A Study on the Structure, Optical Properties and Cellular Localization of Novel 1,3-Benzothiazole-Substituted BODIPYs
Abstract
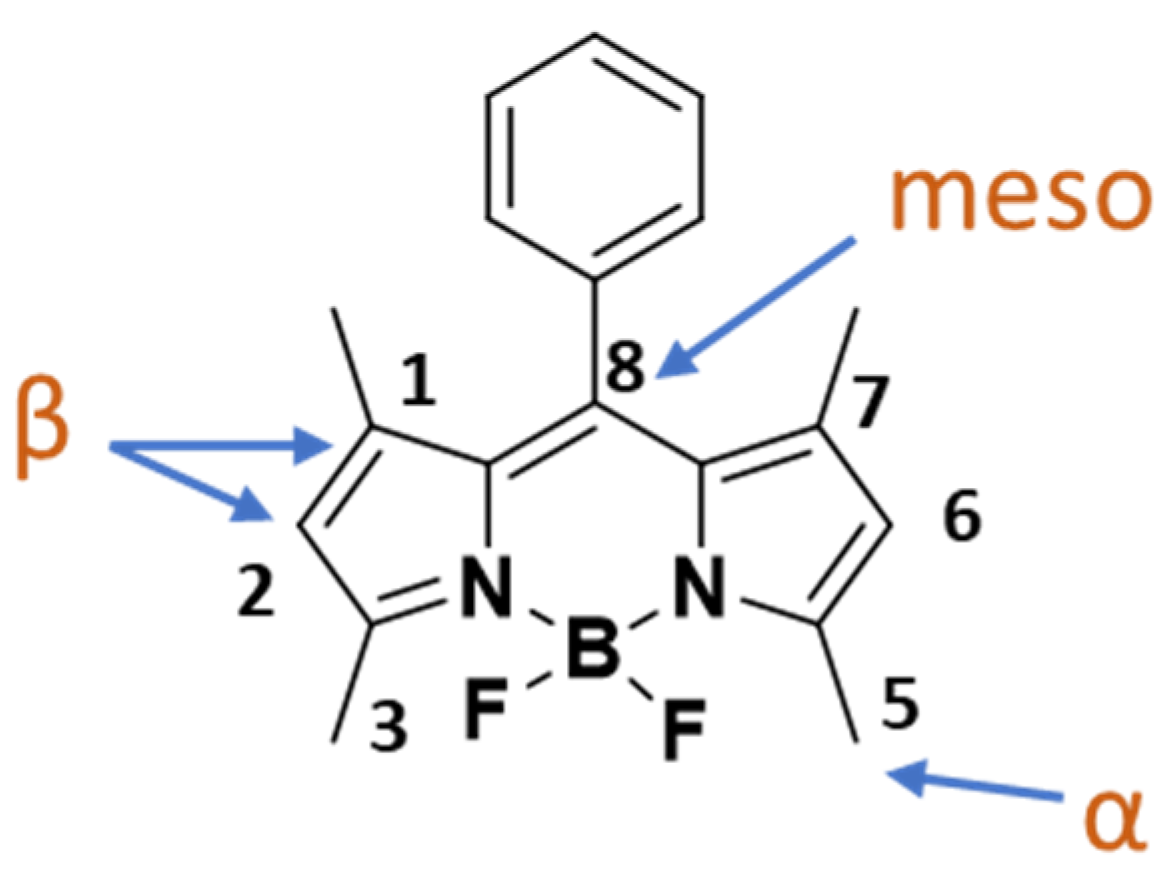
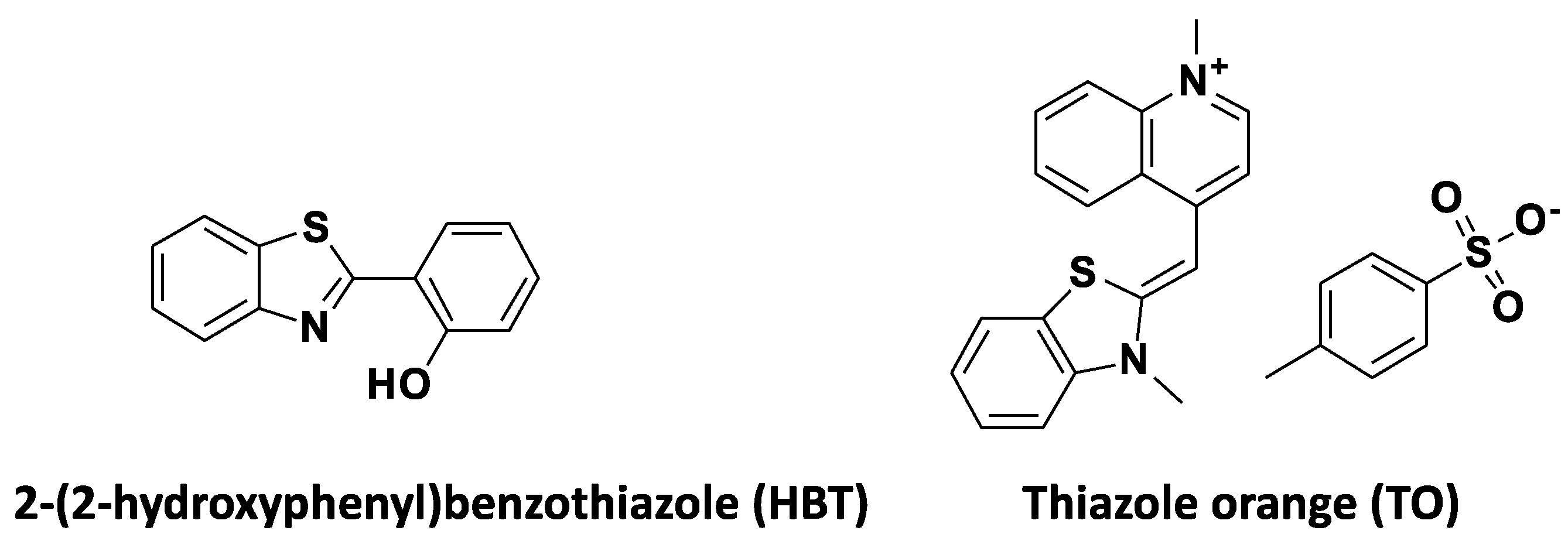
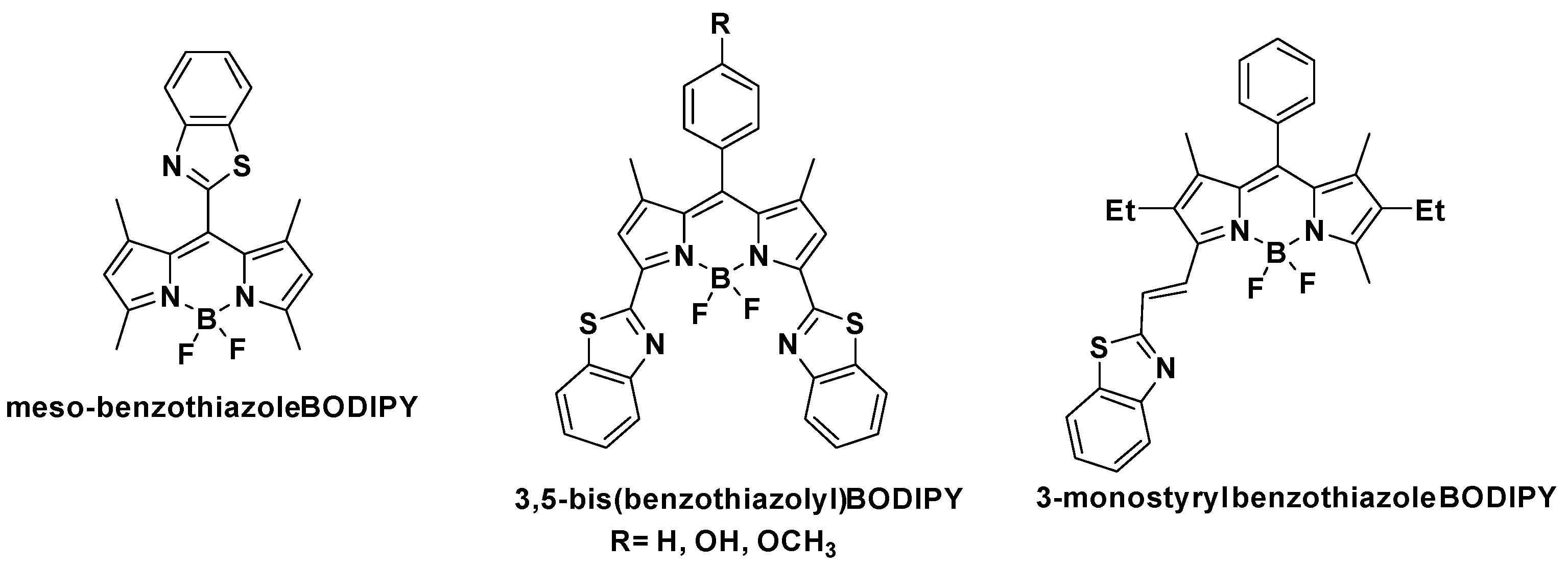
:1. Introduction
2. Materials and Methods
2.1. General Information for Synthesis
2.2. Synthetic Procedures
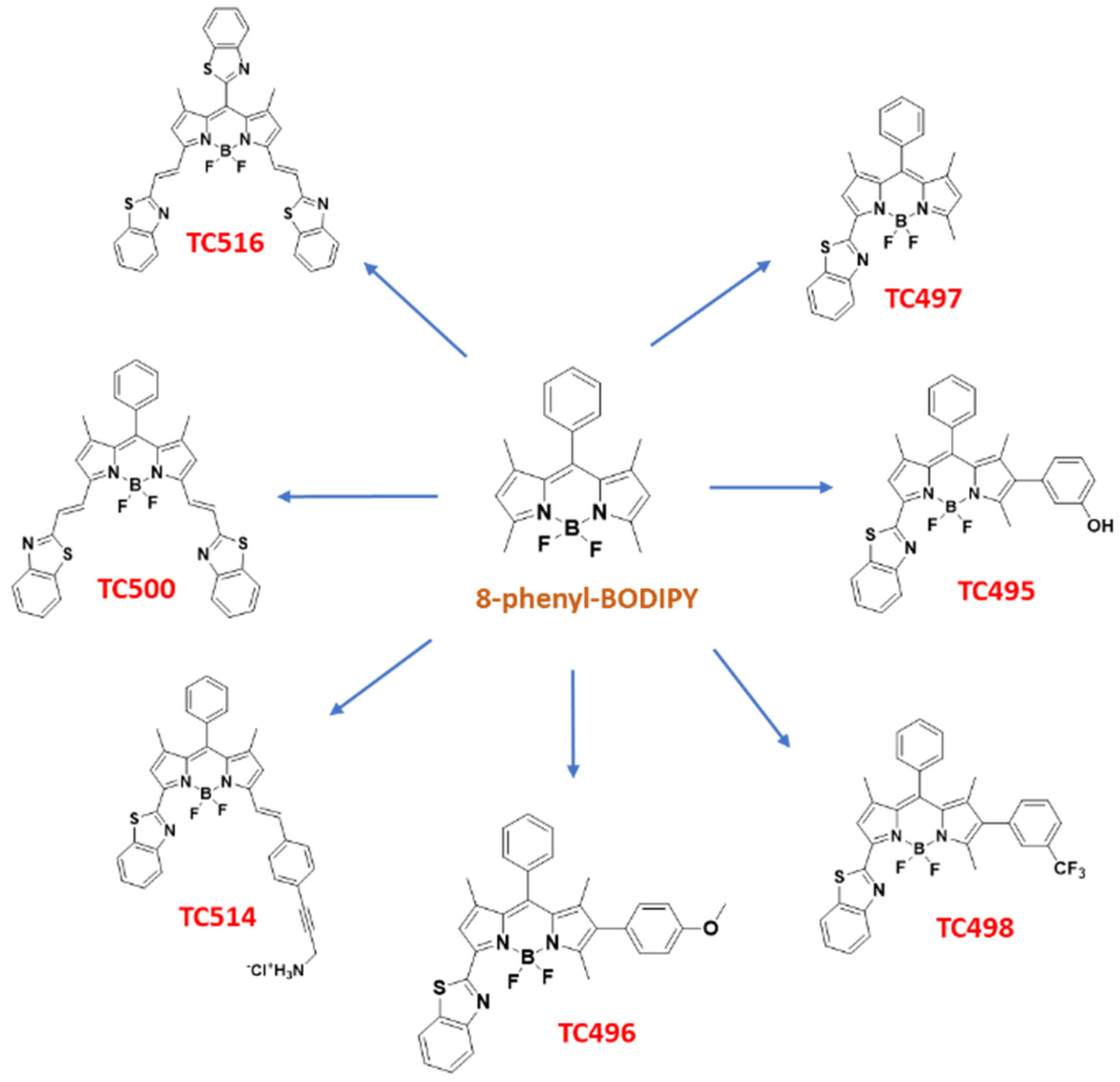
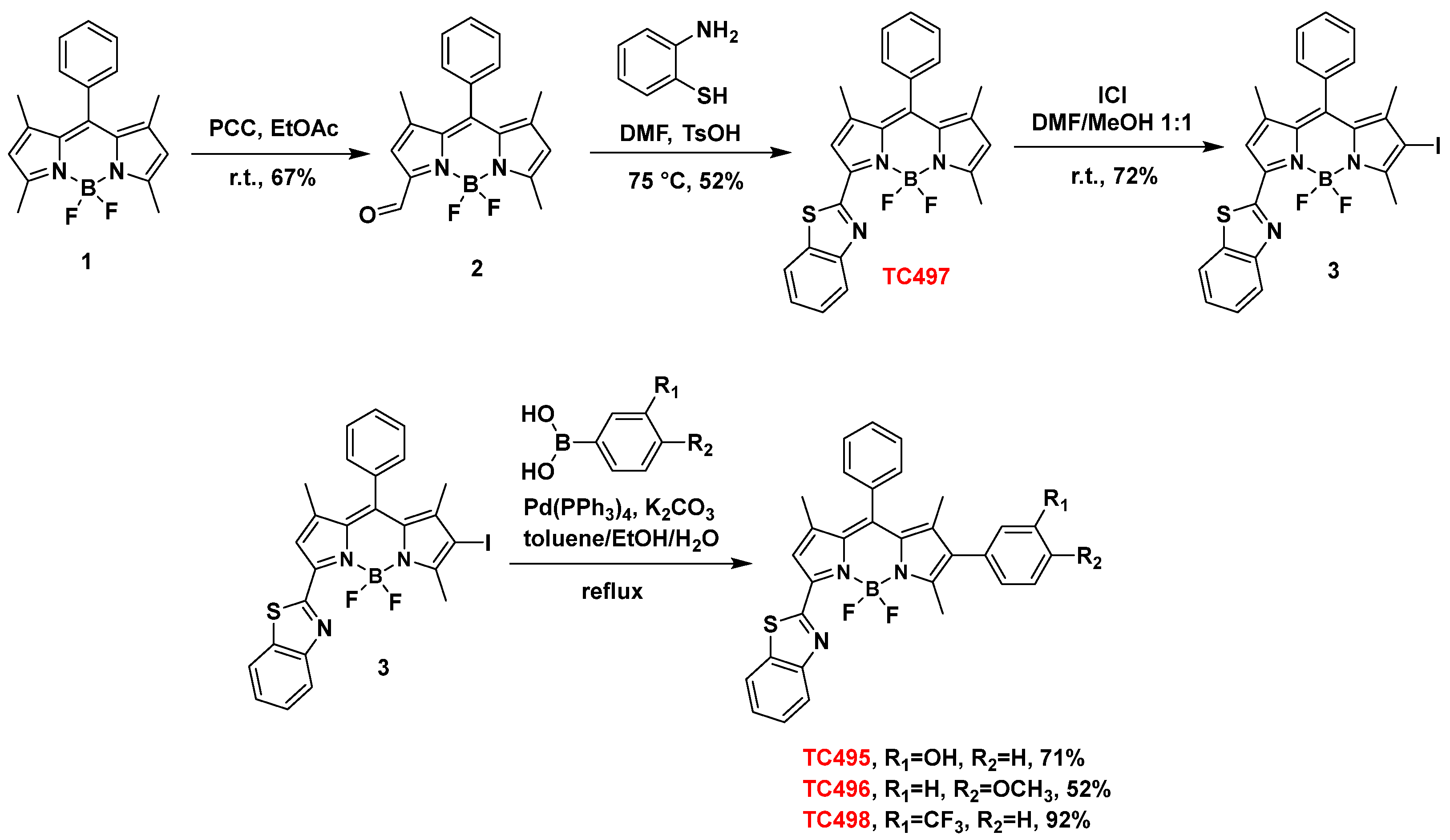
2.2.1. Synthesis of 8-Phenyl-BODIPY (1)
2.2.2. Synthesis of 3-(8-Phenyl-BODIPY)-Carbaldehyde (2)
2.2.3. Synthesis of 3-(Benzo[d]Thiazol-2-yl)-8-Phenyl-BODIPY (TC497)
2.2.4. Synthesis of 3-(Benzo[d]Thiazol-2-yl)-6-Iodo-8-Phenyl-BODIPY (3)
2.2.5. General Procedure for the Suzuki Cross-Coupling Reaction towards Dyes TC495, TC496 and TC498
2.2.6. Synthesis of 3-[(Benzo[d]Thiazol-2-yl)]-6-(2-Hydroxyphenyl)-8-Phenyl-BODIPY (TC495)
2.2.7. Synthesis 3-[(Benzo[d]Thiazol-2-yl)]-6-(4-Methoxyphenyl)-8-Phenyl-BODIPY (TC496)
2.2.8. Synthesis 3-[(Benzo[d]Thiazol-2-yl)]-6-(3-Trifluoromethylphenyl)-8-Phenyl-BODIPY (TC498)
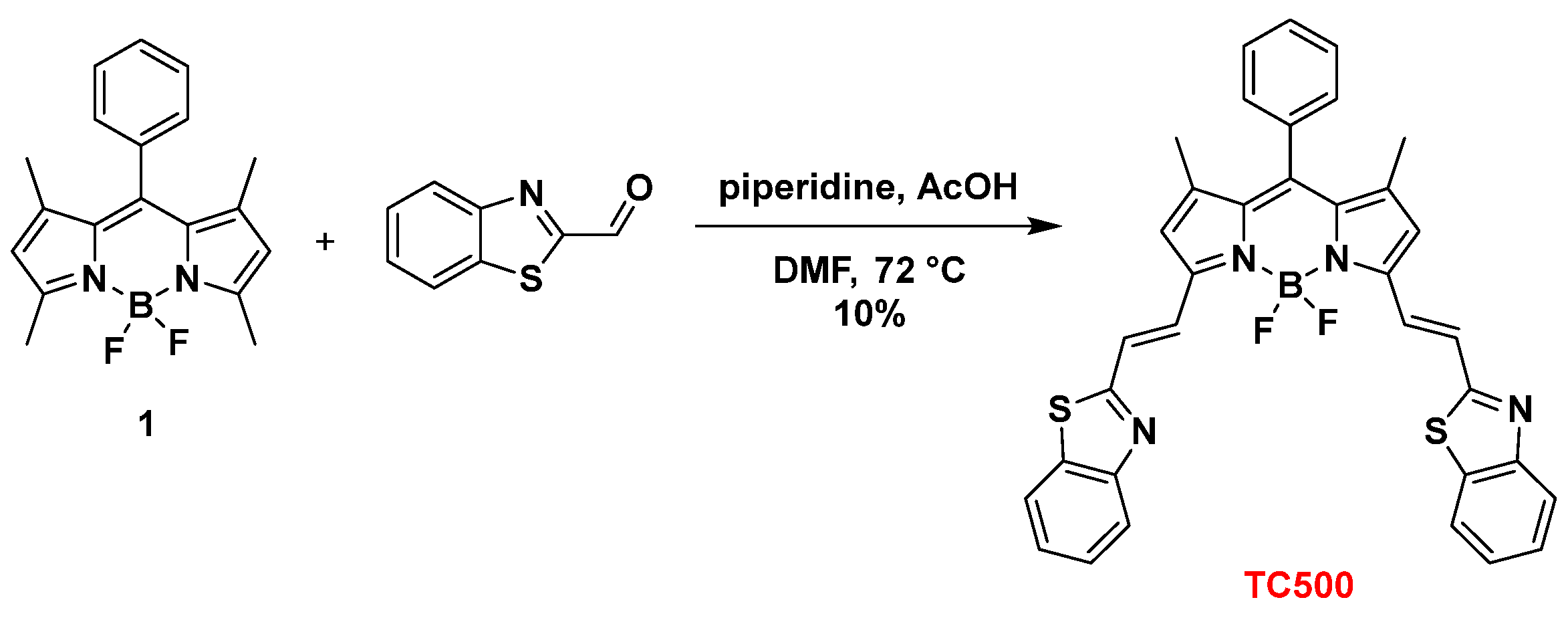
2.2.9. Synthesis of 3,5-bis[(E)2-(Benzo[d]Thiazol-2-yl)Vinyl]-8-Phenyl-BODIPY (TC500)
2.2.10. Synthesis of 3-[(Benzo[d]Thiazol-2-yl)]-(E)-5-4-Iodostyryl)-8-Phenyl-BODIPY (4)
2.2.11. Synthesis of 3-[(Benzo[d]Thiazol-2-yl)]-5-[(E)-2-[Tert-Butyl(4-(Phenyl)prop-2-yn-1-yl)Carbamate]vinyl)-8-Phenyl-BODIPY (5)
2.2.12. Synthesis of 3-[(Benzo[d]Thiazol-2-yl)]-5-[(E)-2(4-(Phenyl)prop-2-yn-1-yl)Chloranimine]Vinyl)-8-Phenyl-BODIPY Hydrochloride (TC514)
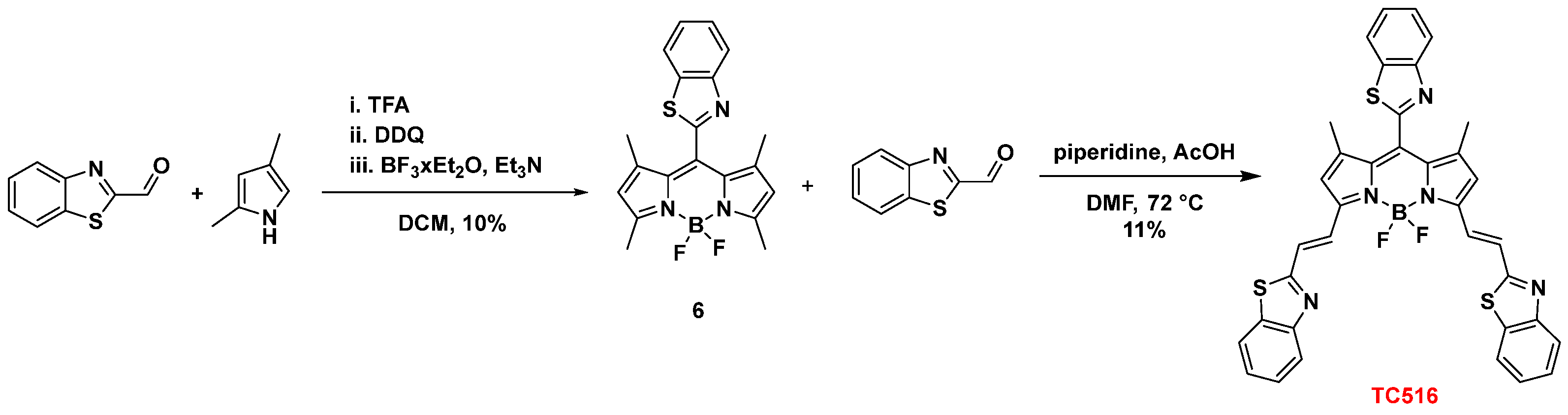
2.2.13. Synthesis of 8-[(Benzo[d]Thiazol-2-yl)-BODIPY (6)
2.2.14. Synthesis of 3,5-bis[(E)2-(Benzo[d]Thiazol-2-yl)Vinyl-8-[(Benzo[d]Thiazol-2-yl)-BODIPY (TC516)
2.3. Computational Studies
2.4. Cellular Assays
2.4.1. Cell Culture
2.4.2. Fluorescence Stability and Cellular Toxicity
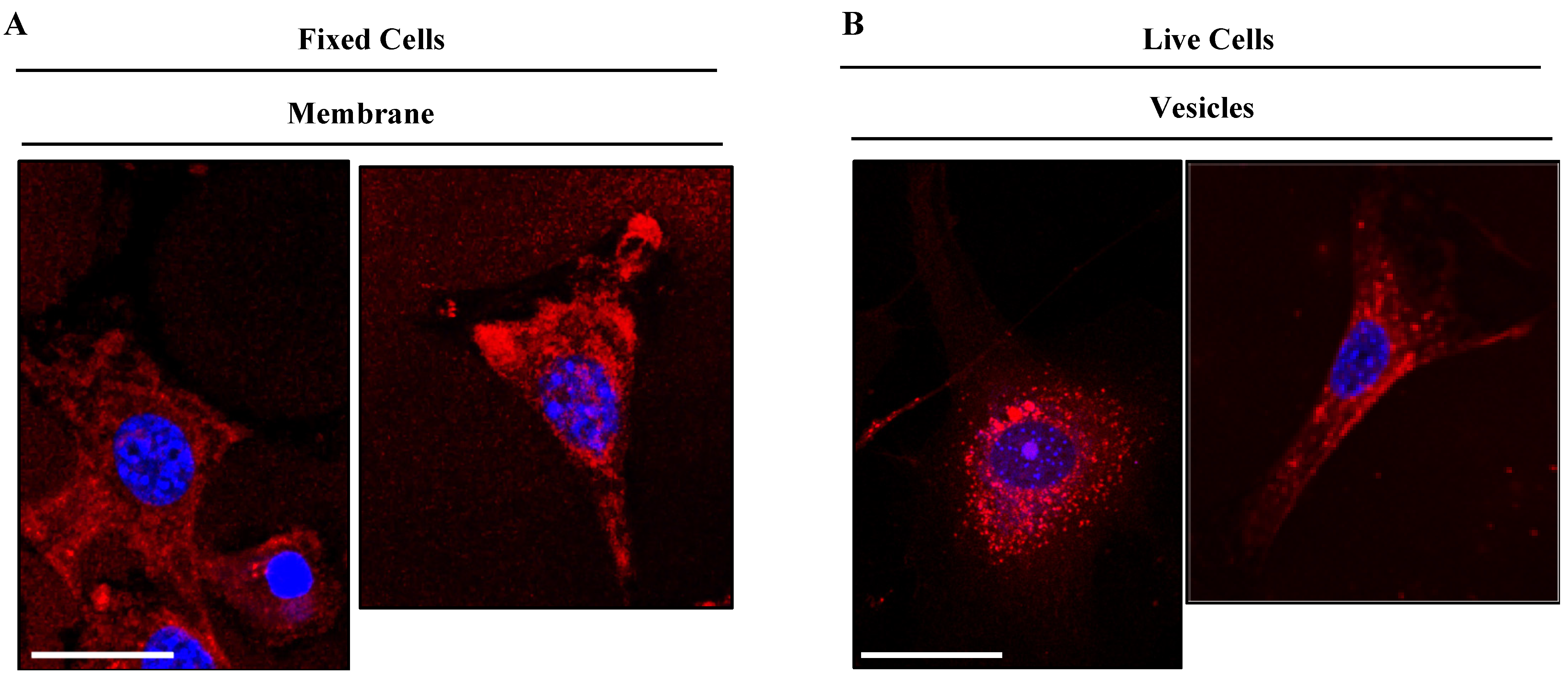
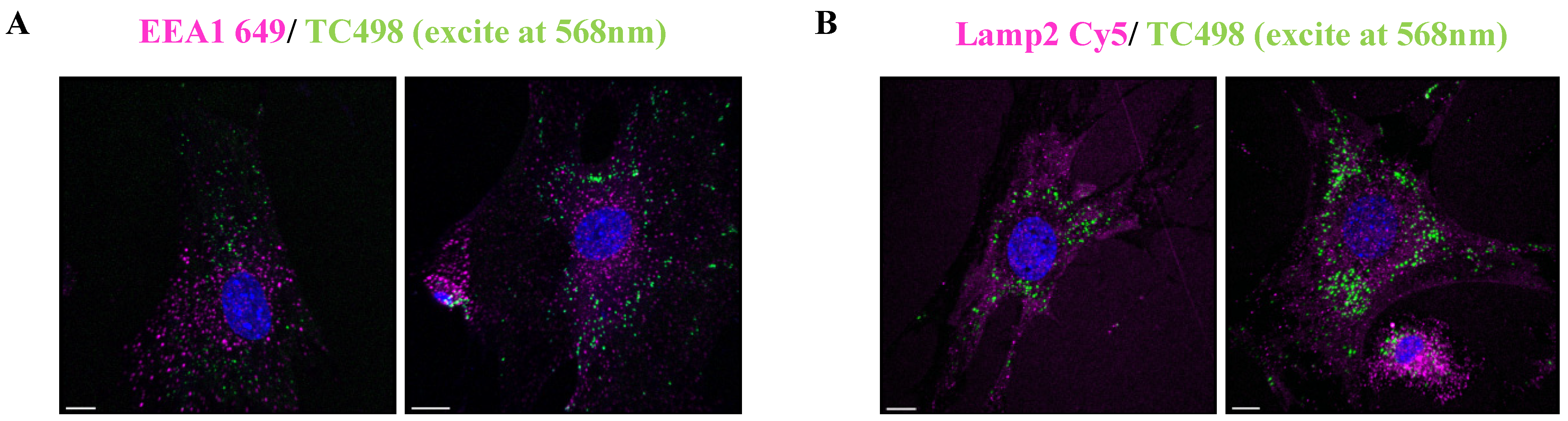
2.4.3. Cellular Biodistribution
2.4.4. Confocal and Lambda Scan Imaging
- TC496: exc 545 nm, em 555–700 nm
- TC497: exc 535 nm, em 545–640 nm
- TC498: exc 568 nm, em 577–640 nm
- TC500: exc 610 nm, em 620–700 nm
- TC514: exc 590 nm, em 598–700 nm
- TC516: exc 635 nm, em 655–710 nm
2.4.5. Time-Lapse Live Imaging
3. Results and Discussion
3.1. Chemistry
3.2. Optical Properties
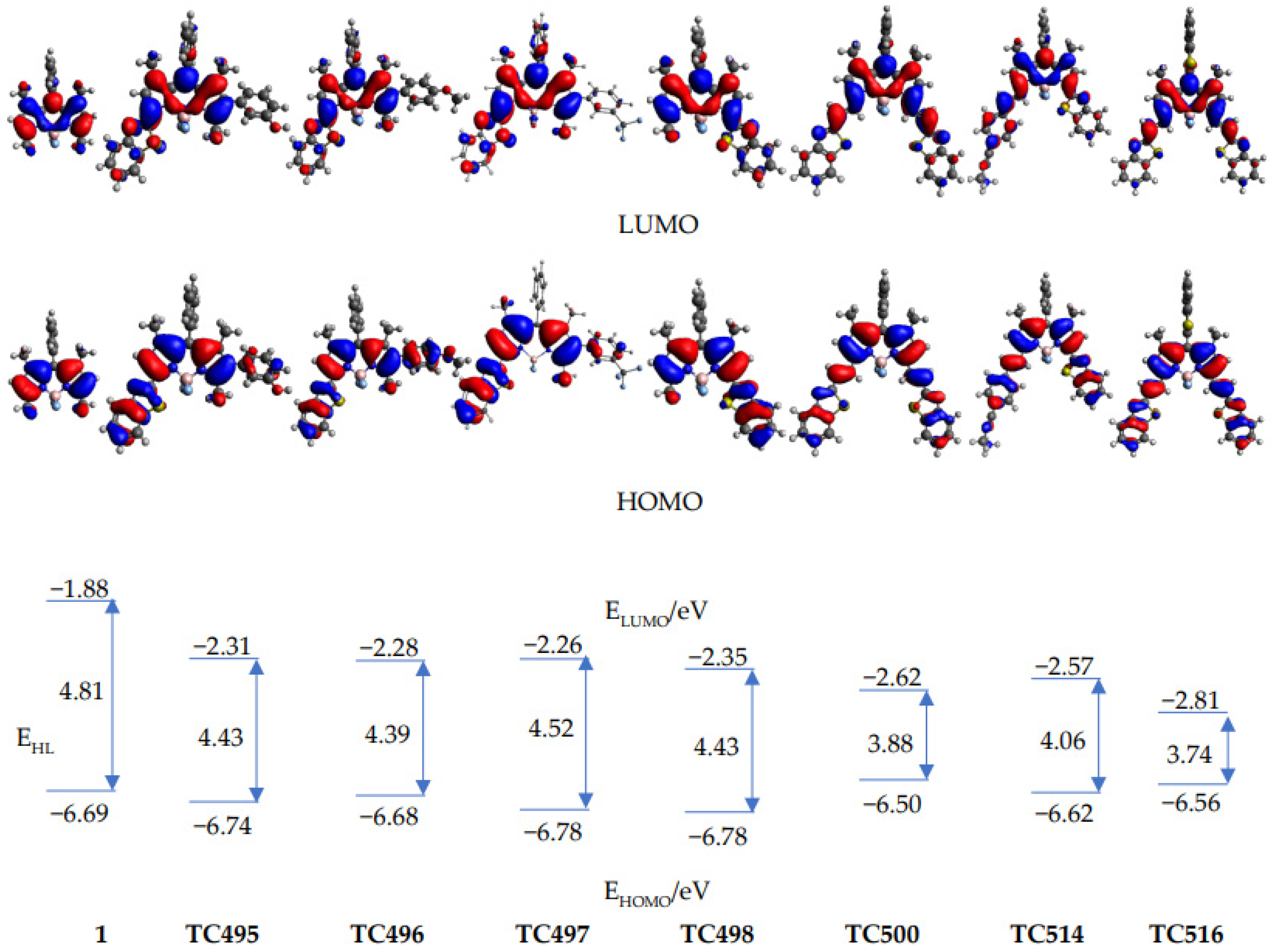
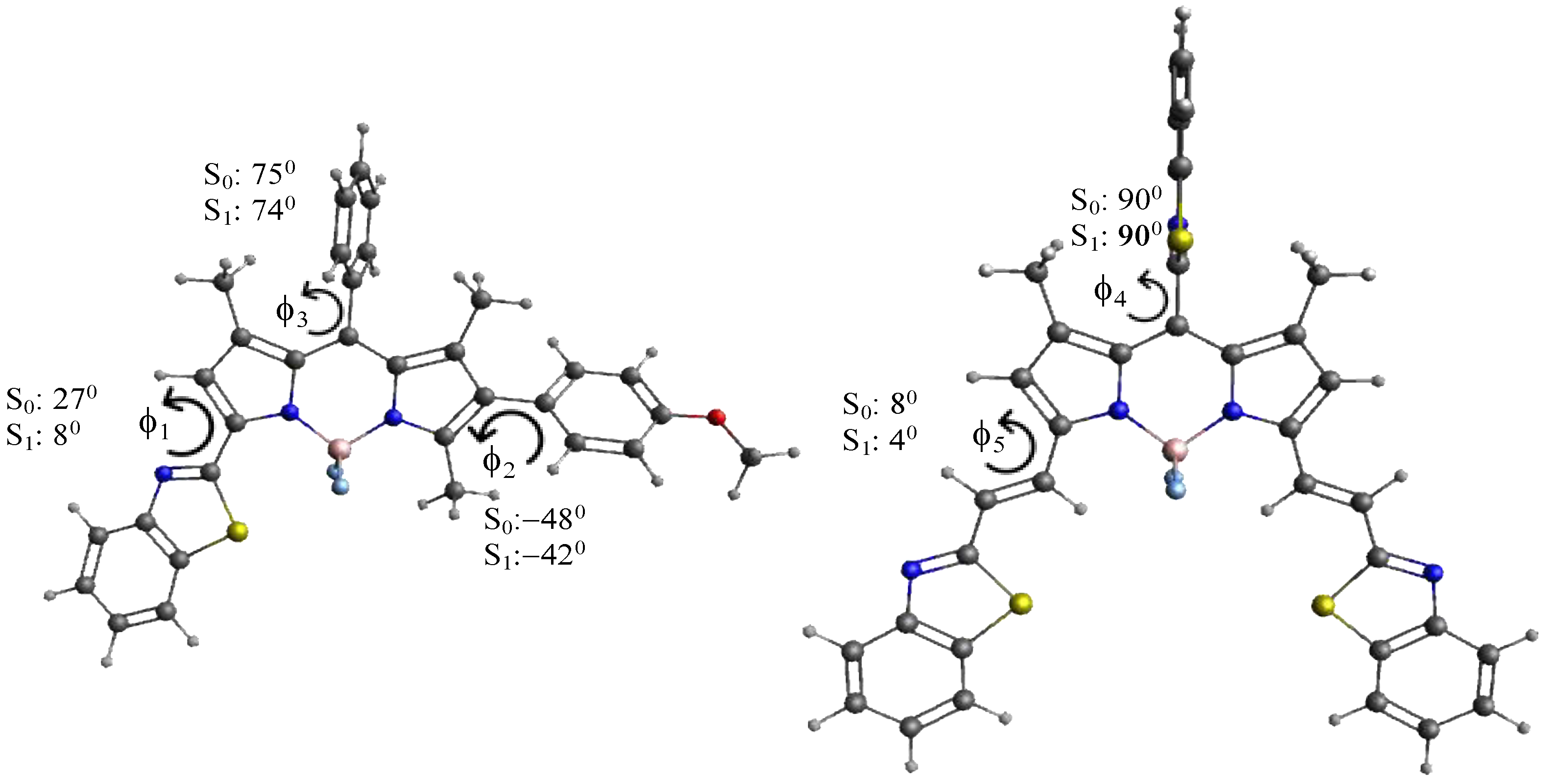
3.3. Theoretical Calculations
