Preliminary in Silico Studies of the Interactions of Certain Genotoxic Azo Dyes with Different Double-Stranded DNA Conformations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Filtering Criteria
- In the Web of Science database, a total of 84 research articles were found by using a combination of keywords “azo dye genotoxicity”.
- In the second step, among these 84 articles, further filtering was performed by using a combination of keywords, “genotoxicity DNA”; therefore, the total number of articles was reduced to 28. Thus, articles on the studies of azo dyes in which the genotoxic effect occurs due to intermolecular interactions with DNA were selected.
- In vivo micronucleus (MN) test result should be positive;
- In vivo chromosome aberration (CA) test result should be positive;
- Both in vitro comet and in vitro MN/CA test results should be positive;
- Both in vivo comet and in vivo MN/CA test result should be positive;
- Capability of forming DNA adducts in DNA-binding assays.
2.2. Receptor/Ligand Retrieval
2.3. Control Dockings
2.4. Receptor/Ligand Preparation and Molecular Docking
3. Results and Discussion
3.1. Control Dockings
3.2. Molecular Docking against Intact dsDNA and dsDNA with Natural Intercalation Gap
3.3. dsDNA Sequence Selectivity of Top-Ranked Azo Dye Conformations
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; De Vito, S.C. Predicting azo dye toxicity. Crit. Rev. Environ. Sci. Technol. 1993, 23, 249–324. [Google Scholar] [CrossRef]
- Morris, P.J.; Travis, A.S. A history of the international dyestuff industry. Am. Dyest. Report. 1992, 81, 59. [Google Scholar]
- Singh, A.L.; Chaudhary, S.; Kumar, S.; Kumar, A.; Singh, A.; Yadav, A. Biodegradation of Reactive Yellow-145 azo dye using bacterial consortium: A deterministic analysis based on degradable Metabolite, phytotoxicity and genotoxicity study. Chemosphere 2022, 300, 134504. [Google Scholar] [CrossRef]
- Khan, S.; Zeyad, M.T.; Malik, A. Genotoxicity assessment of textile waste contaminated soil and characterization of textile dye degradation by a novel indigenous bacterium Ochrobactrum intermedium BS39. Chemosphere 2022, 299, 134082. [Google Scholar] [CrossRef]
- Rajashekarappa, K.K.; Mahadevan, G.D.; Neelagund, S.E.; Sathynarayana, M.; Vijaya, D.; Mulla, S.I. Decolorization of amaranth RI and fast red E azo dyes by thermophilic Geobacillus thermoleovorans KNG 112. J. Chem. Technol. Biotechnol. 2022, 97, 482–489. [Google Scholar] [CrossRef]
