Development of a Carotenoid-Rich Microalgae Colorant by Microencapsulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemicals
2.3. Carotenoid Encapsulation by Spray-Drying Emulsion
2.3.1. Preparation of Oil-in-Water Emulsions
2.3.2. Spray-Drying Process Optimization
2.4. Oil-in-Water Emulsion Characterization
2.4.1. Emulsion Droplet Size
2.4.2. Emulsion Stability
2.4.3. Emulsion Morphology
2.4.4. Emulsion Reconstitution
2.5. Spray-Dried Particle Characterization
2.5.1. Total Carotenoid Content (TCC)
2.5.2. Particle Size Distribution
2.5.3. Particle Morphology
2.5.4. Particle Color Properties
2.6. Incorporation of Dunaliella Salina scCO2 Extract and Spray-Dried Particles in an Aqueous-Based Product
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Encapsulation Process Optimization
3.2. Oil-in-Water Emulsion Characterization
3.3. Spray-Dried Particle Characterization
3.4. Incorporation of Dunaliella Salina scCO2 Extract and Particles in an Aqueous-Based Food Product
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrero, M.; del Pilar Sánchez-Camargo, A.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Hosseini, S.R.P.; Tavakoli, O.; Sarrafzadeh, M.H. Experimental optimization of SC-CO2 extraction of carotenoids from Dunaliella salina. J. Supercrit. Fluids 2017, 121, 89–95. [Google Scholar] [CrossRef]
- Sovová, H.; Stateva, R.P. New developments in the modelling of carotenoids extraction from microalgae with supercritical CO2. J. Supercrit. Fluids 2019, 148, 93–103. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Srinivasan, R.; Chaitanyakumar, A.; Mageswari, A.; Gomathi, A.; Pavan Kumar, J.G.S.; Jayasindu, M.; Bharath, G.; Shravan, J.S.; Gothandam, K.M. Oral administration of lyophilized Dunaliella salina, a carotenoid-rich marine alga, reduces tumor progression in mammary cancer induced rats. Food Funct. 2017, 8, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Mouahid, A.; Crampon, C.; Toudji, S.A.A.; Badens, E. Effects of high water content and drying pre-treatment on supercritical CO2 extraction from Dunaliella salina microalgae: Experiments and modelling. J. Supercrit. Fluids 2016, 116, 271–280. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Krivoshapkina, E.F. Selected Methods of Extracting Carotenoids, Characterization, and Health Concerns: A Review. J. Agric. Food Chem. 2018, 66, 5925–5947. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative assessment on the extraction of carotenoids from microalgal sources: Astaxanthin from H. pluvialis and β-carotene from D. salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Pourkarimi, S.; Hallajisani, A.; Nouralishahi, A.; Alizadehdakhel, A.; Golzary, A. Factors affecting production of beta-carotene from Dunaliella salina microalgae. Biocatal. Agric. Biotechnol. 2020, 29, 101771. [Google Scholar] [CrossRef]
- López, G.D.; Álvarez-Rivera, G.; Carazzone, C.; Ibáñez, E.; Leidy, C.; Cifuentes, A. Bacterial Carotenoids: Extraction, Characterization, and Applications. Crit. Rev. Anal. Chem. 2023, 53, 1239–1262. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Lokesh, V.; Shang, X.; Shin, J. Carotenoids: Dietary Sources, Extraction, Encapsulation, Bioavailability, and Health Benefits—A Review of Recent Advancements. Antioxidants 2022, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Konar, N.; Durmaz, Y.; Gurbuz, B.; Genc Polat, D.; Mert, B. Spray-Drying Optimization for Dunaliella salina and Porphyridium cruentum Biomass. Drying Technology 2023, 41, 2371–2384. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT Food Sci. Technol. 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Lim, A.S.L.; Roos, Y.H. Spray drying of high hydrophilic solids emulsions with layered interface and trehalose-maltodextrin as glass formers for carotenoids stabilization. J. Food Eng. 2016, 171, 174–184. [Google Scholar] [CrossRef]
- Loksuwan, J. Characteristics of microencapsulated β-carotene formed by spray drying with modified tapioca starch, native tapioca starch and maltodextrin. Food Hydrocoll. 2007, 21, 928–935. [Google Scholar] [CrossRef]
- Rocha, G.A.; Fávaro-Trindade, C.S.; Grosso, C.R.F. Microencapsulation of lycopene by spray drying: Characterization, stability and application of microcapsules. Food Bioprod. Process. 2012, 90, 37–42. [Google Scholar] [CrossRef]
- Kha, T.C.; Nguyen, M.H.; Roach, P.D.; Stathopoulos, C.E. Microencapsulation of Gac oil: Optimisation of spray drying conditions using response surface methodology. Powder Technol. 2014, 264, 298–309. [Google Scholar] [CrossRef]