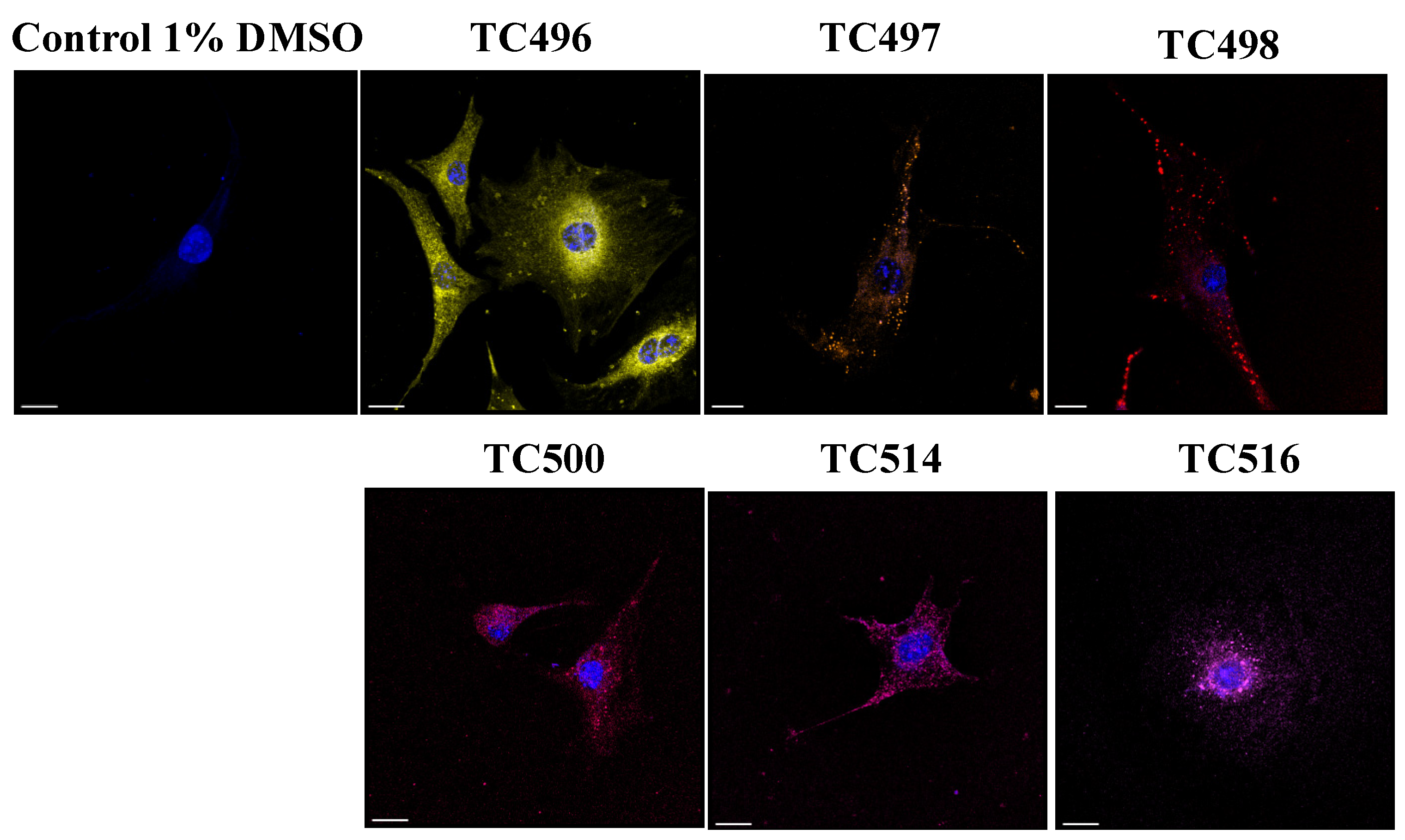
3.4. Cell Biodistribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.-B.; Cao, X.; Zhang, S.; Zhang, K.; Cheng, Y.; Wang, J.; Zhao, J.; Zhou, L.; Liang, X.-J.; Yoon, J. BODIPY as a Multifunctional Theranostic Reagent in Biomedicine: Self-Assembly, Properties, and Applications. Adv. Mater. 2023, 35, 2207546. [Google Scholar] [CrossRef] [PubMed]
- Gurubasavaraj, P.M.; Sajjan, V.P.; Muñoz-Flores, B.M.; Jiménez Pérez, V.M.; Hosmane, N.S. Recent Advances in BODIPY Compounds: Synthetic Methods, Optical and Nonlinear Optical Properties, and Their Medical Applications. Molecules 2022, 27, 1877. [Google Scholar] [CrossRef] [PubMed]
- Antina, E.; Bumagina, N.; Marfin, Y.; Guseva, G.; Nikitina, L.; Sbytov, D.; Telegin, F. BODIPY Conjugates as Functional Compounds for Medical Diagnostics and Treatment. Molecules 2022, 27, 1396. [Google Scholar] [CrossRef] [PubMed]
- Krämer, J.; Kang, R.; Grimm, L.M.; de Cola, L.; Picchetti, P.; Biedermann, F. Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids. Chem. Rev. 2022, 122, 3459–3636. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Bastug, E.; Guler, E. Importance of BODIPY-based Chemosensors for Cations and Anions in Bio-imaging Applications. Curr. Anal. Chem. 2020, 18, 163–175. [Google Scholar] [CrossRef]
- Yan, M.; He, D.; Zhang, L.; Sun, P.; Sun, Y.; Qu, L.; Li, Z. Explorations into the meso-substituted BODIPY-based fluorescent probes for biomedical sensing and imaging. Trends Anal. Chem. 2022, 157, 116771. [Google Scholar] [CrossRef]
- Li, F.Z.; Yin, J.F.; Kuang, G.C. BODIPY-based supramolecules: Construction, properties and functions. Coord. Chem. Rev. 2021, 448, 214157. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Y.; Meana, Y.; Raymo, F.M. BODIPYs with Photoactivatable Fluorescence. Chem. Eur. J. 2021, 27, 11257–11267. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Yim, Y.; Kim, S.; Ryu, B.; Swamy, K.; Kim, G.; Kwon, N.; Kim, C.Y.; Park, S.; Yoon, J. Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging. Angew. Chem. Int. Ed. 2020, 59, 8957–8962. [Google Scholar] [CrossRef]
- Malacarne, M.C.; Gariboldi, M.B.; Caruso, E. BODIPYs in PDT: A Journey through the Most Interesting Molecules Produced in the Last 10 Years. Int. J. Mol. Sci. 2022, 23, 10198. [Google Scholar] [CrossRef]
