Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Gr@ZnO Nanocomposite
2.2. Characterization
2.3. Photocatalytic Experiments
3. Results and Discussion
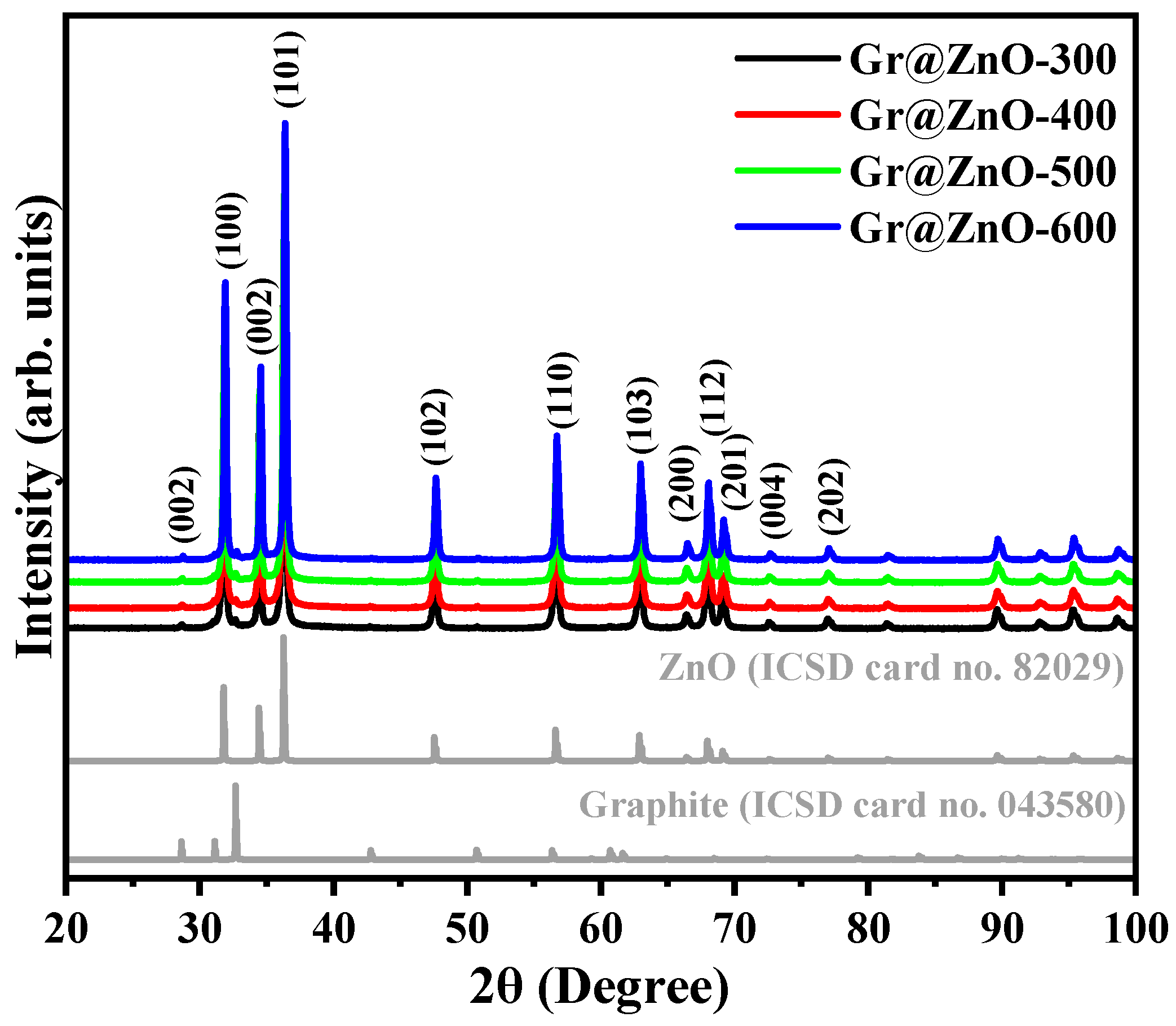
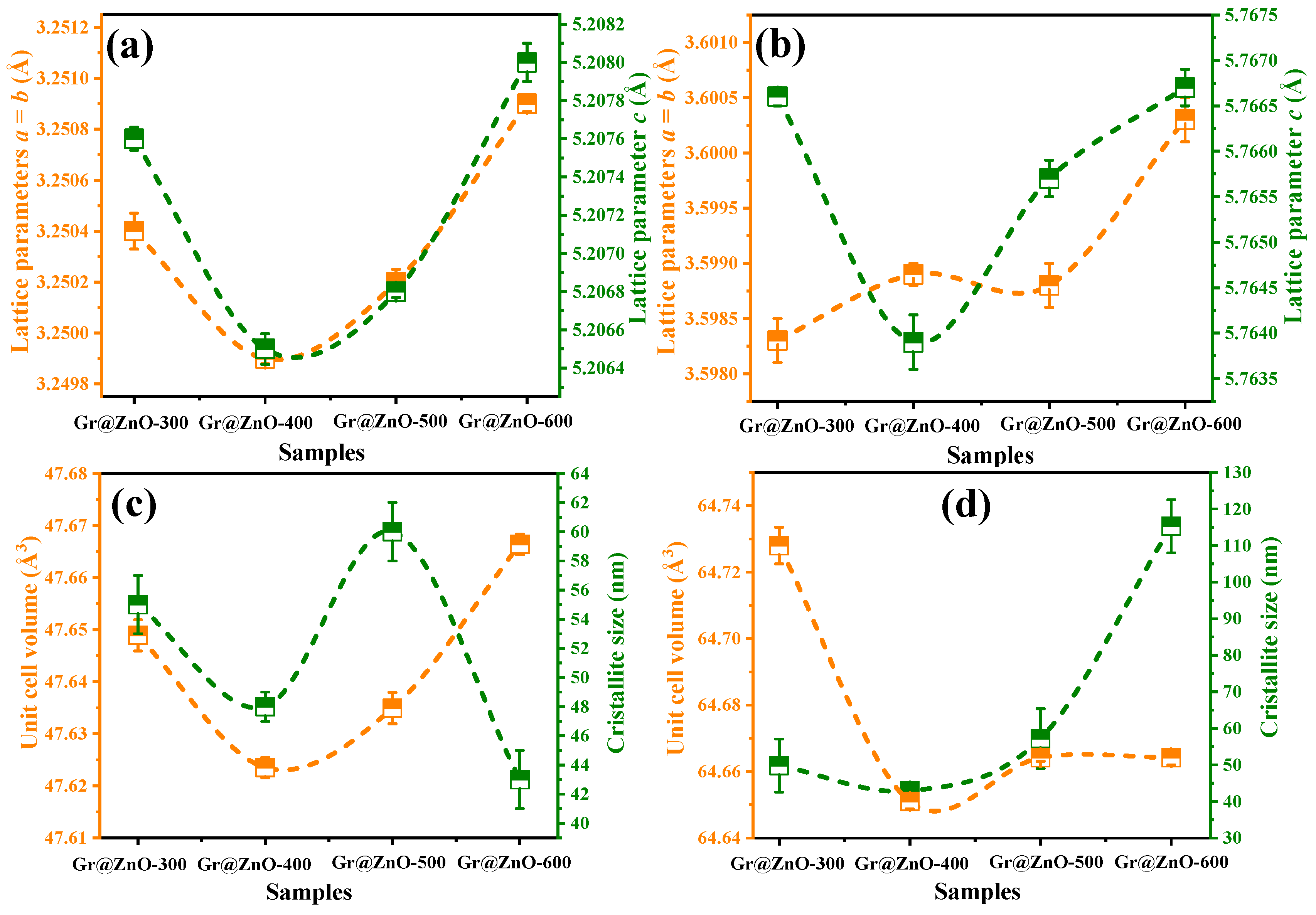
3.1. XRD Diffraction Pattern and Structural Rietveld Refinement
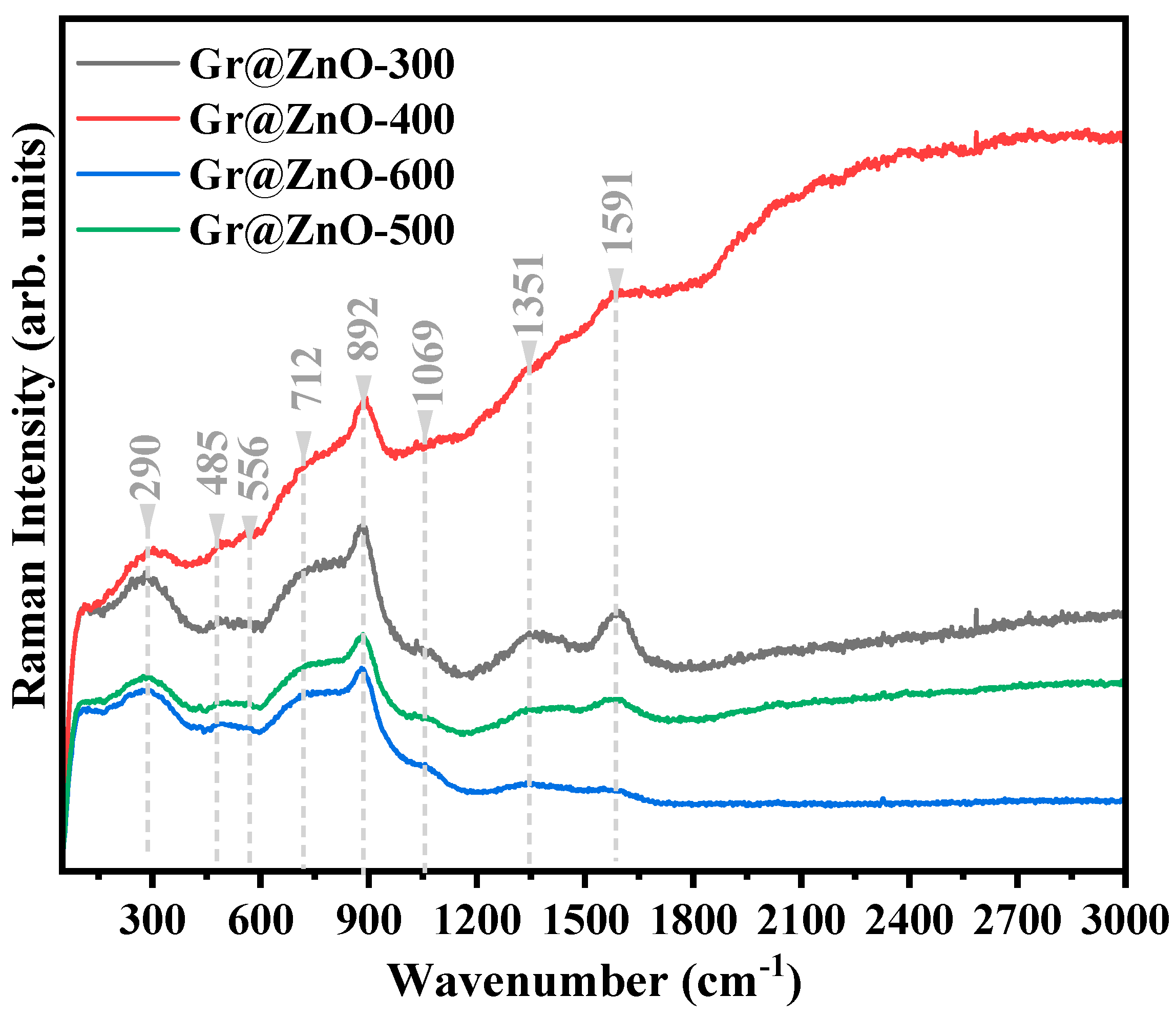
3.2. Vibrational Raman Spectroscopy
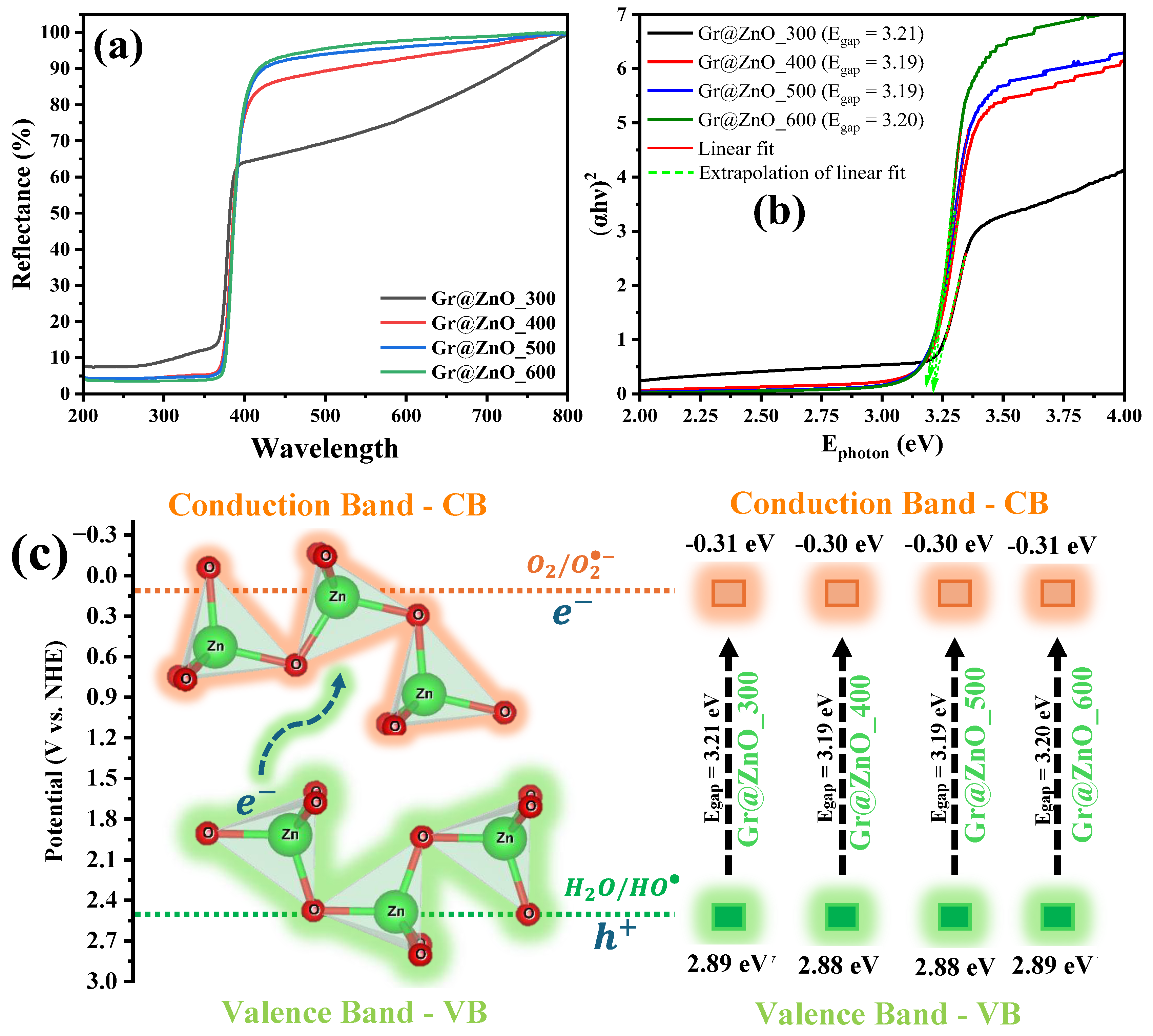
3.3. UV-Vis by Diffuse Reflectance Spectroscopy
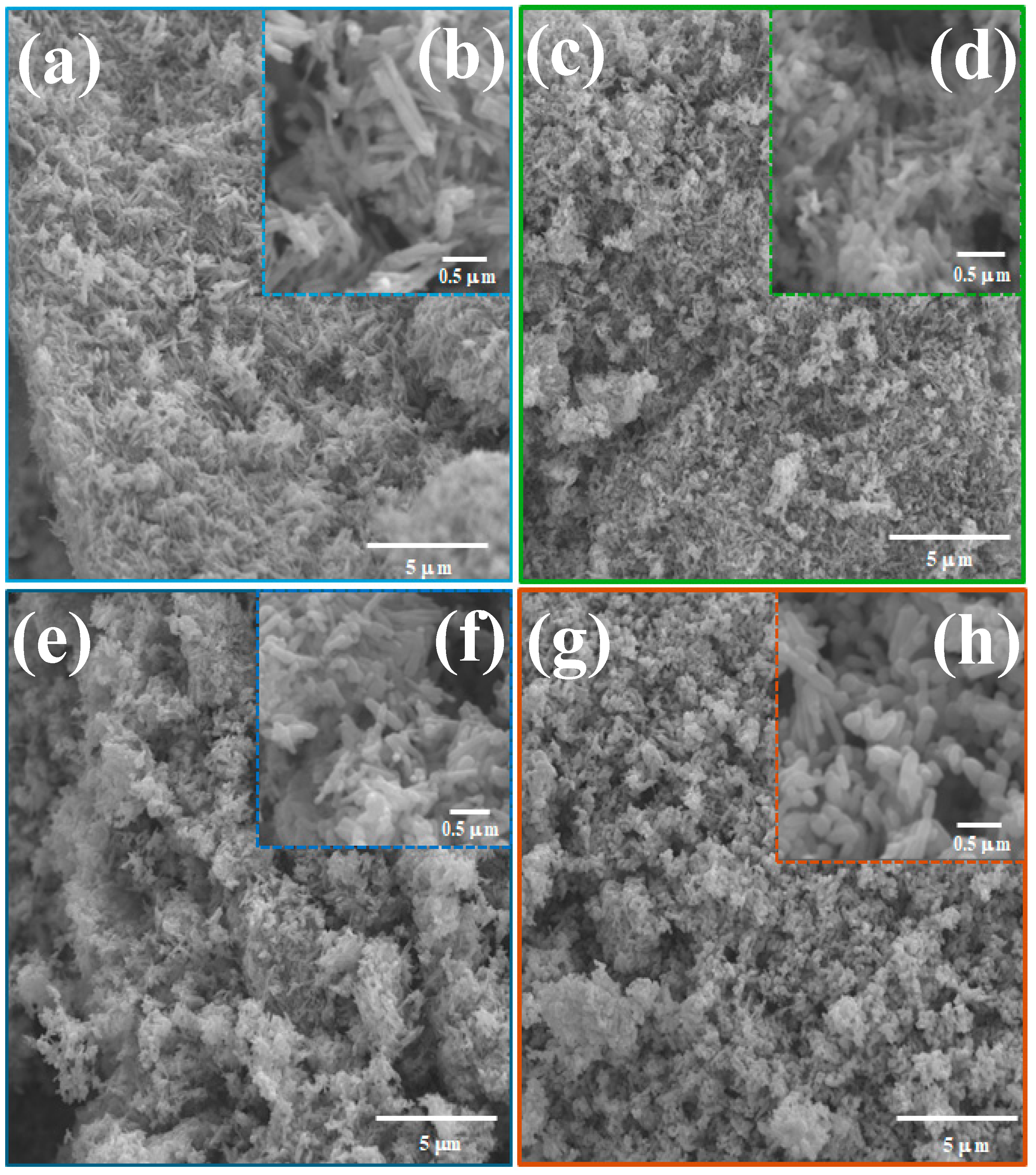
3.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX)
3.5. Colourimetry
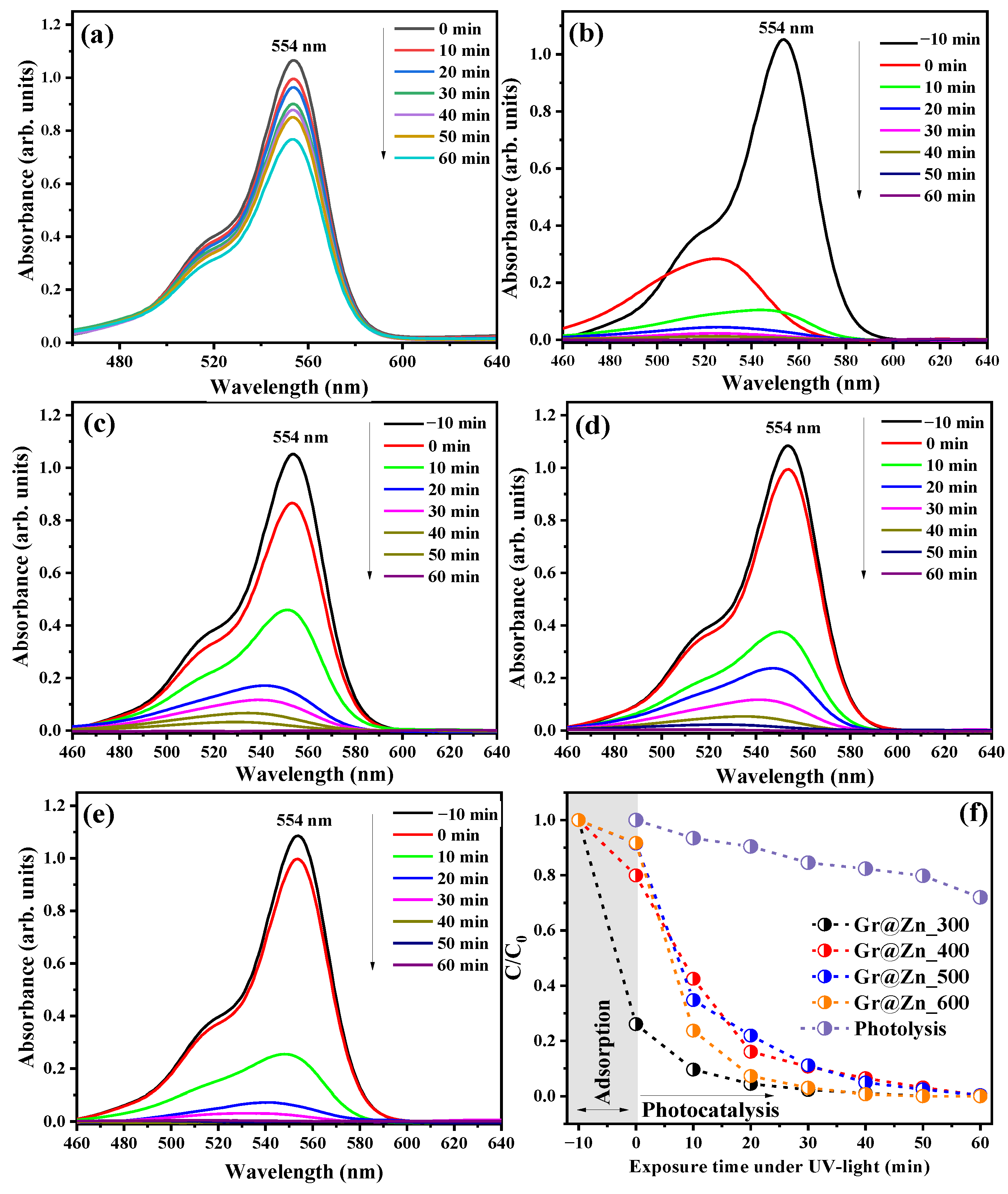
3.6. Photocatalytic Properties of Samples in the Discolouration of RhB Dye Solution
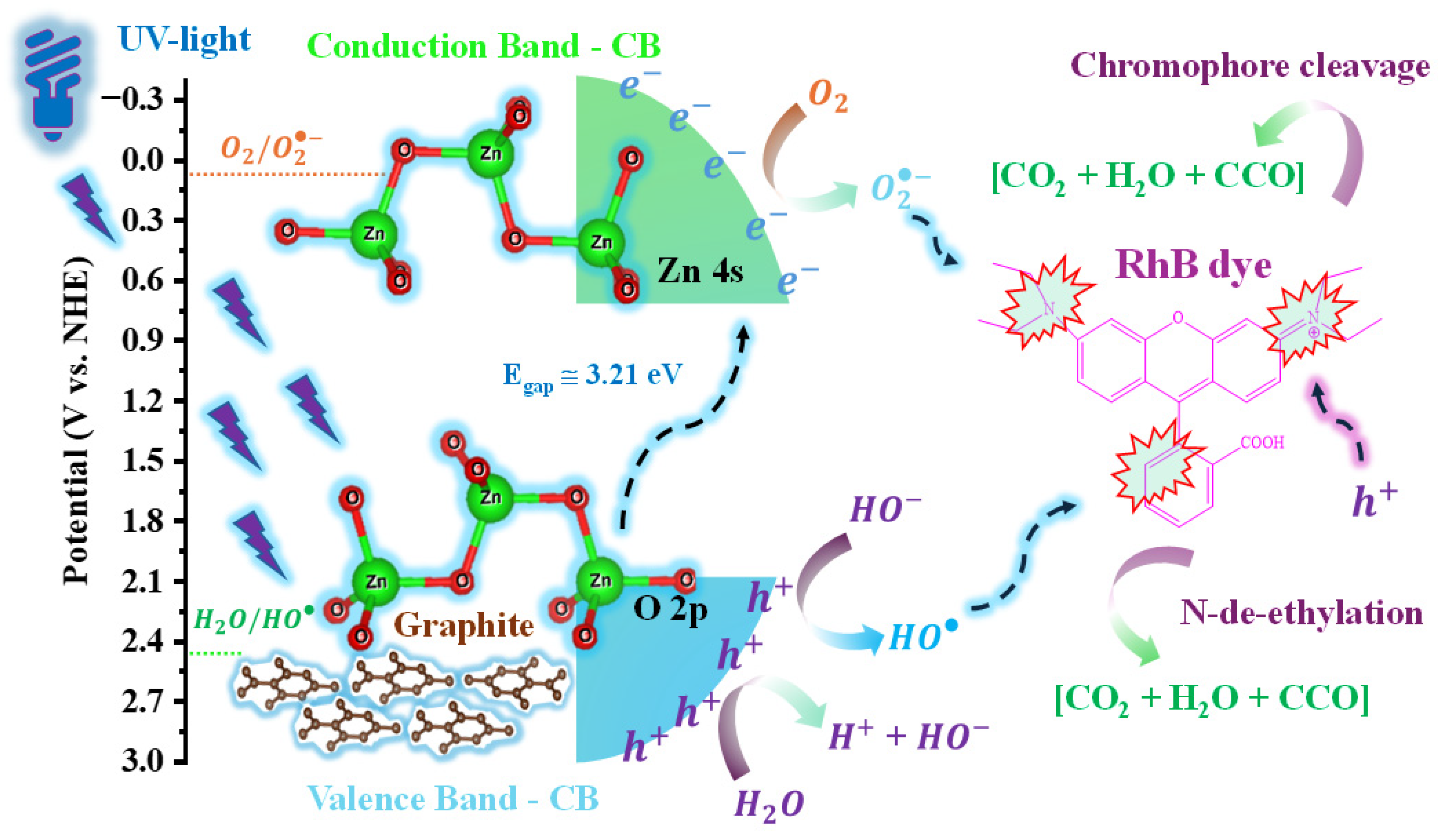
3.7. Proposed Mechanisms for RhB Discolouration by the Gr@ZnO_300 Sample
3.8. Reusability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Wang, Z.; Yang, Y.; Liu, H.; Fang, S.; Liu, S. Research Status and Development Trend of Wastewater Treatment Technology and Its Low Carbonization. Appl. Sci. 2023, 13, 1400. [Google Scholar] [CrossRef]
- Kumar, M.; Shekhar, S.; Kumar, R.; Kumar, P.; Govarthanan, M.; Chaminda, T. Drinking water treatment and associated toxic byproducts: Concurrence and urgence. Environ. Pollut. 2023, 320, 121009. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Kazama, S.; Benyapa, S.; Takizawa, S. Performance Assessment of Household Water Treatment and Safe Storage in Kathmandu Valley, Nepal. Water 2023, 15, 2305. [Google Scholar] [CrossRef]
- Tortajada, C. Contributions of recycled wastewater to clean water and sanitation Sustainable Development Goals. Npj Clean. Water 2020, 3, 22. [Google Scholar] [CrossRef]
- Oliveira, E.D.C.; Castelo-Branco, R.; Silva, L.; Silva, N.; Azevedo, J.; Vasconcelos, V.; Faustino, S.; Cunha, A. First detection of microcystin-lr in the amazon river at the drinking water treatment plant of the municipality of Macapá, Brazil. Toxins 2019, 11, 669. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Rayaroth, M.P.; Aravindakumar, C.T.; Shah, N.S.; Boczkaj, G. Advanced oxidation processes (AOPs) based wastewater treatment—Unexpected nitration side reactions—A serious environmental issue: A review. Chem. Eng. J. 2022, 430, 133002. [Google Scholar] [CrossRef]
- Nobre, F.X.; Mariano, F.A.F.; Santos, F.E.P.; Rocco, M.L.M.; Manzato, L.; De Matos, J.M.E.; Couceiro, P.R.C.; Brito, W.R. Heterogeneous photocatalysis of Tordon 2,4-D herbicide using the phase mixture of TiO2. J. Environ. Chem. Eng. 2019, 7, 103501. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Amirulsyafiee, A.; Khan, M.M.; Harunsani, M.H. Ag3PO4 and Ag3PO4—Based visible light active photocatalysts: Recent progress, synthesis, and photocatalytic applications. Catal. Commun. 2022, 172, 106556. [Google Scholar] [CrossRef]
