1. Introduction
Ceramic glazes, or glassy layers on top of fired clay bodies, were first produced around 1500 BCE in the ancient Near East [
1]. Glaze production became desired because it strengthened fired clay pieces and rendered them nonporous, which made glazed containers ideal for storing liquids, especially for extended periods [
1]. Glazed items also became valued for beautiful decorations and the vibrant colors they could impart on ceramic objects. Since the beginning of glaze production, the brilliant colors that have been achieved in pottery have been mainly due to the addition of metal oxides into the glaze [
1,
2,
3,
4,
5,
6]. For example, Egyptian faience, a self-glazing ceramic material that became popular around 2001–3000 BCE, was most commonly blue from the addition of copper, perhaps in the form of corroded copper metal or copper-containing minerals such as malachite or azurite [
1]. However, the underlying mechanisms for the final glaze color’s development throughout the firing process are not well studied.
Generally, glazes consist of a glass former, fluxes, refractories, and colorants [
2,
3]. The glass former, or silica (SiO
2), has a melting temperature that is too high for ceramic kilns, so fluxes are added to lower the melting temperature via weak intermolecular interactions [
2]. Refractories, usually alumina (Al
2O
3), are added to prevent the molten glaze from running too much during the firing process [
2]. Finally, glaze appearance can be customized with the addition of colorants, which are mostly transition metal oxides [
4,
5,
6]. Even though colorants can impart vibrant colors to the final glaze products, they usually only make up a very small fraction of the glaze mixture (e.g., 1%) [
3]. Additionally, their initial colors can be quite different from the final glaze colors [
2,
4].
The end color of the glaze is also affected by firing conditions and may vary based on whether the firing is performed in an oxidative or reductive kiln [
4]. Since there are so many variables when creating glazed objects, artists often develop glazes empirically and through trial and error [
5]. Thus, a deeper understanding of both the physical and chemical changes that the transition metal oxides in the glaze undergo during firing at a molecular level is needed to better understand, predict, and design final glaze colorings.
Copper-based transition metal oxides are commonly used as colorants in glazes to produce ceramic products with many different colors [
1,
3,
4,
6]. Specifically, copper colorants create blue and green colors in oxidative firing conditions due to a 2+ oxidation state, and they create red colors in reductive firing conditions due to an oxidation state and/or Cu
0 nanostructures [
7,
8,
9,
10,
11].
One relatively simple and commonly used copper-containing glaze, “Robin’s Egg Blue”, is known to make a final blue color, but the exact mechanism of coloration for this and similar glazes throughout the entire firing process is not well understood. Furthermore, artists’ glaze recipes may call for either copper oxide or copper carbonate as the colorant. Although there have been proposed mechanisms to explain the final blue color, the mechanisms are based on analyses of only the end products of the firing process [
5,
7,
11,
12,
13,
14,
15]. A previous study on blue and green ancient Chinese faience found that the Cu
2+ ions are octahedrally coordinated [
15]. Studies such as the one by Schabbach et al. [
14]. have previously investigated different physical characteristics of glaze when fired at different final temperatures, but not throughout the entire firing process [
14]. It is important to study the entire firing process since it enables the examination of the intermediate colorant-glaze interactions that are beneficial for understanding and predicting glaze coloring upon exposure to various temperatures. Thus, a more detailed, systematic study of the colorant and the chemical origin of the glaze color throughout the entire firing process is needed.
Here, we investigated the effect of firing temperature on glazes colored with copper (II) oxide (CuO) and malachite (Cu
2CO
3(OH)
2) transition metal oxides used in the “Robin’s Egg Blue” glaze. Besides preparing blue glazes with each colorant, we also added silicon carbide (SiC) to a glaze with CuO to help create copper reds in an oxidizing atmosphere instead of the normal reduction kiln [
4,
16,
17,
18]. We studied glaze color as a function of increasing firing temperature systematically. Glaze samples taken at different temperature points throughout the firing process were analyzed to better understand the mechanisms behind the production of the final copper glaze colors. Unlike previous studies [
1,
2,
3,
4,
5,
6], a combination of several advanced characterization techniques, such as electron paramagnetic resonance (EPR) and fiber optic reflectance spectrophotometer (FORS), were utilized in this study to probe both the structural and chemical transformations of the glaze during firing at a wide range of temperatures. These results provide a better understanding of the colors of copper-containing pigments and their origins in ceramic glazes at the molecular level.