- Teng, K.-X.; Chen, W.-K.; Niu, L.-Y.; Fang, W.-H.; Cui, G.; Yang, Q.-Z. BODIPY-Based Photodynamic Agents for Exclusively Generating Superoxide Radical over Singlet Oxygen. Angew. Chem. Int. Ed. 2021, 60, 19912–19920. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Montero, R.; Prieto-Castañeda, A.; Sola-Llano, R.; Agarrabeitia, A.R.; García-Fresnadillo, D.; López-Arbeloa, I.; Villanueva, A.; Ortiz, M.J.; de la Moya, S.; Martínez-Martínez, V.; et al. Exploring BODIPY Derivatives as Singlet Oxygen Photosensitizers for PDT. Photochem. Photobiol. 2020, 96, 458–477. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Kalt, M.; Koch, S.; Sithamparanathan, S.; Villiger, V.; Mattiat, J.; Kradolfer, F.; Slyshkina, E.; Luber, S.; Bonmarin, M.; et al. BODIPY-Based Photothermal Agents with Excellent Phototoxic Indices for Cancer Treatment. J. Am. Chem. Soc. 2023, 145, 4534–4544. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Kannadorai, R.K.; Yu, S.W.-K.; Chang, Y.-T.; Wu, J. Push-Pull Type Meso-Ester Substituted BODIPY near-Infrared Dyes as Contrast Agents for Photoacoustic Imaging. Org. Biomol. Chem. 2017, 15, 4531–4535. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Han, X.; Hu, W.; Bai, H.; Peng, B.; Ji, L.; Fan, Q.; Li, L.; Huang, W. Bioapplications of small molecule Aza-BODIPY: From rational structural design to in vivo investigations. Chem. Soc. Rev. 2020, 49, 7533–7567. [Google Scholar] [CrossRef] [PubMed]
- Bodin, J.P.; Gateau, J.; Coïs, J.; Lucas, T.; Lefebvre, F.; Moine, L.; Noiray, M.; Cailleau, C.; Denis, S.; Clavier, G.; et al. Biocompatible and Photostable Photoacoustic Contrast Agents as Nanoparticles Based on Bodipy Scaffold and Polylactide Polymers: Synthesis, Formulation, and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2022, 14, 40501–40512. [Google Scholar] [CrossRef]
- Lu, H.; Mack, J.; Yang, Y.; Shen, Z. Structural modification strategies for the rational design of red/nir region bodipys. Chem. Soc. Rev. 2014, 43, 4778–4823. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. Bodipy Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Harriman, A. The chemistry of fluorescent bodipy dyes: Versatility unsurpassed. Angew. Chem. Int. Ed. 2008, 47, 1184–1201. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. The chemistry of BODIPY: A new El Dorado for fluorescence tools. N. J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Yang, L.; Simionescu, R.; Lough, A.; Yan, H. Some observations relating to the stability of the BODIPY fluorophore under acidic and basic conditions. Dyes Pigm. 2011, 91, 264–267. [Google Scholar] [CrossRef]