- Halilčević, D.; Dautović, E.; Lelić, M.; Husejnović, M.Š.; Smajlović, A.; Srabović, N.; Softić, A. Cytotoxicity and Genotoxicity of Sunset Yellow and Potassium Sorbate in Jurkat Cell Line. Int. J. Biochem. Res. Rev. 2022, 31, 1–9. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, P.; Dhiman, S.K.; Chadha, P.; Saini, H.S. Biochemical, genotoxic, histological and ultrastructural effects on liver and gills of fresh water fish Channa punctatus exposed to textile industry intermediate 2 ABS. Chemosphere 2022, 287, 132103. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Yin, S.; Zhou, C.; Ren, D.; Sun, C. Inedible azo dyes and their analytical methods in foodstuffs and beverages. J. AOAC Int. 2018, 101, 1314–1327. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.A.; Miller, E.C. The carcinogenicity of certain derivatives of p-dimethylaminozobenz in the rat. J. Exp. Med. 1948, 87, 139–156. [Google Scholar] [CrossRef]
- Chung, K.-T.; Cerniglia, C.E. Mutagenicity of azo dyes: Structure-activity relationships. Mutat. Res. Rev. Genet. Toxicol. 1992, 277, 201–220. [Google Scholar] [CrossRef]
- da Cruz Brambilla, C.M.C.; Garcia, A.L.H.; da Silva, F.R.; Taffarel, S.R.; Grivicich, I.; Picada, J.N.; Scotti, A.; Dalberto, D.; Mišík, M.; Knasmüller, S. Amido Black 10B a widely used azo dye causes DNA damage in pro-and eukaryotic indicator cells. Chemosphere 2019, 217, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.; Botasso-Nasciutti, M.O.; Svio, A.L.V.; Souza, L.d.C.M.; Fernandes-Cal, J.R.; Cardoso, F.F.; Fontes, M.R.d.M.; Albuquerque, A.F.; Munari, C.C.; Kummrow, F. In Vivo genotoxicity of a commercial CI Disperse Red 1 dye. Environ. Mol. Mutagenesis 2018, 59, 822–828. [Google Scholar] [CrossRef]
- Oliveira, G.; Ferraz, E.; Chequer, F.; Grando, M.; Angeli, J.; Tsuboy, M.; Marcarini, J.; Mantovani, M.; Osugi, M.E.; Lizier, T.M. Chlorination treatment of aqueous samples reduces, but does not eliminate, the mutagenic effect of the azo dyes Disperse Red 1, Disperse Red 13 and Disperse Orange 1. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2010, 703, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.S.; Mahmood, S.; Anwer, S. Genotoxicity assessment of amaranth and allura red using Saccharomyces cerevisiae. Bull. Environ. Contam. Toxicol. 2013, 90, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Tafurt-Cardona, Y.; Suares-Rocha, P.; Fernandes, T.C.C.; Marin-Morales, M.A. Cytotoxic and genotoxic effects of two hair dyes used in the formulation of black color. Food Chem. Toxicol. 2015, 86, 9–15. [Google Scholar] [CrossRef]