4. Discussion
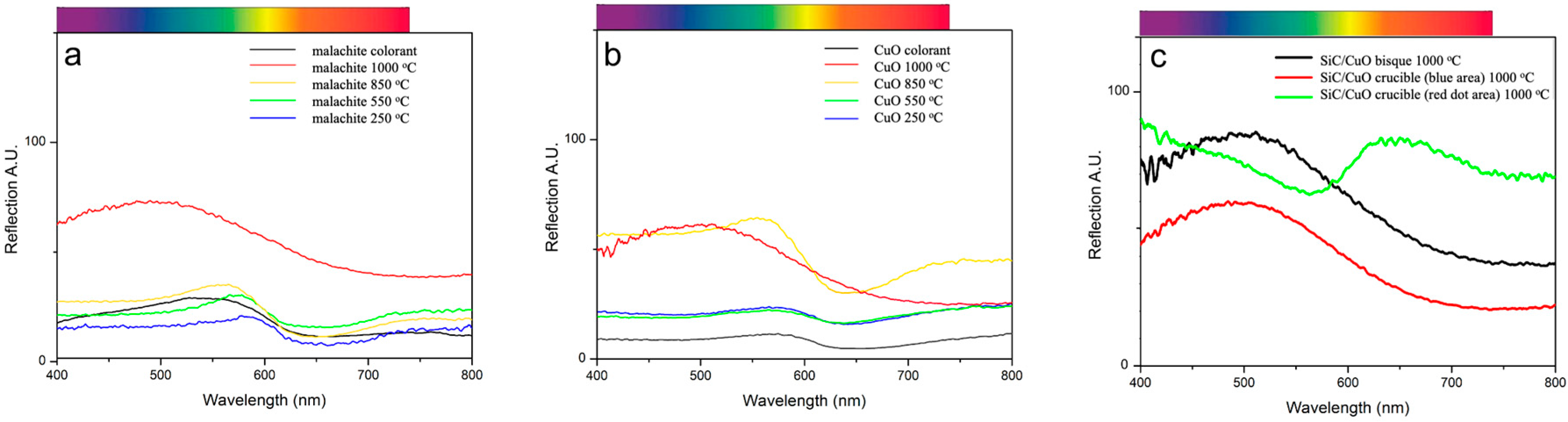
The color of the final glaze product depends on many factors, such as the type of transition metal oxides used as colorants and their oxidation states after firing. The origin of the blue color in the 1% CuO and 1% malachite glaze samples is attributed to the d-d electronic transition involving unfilled d orbitals and the Cu
2+ coordinated complexes. Transition metal ions such as Cu
2+ possess unfilled d orbitals, which degenerate when no ligands are present [
16,
23,
24]. However, when ligands surround and interact with a central transition metal ion, the ligand can donate electrons into the metal ion’s unfilled d orbitals (or vice versa). This causes the metal ion’s d orbitals to split and have different energy levels, resulting in the ligand field effect. Based on the energy gap size, electrons in the lower-energy d orbitals can absorb certain wavelengths in the visible light spectrum and become excited to a higher-level d orbital. It is this absorption of visible light that causes many transition metal complexes to appear colored. The color of the complex depends on the number of d-electrons, the type of coordination ligands around the metal ion, and the geometry of the ligand arrangement. The blue color in the 1% CuO and 1% malachite glaze samples is due to the absorption of the red light with a wavelength between 610 and 650 nm and the subsequent reflection of its complementary blue-green color. However, the origin of the red color in the final glaze (see
Figure 1 and
Figure 2) with SiC as a reducing agent during firing is different from that of the blue color under the oxidative condition. SiC has been used to reduce Cu
2+ to Cu
+1 or Cu
0 [
17]. The red color is certainly not from the d-d transitions because of no unfilled d orbitals in Cu
+1 or Cu
0. Thus the red color is either from the dispersed red-colored Cu
2O (Cu
+1) particles in the final glaze or nanosized metallic copper (Cu
0) [
9,
17].