- Sumanth Kumar, D.; Jai Kumar, B.; Mahesh, H.M. Quantum Nanostructures (QDs): An Overview; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 9780081019757. [Google Scholar]
- Xu, Z.; Zhong, J.; Li, M. Ionic liquid-assisted construction of Z-scheme Ag/Ag3PO4/Ag2MoO4 heterojunctions with enhanced photocatalytic performance. Inorg. Chem. Commun. 2023, 152, 110734. [Google Scholar] [CrossRef]
- Li, X.; Hu, T.; Zhang, J.; Dai, K.; Liang, C. Novel 2D SnNb2O6/Ag3VO4 S-scheme heterojunction with enhanced visible-light photocatalytic activity. Ceram. Int. 2021, 47, 7169–7176. [Google Scholar] [CrossRef]
- Nobre, F.X.; Bastos, I.S.; dos Santos Fontenelle, R.O.; Júnior, E.A.A.; Takeno, M.L.; Manzato, L.; de Matos, J.M.E.; Orlandi, P.P.; de Fátima Souza Mendes, J.; Brito, W.R.; et al. Antimicrobial properties of α-Ag2WO4 rod-like microcrystals synthesized by sonochemistry and sonochemistry followed by hydrothermal conventional method. Ultrason. Sonochem. 2019, 58, 104620. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhang, L.; Xue, Y.; Feng, Y.; Wang, M.; Chen, J.; Ji, B.; Wang, C.; Xue, Y. Effects of shape and particle size on the photocatalytic kinetics and mechanism of nano-CeO2. Int. J. Miner. Metall. Mater. 2022, 29, 2221–2231. [Google Scholar] [CrossRef]
- He, X.; Yang, Y.; Li, Y.; Chen, J.; Yang, S.; Liu, R.; Xu, Z. Effects of structure and surface properties on the performance of ZnO towards photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2022, 599, 153898. [Google Scholar] [CrossRef]
- Tuc Altaf, C.; Colak, T.O.; Rostas, A.M.; Popa, A.; Toloman, D.; Suciu, M.; Demirci Sankir, N.; Sankir, M. Impact on the Photocatalytic Dye Degradation of Morphology and Annealing-Induced Defects in Zinc Oxide Nanostructures. ACS Omega 2023, 8, 14952–14964. [Google Scholar] [CrossRef]
- Ben Amor, I.; Hemmami, H.; Laouini, S.E.; Mahboub, M.S.; Barhoum, A. Sol-Gel Synthesis of ZnO Nanoparticles Using Different Chitosan Sources: Effects on Antibacterial Activity and Photocatalytic Degradation of AZO Dye. Catalysts 2022, 12, 1611. [Google Scholar] [CrossRef]
- García-Casas, X.; Aparicio, F.J.; Budagosky, J.; Ghaffarinejad, A.; Orozco-Corrales, N.; Ostrikov, K.; Sánchez-Valencia, J.R.; Barranco, Á.; Borrás, A. Paper-based ZnO self-powered sensors and nanogenerators by plasma technology. Nano Energy 2023, 114, 108686. [Google Scholar] [CrossRef]
- Manikanika; Chopra, L. Photocatalytic activity of zinc oxide for dye and drug degradation: A review. Mater. Today Proc. 2022, 52, 1653–1656. [Google Scholar] [CrossRef]
- Carmel Aruja, T.; Sharma, R.; Dhiman, V.; Chand, S.; Choudhary, P.; Kondal, N. ZnO based nanoadsorbents for purification of lead contaminated water. Mater. Today Proc. 2022; in press. [Google Scholar] [CrossRef]