- Lima, P.M.; Dacanal, G.C.; Pinho, L.S.; Pérez-Córdoba, L.J.; Thomazini, M.; Moraes, I.C.F.; Favaro-Trindade, C.S. Production of a rich-carotenoid colorant from pumpkin peels using oil-in-water emulsion followed by spray drying. Food Res. Int. 2021, 148, 110627. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Lezama, A.Y.; Dorantes-Alvarez, L.; Jaramillo-Flores, M.E.; Pérez-Alonso, C.; Niranjan, K.; Gutiérrez-López, G.F.; Alamilla-Beltrán, L. Preparation and characterization of non-aqueous extracts from chilli (Capsicum annuum L.) and their microencapsulates obtained by spray-drying. J. Food Eng. 2012, 112, 29–37. [Google Scholar] [CrossRef]
- Rascón, M.P.; Beristain, C.I.; García, H.S.; Salgado, M.A. Carotenoid retention and storage stability of spray-dried encapsulated paprika oleoresin using gum Arabic and Soy protein isolate as wall materials. LWT Food Sci. Technol. 2011, 44, 549–557. [Google Scholar] [CrossRef]
- Liang, R.; Huang, Q.; Ma, J.; Shoemaker, C.F.; Zhong, F. Effect of relative humidity on the store stability of spray-dried beta-carotene nanoemulsions. Food Hydrocoll. 2013, 33, 225–233. [Google Scholar] [CrossRef]
- Coronel-Aguilera, C.P.; San Martín-González, M.F. Encapsulation of spray dried b-carotene emulsion by fluidized bed coating technology. LWT Food Sci. Technol. 2015, 62, 187–193. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of emulsion composition and inlet air temperature on the microencapsulation of flaxseed oil by spray drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Janiszewska-turak, E.; Dellarosa, N.; Tylewicz, U.; Laghi, L.; Romani, S.; Dalla, M.; Witrowa-rajchert, D. The influence of carrier material on some physical and structural properties of carrot juice microcapsules. Food Chem. 2017, 236, 134–141. [Google Scholar] [CrossRef]
- Wu, D.; Lu, J.; Zhong, S.; Schwarz, P.; Chen, B.; Rao, J. Effect of chitosan coatings on physical stability, antifungal and mycotoxin inhibitory activities of lecithin stabilized cinnamon oil-in-water emulsions. LWT 2019, 106, 98–104. [Google Scholar] [CrossRef]
- Orset, S.; Leach, G.C.; Morais, R.; Young, A.J. Spray-drying of the microalga Dunaliella salina: Effects on beta-carotene content and isomer composition. J. Agric. Food Chem. 1999, 47, 4782–4790. [Google Scholar] [CrossRef]
- Leach, G.; Oliveira, G.; Morais, R. Spray-drying of Dunaliella salina to produce a β-carotene rich powder. J. Ind. Microbiol. Biotechnol. 1998, 20, 82–85. [Google Scholar] [CrossRef]
- Morowvat, M.H.; Ghasemi, Y. Spray-Drying Microencapsulation of β-Carotene Contents in Powdered Dunaliella salina Biomass. Int. J. Pharm. Clin. Res. 2016, 8, 1533–1536. [Google Scholar]