- Wang, Q.-X.; Zhang, X.; Ni, J.-C.; Shi, J.-L.; Gao, F.; Chen, G.-L.; Gao, F. Binding mode and photo-cleavage of an azo dye, acid chrome blue K, to double-stranded DNA. J. Solut. Chem. 2012, 41, 1185–1196. [Google Scholar] [CrossRef]
- Wiesmüller, L.; Ford, J.M.; Schiestl, R.H. DNA damage, repair, and diseases. J. Biomed. Biotechnol. 2002, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Maluf, S.W.; Martínez-López, W.; da Silva, J. DNA Damage: Health and Longevity; Hindawi: London, UK, 2018; Volume 2018. [Google Scholar]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The structure and function of DNA. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. [Google Scholar]
- Watson, J.D.; Crick, F.H. The Structure of DNA, Cold Spring Harbor Symposia on Quantitative Biology; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1953; pp. 123–131. [Google Scholar]
- Zimmerman, S.B. The three-dimensional structure of DNA. Annu. Rev. Biochem. 1982, 51, 395–427. [Google Scholar] [CrossRef]
- Ricci, C.G.; Netz, P.A. Docking studies on DNA-ligand interactions: Building and application of a protocol to identify the binding mode. J. Chem. Inf. Modeling 2009, 49, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.J. Supramolecular DNA recognition. Chem. Soc. Rev. 2007, 36, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Winston, C.T.; Boger, D.L. Sequence-selective DNA recognition: Natural products and nature’s lessons. Chem. Biol. 2004, 11, 1607–1617. [Google Scholar]
- Waring, M.J.; Bailly, C. DNA recognition by intercalators and hybrid molecules. J. Mol. Recognit. 1994, 7, 109–122. [Google Scholar] [CrossRef]
- Guo, Y.; Yue, Q.; Gao, B. Molecular docking study investigating the possible mode of binding of CI Acid Red 73 with DNA. Int. J. Biol. Macromol. 2011, 49, 55–61. [Google Scholar] [CrossRef]
- Liman, R.; Ali, M.M.; Ciğerci, İ.H.; İstifli, E.S.; Sarıkurkcu, C. Cytotoxic and genotoxic evaluation of copper oxychloride through Allium test and molecular docking studies. Environ. Sci. Pollut. Res. 2021, 28, 44998–45008. [Google Scholar] [CrossRef]
- Kurt, D.; Acar, A.; Çavuşoğlu, D.; Yalçin, E.; Çavuşoğlu, K. Genotoxic effects and molecular docking of 1, 4-dioxane: Combined protective effects of trans-resveratrol. Environ. Sci. Pollut. Res. 2021, 28, 54922–54935. [Google Scholar] [CrossRef]
- Snyder, R.D.; Holt, P.A.; Maguire, J.M.; Trent, J.O. Prediction of noncovalent Drug/DNA interaction using computational docking models: Studies with over 1350 launched drugs. Environ. Mol. Mutagen. 2013, 54, 668–681. [Google Scholar] [CrossRef]
