1. Introduction
PVC is one of the most common and cheapest synthetic polymers that can be found on the market. The history of PVC started during early 1870 when the first polymer was obtained by the polymerization of vinyl chloride. However, the material obtained from the polymerization of vinyl chloride was stiff and brittle. Hence, it was not ideal for industrial production. By 1926, American chemists discovered how to plasticize PVC and, since then, many PVC based products have been commercialized [
1,
2]. Nowadays, its demand is only lower than those of polyethylene and polypropylene, which are the materials with a higher demand on the market. In 2018, the global demand for PVC was close to 44.3 million tons and is expected to increase to nearly 60 million tons by 2025 [
3]. PVC is used in many different fields, such as in the construction industry (pipes, windows, carpet, plumbing, etc.), electrical and electronic industry (instrument components, housing, sheaths for cables and wires, etc.), automotive industry, food packaging, medical equipment, and many others [
4].
With the fast development of science and technologies, it is necessary to search for new materials with superior properties. In recent decades, nanomaterials have attracted the attention of many researchers due to their properties and applications [
5]. In addition, polymer nanofibers are materials with many advantages and easy fabrication [
6]. Compared to many known materials of the present time, polymer nanofibers have characteristics such as high aspect ratio (length to diameter), small pore size, large surface area, high porosity, high hydrophobic surface, optical transparency, and high mechanical properties (tensile strength, elastic modulus, stiffness, toughness) that make them more suitable in comparison with their macro-scale versions. Various fabrication techniques have been used to produce polymer nanofibers, such as drawing [
7,
8], template synthesis [
9,
10], self-assembly [
11,
12], melt-blown [
13], phase separation [
14], and electrospinning [
15,
16]. Among them, electrospinning is the most efficient method that can produce nanofibers from a variety of polymers. Due to nanofibers morphological characteristics, they exhibit numerous advantages, which made them suitable for their application in various fields such as air filtration systems [
17,
18,
19,
20], sensing devices [
21,
22], reinforcement composite [
23], tissue engineering [
24], energy storage systems [
25], optical [
23], drug delivery [
26], catalyst [
27], water treatment [
28], and so forth.
The development of PVC nanofibers has increasingly attracted the attention of science and industry, thanks to PVC popularity and inexpensiveness [
29]. PVC nanofibers present remarkable characteristics such as small diameter, high porosity, hydrophobicity, and a remarkable affinity for oil. Due to its excellent properties, previously mentioned, PVC nanofibers have expanded the area of application of PVC materials. PVC nanofibers have been used as a material for environmental applications (water filter, air filter, separator for water and oil), energy storage systems, anti-corrosive material, protective clothing, and so on [
30,
31,
32].
This review paper is an overview of the PVC nanofibers fabrication process and the influencing factors of the electrospinning parameters of the morphology of the nanofibers. In addition, the influence that different types of solvent used to dissolve PVC have on the nanofibers’ fabrication is going to be analyzed. Moreover, all technical parameters used for the electrospinning process are going to be specified, such as voltage, injection speed, and nozzle–collector distance. Our article describes the mechanical, morphological, and chemical properties of PVC at a nano-scale dimension. Additionally, we present the applications that electrospun PVC nanofibers have in different fields, such as environment treatment, reinforcement composite, energy storage, and many others. Finally, we propose the prospects and development of materials based on PVC nanofiber for further applications.
2. Fabrication of PVC Nanofibers
2.1. Electrospinning Process
Electrospinning is a process to obtain polymer microfibers or nanofibers from solutions under the influence of the electrostatic force generated by high voltage. Before electrospinning, the polymer powder is dissolved in a solvent, and the homogeneous solution is collected into a syringe. The metallic needle is connected at the tip of the syringe with a controlled diameter. The flow rate of the solution is controlled by a syringe pump. A metallic collector (static plate, rotating drum, parallel electrode, or rotating disk) is set at a distance between 10 and 30 cm from the tip to the needle. Under the influence of a high voltage (from 5 to 50 kV), the electromagnetic forces will pull the polymer solution from the needle to the collector [
33], generating charged jets of the solution, also known as a Taylor cone, which will be formed at the tip of the needle and fabricated fiber on the collector surface [
34]. The Taylor cone is formed when the polymer jet is affected by a high voltage. The Taylor cone formation determines the stability of the nanofiber fabrication process. As the voltage increases, the electric force will eliminate the surface tension of the droplet, and the polymer droplet will be ejected as a jet to the grounded target that acts as a collector. Under the influence of the electromagnetic force, the polymer jet leads to the fabrication of ultrathin fibers. In this process, the solvent evaporates and forms a nanofiber polymer mat on the collector surface [
35].
Figure 1 shows a diagram of an electrospinning process at a laboratory scale.
Several parameters can affect the properties, structure, and morphology of nanofibers. These include parameters of the polymer (molecular weight, glass transition temperature of the polymer, solubility), parameters of the polymer solution (concentration of the solution, viscosity, conductivity, surface tension), technical parameters of the electrospinning process (applied voltage, the flow rate of the solution, the distance between the collector and needle, needle diameter, type of collector), and environmental parameters (temperature, humidity, pressure) [
36,
37]. These factors show the complex influence on the morphology and properties of nanofibers obtained for each different polymer. For example, the increase of applied voltage leads to the decrease [
38,
39], or increase [
40,
41], or not change [
42] of the fiber diameter. On the other hand, the parameters mentioned above are often closely related, so when one parameter is changed, it is necessary to change the range of values of the other parameters to obtain high-quality nanofibers.
2.2. Solvents for PVC and Its Effects on the Electrospinning Process
One of the most important factors that can affect the production of nanofiber during the electrospinning process is the type of solvent used. Solvents directly affect surface tension, conductivity, and the dielectric constant of the polymer solution. Many studies show that solvents with higher conductivity and dielectric constant are more suitable to be used for the electrospinning process [
43]. Polymer solutions with a high dielectric constant tend to be more electrically sensitive when they are subjected to an electrostatic field. The dielectric constant is an essential factor determining the ability to fabricate nanofibers of the electrospinning process [
44]. PVC is well dissolved in a wide variety of solvents, such as tetrahydrofuran (THF), dimethyl formamide (DMF), cyclohexanone, methyl ethyl ketone (MEK), acetylacetone, xylene, dimethylacetamide (DMA) [
45]. Solvents like THF, DMF, and DMA are widely used for the preparation of polymer solutions, which are applied for the fabricating of PVC nanofibers. THF solvent has a low boiling point, meaning that it is quickly evaporated after transmitting and separating an unstable jet, which leads to the fabrication of fibers with defects [
29]. To solve this problem, it was demonstrated that adding DMF, which has a higher boiling point, to the THF solution will reduce the evaporation more slowly, and thereby fabricating the nanofiber is easier. However, to obtain fibers from PVC/ Polyvinylpyrrolidone (PVP) blends, the use of DMA solvents proved to be more effective [
30,
31]. The types of solvents that are used to dissolve PVC for electrospinning are shown in
Table 1. It has been proven that using a DMF:THF solvent in a 1:1 ratio showed the best results for the fabrication of fibers from PVC.