- McClements, D.J. Food Emulsions: Principles, Practice and Techniques, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Donhowe, E.G.; Flores, F.P.; Kerr, W.L.; Wicker, L.; Kong, F. Characterization and invitro bioavailability of β-carotene: Effects of microencapsulation method and food matrix. LWT Food Sci. Technol. 2014, 57, 42–48. [Google Scholar] [CrossRef]
- Frascareli, E.C.; Silva, V.M.; Tonon, R.V.; Hubinger, M.D. Effect of process conditions on the microencapsulation of coffee oil by spray drying. Food Bioprod. Process. 2012, 90, 413–424. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Foo, S.C.; Khong, N.M.H.; Yusoff, F. Physicochemical, microstructure and antioxidant properties of microalgae- derived fucoxanthin rich microcapsules. Algal Res. 2020, 51, 102061. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; Del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions: Principles, Practice and Techniques, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chen, G.; Tao, D. An experimental study of stability of oil–water emulsion. Fuel Process. Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Chung, C.; Mcclements, D.J.; Chung, C.; Mcclements, D.J. Structure–function relationships in food emulsions: Improving food quality and sensory perception. Food Struct. 2014, 1, 106–126. [Google Scholar] [CrossRef]
- Konar, N.; Durmaz, Y.; Genc Polat, D.; Mert, B. Optimization of spray drying for Chlorella vulgaris by using RSM methodology and maltodextrin. J. Food Process. Preserv. 2022, 46, e16594. [Google Scholar] [CrossRef]

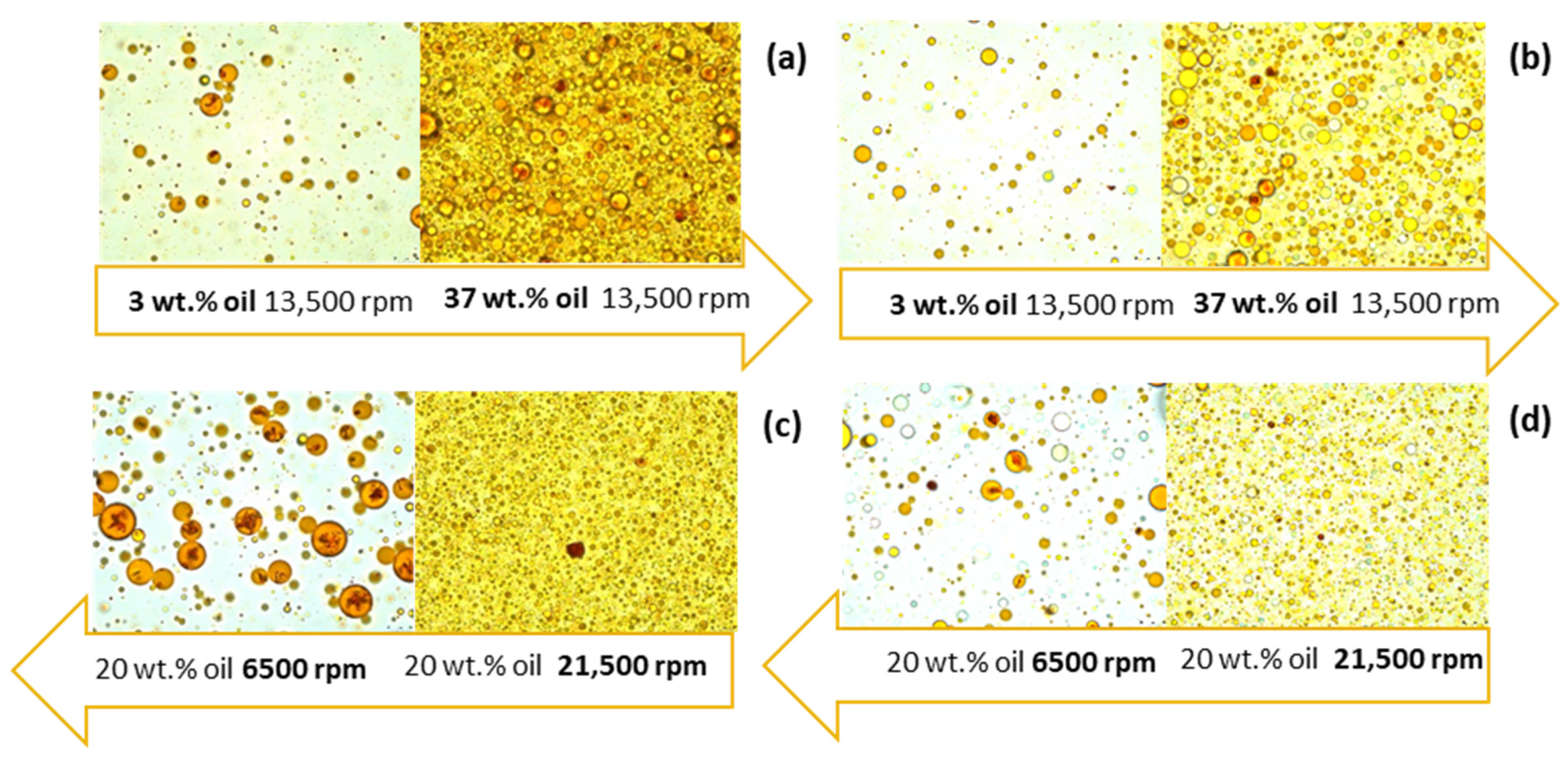
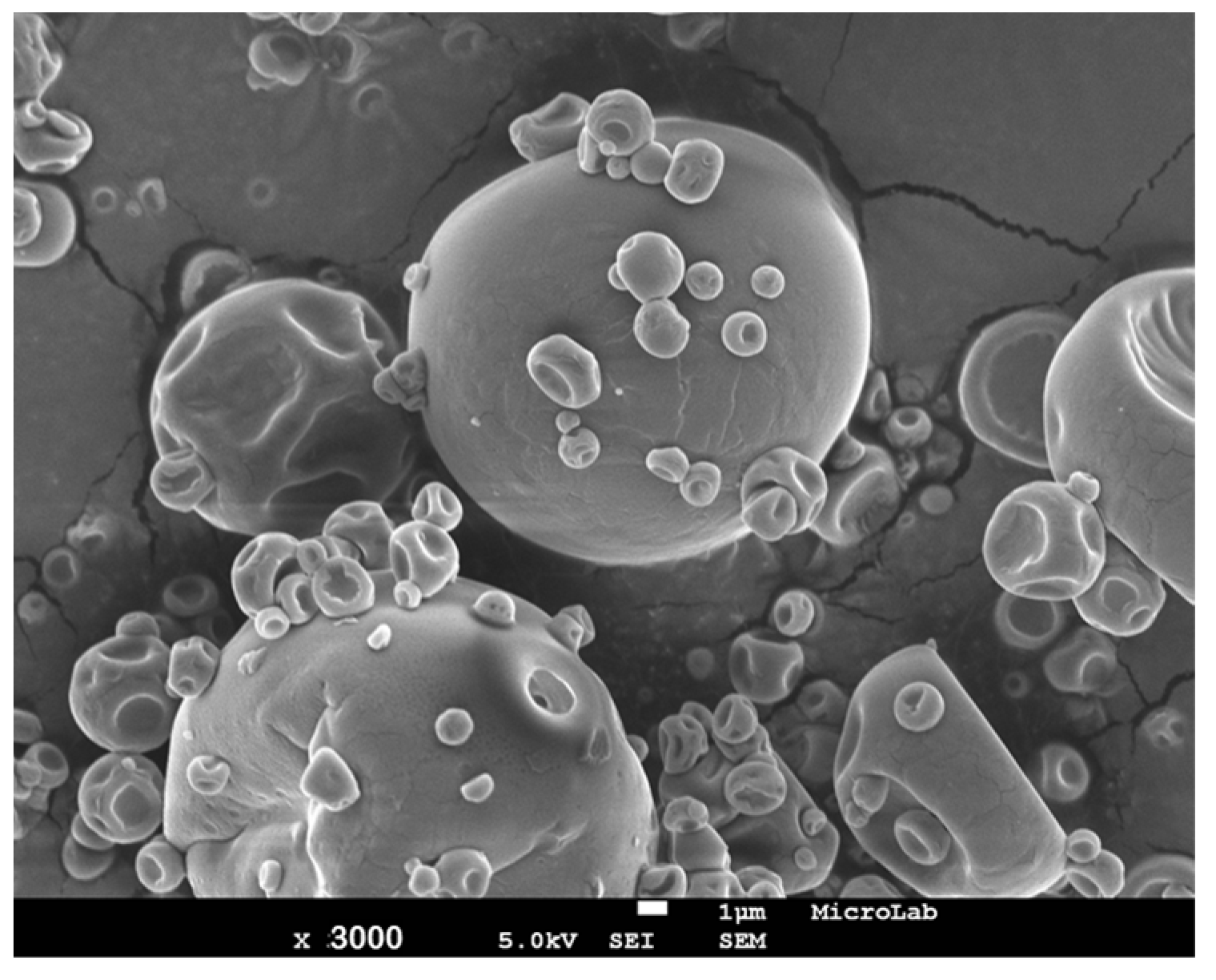

| Encapsulation Process | Emulsion Characterization | Particle Characterization | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fresh | Reconstituted | |||||||||||||||
| Exp. | Oil (wt.%) | Stirring (rpm) | Inlet T 1 (°C) | Outlet T 2 (°C) | EY 3 (wt.%) | EE 4 (wt.%) | Dv50 5 (µm) | PDI 6 | CI 7 1 Day (vol.%) | Dv50 (µm) | PDI | CI 1 Day (vol.%) | Dv50 (µm) | Span | TCC 8 (wt.%) | RGB Color |
| N1 | 10 | 9500 | 130 | 75 | 39 | 31 | 5.33 | 0.06 | 10.0 | 4.9 | 0.26 | 2.50 | 12 | 1.8 | 0.31 |  |
| N2 | 10 | 17,500 | 130 | 85 | 57 | 4 | 4.32 | 0.26 | 2.5 | 4.8 | 0.11 | 0.05 | 8 | 2.0 | 0.04 | |
| N3 | 30 | 9500 | 130 | 76 | 31 | 58 | 9.41 | ND | 10.0 | 8.9 | ND | 10.00 | 16 | 1.8 | 1.75 | |
| N4 | 30 | 17,500 | 130 | 76 | 25 | 80 | 5.22 | 0.26 | 5.0 | 1.4 | 0.34 | 5.00 | 14 | 7.1 | 2.40 | |