- Wang, M.; Vicente, M.G.H.; Mason, D.; Bobadova-Parvanova, P. Stability of a Series of BODIPYs in Acidic Conditions: An Experimental and Computational Study into the Role of the Substituents at Boron. ACS Omega 2018, 3, 5502–5510. [Google Scholar] [CrossRef] [PubMed]
- Jameson, L.P.; Smith, N.W.; Annunziata, O.; Dzyuba, S.V. Interaction of BODIPY dyes with bovine serum albumin: A case study on the aggregation of a click-BODIPY dye. Phys. Chem. Chem. Phys. 2016, 18, 14182–14185. [Google Scholar] [CrossRef] [PubMed]
- Karolin, J.; Johansson, L.B.-A.; Strandberg, L.; Ny, T. Fluorescence and Absorption Spectroscopic Properties of Dipyrrometheneboron Difluoride (BODIPY) Derivatives in Liquids, Lipid Membranes, and Proteins. J. Am. Chem. Soc. 1994, 116, 7801–7806. [Google Scholar] [CrossRef]
- Li, G.; Li, J.; Otsuka, Y.; Zhang, S.; Takahashi, M.; Yamada, K. A BODIPY-Based Fluorogenic Probe for Specific Imaging of Lipid Droplets. Materials 2020, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, S.; She, M.; Chen, J.; Wang, Z.; Liu, P.; Zhang, S.; Li, J. Structural modification of BODIPY: Improve its applicability. Chin. Chem. Lett. 2019, 30, 1815–1824. [Google Scholar] [CrossRef]
- Cherumukkil, S.; Vedhanarayanan, B.; Das, G.; Praveen, V.K.; Ajayaghosh, A. Self-Assembly of Bodipy-Derived Extended π-Systems. Bull. Chem. Soc. Jpn. 2018, 91, 100–120. [Google Scholar] [CrossRef]
- Gemen, J.; Ahrens, J.; Shimon, L.J.W.; Klajn, R. Modulating the Optical Properties of BODIPY Dyes by Noncovalent Dimerization within a Flexible Coordination Cage. J. Am. Chem. Soc. 2020, 142, 17721–17729. [Google Scholar] [CrossRef]
- Blázquez-Moraleja, A.; Álvarez-Fernández, D.; Prieto Montero, R.; García-Moreno, I.; Martínez-Martínez, V.; Bañuelos, J.; Sáenz-de-Santa-María, I.; Chiara, M.D.; Chiara, J.L. A general modular approach for the solubility tagging of BODIPY dyes. Dyes Pigm. 2019, 170, 107545. [Google Scholar] [CrossRef]
- Amendoeira, A.F.; Luz, A.; Valente, R.; Roma-Rodrigues, C.; Ali, H.; van Lier, J.E.; Marques, F.; Baptista, P.V.; Fernandes, A.R. Cell Uptake of Steroid-BODIPY Conjugates and Their Internalization Mechanisms: Cancer Theranostic Dyes. Int. J. Mol. Sci. 2023, 24, 3600. [Google Scholar] [CrossRef] [PubMed]