The properties of the polymer solution, such as the surface tension and the conductivity of the solution, have a strong influence on the formation of fibers. Lee et al. [
46] reported that the surface tension of the PVC solution, using THF:DMF solvent mixture (6:4 in volume), was 33 mN/m, and the electrical conductivity of polymer solutions was 0.11 mS/m. When using THF and DMF solvents, the conductivity value is neither too low nor too high, which made it suitable for the formation of the Taylor cone during PVC nanofiber fabrication.
It is important to mention that in order to high-quality electrospun fibers, the solution to use, the solution should be homogenous. As a result, when fabricating nanofibers from PVC blends, it is important to consider the use of polymers that are soluble in DMF, THF, and DMA. Some of these polymers are PVP [
30], polystyrene (PS) [
32], cellulose acetate (CA) [
47], polyurethane (PU) [
46], and others. In consequence, this narrows the range of applicable polymers that can be electrospun in conjunction with PVC.
2.3. Fabrication of PVC Nanofibers
Table 2 presents the studies conducted on PVC nanofibers fabrication and the applied condition of its electrospinning process. As mentioned above, firstly, PVC is dissolved in solvent until a homogeneous solution is obtained. Then, the polymer solution is poured into a syringe and place in the electrospinning machine, as shown in
Figure 1. Depending on the polymer’s molecular weight, its concentration in the solution will change; ergo, at higher molecular weight, the lowest the concentration of polymer in the solution. In most of the studies on PVC, the appropriate solution concentration is used in the range of 10 to 15% [
29,
31,
49,
50]. The appropriate voltage is in a range between 12 to 20 kV, but the addition of metals, such as silver, iron, and brass, requires a change in the voltage value to 70 kV [
53]. The lowest applicable voltage that has been reported was 8 kV [
29], and the highest applicable voltage reported was 70 kV [
53]. The flow rate for a PVC solution can vary from 0.12 mL/h to 1 mL/h. The distance range between the needle and the collector can go from 6 cm to 30 cm. It has been estimated that the electrospinning process for the spun of a nanofiber mat with thickness from 20 µm to 100 µm can go from a couple of hours to a full working day. An efficient way to remove solvents from the nanofiber mats is by placing the mats at room temperature and low relative humidity in order to let the solvent evaporates.
Medeiros et al. [
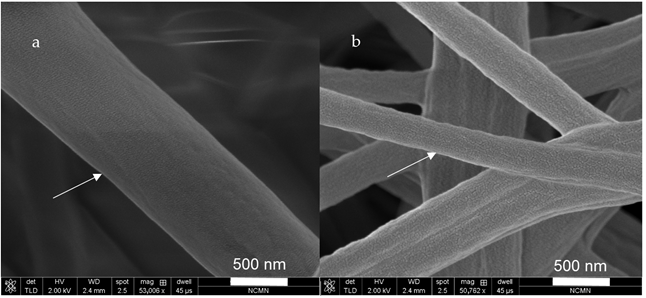
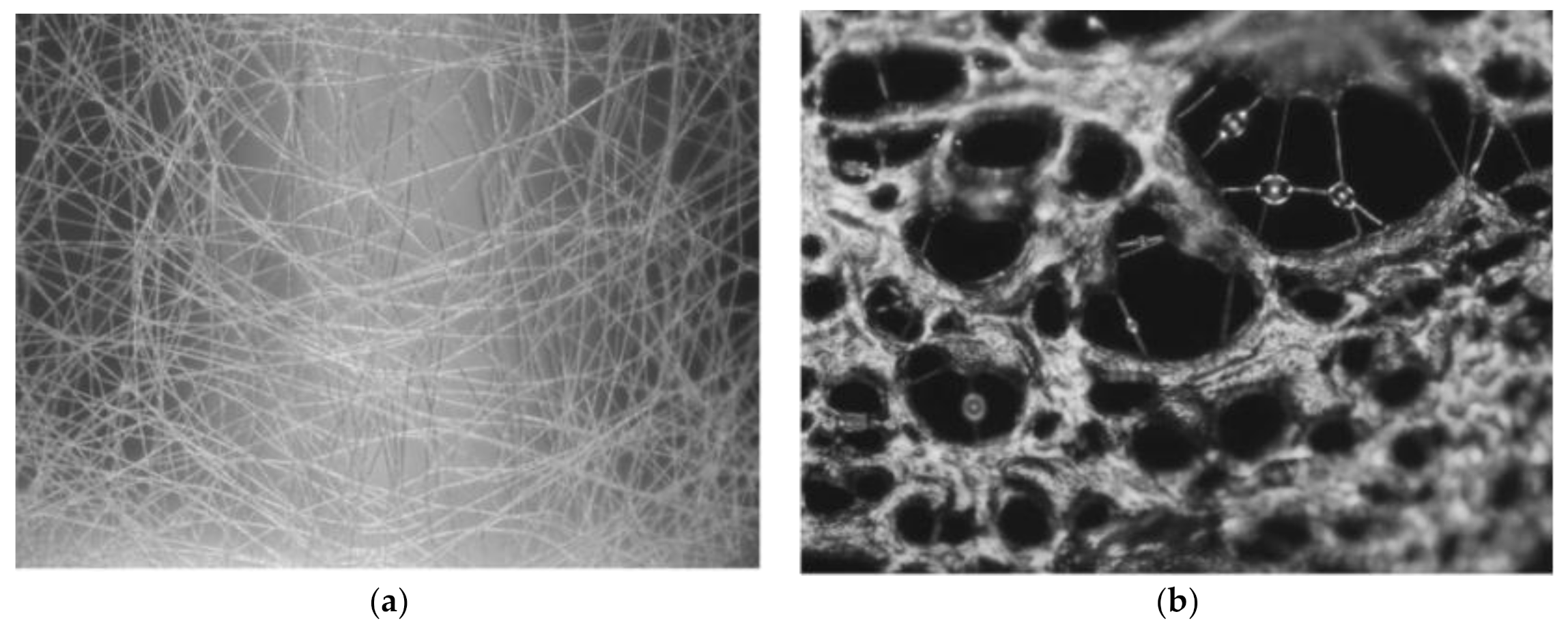
55] studied the influence of relative humidity (RH) on electrospun PVC nanofibers morphology. The electrospun PVC nanofibers were obtained at different relative humidity, varying from 20% RH to 80% RH. The results showed that when the humidity increases, the diameter of nanofibers also increased. In addition, it was reported that fibers showed a wider diameter when ambient humidity was at 60% RH. As well, results showed that the porosity of the fiber surfaces increased in accordance with a high RH. The formation of pores on the surface of the fiber can be explained as a result of the quick evaporation of the solvent and the dynamics of the phase separation. PVC is a hydrophobic polymer. When it is dissolved in solvents like THF and DMF, phase separation occurs between the solvent surface and the humid air. Therefore, when the solvent quickly evaporates from the surface of the nanofibers, it will form nanofiber with a porous structure.