It is clear from our combined results that the colors of copper-containing glazes are not due to the colorant in the physical mixture of the raw glaze but rather to a change in overall glaze structure as the firing temperature approaches and exceeds 850 °C. For example, the color of CuO is black in the raw glaze but blue color in the final glaze product. Our data, especially from the EPR measurements, further confirm that the origin of the final blue colors in the glaze under oxidative conditions is due to the transition metal Cu
2+ ions that are coordinated with the surrounding glass matrix since EPR spectra are sensitive to any changes in either the oxidation state of the central transition metal ions or the ligands that are coordinated to the center ions. EPR data showed that copper in the 1% malachite and 1% CuO glazes had an oxidation state of Cu
2+ throughout the firing process, even when the glazes were not yet blue, indicating that no redox reactions occurred (
Figure 5). Specifically, a broad peak centered at ~3370 G observed in the 1% malachite glaze at both 250 °C and 550 °C is due to the presence of coupled Cu
2+ centers, in agreement with the previous study [
25], while an anisotropic peak observed at 850 °C is attributed to individual Cu
2+ ions within a different structural environment (
Figure 5c). EPR data further confirm that the new coordinated Cu
2+ complexes are formed after heating the glaze to 850 °C due to the interaction with the glass matrix under high temperatures.
Interestingly, we observed that the 1% malachite glaze changed from beige to darker gray, more similar to the 1% CuO glaze, as the firing temperature reached 550 °C (
Figure 1). Natural malachite is known to decompose into CuO at about 375 °C, for which Frost et al. (2002) proposed the decomposition mechanism to be as follows [
26]:
This observation suggests that this decomposition is also occurring within the glaze mixture as well, specifically before the glazes reach a high enough temperature to begin sintering. This decomposition also explains why the final colors of the 1% CuO glaze and 1% malachite glazes were very similar, as shown by the similarities in the FORS spectra of the glazes fired to 1000 °C (
Figure 3a,b). These results indicated that both CuO and malachite can be used as the colorant to create “Robin’s egg blue.” Furthermore, the observation of similar anisotropic Cu
2+ EPR lines (
Figure 5b) for both the 1% malachite and 1% CuO glazes upon heating to 850 °C proves that both colorants give an identical Cu
2+ coordination environment in the final glaze. Thus the origin of the blue colors in both 1% malachite and 1% CuO glaze heated to high temperatures is due to the similar coordinated Cu
2+ complex with the surrounding glass matrix.
The change in glaze color from gray to blue by 850 °C for all of the colored glazes was a surprising observation, as 850 °C was below the flux point of the glaze (
Figure 1). It suggests that even before the glaze became completely molten, Cu
2+ was already well-distributed and coordinated with the other glaze compounds, likely in an octahedral-like and symmetrical structure due to the light blue color [
11]. This was further evidenced by the EPR spectra for samples fired at 850 °C, where an anisotropic Cu
2+ signal strongly appeared at ~3450 G (
Figure 5). Specifically, the fact that g
∥ > g
⊥ >2.0 confirmed that Cu
2+ is located in a square planner (D
4h) geometry due to the Jahn Teller distortion [
21,
25,
27,
28]. Four hyperfine splitting signal as a result of the interaction between the unpaired electrons and the nuclei (I = 3/2) was partially resolved for the parallel component (g
∥ ≈ 2.3, A
∥ ≈ 135 G) but could not be resolved for the perpendicular component (g
⊥ ≈ 2.1) (
Figure 5b). Overall, our signal matches well with other examples of Cu
2+ in square planar coordination [
21,
25,
27,
28,
29]. Additionally, it is probable that a second broad Cu
2+ peak is contributing to the overall signal as a result of some Cu
2+ ions that remain strongly coupled in a manner like Cu
2+ in the raw glaze, similar to previous reports [
21,
28]. Taken together, the significant color change and dramatic sharpening of the EPR signal, even before the glass was molten, validate that the sintering process for this glaze already begins at 850 °C. This can be further seen in the SEM image (
Figure 4) comparisons and in changes in XRD (
Figure S1).
Interestingly, the glazed crucible lids became green immediately after they were taken out of the furnace (
Figure 2a). This may be due to the Cu
2+ being in a more tetrahedral environment with the superheated glaze matrix since aqueous CuCl
4 is known to be green [
30]. Alternatively, the electronic transition or degree of axial elongation may have been different due to the effects of high-temperature heating, causing us to see the unexpected green color. The cooling process may have then changed the structure of the glaze matrix such that square planar geometry coordination was favored, leading to the blue color of the final glaze product at room temperature (
Figure 1 and
Figure 2).
