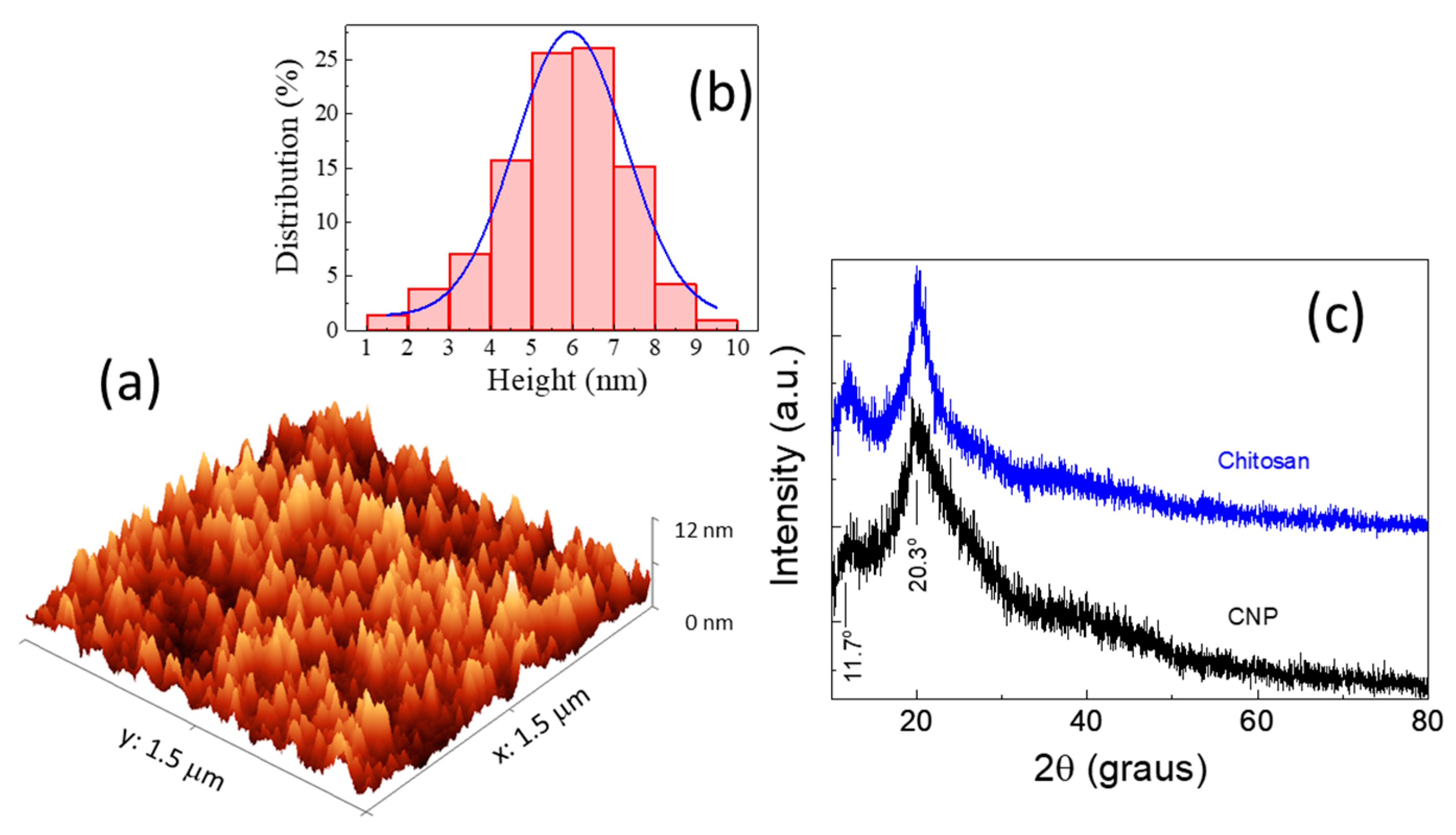
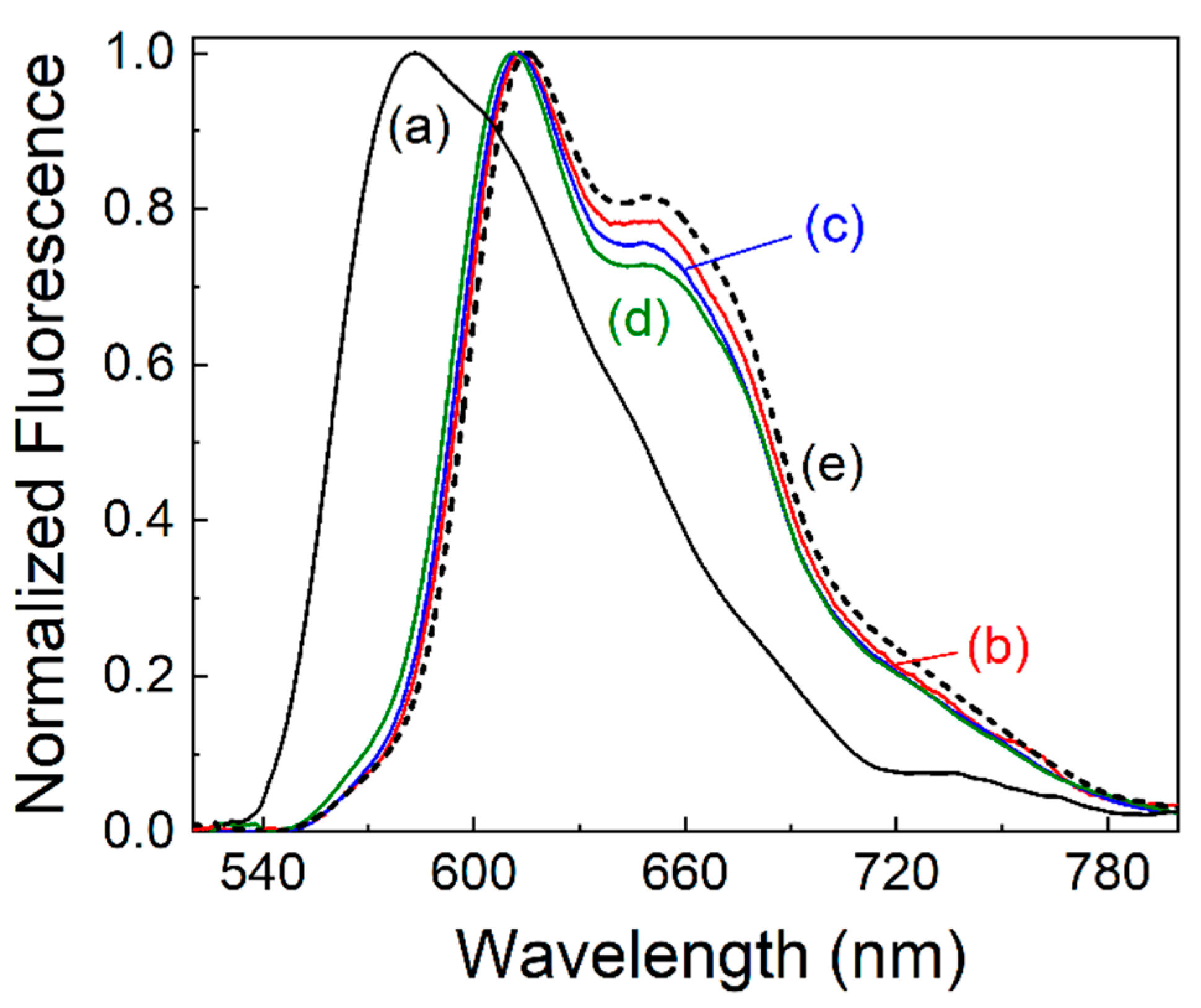
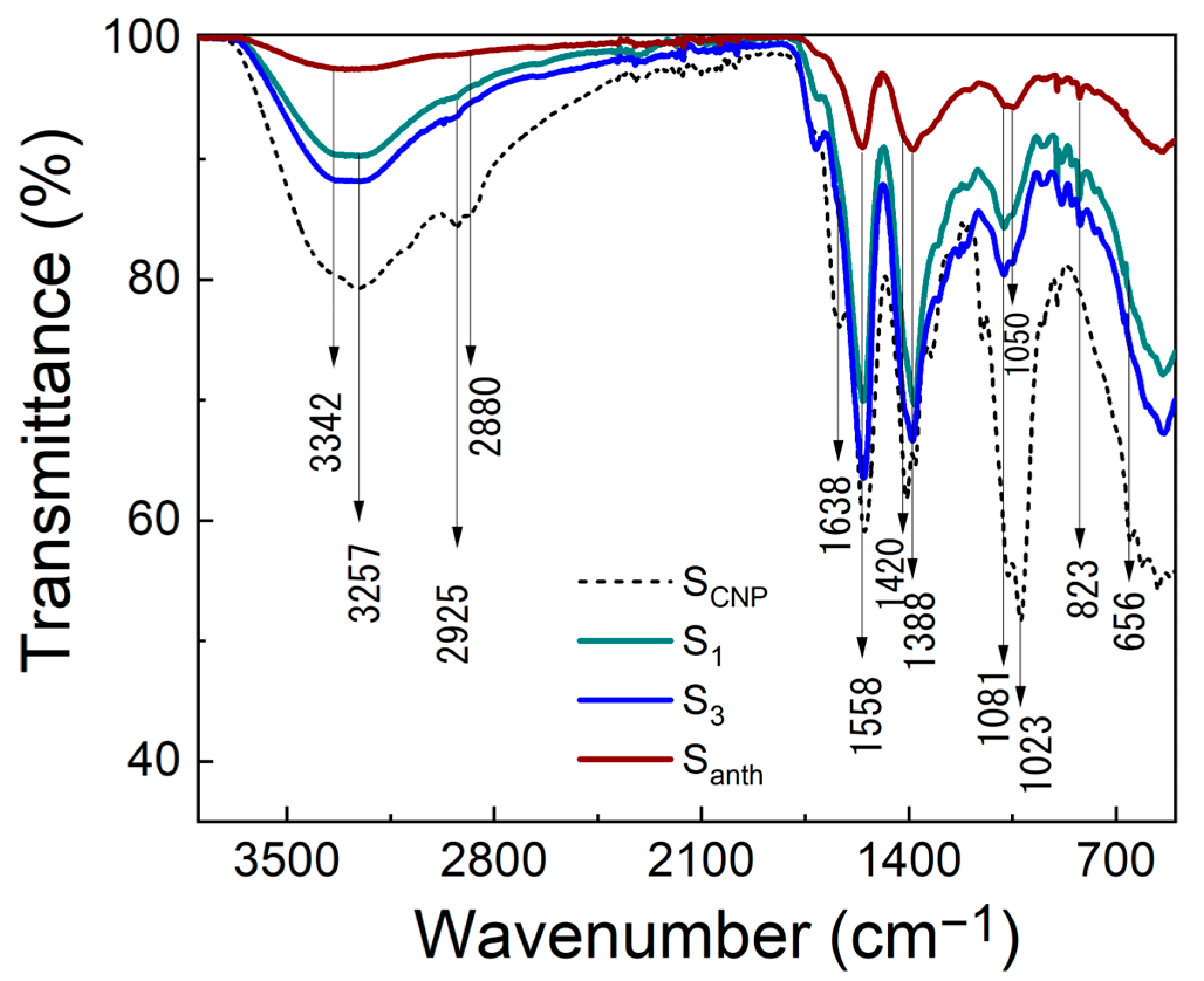
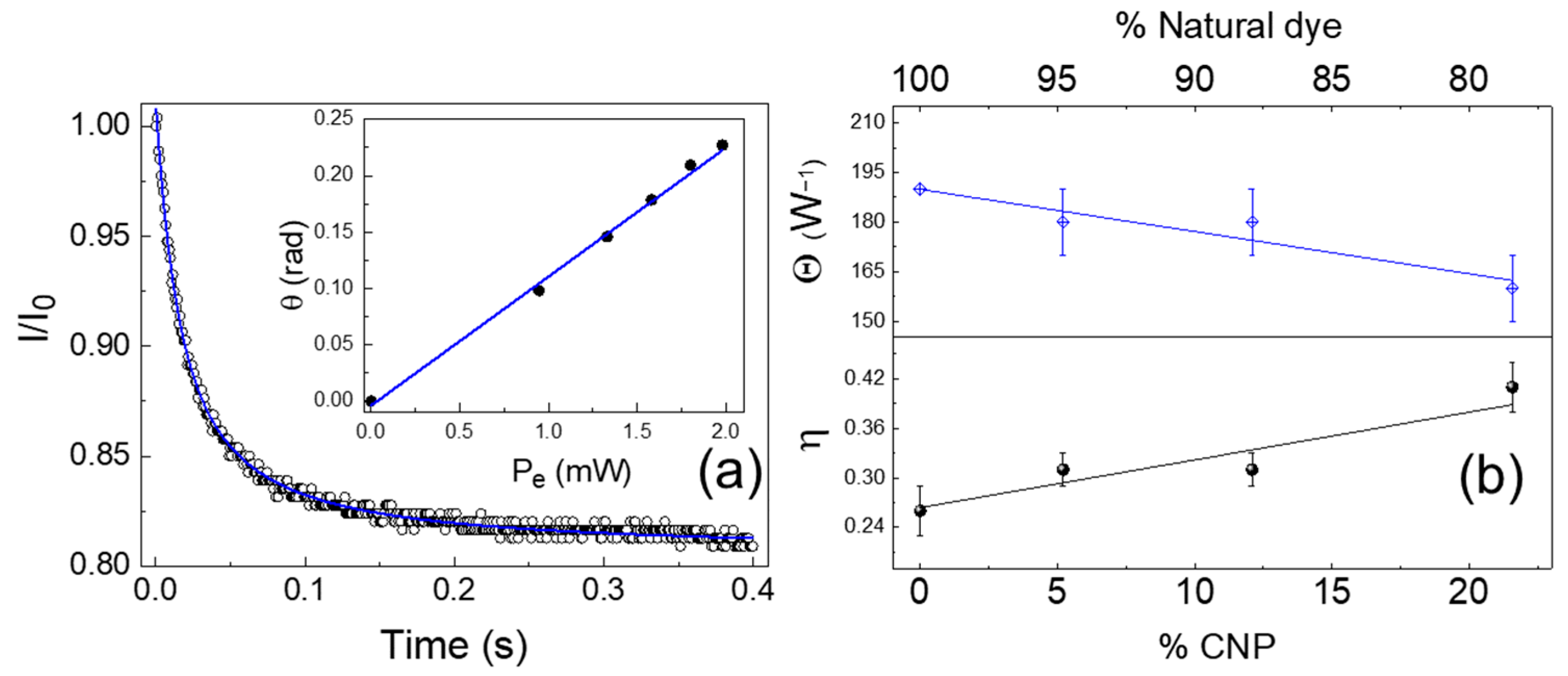
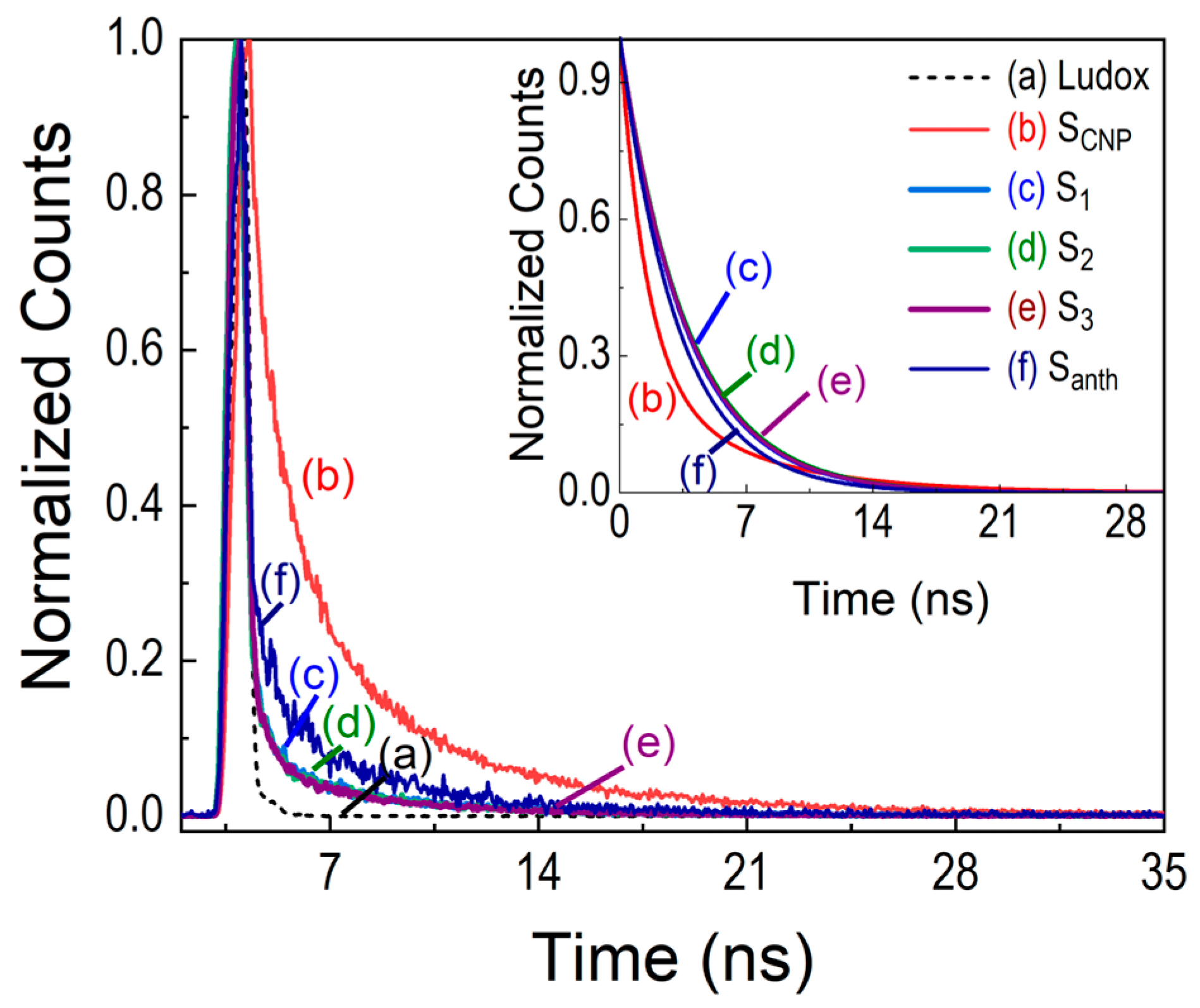
| Sample | <λem> (±1 nm) 1 | D (10−7 m2/s) | Θ (W−1) | η |
|---|---|---|---|---|
| S1 | 653 | (1.42 ± 0.07) | (180 ± 10) | (0.31 ± 0.02) |
| S2 | 650 | (1.41 ± 0.09) | (180 ± 10) | (0.31 ± 0.02) |
| S3 | 648 | (1.4 ± 0.1) | (160 ± 10) | (0.41 ± 0.03) |