- Romieu, A.; Massif, C.; Rihn, S.; Ulrich, G.; Ziessel, R.; Renarda, P.Y. The first comparative study of the ability of different hydrophilic groups to water-solubilise fluorescent BODIPY dyes. N. J. Chem. 2013, 37, 1016–1027. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on bodipy. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gong, Q.; Wang, L.; Hao, E.; Jiao, L. The main strategies for tuning BODIPY fluorophores into photosensitizers. J. Porphyr. Phthalocyanines 2020, 24, 603–635. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wu, J. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bioimaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef] [PubMed]
- Shing, W.; Law, C.; Yoon Yeong, K. Current trends of benzothiazoles in drug discovery: A patent review (2015–2020). Expert. Opin. Ther. Pat. 2022, 32, 299–315. [Google Scholar] [CrossRef]
- Xuan, S.; Zhao, N.; Ke, X.; Zhou, Z.; Fronczek, F.R.; Kadish, K.M.; Smith, K.M.; Graca, M.; Vicente, H. Synthesis and Spectroscopic Investigation of a Series of Push-Pull Boron Dipyrromethenes (BODIPYs). J. Org. Chem. 2017, 82, 2545–2557. [Google Scholar] [CrossRef]
- Henary, M.; Paranjpe, S.; Owens, E.A. Synthesis and applications of benzothiazole containing cyanine dyes. Heterocycl. Commun. 2013, 19, 1–11. [Google Scholar] [CrossRef]
- Ren, M.; Wang, L.; Lv, X.; Liu, J.; Chen, H.; Wang, J.; Guo, W. Development of a benzothiazole-functionalized red-emission pyronin dye and its dihydro derivative for imaging lysosomal viscosity and tracking endogenous peroxynitrite. J. Mater. Chem. B. 2019, 7, 6181–6186. [Google Scholar] [CrossRef]
- Aktan, E.; Uyar, T. Hetarylazopyrazolone Dyes Based on Benzothiazole and Benzimidazole Ring Systems: Synthesis, Spectroscopic Investigation, and Computational Study. J. Chem. 2017, 2017, 8659346. [Google Scholar] [CrossRef]
- Das, S.; Indurthi, H.K.; Asati, P.; Saha, P.; Sharma, D.K. Benzothiazole based fluorescent probes for the detection of biomolecules, physiological conditions, and ions responsible for diseases. Dyes Pigm. 2022, 199, 110074. [Google Scholar] [CrossRef]
- Shi, W.-J.; Chen, R.; Yang, J.; Wei, Y.-F.; Guo, Y.; Wang, Z.-Z.; Yan, J.-W.; Niu, L. Novel Meso-Benzothiazole-Substituted BODIPY-Based AIE Fluorescent Rotor for Imaging Lysosomal Viscosity and Monitoring Autophagy. Anal. Chem. 2022, 94, 14707–14715. [Google Scholar] [CrossRef] [PubMed]
- Poronik, Y.M.; Yakubovskyi, V.P.; Shandura, M.P.; Vlasenko, Y.G.; Chernega, A.N.; Kovtun, Y.P. 3,5-Bis(benzothiazolyl)-Substituted BODIPY Dyes. Eur. J. Org. Chem. 2010, 14, 2746–2752. [Google Scholar] [CrossRef]