Figure 2 shows the porous structure formed on the surface of the nanofibers, marked by the arrows.
3. Properties of Electrospun PVC Nanofibers
3.1. Fibers Diameter
The diameter of the nanofiber is an important factor when studying its morphology. It affects many properties of nanofiber mats, such as durability, porosity, as well as surface area. The diameter of the nanofiber depends greatly on the electrospinning conditions, such as the power supply (kV), the distance between the needle and collector, the injection speed of the solution, and the type of solvent. In order to study the morphology of the nanofibers, scanning electron microscopy (SEM) images are used, which allows a more deeply analysis of materials at nano-scale. When talking about fibers, SEM images will provide more detailed information about the fiber’s diameter and surface structure. Before any morphological studies are conducted by the use of SEM, it is common to coat with a thin layer of conductive material, like gold [
53], the nanofibers sample’s surface, mainly because almost all nanofibers presents low or non-conductive properties. The nanofibers collected from the surface of the collector have different diameters. The diameter is measured by calculating the average over a set of different nanofiber diameters samples. Lee et al. [
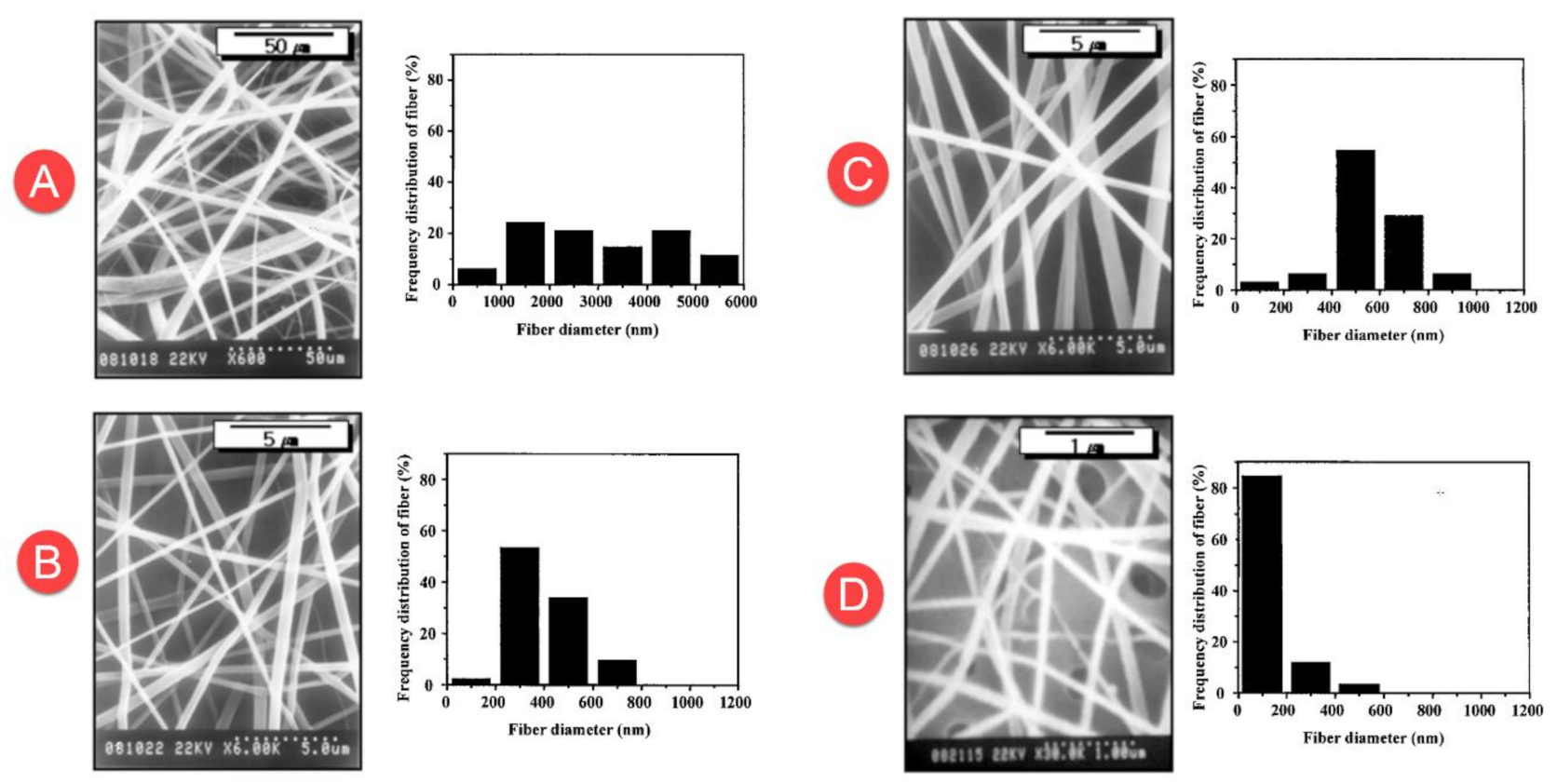
29] obtained PVC nanofibers with a diameter range between 500 nm to 6 μm by the use of THF solvent. On the other hand, the use of DMF solvent generated nanofibers with an average diameter of 200 nm (
Figure 3).
It has been demonstrated that the diameter of the nanofibers decreases from 350 nm to 270 nm when the applied voltage increases from 15 kV to 30 kV. The diameter of the nanofiber increases from 320 nm to 450 nm when increasing the feed rate of the solution from 0.3 mL/h to 1 mL/h. The diameter of the nanofiber did not show any change by modifying the distance between the nozzle and collector, but it decreases the defects on the nanofiber mats [
54]. Adding another polymer or a metal filler to PVC leads to a change in the diameter of the nanofibers. Elmessiry et al. [
47] added CA into PVC, and when increasing the CA content from 2% to 8%, the nanofiber diameter was gradually reduced. Moreover, adding Fe, Fe
3O
4 [
51,
62], Al, steel, brass [
53] into PVC also leads to a decrease in fibers diameter. However, Lee et al. [
46] have reported that increasing PU into PVC leads to an increase in the diameter of the nanofibers.