- Wiedemann, P.M.; Schütz, H. The Role of Evidence in Risk Characterization: Making Sense of Conflicting Data; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Topaktas, M.; Kafkas, N.; Sadighazadi, S.; Istifli, E. In vitro cytogenetic toxicity of bezafibrate in human peripheral blood lymphocytes. Cytotechnology 2017, 69, 579–589. [Google Scholar] [CrossRef]
- Istifli, E.S.; Çelik, R.; Hüsunet, M.T.; Çetinel, N.; Demirhan, O.; Ila, H.B. cytogenotoxic evaluation of sertraline. Interdiscip. Toxicol. 2018, 11, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Bender, B.J.; Gahbauer, S.; Luttens, A.; Lyu, J.; Webb, C.M.; Stein, R.M.; Fink, E.A.; Balius, T.E.; Carlsson, J.; Irwin, J.J. A practical guide to large-scale docking. Nat. Protoc. 2021, 16, 4799–4832. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, J.; Zhang, Y. Probing the characterization of the interaction of aflatoxins B1 and G1 with calf thymus DNA in vitro. Toxins 2017, 9, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, A. The genetic toxicology of paracetamol and aspirin: A review. Mutat. Res. Rev. Genet. Toxicol. 1993, 296, 199–210. [Google Scholar] [CrossRef]
- Watanabe, M. The cytogenetic effects of aspirin and acetaminophen on in vitro human lymphocytes. Nippon. Eiseigaku Zasshi 1982, 37, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Husain, M.A.; Rehman, S.U.; Ishqi, H.M.; Sarwar, T.; Tabish, M. Spectroscopic and molecular docking evidence of aspirin and diflunisal binding to DNA: A comparative study. RSC Adv. 2015, 5, 64335–64345. [Google Scholar] [CrossRef]
- Loechler, E.L.; Teeter, M.M.; Whitlow, M.D. Mapping the binding site of aflatoxin B1 in DNA: Molecular modeling of the binding sites for the N (7)-guanine adduct of aflatoxin B1 in different DNA sequences. J. Biomol. Struct. Dyn. 1988, 5, 1237–1257. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Lewis-Atwell, T.; Townsend, P.A.; Grayson, M.N. Comparisons of different force fields in conformational analysis and searching of organic molecules: A review. Tetrahedron 2021, 79, 131865. [Google Scholar] [CrossRef]
- Vela, S.; Fabrizio, A.; Briling, K.R.; Corminboeuf, C. Learning the Exciton Properties of Azo-dyes. J. Phys. Chem. Lett. 2021, 12, 5957–5962. [Google Scholar] [CrossRef]
- Brüschweiler, B.J.; Merlot, C. Azo dyes in clothing textiles can be cleaved into a series of mutagenic aromatic amines which are not regulated yet. Regul. Toxicol. Pharmacol. 2017, 88, 214–226. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and Python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, S.; Matsusaka, N.; Madarame, H.; Ueno, S.; Susa, N.; Ishida, K.; Kawamura, N.; Sekihashi, K.; Sasaki, Y.F. The comet assay in eight mouse organs: Results with 24 azo compounds. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2000, 465, 11–26. [Google Scholar] [CrossRef]