| N5 | 10 | 9500 | 200 | 98 | 54 | 32 | 5.33 | 0.06 | 10.0 | 5.1 | 0.13 | 5.00 | 10 | 1.9 | 0.32 | |
| N6 | 10 | 17,500 | 200 | 105 | 58 | 4 | 4.32 | 0.22 | 2.5 | 4.7 | 0.13 | 0.05 | 9 | 2.0 | 0.04 | |
| N7 | 30 | 9500 | 200 | 105 | 28 | 72 | 9.41 | ND | 10.0 | 10.5 | ND | 10.00 | 39 | 11.2 | 2.16 | |
| N8 | 30 | 17,500 | 200 | 110 | 39 | 74 | 5.22 | 0.26 | 5.0 | 8.2 | 0.42 | 5.00 | 21 | 16.7 | 2.22 | |
| N9 | 20 | 13,500 | 110 | 75 | 42 | 52 | 5.05 | 0.07 | 10.0 | 6.6 | 0.39 | 5.00 | 11 | 1.9 | 1.04 | |
| N10 | 20 | 13,500 | 220 | 115 | 48 | 27 | 5.05 | 0.07 | 10.0 | 5.0 | 0.11 | 5.00 | 13 | 2.0 | 0.54 | |
| N11 | 3 | 13,500 | 165 | 92 | 69 | 13 | 4.37 | 0.21 | 5.0 | 4.6 | 0.12 | 2.50 | 9 | 1.9 | 0.04 | |
| N12 | 37 | 13,500 | 165 | 98 | 16 | 79 | 10.00 | ND | 15.0 | 7.0 | 0.34 | 7.50 | 26 | 17.6 | 2.92 | |
| N13 | 20 | 6500 | 165 | 90 | 40 | 37 | 16.87 | ND | 15.0 | 9.4 | ND | 7.50 | 14 | 1.9 | 0.74 | |
| N14 | 20 | 21,500 | 165 | 85 | 46 | 37 | 4.56 | 0.18 | 5.0 | 2.0 | 0.39 | 1.25 | 9 | 2.2 | 0.74 | |
| N15 | 20 | 13,500 | 165 | 88 | 42 | 34 | 5.05 | 0.07 | 10.0 | 2.3 | 0.45 | 5.00 | 10 | 1.7 | 0.67 | |
| N16 | 20 | 13,500 | 165 | 76 | 42 | 47 | 5.05 | 0.07 | 10.0 | 5.0 | 0.20 | 5.00 | 9 | 1.8 | 0.93 | |
| N17 | 20 | 13,500 | 165 | 88 | 43 | 44 | 5.05 | 0.07 | 10.0 | 2.1 | 0.45 | 5.00 | 10 | 1.7 | 0.88 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, A.N.; Monte, J.; Rodríguez-Rojo, S.; Nogueira, I.D.; Gouveia, L.F.; Brazinha, C.; Matias, A.A. Development of a Carotenoid-Rich Microalgae Colorant by Microencapsulation. Colorants 2024, 3, 39-52. https://doi.org/10.3390/colorants3010003
Nunes AN, Monte J, Rodríguez-Rojo S, Nogueira ID, Gouveia LF, Brazinha C, Matias AA. Development of a Carotenoid-Rich Microalgae Colorant by Microencapsulation. Colorants. 2024; 3(1):39-52. https://doi.org/10.3390/colorants3010003
Chicago/Turabian StyleNunes, Ana N., Joana Monte, Soraya Rodríguez-Rojo, Isabel D. Nogueira, Luís F. Gouveia, Carla Brazinha, and Ana A. Matias. 2024. "Development of a Carotenoid-Rich Microalgae Colorant by Microencapsulation" Colorants 3, no. 1: 39-52. https://doi.org/10.3390/colorants3010003
APA StyleNunes, A. N., Monte, J., Rodríguez-Rojo, S., Nogueira, I. D., Gouveia, L. F., Brazinha, C., & Matias, A. A. (2024). Development of a Carotenoid-Rich Microalgae Colorant by Microencapsulation. Colorants, 3(1), 39-52. https://doi.org/10.3390/colorants3010003