- Sansalone, L.; Zhang, Y.; Mazza, M.M.A.; Davis, J.L.; Song, K.H.; Captain, B.; Zhang, H.F.; Raymo, F.M. High-Throughput Single-Molecule Spectroscopy resolves the conformational isomers of BODIPY chromophores. J. Phys. Chem. Lett. 2019, 10, 6807–6812. [Google Scholar] [CrossRef] [PubMed]
- Prousis, K.C.; Canton-Vitoria, R.; Pagona, G.; Goulielmaki, M.; Zoumpourlis, V.; Tagmatarchis, N.; Calogeropoulou, T. New cationic heptamethinecyanine-graphene hybrid materials. Dyes Pigment. 2020, 17, 108047. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView 5.0.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Ramos-Torres, Á.; Avellanal-Zaballa, E.; Prieto-Castañeda, A.; García-Garrido, F.; Bañuelos, J.; Agarrabeitia, A.R.; Ortiz, M.J. Formyl BODIPYs by PCC-Promoted Selective Oxidation of α-MethylBODIPYs. Synthetic Versatility and Applications. Org. Lett. 2019, 21, 4563–4566. [Google Scholar] [CrossRef]
- Kaur, G.; Moudgil, R.; Shamim, M.; Gupta, V.K.; Banerjee, B. Camphor sulfonic acid catalyzed a simple, facile, and general method for the synthesis of 2-arylbenzothiazoles, 2-arylbenzimidazoles, and 3H-spiro[benzo[d]thiazole-2,3′-indolin]-2′-ones at room temperature. Synth. Commun. 2021, 51, 1100–1120. [Google Scholar] [CrossRef]
- Wang, J.B.; Fang, X.Q.; Pan, X.; Dai, S.Y.; Song, Q.H. New 2,6-Modified Bodipy Sensitizers for Dye-Sensitized Solar Cells. Chem. Asian J. 2012, 7, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cui, X.; Therrien, B.; Zhao, J. Energy-Funneling-Based Broadband Visible-Light-Absorbing Bodipy–C60 Triads and Tetrads as Dual Functional Heavy-Atom-Free Organic Triplet Photosensitizers for Photocatalytic Organic Reactions. Chem. Eur. 2013, 19, 17472–17482. [Google Scholar] [CrossRef]
- Huang, L.; Yu, X.; Wu, W.; Zhao, J. Styryl Bodipy-C60 Dyads as Efficient Heavy-Atom-Free Organic Triplet Photosensitizers. Org. Lett. 2012, 14, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhao, J.; Wu, W.; Yu, X.; Liu, Y. Accessing the Long-Lived Triplet Excited States in Bodipy-Conjugated 2-(2-Hydroxyphenyl) Benzothiazole/Benzoxazoles and Applications as Organic Triplet Photosensitizers for Photooxidations. J. Org. Chem. 2012, 77, 6166–6178. [Google Scholar] [CrossRef] [PubMed]