- Mikhailov, M.M.; Yuryev, S.A.; Lapin, A.N.; Goronchko, V.A. Reflective thermal control coating for spacecraft based on ZnO pigment and Li2SiO3 silicate modified by SiO2 nanoparticles. Ceram. Int. 2023, 49, 20817–20821. [Google Scholar] [CrossRef]
- Smaoui, S.; Chérif, I.; Ben Hlima, H.; Khan, M.U.; Rebezov, M.; Thiruvengadam, M.; Sarkar, T.; Shariati, M.A.; Lorenzo, J.M. Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packag. Shelf Life 2023, 36, 101045. [Google Scholar] [CrossRef]
- Youn, S.-M.; Choi, S.-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Meng, Y.; Yu, M.; Yang, H. Modulating the antibacterial activity of ZnO/talc by balancing the monodispersity of ZnO nanoparticles. Appl. Clay Sci. 2023, 242, 107024. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Baranov, A.N.; Sokolov, P.S.; Solozhenko, V.L. ZnO under Pressure: From Nanoparticles to Single Crystals. Crystals 2022, 12, 744. [Google Scholar] [CrossRef]
- Zarhri, Z.; Cocom Dzul, M.B.; Ziat, Y.; Jiménez Torrez, L.F.; Oubram, O.; Ifguis, O. Comparative study of optical properties of ZnO Zinc Blend and Rock Salt structures, TB-mBJ and GGA approximations. Phys. B Condens. Matter 2022, 634, 413798. [Google Scholar] [CrossRef]
- Koster, R.S.; Fang, C.M.; Dijkstra, M.; Van Blaaderen, A.; Van Huis, M.A. Stabilization of rock salt ZnO nanocrystals by low-energy surfaces and Mg additions: A first-principles study. J. Phys. Chem. C 2015, 119, 5648–5656. [Google Scholar] [CrossRef]
- Ejsmont, A.; Goscianska, J. Hydrothermal Synthesis of ZnO Superstructures with Controlled Morphology via Temperature and pH Optimization. Materials 2023, 16, 1641. [Google Scholar] [CrossRef]
- Karkeh-Abadi, F.; Ghiyasiyan-Arani, M.; Salavati-Niasari, M. Sonochemical synthesized BaMoO4/ZnO nanocomposites as electrode materials: A comparative study on GO and GQD employed in hydrogen storage. Ultrason. Sonochem. 2022, 90, 106167. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.B.; Saeed, F.R.; Gbashi, K.R.; Mahmood, U.S. Synthesis and characterization of zinc oxide nanoparticles via oxalate co-precipitation method. Mater. Lett. X 2022, 13, 100126. [Google Scholar] [CrossRef]
- Zhao, D.; Sathasivam, S.; Wang, M.; Carmalt, C.J. Transparent and conducting boron doped ZnO thin films grown by aerosol assisted chemical vapor deposition. RSC Adv. 2022, 12, 33049–33055. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Kaurav, N. The structural and optical properties of ZnO nanoparticles synthesized via thermal decomposition. Mater. Today Proc. 2022, 54, 624–627. [Google Scholar] [CrossRef]
- Murcia, J.J.; Hernández Niño, J.S.; Rojas, H.; Brijaldo, M.H.; Martín-Gómez, A.N.; Sánchez-Cid, P.; Navío, J.A.; Hidalgo, M.C.; Jaramillo-Paez, C. ZnO/Ag3PO4 and ZnO–Malachite as Effective Photocatalysts for the Removal of Enteropathogenic Bacteria, Dyestuffs, and Heavy Metals from Municipal and Industrial Wastewater. Water 2021, 13, 2264. [Google Scholar] [CrossRef]
