Near-Infrared Absorbing Molecule Based on Triphenylamine Radical Cation with Extended Homoaryl π-System
Abstract
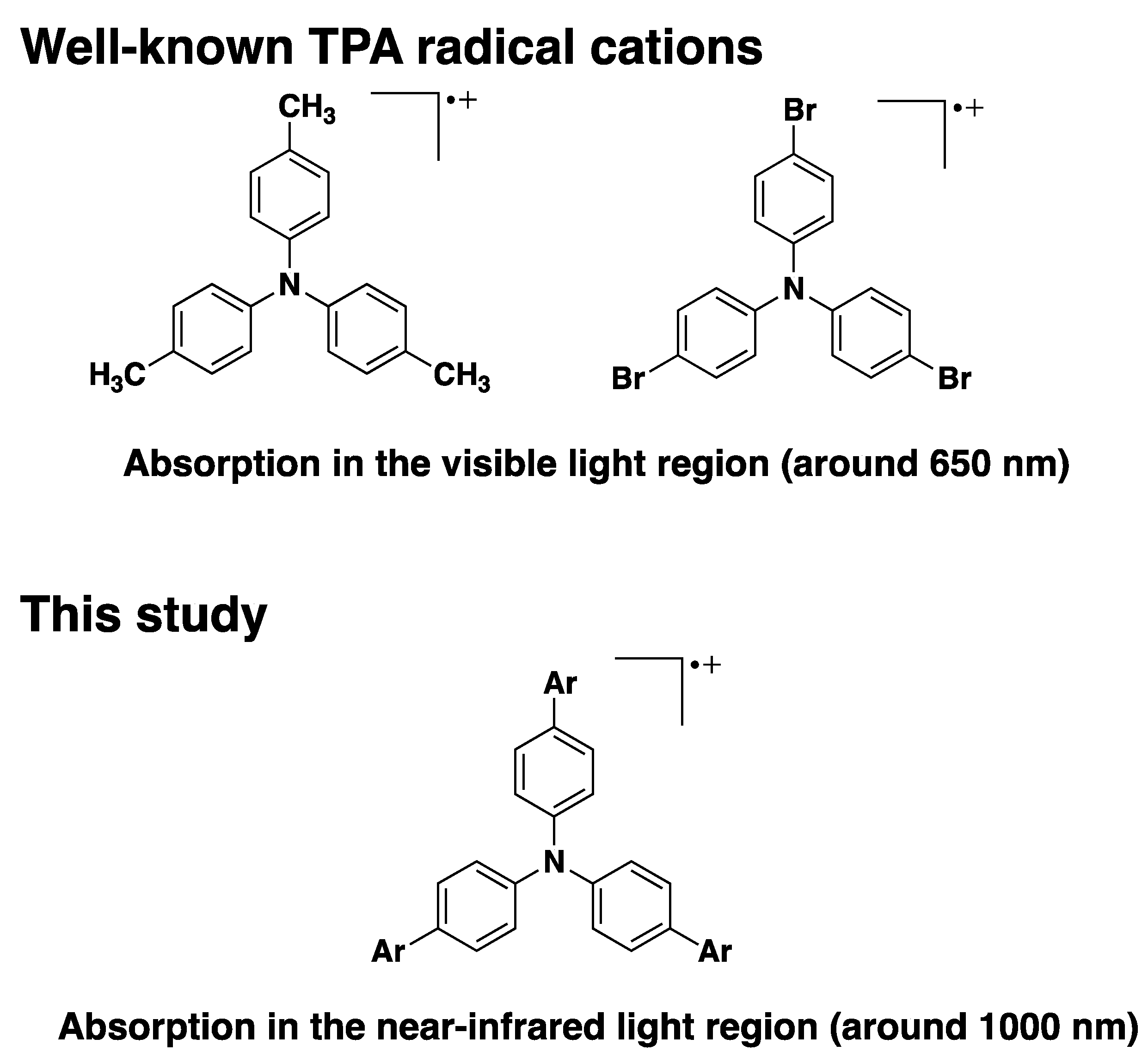
1. Introduction
2. Results and Discussion
2.1. Theoretical Calculations
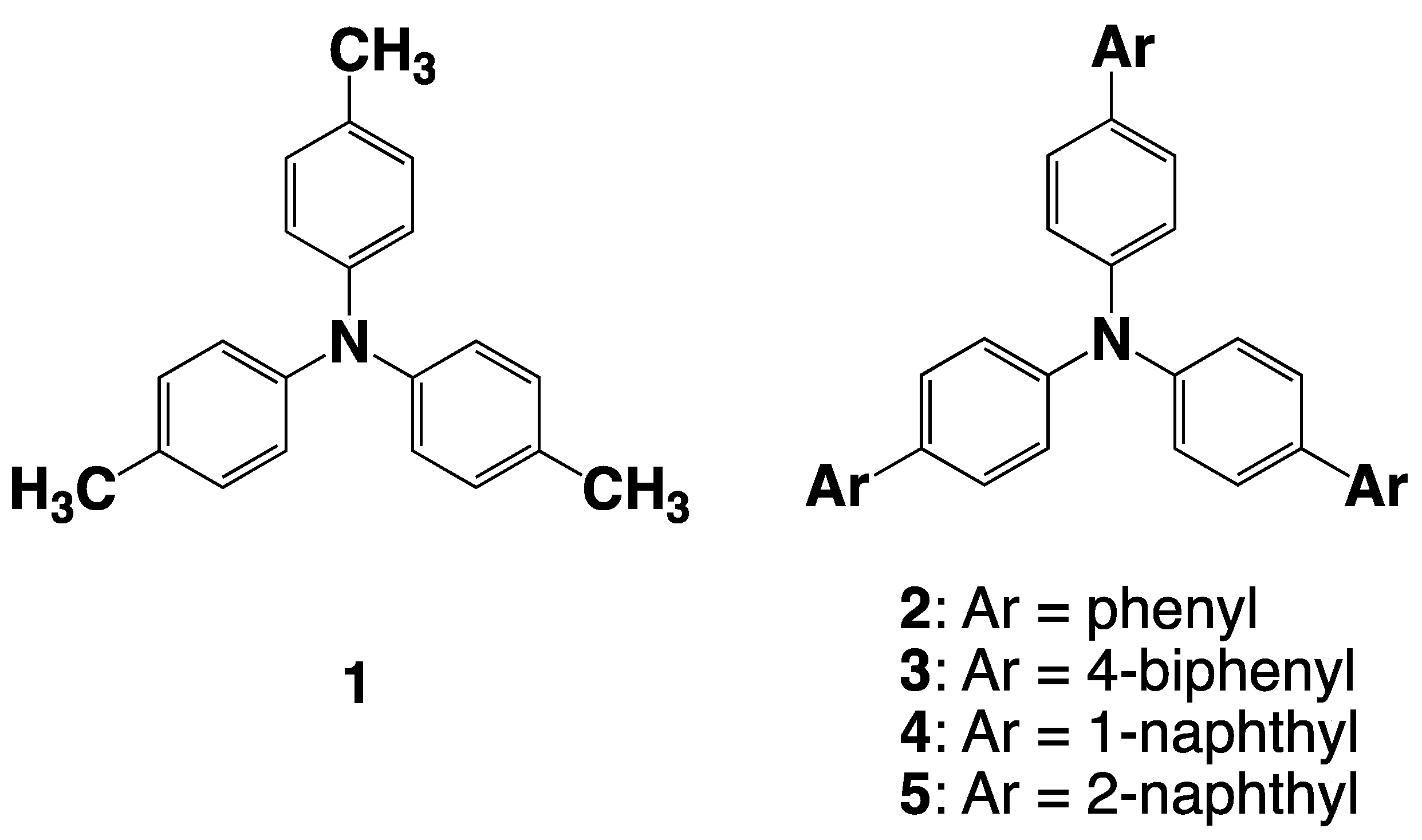
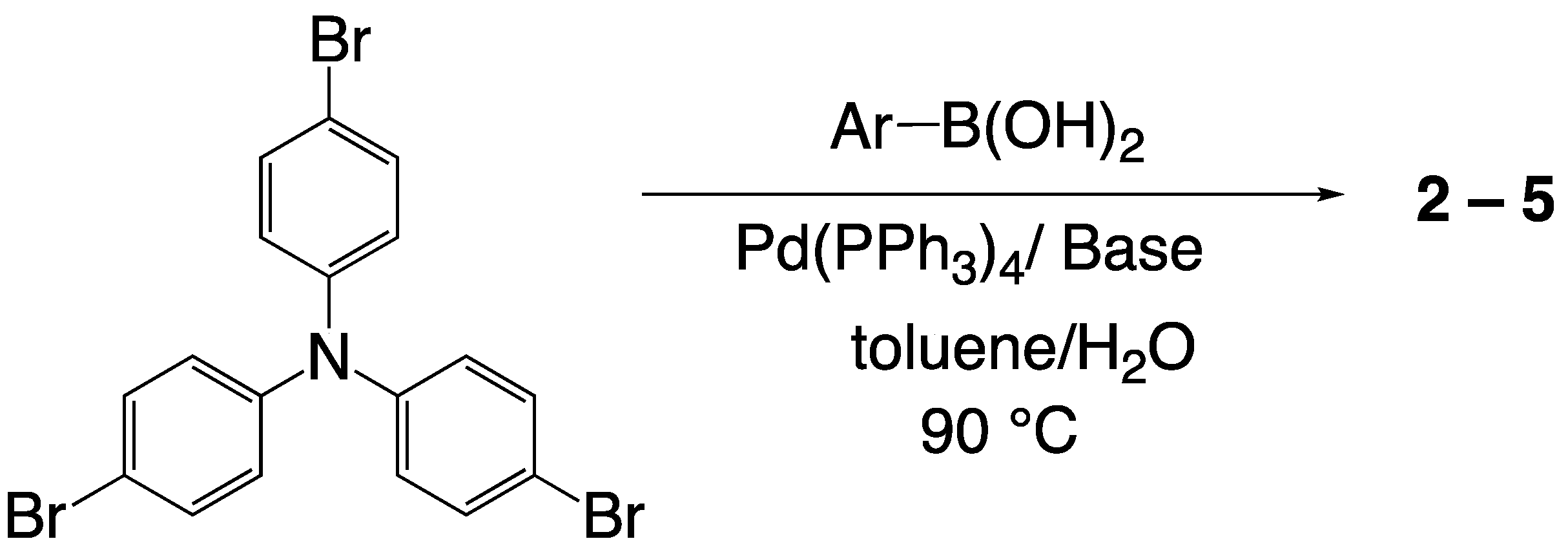
2.2. Synthesis
2.3. Solubility
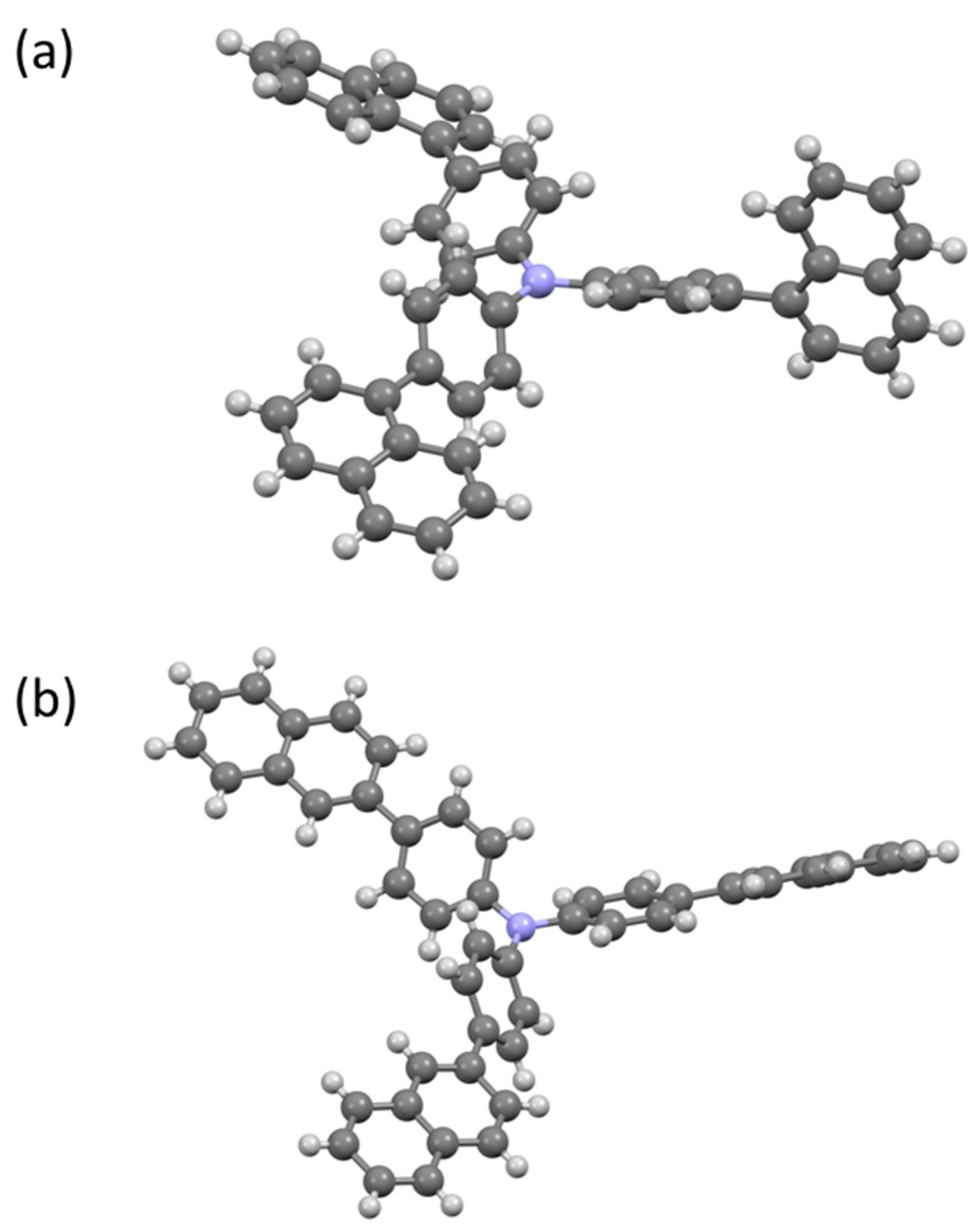
2.4. Crystal Structures of 4 and 5
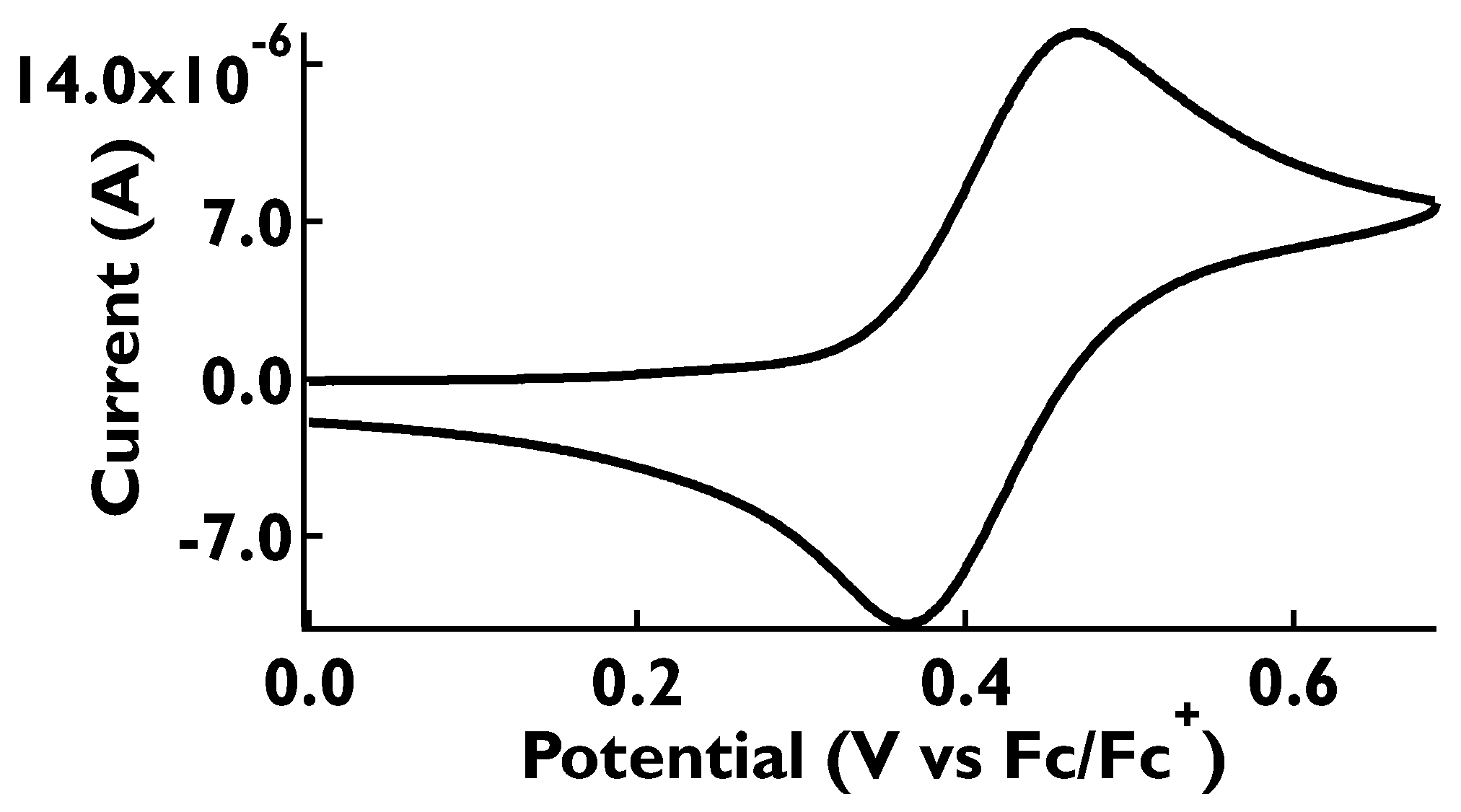
2.5. Cyclic Voltammetry Measurements
2.6. Absorption and Fluorescence Spectra of the Neutral Species, 1–5
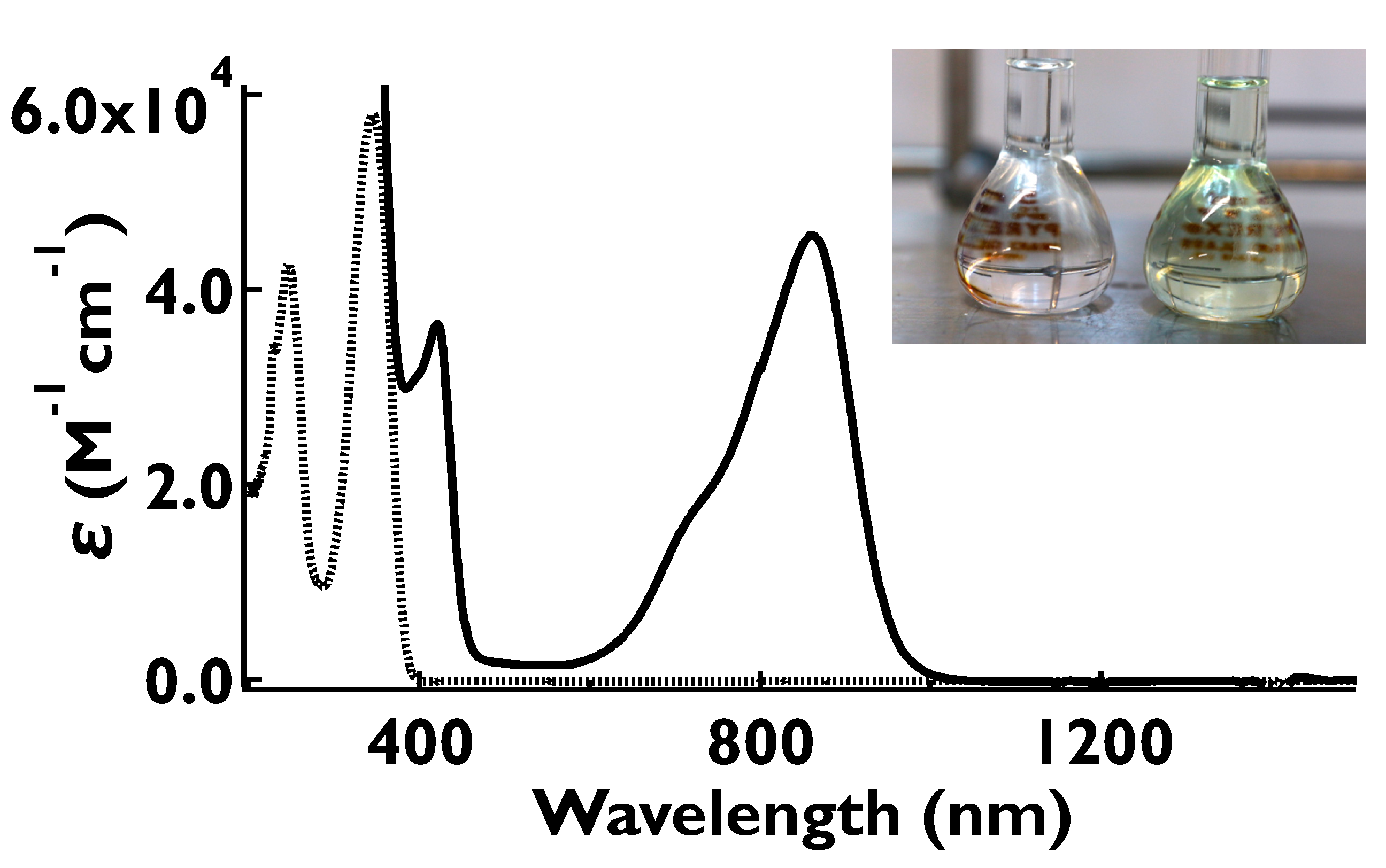
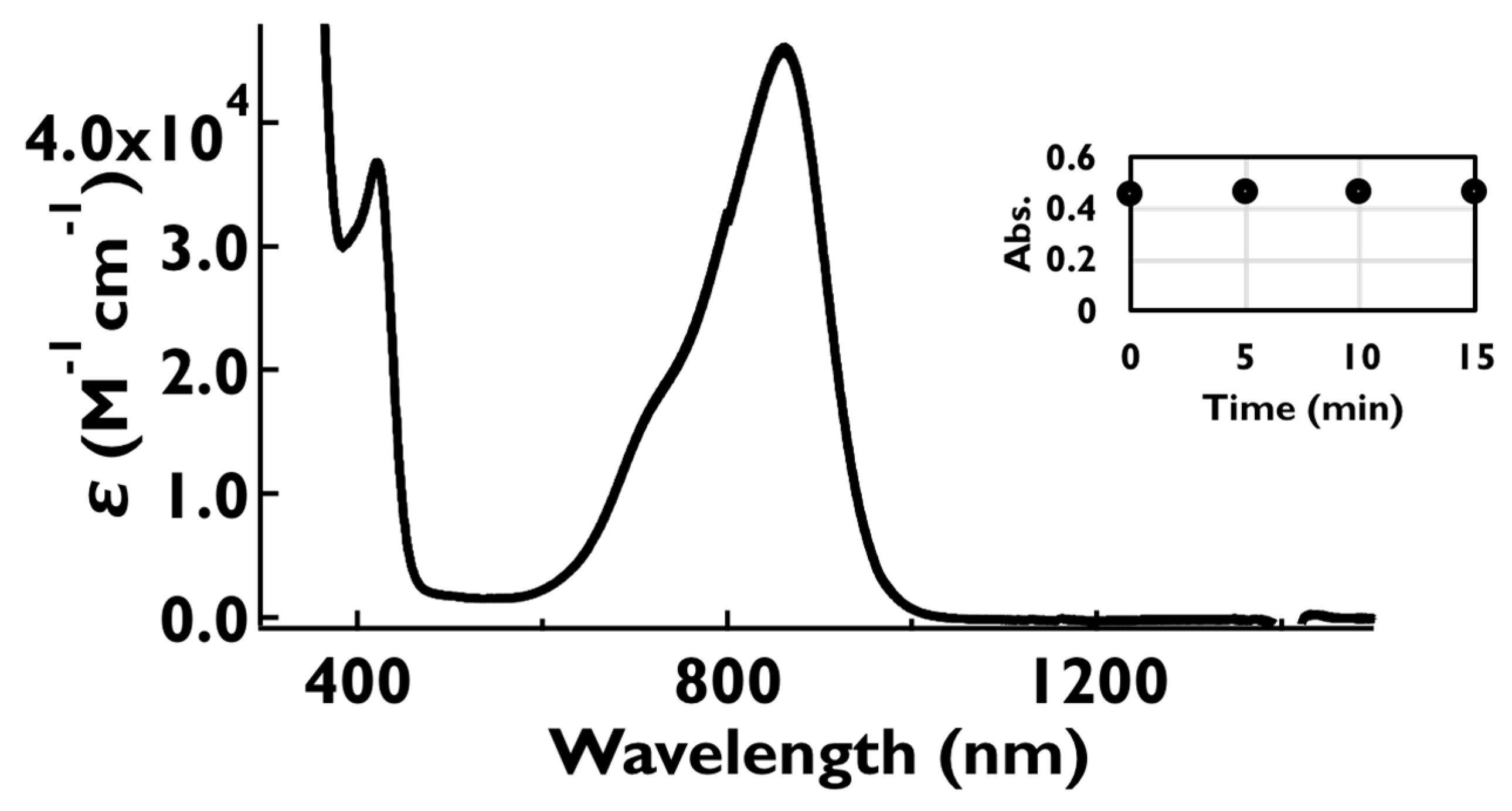
2.7. UV-Vis-NIR Absorption and Fluorescence Spectra of the Oxidized Species, 1•+–5•+
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- Wang, Z. Near-Infrared Organic Materials and Emerging Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Matsuoka, M. Infrared Absorbing Dyes (Topics in Applied Chemistry); Springer: Berlin/Heidelberg, Germany, 1990; ISBN 0306434784. [Google Scholar]