- Levine, W.G. Metabolism of azo dyes: Implication for detoxication and activation. Drug Metab. Rev. 1991, 23, 253–309. [Google Scholar] [CrossRef]
- Stiborová, M.; Frei, E.; Schmeiser, H.H.; Wiessler, M.; Hradec, J. Formation and 32P-postlabeling of DNA and tRNA adducts derived from peroxidative activation of carcinogenic azo dye N, N-dimethyl-4-aminoazobenzene. Carcinogenesis 1992, 13, 1657–1662. [Google Scholar] [CrossRef]
- Stiborová, M.; Asfaw, B.; Frei, E. Peroxidase-activated carcinogenic azo dye Sudan I (Solvent Yellow 14) binds to guanosine in transfer ribonucleic acid. Gen. Physiol. Biophys. 1995, 14, 39. [Google Scholar]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef]
- de Aragão Umbuzeiro, G.; Freeman, H.; Warren, S.H.; Kummrow, F.; Claxton, L.D. Mutagenicity evaluation of the commercial product CI Disperse Blue 291 using different protocols of the Salmonella assay. Food Chem. Toxicol. 2005, 43, 49–56. [Google Scholar] [CrossRef]
- Uliana, C.V.; Garbellini, G.S.; Yamanaka, H. Evaluation of the interactions of DNA with the textile dyes Disperse Orange 1 and Disperse Red 1 and their electrolysis products using an electrochemical biosensor. Sens. Actuators B Chem. 2013, 178, 627–635. [Google Scholar] [CrossRef]
- Weisburger, J.H. A perspective on the history and significance of carcinogenic and mutagenic N-substituted aryl compounds in human health. Mutat. Res. 1997, 376, 261–266. [Google Scholar] [CrossRef]
- Stallings, R.; Ford, A.; Nelson, D.; Torney, D.; Hildebrand, C.; Moyzis, R. Evolution and distribution of (GT) n repetitive sequences in mammalian genomes. Genomics 1991, 10, 807–815. [Google Scholar] [CrossRef]
- Kuwahara, J.; Sugiura, Y. Sequence-specific recognition and cleavage of DNA by metallobleomycin: Minor groove binding and possible interaction mode. Proc. Natl. Acad. Sci. 1988, 85, 2459–2463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, D.S.; Garrard, W.T. The ubiquitous potential Z-forming sequence of eucaryotes,(dT-dG) n.(dC-dA) n, is not detectable in the genomes of eubacteria, archaebacteria, or mitochondria. Mol. Cell. Biol. 1986, 6, 3010–3013. [Google Scholar] [PubMed] [Green Version]
- Umezawa, Y.; Nishio, M. Thymine-methyl/π interaction implicated in the sequence-dependent deformability of DNA. Nucleic Acids Res. 2002, 30, 2183–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ooka, M.; Kobayashi, K.; Abe, T.; Akiyama, K.; Hada, M.; Takeda, S.; Hirota, K. Determination of genotoxic potential by comparison of structurally related azo dyes using DNA repair-deficient DT40 mutant panels. Chemosphere 2016, 164, 106–112. [Google Scholar] [CrossRef]