Table 3 presents the diameter of electrospun PVC nanofiber through numerous studies. It can be seen that the diameters of PVC nanofibers obtained from the studies are different and range from 50 nm to 6 µm. The different diameters are obtained due to the use of different precursors and different conditions of the electrospinning process.
3.2. Porosity of the Nanofiber Mat
The porosity of a material is defined as the content of air volume (void) compared to the total volume. The void in the nanofibers mat is understood as the total space between the nanofibers and the volume size of all pores on the surface of the nanofibers. High porosity materials are stunning candidates for many different applications, such as tissue engineering, catalysts, sensing devices, energy storage, oil sorbent, water treatment materials, air filtration systems, medical applications, solar cells [
67,
68]. Zhu et al. have reported that the porosity of the electrospun PVC nanofiber mat is about 99.7% [
32]. Hussain et al. [
48] obtained silver nanofibers by employing electrospun PVC fibers as a template. The process to obtain such silver nanofibers was, PVC fibers with diameters from 0.35 μm to 3.5 μm with pores sizes from 200 to 350 nm were fabricated. Silver was deposited on the PVC fibers by thermal evaporation. To obtain the silver nanofibers, the PVC fibers with silver were immersed and washed in THF to completely remove the polymer, leaving only the silver nanofibers.
3.3. Hydrophobicity
The hydrophobicity of nanofiber is understood as when the fiber is put into water, and the fiber will not absorb water. Hydrophobic phenomena can be observed in the wild, such as lotus leaves and in duck feathers. The hydrophobicity is determined by the angle of contact between the surface of the material, air, and water. PVC is a highly hydrophobic material due to the intrinsic properties of the chloride functional group [
69]. In
Table 4, it is possible to observe the contact angle that water has on PVC nanofibers surface. Analyzing this data, it is possible to conclude that PVC nanofibers present the characteristics of a hydrophobic material. This property is well-suited for making materials for waterproof and breathable applications, such as protective clothing. However, hydrophobic materials are not suitable for filtration. Therefore, hydrophilic material, e.g., PVP, polyacrylonitrile (PAN), and poly(ethylene glycol) (PEG), can be added to PVC in order to reduce the water contact angle, which means that its hydrophobicity will be reduced [
70,
71,
72]. It was found that when the number of particles in the nanofiber mat increased, it increased the roughness of the mat and eventually increased the water contact angle [
73].
3.4. Mechanical Properties
One of the important properties of nanofibers is their mechanical strength. Nanofiber mats with high strength will increase their applicability. Mechanical properties of the nanofiber mats are often inferior to textile fibers fabricate from the same polymer. The nanofiber mat, which is obtained from electrospinning, constitutes randomly distributed nanofibers. Therefore, it often has low mechanical properties. However, some reports have shown that when the diameter of nanofiber decreases to a critical value, the mechanical properties of nanofiber increase exponentially [
76,
77,
78]. The mechanical properties of a nanofiber mat depend on the orientation of the nanofibers, the orientation of the polymer chain, and the expansion of the polymer chain [
79]. Therefore, to improve nanofiber’s durability, it is possible by correcting the orientation of the fibers and polymer molecules. It is possible to obtain PVC nanofiber mats with high orientated nanofibers by using a rotating drum collector and increase its rotation speed. In our recent work [
78], it was shown that increasing the drum collector’s rotation speed leads to an increase in the orientation of nanofibers and enhances the mechanical properties of the nanofiber mats. The tensile strength increased from 2.2 ± 0.2 MPa to 9.1 ± 0.3 MPa, and Young’s modulus increased from 53 ± 14 to 308 ± 19 MPa. The increase in mechanical properties is due to the alignment of nanofibers in the mat, the decrease of fibers diameter, and the molecular orientation in the amorphous phase of polymer.
Lee et al. [
29] reported that the mechanical tensile strength of PVC nanofibers was around 1.8 MPa. Carrizales et al. [
57] reported that elastic modulus (Young’s modulus), tensile strength, elongation at break of were 12.3 MPa, 2.2 MPa, and 0.9 mm/mm, respectively. In our work [
80], the tensile strength obtained was 2.2 MPa, and the elastic modulus was 53.2 MPa, the elongation at break was 25.56%.
Table 5 presents the mechanical properties of PVC nanofiber mats from different works.
To improve the mechanical properties of PVC nanofiber mats, other polymers can be added. Lee et al. [
46] studied the mechanical properties of nanofiber mats from PVC blended with PU. The results showed that tensile strength and elongation at break increased as PU concentration increased from 0 to 100%, while elastic modulus reached the highest value when PVC/PU content was 50/50 (
Table 6). Namsaeng et al. [
65] reported that the tensile strength and Young’s modulus of electrospun PVC/PAN nanofiber mats were increased by 127% and 175%, respectively, when using 1 wt% multiwalled carbon nanotubes (MWCNTs) tensile strength and Young’s modulus, increased by 205% and 314%, respectively. Magdi et al. [
47] reported that adding small amounts of CA to PVC also represented significantly increased the durability of the nanofiber mats. Adding 8% CA led to an increase in PVC nanofibers mat breaking strength by 350%, breaking extension by 210%, first modulus by 164%, second modulus by 227%, and work of rupture by 753%.
In addition, Jin et al. [
82] reported a core-shell structure PVC/ Poly(3,4-ethylenedioxythiophene) (PEDOT) nanofiber mat. PVC nanofibers were fabricated by electrospinning process, then PEDOT was coated on the surface of PVC nanofibers by a fast in situ interfacial polymerization using FeCl
3 as an oxidant. Results showed that the strength of core-shell PVC/PEDOT nanofiber mats had a higher tensile strength of 5.6 MPa and 8.9 MPa, respectively, compared to 1.2 MPa of PVC nanofiber mats.