- Saeed, M.; Albalawi, K.; Khan, I.; Akram, N.; Abd El-Rahim, I.H.A.; Alhag, S.K.; Ezzat Ahmed, A. Faiza Synthesis of p-n NiO-ZnO heterojunction for photodegradation of crystal violet dye. Alexandria Eng. J. 2023, 65, 561–574. [Google Scholar] [CrossRef]
- Kara, R.; Mentar, L.; Azizi, A. Synthesis and characterization of Mg-doped ZnO thin-films electrochemically grown on FTO substrates for optoelectronic applications. RSC Adv. 2020, 10, 40467–40479. [Google Scholar] [CrossRef]
- Shivananjaiah, H.N.; Sailaja Kumari, K.; Geetha, M.S. Green mediated synthesis of lanthanum doped zinc oxide: Study of its structural, optical and latent fingerprint application. J. Rare Earths 2020, 38, 1281–1287. [Google Scholar] [CrossRef]
- Wang, C.C.; Lo, A.Y.; Cheng, M.C.; Chang, Y.S.; Shih, H.C.; Shieu, F.S.; Tsai, H.T. Zinc oxide nanostructures enhanced photoluminescence by carbon-black nanoparticles in Moiré heterostructures. Sci. Rep. 2023, 13, 9704. [Google Scholar] [CrossRef]
- Lam, E.; Luong, J.H.T. Carbon materials as catalyst supports and catalysts in the transformation of biomass to fuels and chemicals. ACS Catal. 2014, 4, 3393–3410. [Google Scholar] [CrossRef]
- Xu, J.J.; Lu, Y.N.; Tao, F.F.; Liang, P.F.; Zhang, P.A. ZnO Nanoparticles Modified by Carbon Quantum Dots for the Photocatalytic Removal of Synthetic Pigment Pollutants. ACS Omega 2023, 8, 7845–7857. [Google Scholar] [CrossRef] [PubMed]
- Jayababu, N.; Kim, D. ZnO nanorods@conductive carbon black nanocomposite based flexible integrated system for energy conversion and storage through triboelectric nanogenerator and supercapacitor. Nano Energy 2021, 82, 105726. [Google Scholar] [CrossRef]
- Saranya, M.; Ramachandran, R.; Wang, F. Graphene-zinc oxide (G-ZnO) nanocomposite for electrochemical supercapacitor applications. J. Sci. Adv. Mater. Devices 2016, 1, 454–460. [Google Scholar] [CrossRef]
- Sawada, H.; Wang, R.; Sleight, A.W. An Electron Density Residual Study of Zinc Oxide. J. Solid State Chem. 1996, 122, 148–150. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the “Debye-Scherrer equation”. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef]
- Sankara Reddy, B.; Venkatramana Reddy, S.; Koteeswara Reddy, N. Physical and magnetic properties of (Co, Ag) doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2013, 24, 5204–5210. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Noor, N.A.M.; Kamarudin, S.K.; Darus, M.; Diyana, N.F.; Yunos, M.; Idris, M.A. Photocatalytic properties and graphene oxide additional effects in TiO2. Solid State Phenom. 2018, 280, 65–70. [Google Scholar] [CrossRef]