- Fabian, J.; Nakazumi, H.; Matsuoka, M. Near-infrared absorbing dyes. Chem. Rev. 1992, 92, 1197–1226. [Google Scholar] [CrossRef]
- Rao, R.S.; Suman; Singh, S.P. Near-Infrared (>1000 nm) Light-Harvesters: Design, Synthesis and Applications. Chem. A Eur. J. 2020, 26, 16582–16593. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, X.; Li, J.; Wei, J. A short review on NIR-II organic small molecule dyes. Dye. Pigment. 2020, 183, 108756. [Google Scholar] [CrossRef]
- Li, B.; Zhao, M.; Zhang, F. Rational Design of Near-Infrared-II Organic Molecular Dyes for Bioimaging and Biosensing. ACS Mater. Lett. 2020, 2, 905–917. [Google Scholar] [CrossRef]
- Qi, J.; Qiao, W.; Wang, Z.Y. Advances in Organic Near-Infrared Materials and Emerging Applications. Chem. Rec. 2016, 16, 1531–1548. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, J. Higher order acenes and fused acenes with near-infrared absorption and emission. Aust. J. Chem. 2011, 64, 519–528. [Google Scholar] [CrossRef]
- Seo, E.T.; Nelson, R.F.; Fritsch, J.M.; Marcoux, L.S.; Leedy, D.W.; Adams, R.N. Anodic Oxidation Pathways of Aromatic Amines. Electrochemical and Electron Paramagnetic Resonance Studies. J. Am. Chem. Soc. 1966, 88, 3498–3503. [Google Scholar] [CrossRef]
- Nelson, R.R.; Adams, R.N. Anodic oxidation pathways of substituted triphenylamines. II. Quantitative studies of benzidine formation. J. Am. Chem. Soc. 1968, 90, 3925–3930. [Google Scholar] [CrossRef]
- Nelson, R.F.; Philp, R.H. Electrochemical and spectroscopic studies of cation radicals. 4. Stopped-flow determination of triarylaminium radical coupling rate constants. J. Phys. Chem. 1979, 83, 713–716. [Google Scholar] [CrossRef]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Miomandre, F.; Audebert, P. Tetrazine-triphenylamine dyads: Influence of the nature of the linker on their properties. Electrochim. Acta 2013, 110, 693–701. [Google Scholar] [CrossRef]