3.5. Thermal Properties
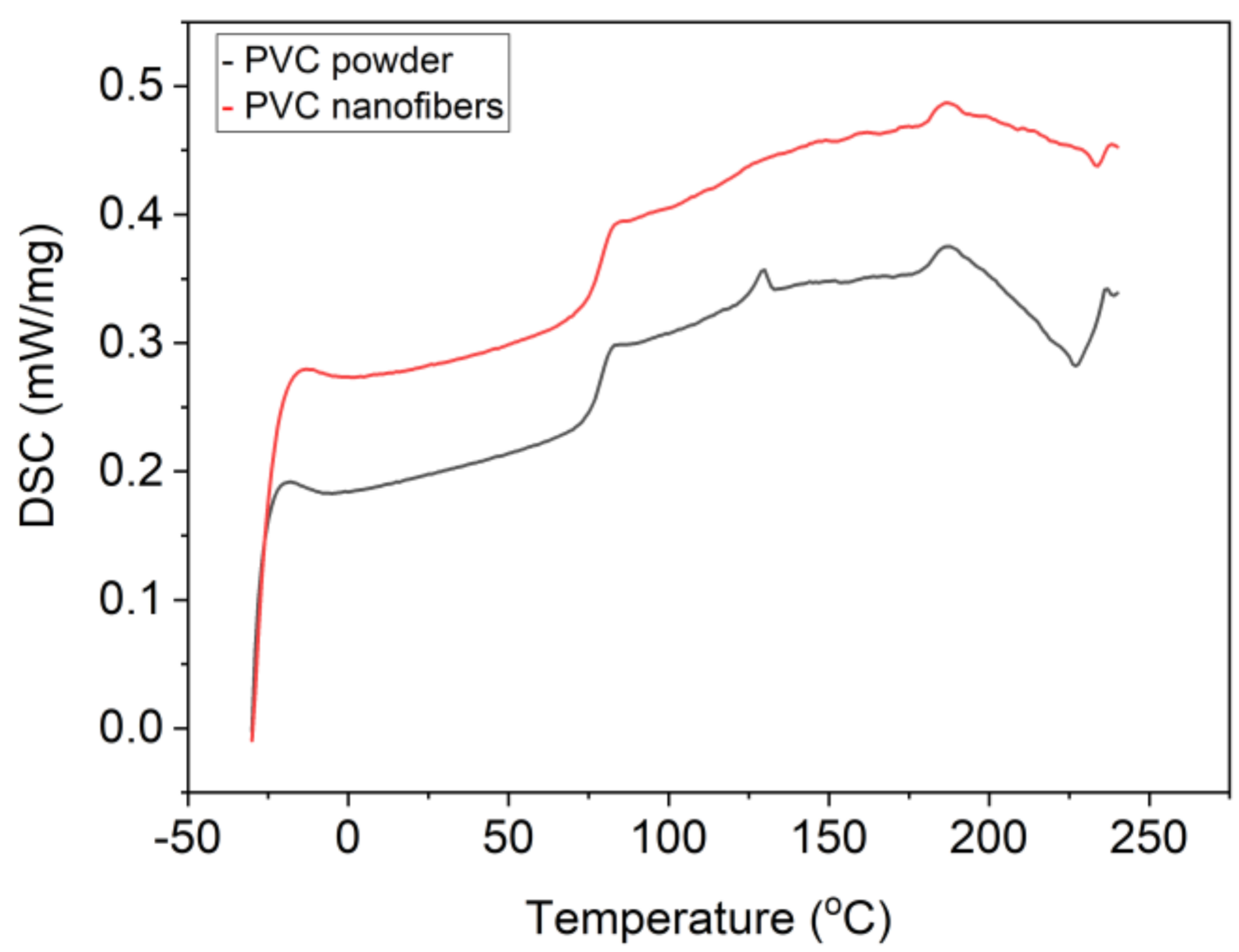
Fourier transform infrared spectroscopy (FI-IR) analysis shows that there is no structural change between PVC nanofiber and PVC powder. The characteristic peaks of PVC powder at 1328, 1253, 964, and 613 cm
−1 are found to be the same as PVC nanofiber [
64]. PVC is an amorphous polymer; hence DSC analysis results do not show a melting point. Glass transition temperatures of PVC nanofiber and PVC powder were shown to be similar at a temperature of 83 °C [
57,
83].
Figure 4 shows a differential scanning calorimetry (DSC) thermogram of PVC powder and PVC nanofibers.
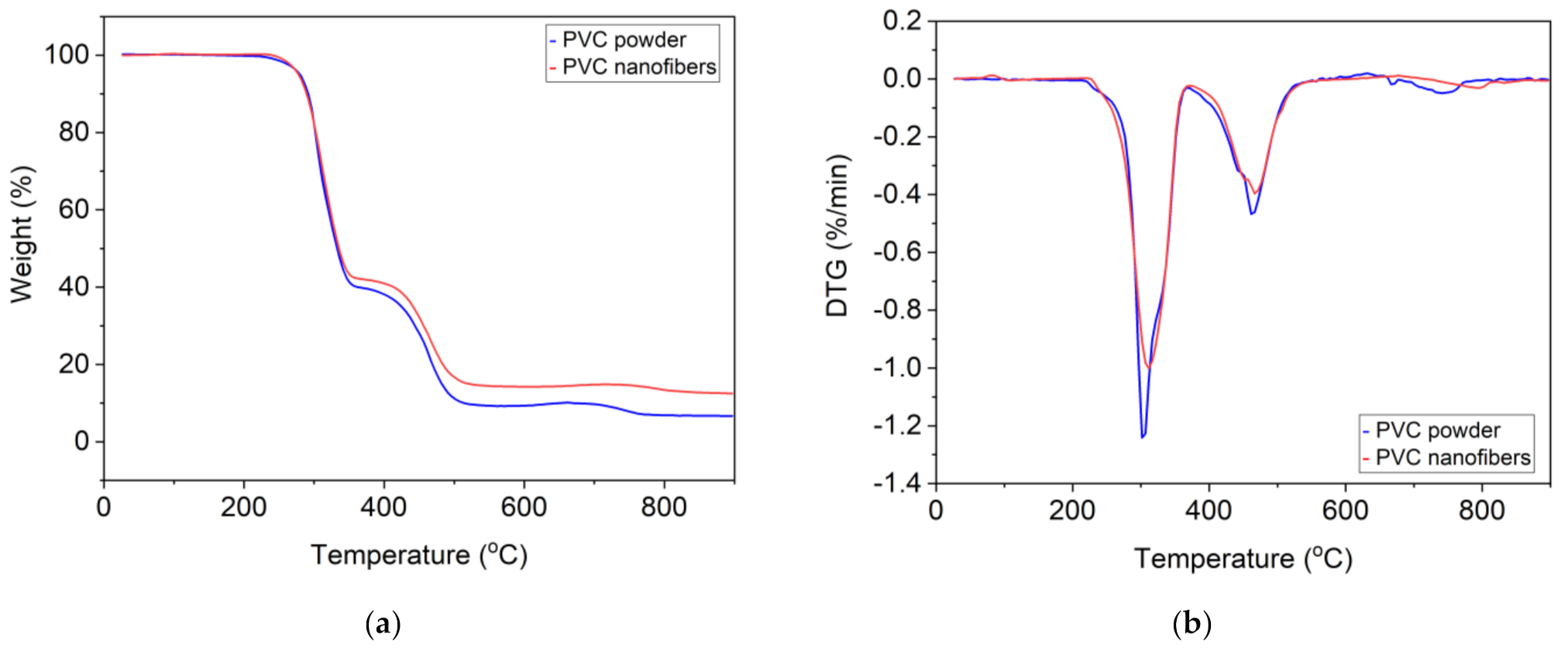
Carrizales et al. [
57] reported that PVC powder and PVC nanofiber presented three degradation steps, with the nanofibers retaining a little more mass during each step. PVC nanofibers started decomposition at 276 °C, 6 °C lower than PVC powder (282 °C). However, our recent work [
81] showed that the TGA curve for the initial PVC powder and the nanofibers is the same and has two thermal degradation stages. The first stage of thermal degradation starts at 233 °C and the second stage starts at 320 °C.