- Refat, N.M.; Nassar, M.Y.; Sadeek, S.A. A controllable one-pot hydrothermal synthesis of spherical cobalt ferrite nanoparticles: Synthesis, characterization, and optical properties. RSC Adv. 2022, 12, 25081–25095. [Google Scholar] [CrossRef]
- Rahman, M.; Kamruzzaman, M.; Zapien, J.A.; Afrose, R.; Anam, T.K.; Liton, M.N.H.; Helal, M.A.; Khan, M.K.R. Conversion of n-type to p-type conductivity in ZnO by incorporation of Ag and Ag-Li. Mater. Today Commun. 2022, 33, 104278. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P. Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System. Phys. Status Solid Basic Res. 2018, 255, 1700393. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, J.; Jhun, C.G. Zno nanospheres fabricated by mechanochemical method with photocatalytic properties. Catalysts 2021, 11, 572. [Google Scholar] [CrossRef]
- Otis, G.; Ejgenberg, M.; Mastai, Y. Solvent-free mechanochemical synthesis of zno nanoparticles by high-energy ball milling of ε-zn(Oh)2 crystals. Nanomaterials 2021, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Masula, K.; Bhongiri, Y.; Raghav Rao, G.; Vijay Kumar, P.; Pola, S.; Basude, M. Evolution of photocatalytic activity of CeO2–Bi2O3 composite material for wastewater degradation under visible-light irradiation. Opt. Mater. 2022, 126, 112201. [Google Scholar] [CrossRef]
- Safartoobi, A.; Mazloom, J.; Ghodsi, F.E. Silver/molybdenum metal-organic framework derived Ag2MoO4 nanoparticles as novel electrode for high-performance supercapacitor. J. Energy Storage 2023, 68, 107818. [Google Scholar] [CrossRef]
- Farooq, U.; Phul, R.; Alshehri, S.M.; Ahmed, J.; Ahmad, T. Electrocatalytic and Enhanced Photocatalytic Applications of Sodium Niobate Nanoparticles Developed by Citrate Precursor Route. Sci. Rep. 2019, 9, 4488. [Google Scholar] [CrossRef]
- Khadim, H.J.; Al-Farraji, A.; Ammar, S.H. Boosted visible-light-driven photocatalytic degradation of lomefloxacin over α-Ag2WO4/NiSx nanocomposites. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100722. [Google Scholar] [CrossRef]
- Mar, G.L.; Timbrell, P.Y.; Lamb, R.N. Formation of Zinc Oxide Thin Films by the Thermal Decomposition of Zinc Acetate. Surf. Sci. Princ. Appl. 1993, 73, 177–192. [Google Scholar] [CrossRef]
- Oliveira, Y.L.; Costa, M.J.S.; Jucá, A.C.S.; Silva, L.K.R.; Longo, E.; Arul, N.S.; Cavalcante, L.S. Structural characterization, morphology, optical and colorimetric properties of NiWO4 crystals synthesized by the co-precipitation and polymeric precursor methods. J. Mol. Struct. 2020, 1221, 128774. [Google Scholar] [CrossRef]
- Nobre, F.X.; Mendes, O.C.; da Silva, A.P.J.; Junior, J.L.S.; do Nascimento, M.V.B.; Pessoa Junior, W.A.G.; Manzato, L.; Brandim, A.S.; Matos, J.M.E.; Brito, W.R.; et al. Fast and efficient green synthesis of CaWO4 NPs using eggshells as a biogenic calcium source: Structure, optical property, and morphology. J. Photochem. Photobiol. A Chem. 2023, 439, 114589. [Google Scholar] [CrossRef]