| Santh | 655 | (1.41 ± 0.03) | (190 ± 20) | (0.26 ± 0.03) |
| Carbon Source | Sample Name | τ (ns) | η (%) |
|---|---|---|---|
| Bovine Serum Albumin [58] | FC-NPs-2 | 7.6 | 16.5 |
| Jinhua Bergamot [59] | C-dots | 3.84 | 50.78 |
| Grape Seed [60] | sGQDs | 10.04 | 31.79 |
| Tamarindus indica Leaves [61] | CQDs | 5.98 | 46.6 |
| Chitosan (this work) | S3 CNPs | (3.65 ± 0.04) | (41 ± 3) |
| Sample | τ1 (ns) (A1 %) | τ2 (ns) (A2 %) | χ2 | τAVE (ns) |
|---|---|---|---|---|
| SCNP | 1.4 ± 0.2 (73.68) | 6.2 ± 0.3 (26.32) | 1.00 ± 0.01 | 4.34 |
| S1 | 3.58 ± 0.05 (100) | - | 1.01 ± 0.01 | - |
| S2 | 3.71 ± 0.04 (100) | - | 1.18 ± 0.08 | - |
| S3 | 3.65 ± 0.04 (100) | - | 1.20 ± 0.06 | - |
| Santh | 3.2 ± 0.2 (100) | - | 1.03 ± 0.05 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lima, S.R.; Costa, T.V.; Santos, T.T.S.; Felipe, D.G.; Serna, T.B.; Andrade, A.A.; Pilla, V. Optical Characterization of Fluorescent Chitosan-Based Carbon Dots Embedded in Aqueous Natural Dye. Colorants 2024, 3, 269-281. https://doi.org/10.3390/colorants3040019
De Lima SR, Costa TV, Santos TTS, Felipe DG, Serna TB, Andrade AA, Pilla V. Optical Characterization of Fluorescent Chitosan-Based Carbon Dots Embedded in Aqueous Natural Dye. Colorants. 2024; 3(4):269-281. https://doi.org/10.3390/colorants3040019
Chicago/Turabian StyleDe Lima, Sthanley R., Thiago V. Costa, Tácio T. S. Santos, Dora G. Felipe, Teófanes B. Serna, Acácio A. Andrade, and Viviane Pilla. 2024. "Optical Characterization of Fluorescent Chitosan-Based Carbon Dots Embedded in Aqueous Natural Dye" Colorants 3, no. 4: 269-281. https://doi.org/10.3390/colorants3040019
APA StyleDe Lima, S. R., Costa, T. V., Santos, T. T. S., Felipe, D. G., Serna, T. B., Andrade, A. A., & Pilla, V. (2024). Optical Characterization of Fluorescent Chitosan-Based Carbon Dots Embedded in Aqueous Natural Dye. Colorants, 3(4), 269-281. https://doi.org/10.3390/colorants3040019