Figure 5 shows the thermogravimetric analysis (TGA) and differential thermal analysis (DTA) curves of PVC powder and PVC nanofibers. Phatcharasite et al. [
50] reported that the thermal properties of PVC nanofiber depend on the type of solvent used in electrospinning. When using THF, a high crystalline membrane with a high exothermal peak was obtained at a temperature of −30 °C. Taweepreda et al. [
49] reported that, when adding PU, epoxidized natural rubber (ENR) to PVC, the nanofibers exhibited higher thermal stability. In addition, when adding ENR to PVC, the resulting nanofibers became more flexible and were shown to have a lower glass transition temperature than nanofiber from pure PVC.
Zhang et al. [
84] studied the thermal conductivity of various vinyl polymers. They reported that the thermal conductivity of PVC nanofiber is lower than other nanofiber polymers, such as polyester (PE), polyvinyl alcohol (PVA), and poly(vinylidene fluoride) (PVDF). This is explained by the fact that vibrational energy can travel along the molecular chain more efficiently when side groups are either lighter or more symmetric.
4. Application of PVC Nanofiber
4.1. Water Filtration and Treatment
Because of pollution in freshwater sources all over the world, the problem of having a clean water supply has become more essential than ever. Industrial and agricultural activities are leading to water contamination by releasing heavy metals such as Pb, Cr, As, Hg, etc. [
85]. As a result, freshwater cannot be used without any filtration treatment [
86]. Materials for nanofiltration are being applied to obtain water with a high degree of purity. Electrospun nanofiber membranes present great potential for environmental applications. Based on some useful properties such as high surface area, high porosity (up to 80%) compared to conventional polymer membranes (5–35%), excellent functionalization ability, and good mechanical behavior, they are more effective when used for liquids separation and filtration [
87]. PVC is a material that is insoluble in water, bases, and acids. Thus, it has great potential for use as a filter material.
Asmatulu et al. [
31] have shown that PVC nanofiber membranes have a high hydrophobic level that interferes with their ability to be used as a filter media. To solve this problem, researchers added small amounts of PVP into the PVC solution before fabricating the fibers. Adding PVP into PVC results in a significant reduction in water contact angle (
Table 7) and accelerates the formation of the Wenzel state of the fibers membranes, which improved the filtration process [
88,
89]. The assessment of the filtration efficiency of different water samples (lake water, abrasive particles from a water jet cutter, a magnetite solution, and six sub-variants of these three water samples treated by the coagulants Alum and Tanfloc) was done by checking turbidity, pH, and total dissolved solids (TDS) indexes. The results showed that the membrane from PVC and 4% PVP exhibit good filtration ability. However, there was no report on the filtration efficiency of PVC fibers when adding PVP with different concentrations.
In another publication, Alarifi et al. [
30] showed that hydrophobic PVC nanofibers did not affect the membrane filtration capacity. Adding PVP to PVC does not change the water contact angle (
Table 8). They came to this conclusion by evaluating membrane filtration capacity for different water samples (dam water and city wastewater). To ensure the quality of water filtration, the turbidity of water must be measurable in order to know the values of purification. According to the evaluation results, the PVC sample with a higher concentration of PVP shows better filtering ability. Nevertheless, the PVC fiber itself, without PVP, also shows good filtering ability. However, one disadvantage of the nanofibers membrane that limits its applicability as a filter material is that it has a low mechanical strength.
4.2. Air Filtration
Due to the influence of the rapid growth of urban areas, and the development of industries, global air quality has been seriously degraded. The fine particles of particulate matter 2.5 (PM 2.5) seriously affect human health. Many types of filtration technology have been developed. Nanofibers have small pores size, large surface area, small diameter, high porosity, and the ability to incorporate active chemical agents on a nano-scale surface, making them a potential candidate for air filtration technology [
90]. Electrospun PVC/PU (8/2 w/w) nanofiber mats with high abrasion resistance (134 cycles) and comparable air permeability (154.1 mm/s) showed fascinating filtration efficiency (99.5%). In addition, the low-pressure drop (144 Pa) performance for 300–500 nm sodium chloride aerosol particles suggests their use as a promising medium for a variety of potential applications in air filtration [
91]. Lackowski et al. [
92] obtained electrospun PVC/PVDF nanofibers for the filtration of smoke and nanoparticles. The results showed that PVC/PVDF nanofibers have a diameter of about 400–800 nm and offer better filtration efficiency compared with HEPA filtration (
Figure 6).
Shao et al. [
93] have been combining PVC nanofiber mats and PA6 nanofiber mats to prepare a self-powered air filtration composite membrane. The principal work of this filter is that PVC nanofiber mats will be positively charged and PA6 nanofiber mats will be negatively charged under the action of air currents. The filtration efficiency of the composite increases as it is fabricated based on mechanical filtration and electrostatic adsorption. The removal efficiency of the PVC/PA6 nanofiber composite was evaluated at 98.75%, and the pressure drop was evaluated at 67.5 Pa for sodium chloride aerosol particles with a diameter of 0.3 µm.
On the market, we can find that the company RESPILON
® (Brno, Czech Republic) has used PVC nanofibers to fabricate a window membrane. Such membranes are attached to the windows for air purification, dust resistance, and insulation. The membrane can block up to 90% of smoke, dust, and allergens and can remove particles larger than 150 nm. In addition, it is important to mention that the membrane is made from nanofibers. Hence, there is no release of harmful substances or particles [
94].
4.3. Oil Spill Cleanup
Oil spills have serious and long-lasting effects on marine ecosystems and the environment in general. Removing oil spills quickly and efficiently is an issue that has never been more urgent. Recently, the research on using nanomaterials as sorbent membranes to separate oil and water has been increasingly concerned [
95,
96]. Using sorbents to concentrate and transform oil from the liquid phase to the semi-solid or solid phase and then removing this oil from water is an effective and economical mechanical method [
97,
98]. Electrospun nanofibers have many suitable properties for oil and water separation, such as superior hydrophobic–oleophilic behavior (no water sorption but oil sorption), high porosity, low density, small diameter, and fibrillary structure [
99,
100]. Therefore, nanofiber membranes are a potential material that can be used to separate oil from water [
101,
102].