- Erten-Ela, S.; Yilmaz, M.D.; Icli, B.; Dede, Y.; Icli, S.; Akkaya, E.U. A panchromatic boradiazaindacene (BODIPY) sensitizer for dye-sensitized solar cells. Org. Lett. 2008, 10, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, J.; Guo, H.; Xie, L. Geometry Relaxation-Induced Large Stokes Shift in Red-Emitting Borondipyrromethenes (BODIPY) and Applications in Fluorescent Thiol Probes. J. Org. Chem. 2012, 77, 2192–2206. [Google Scholar] [CrossRef]
- Bittel, A.M.; Davis, A.M.; Wang, L.; Nederlof., M.A.; Escobedo, J.O.; Strongin, R.M.; Gibbs, S.L. Varied Length Stokes Shift BODIPY-Based Fluorophores for Multicolor Microscopy. Sci. Rep. 2018, 8, 4590. [Google Scholar] [CrossRef]
- Marvin 14.9.29, ChemAxon. 2014. Available online: http://www.chemaxon.com (accessed on 1 January 2023).
- Würth, C.; Grabolle, M.; Pauli, J.; Spieles, M.; Resch-Genger, U. Relative and absolute determination of fluorescence quantum yields of transparent samples. Nat. Protoc. 2013, 8, 1535–1550. [Google Scholar] [CrossRef]
- Chibani, S.; Laurent, A.D.; Le Guennic, B.; Jacquemin, D. Improving the Accuracy of Excited-State Simulations of BODIPY and Aza-BODIPY Dyes with a Joint SOS-CIS(D) and TD-DFT Approach. J. Chem. Theory Comput. 2014, 10, 4574–4582. [Google Scholar] [CrossRef] [PubMed]
- Chibani, S.; Le Guennic, B.; Charaf-Eddin, A.; Maury, O.; Andraud, C.; Jacquemin, D. On the Computation of Adiabatic Energies in Aza-Boron-Dipyrromethene Dyes. J. Chem. Theory Comput. 2012, 8, 3303–3313. [Google Scholar] [CrossRef] [PubMed]
- Chibani, S.; Le Guennic, B.; Charaf-Eddin, A.; Laurent, A.D.; Jacquemin, D. Revisiting the optical signatures of BODIPY with ab initio tools. Chem. Sci. 2013, 4, 1950–1963. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Martin, R.L. Natural Transition Orbitals. J.Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Ngoy, B.P.; Molupe, N.; Harris, J.; Fomo, G.; Mack, J.; Nyokong, T. Photophysical studies of 2,6-dibrominated BODIPY dyes substituted with 4-benzyloxystyryl substituents. J. Porphyr. Phtalocyanines 2017, 21, 431–438. [Google Scholar] [CrossRef]
- Ghai, L.; Mack, J.; Lu, H.; Yamada, H.; Kuzuhara, D.; Lai, G.; Li, Z.; Shen, Z. New 2,6-Distyryl-Substituted BODIPY Isomers: Synthesis, Photophysical Properties, and Theoretical Calculations. Chem. Eur. J. 2014, 20, 1091–1102. [Google Scholar] [CrossRef]
- Ni, Y.; Zeng, L.; Kang, N.-Y.; Huang, K.-W.; Wang, L.; Zeng, Z.; Chang, Y.-T.; Wu, J. meso-Ester and Carboxylic Acid Substituted BODIPYs with Far-Red and Near-Infrared Emission for Bioimaging Applications. Chem. Eur. J. 2014, 20, 2301–2310. [Google Scholar] [CrossRef]