- Basu, A.; Suresh Kumar, G. Minor groove binding of the food colorant carmoisine to DNA: Spectroscopic and calorimetric characterization studies. J. Agric. Food Chem. 2014, 62, 317–326. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Chung, K.-T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C 2016, 34, 233–261. [Google Scholar] [CrossRef]
- Moosvi, S.; Kher, X.; Madamwar, D. Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dye. Pigment. 2007, 74, 723–729. [Google Scholar] [CrossRef]
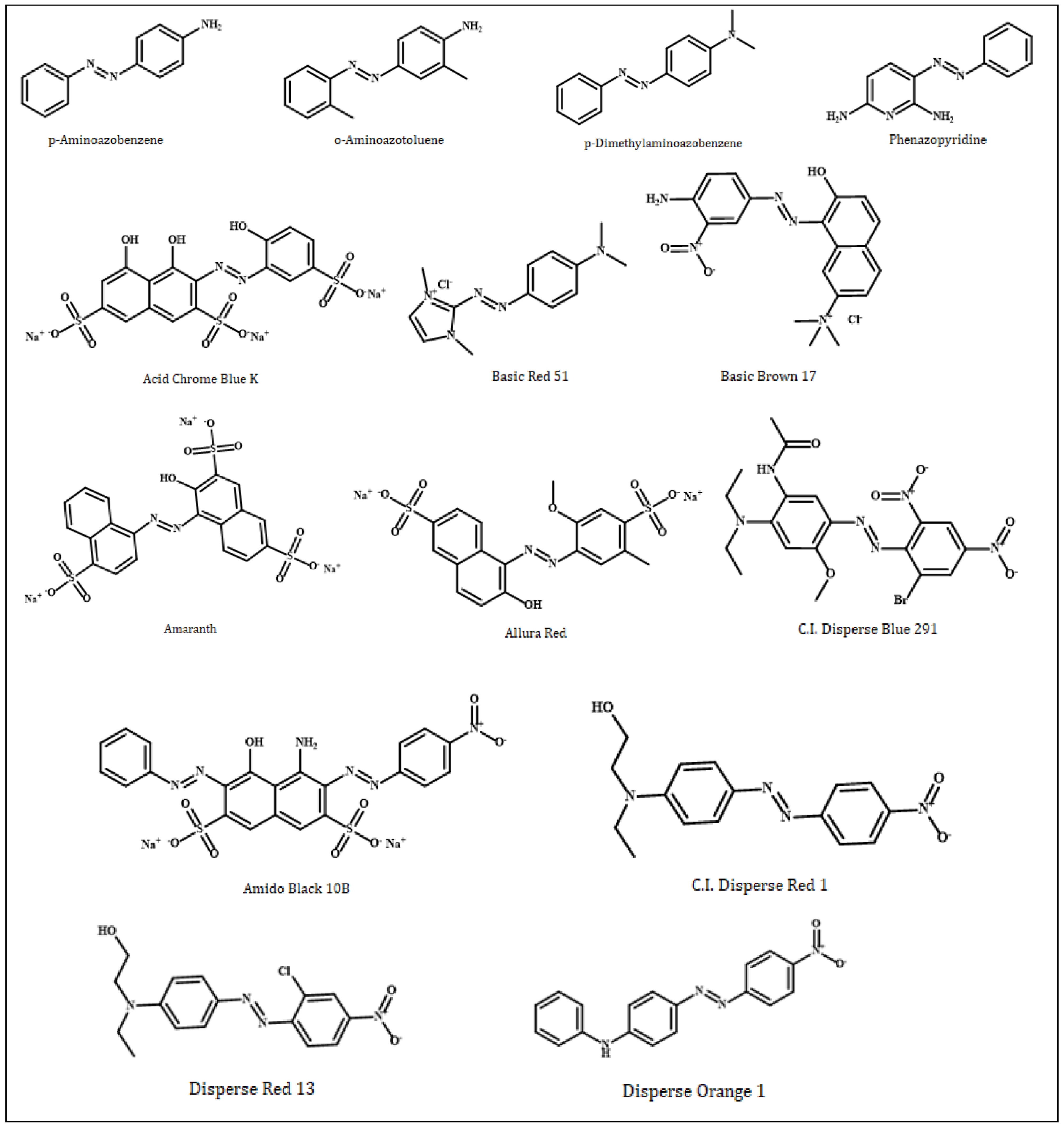




| No | Compound | PubChem CID | Molecular Weight (g/mol) | Molecular Formula |
|---|---|---|---|---|
| Genotoxic azo dyes | ||||
| 1 | p-Aminoazobenzene | 6051 | 197.24 | C12H11N3 |
| 2 | o-Aminoazotoluene | 7340 | 225.29 | C14H15N3 |
| 3 | p-Dimethylaminoazobenzene | 6053 | 225.29 | C14H15N3 or C6H5N=NC6H4N(CH3)2 |
| 4 | Phenazopyridine | 4756 | 213.24 | C11H11N5 |
| 5 | Acid Chrome Blue K | 135659037 | 586.4 | C16H9N2Na3O12S3 |
| 6 | Basic Red 51 | 166491 | 279.77 | C13H18ClN5 |
| 7 | Basic Brown 17 | 135515517 | 401.8 | C19H20ClN5O3 |
| 8 | Amaranth | 13506 | 604.5 | C20H11N2O10S3.3Na or C20H11N2Na3O10S3 |
| 9 | Allura Red | 33258 | 496.4 | C18H14N2Na2O8S2 |
| 10 | C.I. Disperse Blue 291 | 92446 | 509.3 | C19H21BrN6O6 |
| 11 | Amido Black 10B | 135442942 | 616.5 | C22H14N6Na2O9S2 |
| 12 | C.I. Disperse Red 1 | 17886 | 314.34 | C16H18N4O3 |
| 13 | Disperse Orange 1 | 17414 | 318.3 | C18H14N4O2 |
| 14 | Disperse Red 13 | 18516 | 348.78 | C16H17ClN4O3 |
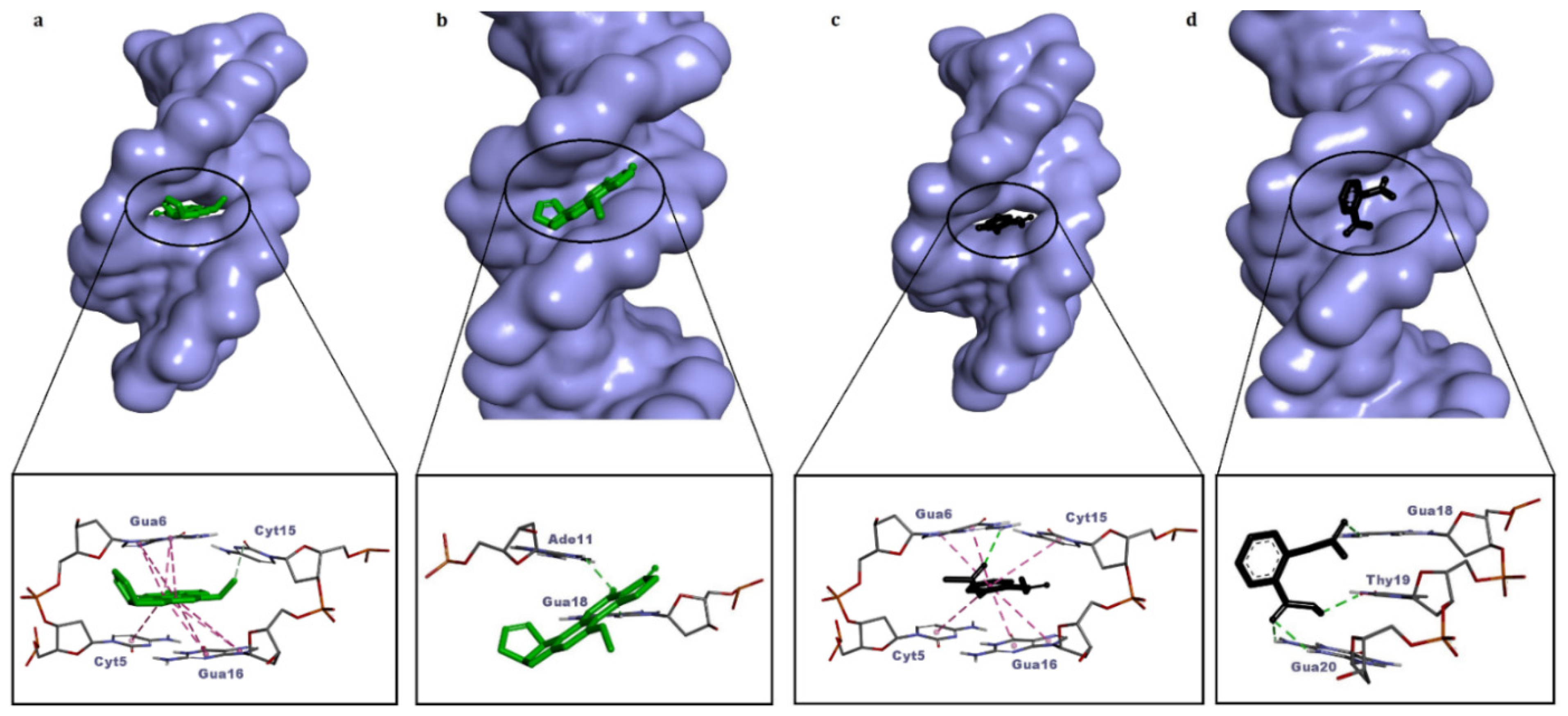
| Dye (Ligand) | BFE (kcal/mol) | Binding Mode | Intermolecular Interactions |
|---|---|---|---|
| AFB1 (positive control) | −8.64 | Intercalation | 1 carbon–hydrogen bond (Cyt15), 9 pi–pi stacked (Cyt5, Gua6, Gua16) |
| −8.12 | Minor groove | 3 H bonds (Ade11, Gua18) | |
| Aspirin (negative control) | −6.10 | Intercalation | 1 H bond (Gua6), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −5.77 | Minor groove | 4 H bonds (Gua18, Thy19, Gua20) | |
| p-Aminoazobenzene | −6.49 | Intercalation | 2 H bonds (Gua6), 6 pi–pi stacked (Cyt5, Gua6, Gua16, Cyt15) |
| −6.45 | Minor groove | 3 H bonds (Gua12, Cyt16, Thy17), 1 pi–donor H bond (Gua12) | |
| o-Aminoazotoluene | −6.92 | Threading intercalation | 1 H bond (Gua16), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 2 pi–pi T-shaped (Gua6, Ade7), 5 pi–alkyl (Cyt5, Gua6, Cyt15, Gua16) |