- Golba, S.; Starczewska, O.; Idzik, K. Electrochemical and spectrophotometric properties of polymers based on derivatives of di- and triphenylamines as promising materials for electronic applications. Des. Monomers Polym. 2015, 18, 770–779. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable triarylamine-based electroactive high performance polymers for anodically electrochromic applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, N.; Jia, X.; Liu, X.; Wang, C.; Chao, D. Electrochromic and electrofluorochromic behavior of novel polyurea bearing oligoaniline and triphenylamine units. Polymer 2018, 134, 1–7. [Google Scholar] [CrossRef]
- Vamvounis, G.; Shaw, P.E.; Burn, P.L. Design protocols in triarylamine cored dendrimer-based explosive sensors. J. Mater. Chem. C 2013, 1, 1322–1329. [Google Scholar] [CrossRef]
- Higuchi, A.; Ohnishi, K.; Nomura, S.; Inada, H.; Shirota, Y. Tri(biphenyl-4-yl)amine and tri(p-terphenyl-4-yl)amine as a novel class of molecules for amorphous molecular materials. J. Mater. Chem. 1992, 2, 1109–1110. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, M.K.; Hong, J.-P.; Lee, W.; Noh, S.; Lee, C.; Lee, S.; Hong, J.-I. 4,4′,4″-Tris(4-naphthalen-1-yl-phenyl)amine as a multifunctional material for organic light-emitting diodes, organic solar cells, and organic thin-film transistors. Org. Electron. 2010, 11, 1288–1295. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, M.K.; Hong, J.-P.; Lee, W.; Lee, S.; Hong, J.-I. A Multifunctional Material Based on Triphenylamine and a Naphthyl Unit for Organic Light-Emitting Diodes, Organic Solar Cells, and Organic Thin-Film Transistors. Bull. Korean Chem. Soc. 2013, 34, 1355–1360. [Google Scholar] [CrossRef][Green Version]
- Amthor, S.; Noller, B.; Lambert, C. UV/Vis/NIR spectral properties of triarylamines and their corresponding radical cations. Chem. Phys. 2005, 316, 141–152. [Google Scholar] [CrossRef]
- Rim, Y.S.; Bae, S.; Chen, H.; De Marco, N.; Yang, Y. Recent Progress in Materials and Devices toward Printable and Flexible Sensors. Adv. Mater. 2016, 28, 4415–4440. [Google Scholar] [CrossRef]
- Okamoto, T.; Mitsui, C.; Yamagishi, M.; Nakahara, K.; Soeda, J.; Hirose, Y.; Miwa, K.; Sato, H.; Yamano, A.; Matsushita, T.; et al. V-shaped organic semiconductors with solution processability, high mobility, and high thermal durability. Adv. Mater. 2013, 25, 6392–6397. [Google Scholar] [CrossRef]
- Yano, M.; Kashiwagi, Y.; Inada, Y.; Hayashi, Y.; Mitsudo, K.; Kubono, K. Crystal structure of tris [4-(naphthalen-1-yl)phenyl]amine. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1649–1652. [Google Scholar] [CrossRef] [PubMed]
- CCDC 2164002 (5) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-1223-336033.