For the 1% CuO/1% SiC glaze, it was observed that the red spots did not appear until after the glazes were fired to 1000 °C and above (
Figure 1 and
Figure 2b). At 850 °C, the glaze was light blue with gray particulates, which were most likely the unreacted SiC particles (
Figure 1). The red dots were more significant when the glaze was held for a longer time and at a higher temperature than 1000 °C, as seen when comparing the glazed crucible lid to the glazed bisque disk (
Figure 2b). These results suggest that longer soaking times, a.k.a. the time the glazes are held at the highest temperature, are needed for artists who hope to create full, copper red glazes in an oxidative environment using SiC as a reducing agent. Comparatively, because the final color for our copper blue glazes (no SiC) was achieved before total vitrification to glass, an artist’s soaking time for copper blue glazes is only dependent on the desired final surface texture (i.e., smoother and glassy vs. rougher). A mechanism for the reduction in copper using SiC was previously proposed by Orna and Goodstein [
16]:
This proposed mechanism was supported by our observations of the reduction in red spots we observed in glaze with SiC, as Cu
2O (Cu has a 1+ oxidation state) is known to be red [
16]. Additionally, the non-red glaze areas appeared greener than the blue 1% CuO and 1% malachite glazes, suggesting that Cu
+ was also distributed along with the Cu
2+ in the non-visible red areas (
Figure 2b and
Figure 3c) [
31]. In addition, the final glaze was not as reflective as the other glazes without SiC due to the presence of small bubbles, which were most likely caused by CO
2 production during the copper reduction process. The high temperature that the reaction required to take place was likely influenced by the fact that SiC is a refractory material. Nevertheless, there is still disagreement in the literature as to whether copper reds are due to the presence of Cu
2O or Cu
0 nanoparticles after reduction [
7,
9,
10,
11,
17]. More careful analyses, such as XANES, would help to confirm the underlying chemical changes that result in these red areas.
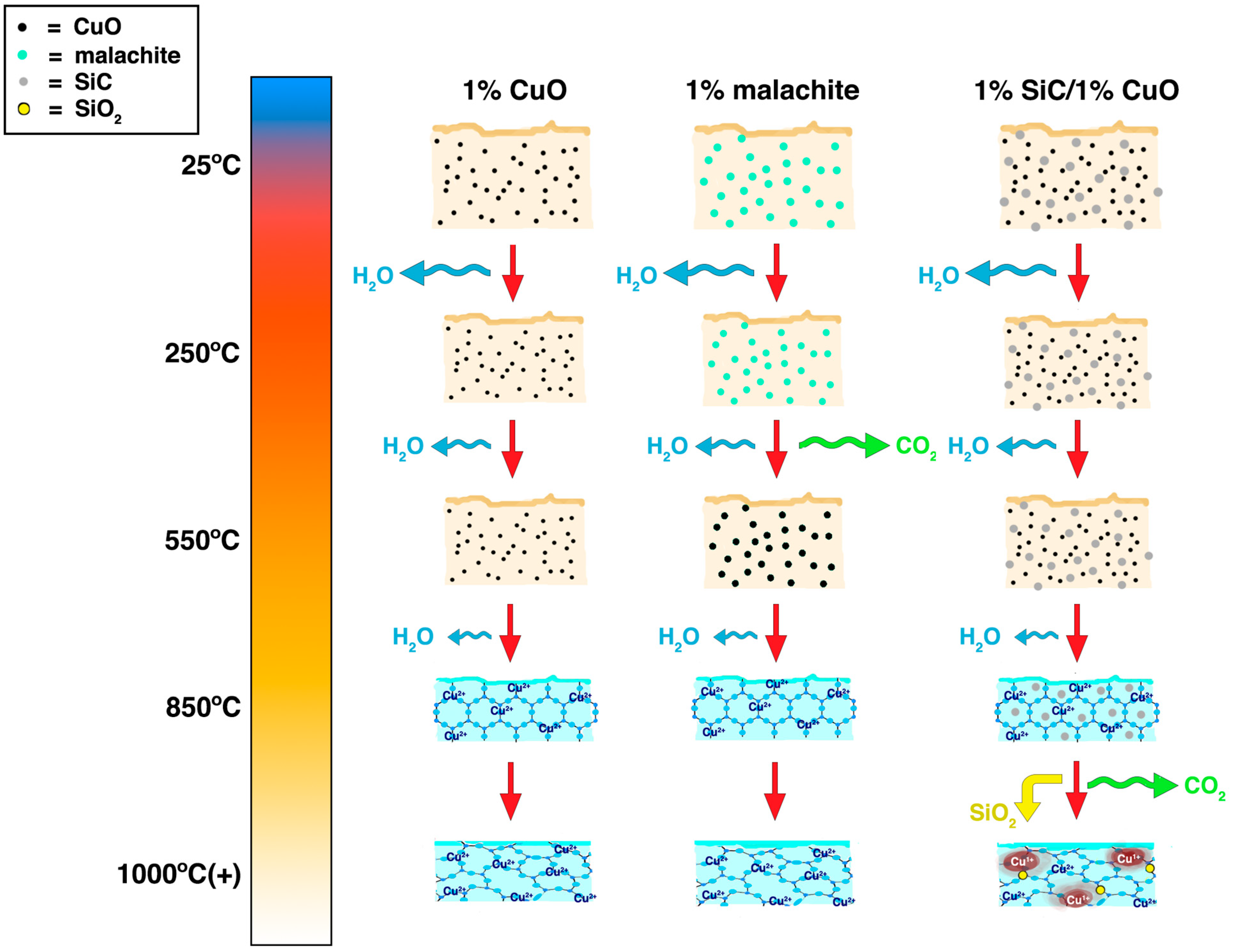
Figure 6 is an illustration that summarizes the proposed mechanism and the timelines of the firing process for our “Robin’s egg blue” glaze variants based on the previous literature and the data we collected in this study [
16,
23,
26]. It illustrates the loss of water in all glazes mostly by 250 °C, as indicated by the size of the blue arrows, but water may have still been continuously lost with higher temperatures as other glaze components begin to decompose and sinter, as indicated by the increasingly smaller blue arrows. The water loss and loss of volume of the glaze were reflected in the gradually decreasing glaze square size and thickness as the furnace temperatures increased. By 850 °C, all glaze samples appeared blue in color (