PVC nanofibers are oleophilic, which means, is a material with a high affinity for oil; in other words, when oils are exposed to PVC nanofibers, they will absorb the oil [
103,
104]. Materials with hydrophobic and oleophilic characteristics are suitable when used to remove oil from water. Furthermore, these materials do not pollute the environment.
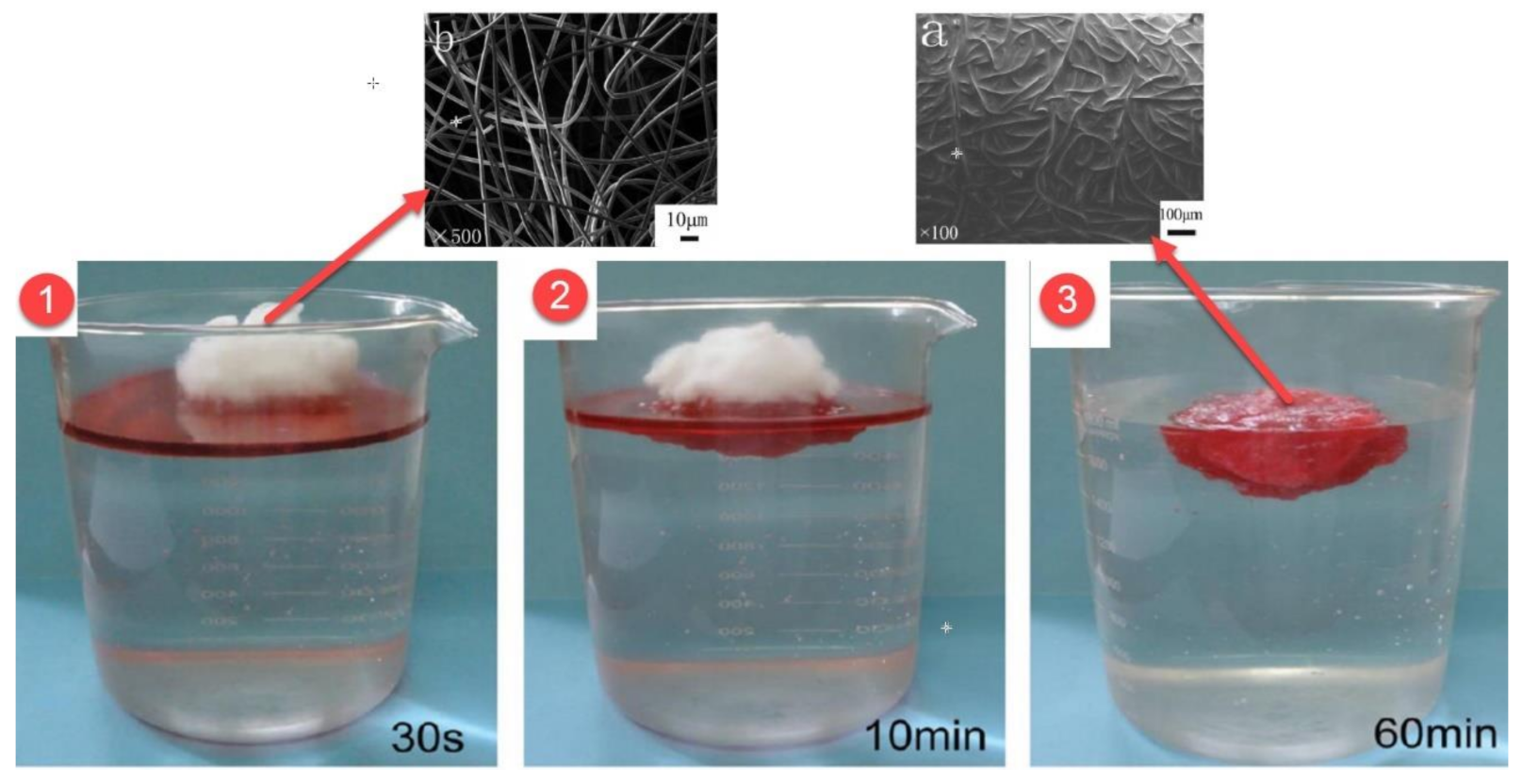
Messiry et al. [
74] evaluated the oil absorption capacity of fibers from PVC and a mixture of PVC and CA with different concentrations (2%, 4%, 6%, 8%). The water contact angle of the nanofiber decreases as CA content increases. The nanofibers obtained from a PVC blend with 8% CA have diameters of 89 nm, which is significantly smaller than those from pure PVC nanofibers of (207 nm), thus, providing a better oil absorption. The oil sorption of the nanofiber sorbent using PVC and PVC/CA 8% was 17.4 g oil/1 g nanofiber and 25.8 oil/1 g nanofiber, respectively (
Table 9).
Figure 7 shows the nanofiber mat before and after the absorption of the oil, for PVC and PVC/CA 8% nanofiber mats. As we can observe, after absorption, the nanofiber surface is covered with oil. However, the authors did not specify which oils were used in their experiments.
Zhu et al. [
32] researched the oil sorption capacity of nanofibers obtained from PVC and PS polymer mixture in a weight ratio of 1:9. The results showed that the sorption capacities of the PVC/PS sorbent for motor oil, peanut oil, diesel, and ethylene glycol were 146, 119, 38, and 81 g/g, respectively. Its sorption capacity is rated to be 5 to 9 times higher than commercial polypropylene (PP) sorbent. The mechanism of oil sorption is explained by high porosity and appropriated void size, and the large surface area of the nanofibers. However, the adsorption capacity for oils can differ depending on the characteristic of oil, such as oil viscosity, density, and surface tension. Additionally, nanofiber morphology, such as fiber diameter, void in the mat, and surface roughness, has a direct effect on the adsorption capacity for oil.
Figure 8 shows the experiment separation of motor oil on the water and SEM images.
4.4. PVC Nanofiber for Energy Application
Fossil energy sources are limited, while natural sources such as solar, wind, water, etc., are quasi-infinite. Development of energy storage materials such as solar cells, lithium batteries, and accumulators to store energy is needed. Electrospun nanofiber can be used in electrodes and separators in lithium-based batteries, fuel cells, electrocatalysts for electrode materials, electrolyte membranes, photoelectrodes in dye-sensitized solar cells, etc. [
105].
Because PVDF is a semi-crystalline material, the crystal part of PVDF interferes with the movement of lithium-ion. Therefore batteries with PVDF-based electrolytes polymer have low ion conductivity and charge/discharge capability [
106]. Adding PVC to PVDF eliminates crystallinity and enhances ionic conductivity [
107]. Electrospun PVDF/PVC nanofibers (8/2 w/w) can be used for electrolytes polymer in pin lithium-ion polymer [
64]. The presence of PVC in the nanofiber membranes has increased electrolyte uptake and ionic conductivity of the composite polymer electrolytes. The composite PVDF–PVC PEs had a high ionic conductivity up to 2.25 × 10
−3 S cm
−1 at 25 °C.