- Momeni, M.R.; Brown, A. Why do TD-DFT excitation energies of BODIPY/Aza-BODIPY families largely deviate from experiment? Answers from electron correlated and multireference methods. J. Chem. Theory Comput. 2015, 11, 2619–2632. [Google Scholar] [CrossRef]
- Postils, V.; Ruiperez, F.; Casanova, D. Mild Open-Shell Character of BODIPY and Its Impact on Singlet and Triplet Excitation Energies. J. Chem. Theory Comput. 2021, 17, 5825–5838. [Google Scholar] [CrossRef]

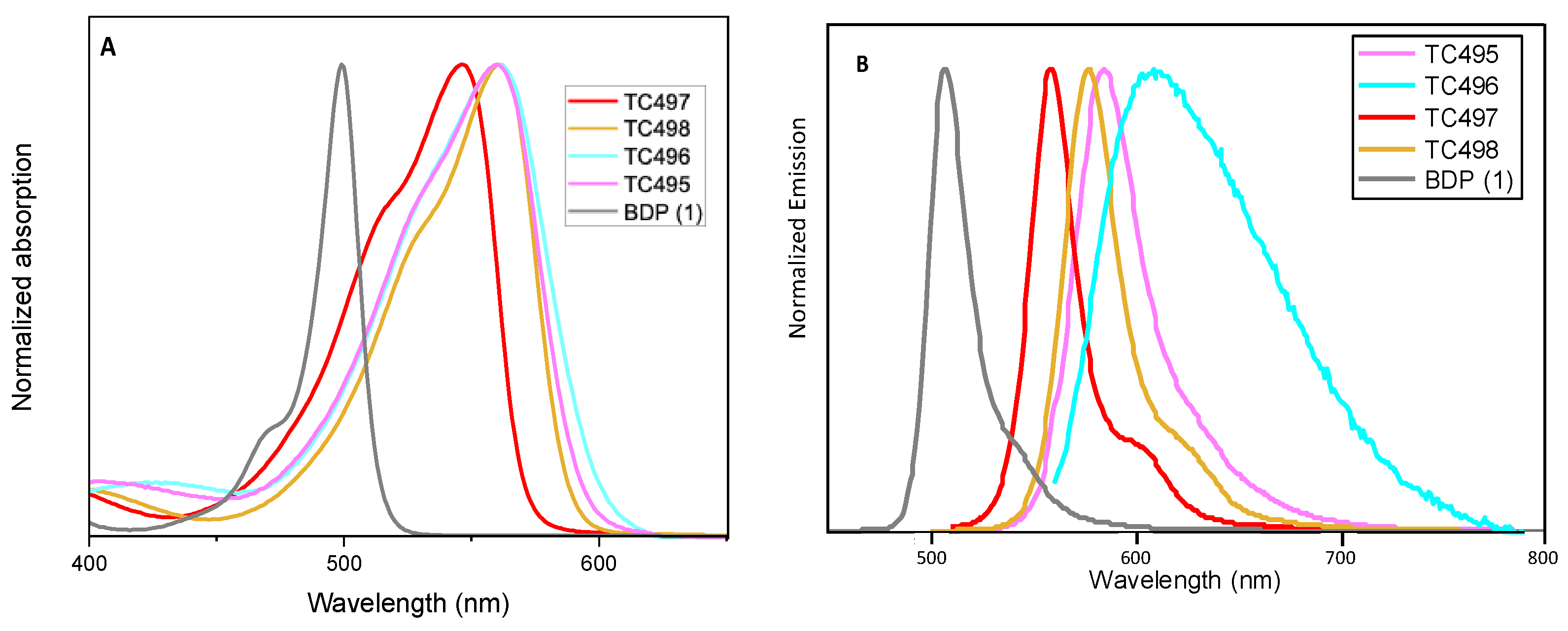
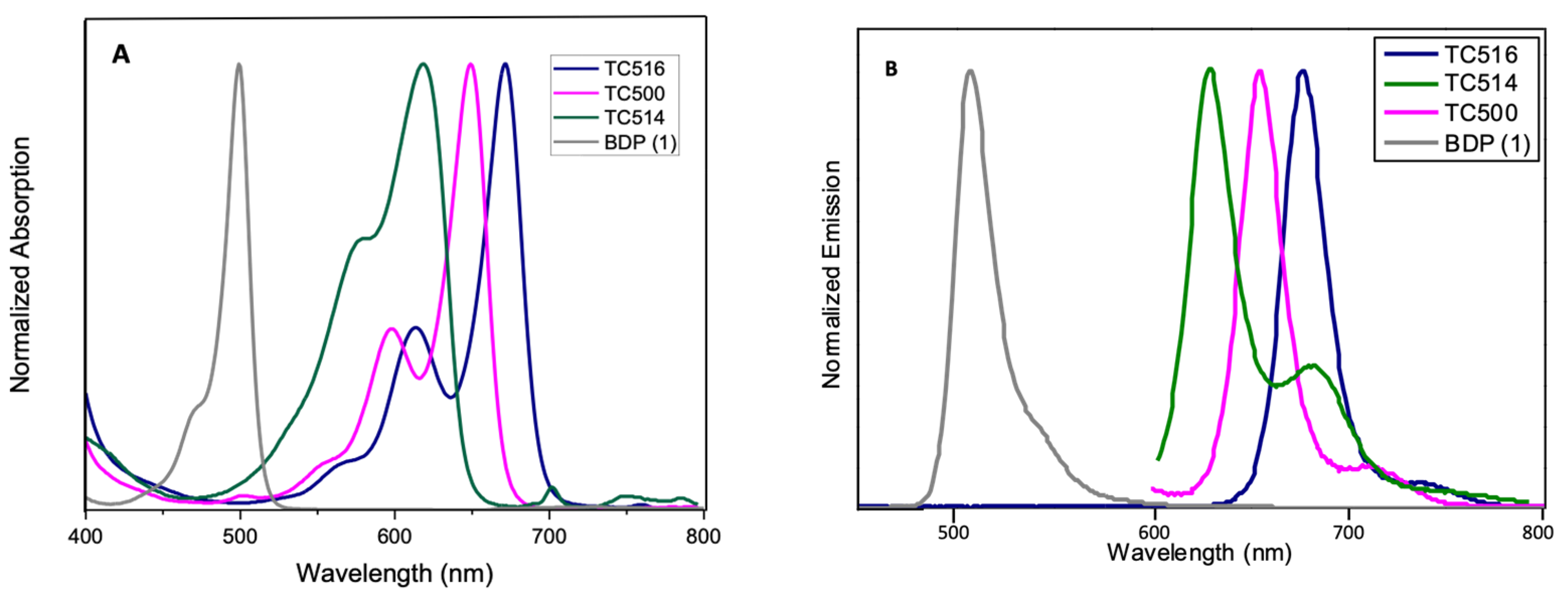

| Dye | λabs a (nm) | λem b (nm) | Δλ c (nm) | Δʋ (cm−1) | φ | Fluorescence Lifetime τ (ns) | ε (M−1cm−1) | Brightness (φ × ε) |
|---|---|---|---|---|---|---|---|---|
| 1 | 499 | 508 | 9 | 355 | 0.61 | 3.5 | 55,500 | 33,850 |
| TC495 | 555 | 586 | 31 | 954 | 0.11 d | 1.58 | 97,800 | 10,758 |
| TC496 | 555 | 605 | 50 | 1490 | 0.01 d | 0.39 | 16,600 | 166 |
| TC497 | 541 | 560 | 19 | 627 | 0.73 d | 4.95 | 54,400 | 39,712 |
| TC498 | 561 | 580 | 19 | 584 | 0.56 d | 4.82 | 62,000 | 34,720 |
| TC500 | 647 | 656 | 9 | 212 | 0.36 d | 2.98 | 28,750 | 10,350 |
| TC514 | 612 | 629 | 17 | 442 | 0.67 e | 4.59 | 24,450 | 16,382 |
| TC516 | 670 | 677 | 7 | 154 | 0.31 e | 2.32 | 34,150 | 10,586 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirkilessi, O.; Arapatzi, C.; Reis, H.; Kostourou, V.; Prousis, K.C.; Calogeropoulou, T. A Study on the Structure, Optical Properties and Cellular Localization of Novel 1,3-Benzothiazole-Substituted BODIPYs. Colorants 2024, 3, 17-38. https://doi.org/10.3390/colorants3010002
Kirkilessi O, Arapatzi C, Reis H, Kostourou V, Prousis KC, Calogeropoulou T. A Study on the Structure, Optical Properties and Cellular Localization of Novel 1,3-Benzothiazole-Substituted BODIPYs. Colorants. 2024; 3(1):17-38. https://doi.org/10.3390/colorants3010002
Chicago/Turabian StyleKirkilessi, Olga, Christina Arapatzi, Heribert Reis, Vassiliki Kostourou, Kyriakos C. Prousis, and Theodora Calogeropoulou. 2024. "A Study on the Structure, Optical Properties and Cellular Localization of Novel 1,3-Benzothiazole-Substituted BODIPYs" Colorants 3, no. 1: 17-38. https://doi.org/10.3390/colorants3010002
APA StyleKirkilessi, O., Arapatzi, C., Reis, H., Kostourou, V., Prousis, K. C., & Calogeropoulou, T. (2024). A Study on the Structure, Optical Properties and Cellular Localization of Novel 1,3-Benzothiazole-Substituted BODIPYs. Colorants, 3(1), 17-38. https://doi.org/10.3390/colorants3010002