| −6.73 | Minor groove | 2 H bonds (Gua12, Thy17) | |
| p-Dimethylaminoazobenzene | −6.50 | Threading intercalation | 1 carbon–hydrogen bond (Gua16), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 1 pi–pi T-shaped (Gua6) |
| −6.66 | Minor groove | 3 H bonds (Ade11, Gua18, Thy19), 1 carbon–hydrogen bond (Ade11) | |
| Phenazopyridine | −6.69 | Intercalation | 2 H bonds (Cyt15, Gua16), 1 pi–donor H bond (Gua16), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −6.94 | Minor groove | 4 H bonds (Gua18, Thy19, Gua20), 1 carbon–hydrogen bond (Ade11) | |
| Acid Chrome Blue K | −8.71 | Threading intercalation | 4 H bonds (Cyt5, Gua6, Ade7, Cyt15), 1 carbon–hydrogen bond (Cyt15), 11 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −9.36 | Minor groove | 6 H bonds (Ade9, Cyt10, Ade13, Gua18, Gua20), 3 carbon–hydrogen bonds (Gua12, Thy19, Gua20), 1 pi–sulfur (Ade9) | |
| Basic Red 51 | −6.94 | Threading intercalation | 2 carbon–hydrogen bonds (Ade7, Gua16), 1 pi–donor hydrogen bond (Gua6), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −6.35 | Minor groove | 1 H bond (Gua12), 1 carbon–hydrogen bond (Cyt16) | |
| Basic Brown 17 | −8.50 | Threading intercalation | 2 H bonds (Ade7, Cyt15), 2 carbon–hydrogen bonds (Gua6), 1 pi–donor hydrogen bond (Gua6), 9 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −8.79 | Minor groove | 1 attractive charge (Thy19), 6 H bonds (Ade11, Ade13, Cyt16, Thy17, Gua18), 2 carbon–hydrogen bonds (Gua18) | |
| Amaranth | −8.74 | Threading intercalation | 4 H bonds (Gua6, Cyt15, Gua16), 1 pi–sulfur (Ade7), 11 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 1 pi–pi T-shaped (Ade7) |
| −9.42 | Minor groove | 5 H bonds (Ade9, Gua12, Thy17, Gua20, Gua21), 1 carbon–hydrogen bond (Gua12), 1 pi–donor hydrogen bond (Ade11), 1 pi–sulfur (Gua20) | |
| Allura Red | −8.19 | Intercalation | 1 H bond (Cyt15), 1 carbon–hydrogen bond (Gua16), 1 pi–donor hydrogen bond (Gua6), 10 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 2 pi–alkyl (Cyt5, Gua6) |
| −8.80 | Minor groove | 5 H bonds (Ade9, Ade11, Gua12, Gua20, Gua21), 1 carbon–hydrogen bond (Gua12), 1 pi–sulfur (Gua20) | |
| C.I. Disperse Blue 291 | −7.39 | Threading intercalation | 2 H bonds (Gua6, Cyt15), 3 carbon–hydrogen bond (Cyt15, Ade17), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 3 pi–alkyl (Cyt15, Gua16) |
| −6.90 | Major groove | 4 H bonds (Ade13, Cyt14), 1 pi–pi T-shaped (Gua14), 1 Pi–alkyl (Gua14) | |