- van Brenk, B.; Wösten, H.A.B. A screening method for decoloration of xenobiotic dyes by fungi. J. Microbiol. Methods 2021, 188, 106301. [Google Scholar] [CrossRef] [PubMed]
- Kusmierek, E. A CeO2 semiconductor as a photocatalytic and photoelectrocatalytic material for the remediation of pollutants in industrial wastewater: A review. Catalysts 2020, 10, 1435. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Tseng, C.-S.; Tseng, P.-J.; Huang, C.-W.; Wu, T.; Lin, Y.-W. Synthesis and characterization of Ag/Ag3PO4 nanomaterial modified BiPO4 photocatalyst by sonochemical method and its photocatalytic application. J. Mater. Sci. Mater. Electron. 2017, 28, 11886–11899. [Google Scholar] [CrossRef]
- Kumar, S.; AlOmar, S.Y.; Kumari, K.; Albalawi, F.; Kumar, R.; Ahmed, F.; Ahmad, N.; Dwivedi, S.; Alvi, P.A. Structural, Optical, Electrical and Antibacterial Properties of Fe-Doped CeO2 Nanoparticles. Crystals 2021, 11, 1594. [Google Scholar] [CrossRef]
- Cho, I.S.; Kwak, C.H.; Kim, D.W.; Lee, S.; Hong, K.S. Photophysical, photoelectrochemical, and photocatalytic properties of novel SnWO4 oxide semiconductors with narrow band gaps. J. Phys. Chem. C 2009, 113, 10647–10653. [Google Scholar] [CrossRef]
- Cho, I.S.; Kim, D.W.; Cho, C.M.; An, J.S.; Roh, H.S.; Hong, K.S. Synthesis, characterization and photocatalytic properties of CaNb2O6 with ellipsoid-like plate morphology. Solid. State Sci. 2010, 12, 982–988. [Google Scholar] [CrossRef]
- Assis, M.; Robeldo, T.; Foggi, C.C.; Kubo, A.M.; Mínguez-Vega, G.; Condoncillo, E.; Beltran-Mir, H.; Torres-Mendieta, R.; Andrés, J.; Oliva, M.; et al. Ag Nanoparticles/α-Ag2WO4 Composite Formed by Electron Beam and Femtosecond Irradiation as Potent Antifungal and Antitumor Agents. Sci. Rep. 2019, 9, 9927. [Google Scholar] [CrossRef]
- Takeno, M.L.; Nobre, F.X.; da Costa, F.F.; Botelho do Nascimento, M.V.; Pessoa Júnior, W.A.G.; Araújo Júnior, E.A.; Sousa, G.d.S.; de Sá, M.L.; Gurgel, R.S.; Albuquerque, P.M.; et al. Solvent Effect on the Structural, Optical, Morphology, and Antimicrobial Activity of Silver Phosphate Microcrystals by Conventional Hydrothermal Method. ACS Omega 2024, 9, 23069–23085. [Google Scholar] [CrossRef]
- do Nascimento, M.V.; Nobre, F.X.; de Araújo, E.N.; de Araujo, C.I.; Couceiro, P.R.; Manzato, L. Ag2−xCuxWO4 Solid Solution: Structure, Morphology, Optical Properties, and Photocatalytic Performance in the Degradation of RhB under Blue Light-Emitting Device Irradiation. J. Phys. Chem. C 2021, 125, 11875–11890. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.K.; Kaushik, R.D.; Purohit, L.P. Chalcogen-doped zinc oxide nanoparticles for photocatalytic degradation of Rhodamine B under the irradiation of ultraviolet light. Mater. Today Chem. 2021, 20, 100464. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Ramaraghavulu, R.; Devarayapalli, K.C.; Yoo, K.; Vattikuti, S.V.P.; Shim, J. Photocatalytic dye degradation and hydrogen production activity of Ag3PO4/g-C3N4 nanocatalyst. J. Mater. Sci. Mater. Electron. 2019, 30, 14890–14901. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, H.M.; Wang, L.; Kim, I.; Yoon, Y.; Lee, H. Solar-light photocatalytic disinfection using crystalline/amorphous low energy bandgap reduced TiO2. Sci. Rep. 2016, 6, 25212. [Google Scholar] [CrossRef] [PubMed]
- Nobre, F.X.; Muniz, R.; do Nascimento, E.R.; Amorim, R.S.; Silva, R.S.; Almeida, A.; Moreira, J.A.; Tavares, P.B.; Brito, W.R.; Couceiro, P.R.C.; et al. Hydrothermal temperature dependence of CaWO4 nanoparticles: Structural, optical, morphology and photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021, 32, 9776–9794. [Google Scholar] [CrossRef]
- Sousa, G.d.S.; Nobre, F.X.; Araújo Júnior, E.A.; Sambrano, J.R.; Albuquerque, A.d.R.; Bindá, R.d.S.; Couceiro, P.R.d.C.; Brito, W.R.; Cavalcante, L.S.; Santos, M.R.d.M.C.; et al. Hydrothermal synthesis, structural characterization and photocatalytic properties of β-Ag2MoO4 microcrystals: Correlation between experimental and theoretical data. Arab. J. Chem. 2020, 13, 2806–2825. [Google Scholar] [CrossRef]
- Nobre, F.X.; Gil Pessoa, W.A.; Ruiz, Y.L.; Imbiriba Bentes, V.L.; Silva-Moraes, M.O.; Costa Silva, T.M.; Rocco, M.L.M.; Larrudé, D.R.G.; de Matos, J.M.E.; Couceiro, P.R.d.C.; et al. Facile synthesis of nTiO2 phase mixture: Characterization and catalytic performance. Mater. Res. Bull. 2019, 109, 60–71. [Google Scholar] [CrossRef]
- Chang, H.; Yi, H.; Ke, Q.; Zhang, J. Preparation of a AgCl/PbMoO4 Composite and Investigation of Its Photocatalytic Oxidative Desulfurization Performance. ACS Omega 2020, 5, 10927–10938. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Singh, L.; Ram, C. Photocatalytic degradation of hazardous Rhodamine B dye using sol-gel mediated ultrasonic hydrothermal synthesized of ZnO nanoparticles. Results Eng. 2023, 17, 100890. [Google Scholar] [CrossRef]
- Kumar, A.; Choudhary, P.; Kumar, A.; Camargo, P.H.C.; Krishnan, V. Recent Advances in Plasmonic Photocatalysis Based on TiO2 and Noble Metal Nanoparticles for Energy Conversion, Environmental Remediation, and Organic Synthesis. Small 2022, 18, 2101638. [Google Scholar] [CrossRef]
- Santiago, A.A.G.; Andrade Neto, N.F.; Longo, E.; Paskocimas, C.A.; Motta, F.V.; Bomio, M.R.D. Fast and continuous obtaining of Eu3+ doped CeO2 microspheres by ultrasonic spray pyrolysis: Characterization and photocatalytic activity. J. Mater. Sci. Mater. Electron. 2019, 30, 11508–11519. [Google Scholar] [CrossRef]