Figure 1 and
Figure 2), indicating that complexation with the Cu
2+ ions had occurred, which may have been due to the formation of silica-alumina matrix as the glaze components began to sinter. Finally, by 1000 °C and above, the glazes vitrified into amorphous glass, resulting in disorder in the silica-alumina matrix that was indicated by the loss of defined peaks in the XRD pattern (
Figure S1). Specifically for the 1% malachite glaze, the malachite likely decomposed into CuO by 550 °C, as represented by the darkening of the turquoise-colored colorant dots into black dots along with a loss of H
2O and CO
2. Meanwhile, for the 1% SiC/1% CuO glaze, the SiC remained unreacted until around 1000 °C, before it reduced CuO to likely form Cu
2O, SiO
2, and CO
2, represented by the red spots and yellow particles at the right bottom of
Figure 6.
To further elucidate the chemistry of glaze firing, X-ray absorption near edge structure (XANES) could be performed to confirm the electronic transitions that are responsible for the color of our blue glazes. Other methods, such as X-ray photoelectron spectroscopy (XPS), can also be used to further investigate the form(s) of copper responsible for our copper red glaze.
This study includes a novel, systematic study of glaze coloration and chemistry as a function of firing temperature, particularly for an artistic ceramic glaze that only contains 1 wt. % copper colorant. We unexpectedly observed that final glaze colors were achieved below the glaze flux range, indicating that copper complexation likely occurred at the very beginning of the vitrification stage. Additionally, the decomposition of malachite into CuO may have been observed by the midpoint of the firing process, explaining why either CuO or malachite can be used by artists to achieve the same color.
Our results have provided a better understanding of how only 1 wt. % copper compound can produce vibrant glaze colors that are not due to the initial, intrinsic color of the colorant but rather an overall structural change in the glaze that causes the surrounding glaze matrix to interact with the copper centers. Additionally, we gain a better understanding of the chemical reactions behind the color changes and when the reactions take place during the firing process. These results can be applied to better-controlled glaze production for artists and a deeper appreciation of ceramic glaze chemistry and aesthetics.