- Wang, K.; Deng, Z.H.; Xie, S.J.; Zhai, D.D.; Fang, H.Y.; Shi, Z.J. Synthesis of arylamines and N-heterocycles by direct catalytic nitrogenation using N2. Nat. Commun. 2021, 12, 248. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1380. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
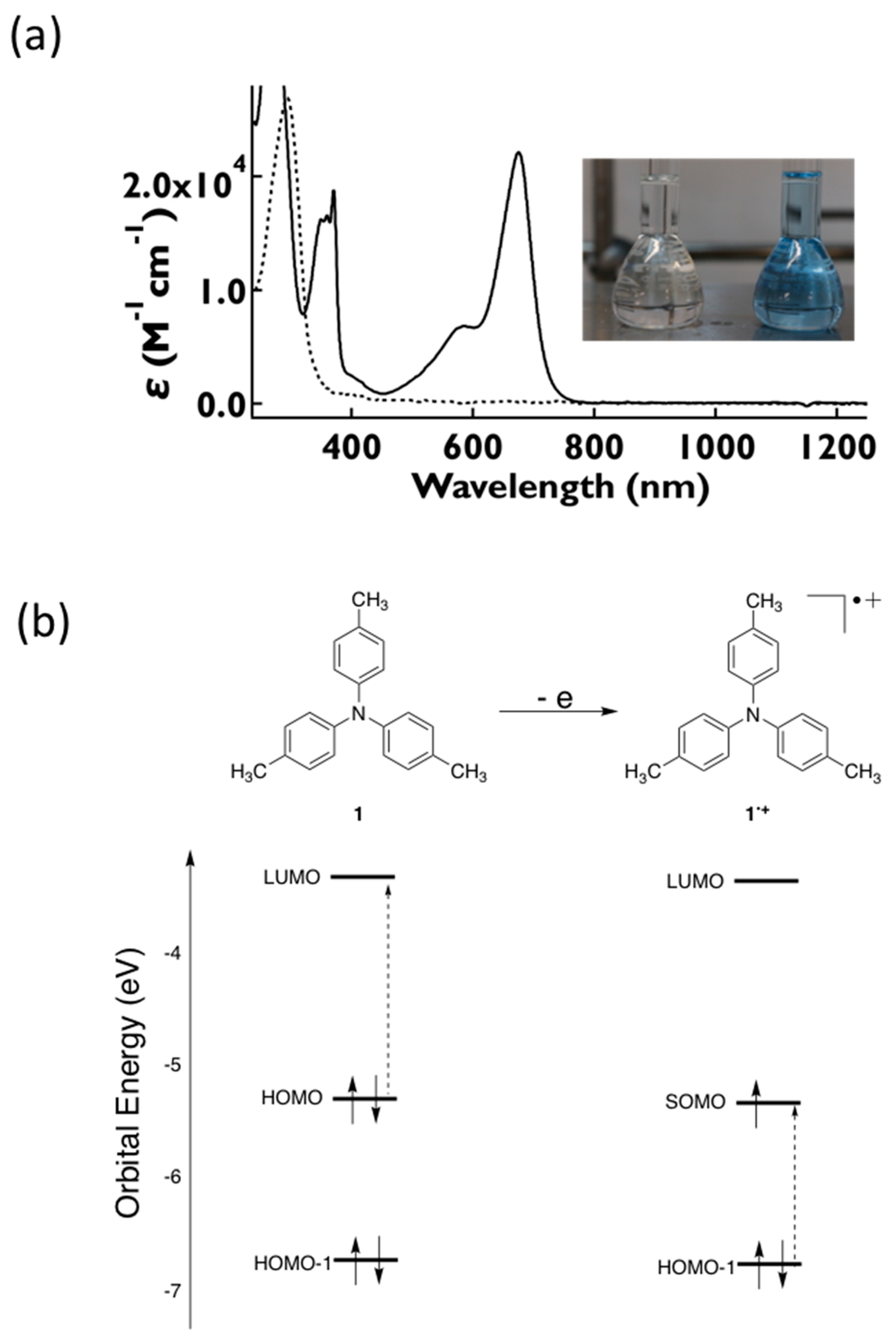
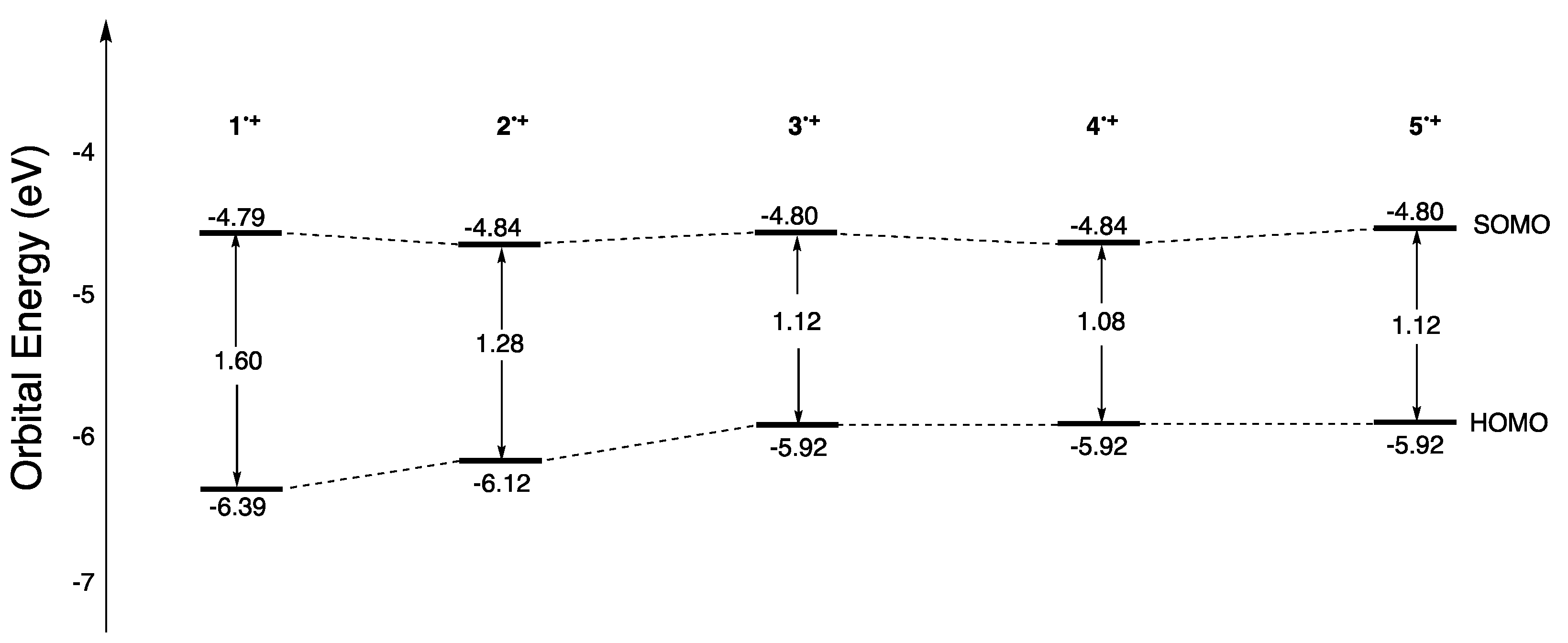

| Compound | E0 (V vs. Fc/Fc+) | EHOMO (eV) |
|---|---|---|
| 1 | 0.33 | −4.87 |
| 2 | 0.42 | −4.97 |
| 3 | 0.42 | −4.95 |
| 4 | 0.47 | −5.00 |
| 5 | 0.42 | −4.96 |
| Compound | Absorption Spectra | Fluorescence Spectra | ||
|---|---|---|---|---|
| Obsd. | Calcd. | Obsd. | ||
| λmax (nm) | log ε | λmax (nm) | λmax (nm) | |
| 1 | 294 | 4.43 | 326 | - |
| 2 | 344 | 4.78 | 363 | 416 |
| 3 | 361 | 4.71 | 390 | 442 |
| 4 | 341 | 4.65 | 379 | 437 |
| 5 | 363 | 4.84 | 393 | 440 |
| Absorption Spectra | DFT Calculation | |||||
|---|---|---|---|---|---|---|
| Obsd. | Calcd. | HOMO (eV) | SOMO (eV) | ΔE (eV) | ||
| λmax (nm) | log ε | λmax (nm) | ||||
| 1•+ | 675 | 4.34 | 633 | −6.39 | −4.79 | 1.60 |
| 2•+ | 862 | 4.66 | 857 | −6.12 | −4.84 | 1.28 |
| 3•+ | 991 | 4.46 | 1113 | −5.92 | −4.80 | 1.12 |