| Amido Black 10B | −8.30 | Threading intercalation | 4 H bonds (Cyt15, Gua16), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −9.23 | Minor groove | 7 H bonds (Ade5, Thy22, Thy23, Gua24), 2 pi–pi T-shaped (Gua21, Gua24) | |
| C.I. Disperse Red 1 | −6.94 | Threading intercalation | 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −7.67 | Minor groove | 3 H bonds (Ade9, Gua20), 1 pi–donor hydrogen bond (Ade11) | |
| Disperse Orange 1 | −8.18 | Threading intercalation | 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16) |
| −8.23 | Minor groove | 3 H bonds (Ade9, Gua20) | |
| Disperse Red 13 | −7.27 | Threading intercalation | 1 pi–donor hydrogen bond (Gua6), 1 pi–sigma (Gua16), 6 pi–pi stacked (Cyt5, Gua6, Cyt15, Gua16), 2 pi–alkyl (Cyt15, Gua16) |
| −7.68 | Minor groove | 4 H bonds (Ade9, Gua12, Gua20) |
| Dye (Ligand) | Most Favorable Binding Mode | BFE (kcal/mol) | dsDNA Sequence Selectivity |
|---|---|---|---|
| P-aminoazobenzene | Intercalation | −6.49 |  |
| O-aminoazotoluene | Threading intercalation | −6.92 |  |
| P-dimethylaminoazobenzene | Minor groove | −6.66 |  |
| Phenazopyridine | Minor groove | −6.94 |  |
| Acid Chrome Blue K | Minor groove | −9.36 |  |
| Basic Red 51 | Threading intercalation | −6.94 |  |
| Basic Brown 17 | Minor groove | −8.79 |  |
| Amaranth | Minor groove | −9.42 |  |
| Allura Red | Minor groove | −8.80 |  |
| C.I. Disperse Blue 291 | Threading intercalation | −7.39 |  |
| Amido Black 10B | Minor groove | −9.23 | 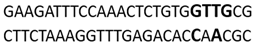 |
| C.I. Disperse Red 1 | Minor groove | −7.67 | 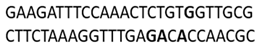 |
| Disperse Orange 1 | Minor groove | −8.23 |  |
| Disperse Red 13 | Minor groove | −7.68 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
İstifli, E.S. Preliminary in Silico Studies of the Interactions of Certain Genotoxic Azo Dyes with Different Double-Stranded DNA Conformations. Colorants 2022, 1, 236-255. https://doi.org/10.3390/colorants1020015
İstifli ES. Preliminary in Silico Studies of the Interactions of Certain Genotoxic Azo Dyes with Different Double-Stranded DNA Conformations. Colorants. 2022; 1(2):236-255. https://doi.org/10.3390/colorants1020015
Chicago/Turabian Styleİstifli, Erman Salih. 2022. "Preliminary in Silico Studies of the Interactions of Certain Genotoxic Azo Dyes with Different Double-Stranded DNA Conformations" Colorants 1, no. 2: 236-255. https://doi.org/10.3390/colorants1020015
APA Styleİstifli, E. S. (2022). Preliminary in Silico Studies of the Interactions of Certain Genotoxic Azo Dyes with Different Double-Stranded DNA Conformations. Colorants, 1(2), 236-255. https://doi.org/10.3390/colorants1020015