- Gomes, E.O.; Gracia, L.; Santiago, A.A.G.; Tranquilin, R.L.; Motta, F.V.; Amoresi, R.A.C.; Longo, E.; Bomio, M.R.D.; Andres, J. Structure, electronic properties, morphology evolution, and photocatalytic activity in PbMoO4 and Pb1-2: XCaxSrxMoO4(x = 0.1, 0.2, 0.3, 0.4 and 0.5) solid solutions. Phys. Chem. Chem. Phys. 2020, 22, 25876–25891. [Google Scholar] [CrossRef]
- Ayappan, C.; Palanivel, B.; Jayaraman, V.; Maiyalagan, T.; Mani, A. One-step hydrothermal synthesis of CaWO4/α-Ag2WO4 heterojunction: An efficient photocatalyst for removal of organic contaminants. Mater. Sci. Semicond. Process. 2019, 104, 104693. [Google Scholar] [CrossRef]
- Sayem, M.A.; Hossen Suvo, M.A.; Syed, I.M.; Bhuiyan, M.A. Effective adsorption and visible light driven enhanced photocatalytic degradation of rhodamine B using ZnO nanoparticles immobilized on graphene oxide nanosheets. Results Phys. 2024, 58, 107471. [Google Scholar] [CrossRef]





| Sample | Hex | Colourimetric Coordinates | Colour | RGB | Egap | ||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | R | G | B | ||||
| A | 7C756D | 49.58 | 1.79 | 5.12 | 124 | 117 | 109 | 3.21 | |
| B | CFCBC2 | 81.66 | 0.36 | 4.62 | 207 | 203 | 194 | 3.19 | |
| C | C2BEB9 | 77.32 | 0.65 | 3.04 | 194 | 190 | 185 | 3.19 | |
| D | B0AEAB | 71.15 | 0.23 | 1.77 | 176 | 174 | 171 | 3.21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, D.A.; da Silva, P.H.d.A.; da Silva, F.P.; Romaguera-Barcelay, Y.; Ferreira, R.D.; Araujo Junior, E.A.; Nascimento, J.F.d.L.; da Costa, F.F.; Takeno, L.L.; Leyet Ruiz, Y.; et al. Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye. Colorants 2024, 3, 229-252. https://doi.org/10.3390/colorants3030016
de Souza DA, da Silva PHdA, da Silva FP, Romaguera-Barcelay Y, Ferreira RD, Araujo Junior EA, Nascimento JFdL, da Costa FF, Takeno LL, Leyet Ruiz Y, et al. Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye. Colorants. 2024; 3(3):229-252. https://doi.org/10.3390/colorants3030016
Chicago/Turabian Stylede Souza, Dalete Araujo, Pedro Hyug de Almeida da Silva, Francisco Paulino da Silva, Yonny Romaguera-Barcelay, Robson Dantas Ferreira, Edgar Alves Araujo Junior, José Fábio de Lima Nascimento, Fagner Ferreira da Costa, Litiko Lopes Takeno, Yurimiler Leyet Ruiz, and et al. 2024. "Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye" Colorants 3, no. 3: 229-252. https://doi.org/10.3390/colorants3030016
APA Stylede Souza, D. A., da Silva, P. H. d. A., da Silva, F. P., Romaguera-Barcelay, Y., Ferreira, R. D., Araujo Junior, E. A., Nascimento, J. F. d. L., da Costa, F. F., Takeno, L. L., Leyet Ruiz, Y., Dominguez, L. A., Brito, W. R., & Nobre, F. X. (2024). Easy and Fast Obtention of ZnO by Thermal Decomposition of Zinc Acetate and Its Photocatalytic Properties over Rhodamine B Dye. Colorants, 3(3), 229-252. https://doi.org/10.3390/colorants3030016