| 4•+ | 1071 | 4.46 | 1349 | −5.92 | −4.84 | 1.08 |
| 5•+ | 1028 | 4.56 | 1244 | −5.92 | −4.80 | 1.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, M.; Tamada, K.; Nakai, M.; Mitsudo, K.; Kashiwagi, Y. Near-Infrared Absorbing Molecule Based on Triphenylamine Radical Cation with Extended Homoaryl π-System. Colorants 2022, 1, 226-235. https://doi.org/10.3390/colorants1020014
Yano M, Tamada K, Nakai M, Mitsudo K, Kashiwagi Y. Near-Infrared Absorbing Molecule Based on Triphenylamine Radical Cation with Extended Homoaryl π-System. Colorants. 2022; 1(2):226-235. https://doi.org/10.3390/colorants1020014
Chicago/Turabian StyleYano, Masafumi, Kohei Tamada, Misaki Nakai, Koichi Mitsudo, and Yukiyasu Kashiwagi. 2022. "Near-Infrared Absorbing Molecule Based on Triphenylamine Radical Cation with Extended Homoaryl π-System" Colorants 1, no. 2: 226-235. https://doi.org/10.3390/colorants1020014
APA StyleYano, M., Tamada, K., Nakai, M., Mitsudo, K., & Kashiwagi, Y. (2022). Near-Infrared Absorbing Molecule Based on Triphenylamine Radical Cation with Extended Homoaryl π-System. Colorants, 1(2), 226-235. https://doi.org/10.3390/colorants1020014