Wei et al. [
66] studied the hydrogen storage capacity of PVC/Ni nanofibers using the volumetric method. The hydrogen storage of the obtained material carbonized at 1000 °C is determined to be 0.30 wt%, higher than the material based on PAN nanofibers at the same temperature (0.15 wt%) [
108]. According to the author’s assessment, PVC/Ni nanofibers are a potential energy storage material.
Angulakshmi et al. [
109] have fabricated a membrane consisting of 3 layers in which a layer of PVC nanofiber mat is between 2 layers of poly(vinylidene fluoride- hexafluoropropylene) (PVDF-HFP) nanofiber mats. PVDF-HFP membrane exhibited high porosity and uptake of electrolyte, but its mechanical integrity is poor. In this case, PVC nanofiber mats enhanced the mechanical properties of the membrane. The obtained PVF-HFP/PVC/PVF-HFP membrane has the potential to be used as a component for lithium battery applications. Bai et al. [
110] synthesized hard carbon materials through the pyrolysis of PVC nanofibers at 600–800 °C. The results showed that the material has excellent cycle performance, high reversible capacity, and good rate capability. The obtained material can be used to make anodes for Na-ion battery application.
4.5. Protective Clothing
Fibers from PVC are accessible and inexpensive materials. PVC nanofibers are characterized by their hydrophobicity, not water expansion, and not hygroscopicity. In addition, PVC nanofibers present high chemical-resistance to agents such as acids, bases, salts, and they are insoluble in most organic solvents; hence they can be used to manufacture protective clothing and lab coats for medical applications [
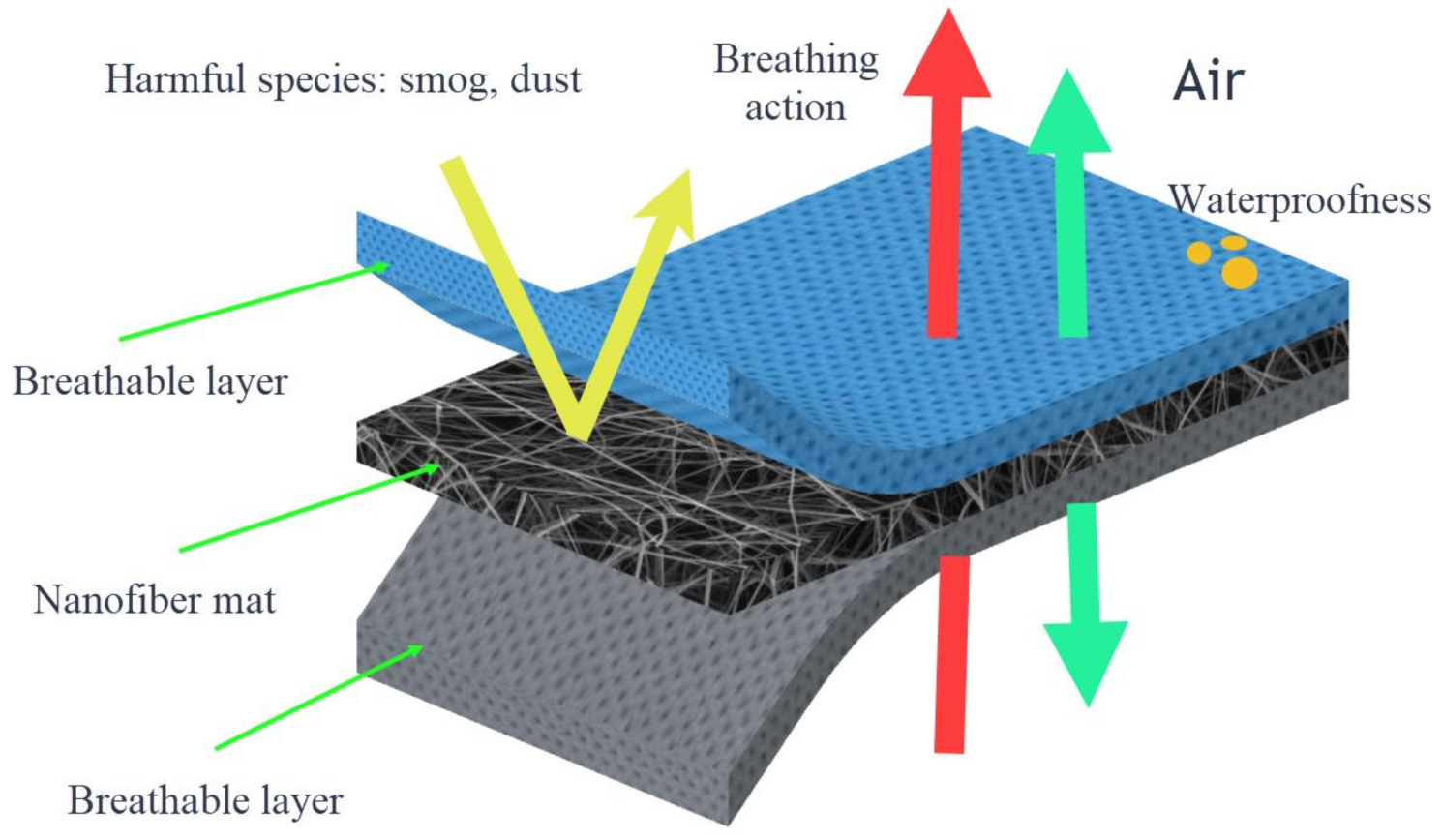
111]. Having inherited these characteristic properties, PVC nanofibers have the potential to be used in the field of protective clothing. When using nanofibers as a part of protective clothing enhances the clothing’s filtration ability from harmful environmental agents, good air exchange, and water repellency.
Figure 9 illustrates how nanofiber mats work when they are used as protective clothing.
Sundarrajan and Ramakrishna [
112] have studied nanofiber mats based on PVC, in combination with nano magnesium oxide to protect against paraoxon nerve agents. The results showed that PVC/MgO nanofiber mat has hydrolysis of paraoxon. PVC/MgO nanofiber mats had put together along with the woven textile media for the protective clothing.
Ramaseshan et al. [
113] have fabricated functional membranes from PVC polymer with β-cyclodextrin (β-CD) and o-iodosobenzoic acid (IBA), a blend of β-CD+IBA, and the synthesized catalyst. These functional membranes are used to eliminate paraoxon nerve stimulants. The highest paraoxon hydrolytic efficiency of functional membranes is 11.5 times faster than the activated charcoal used in protective clothing.
4.6. Protection from Corrosion
Protecting surfaces from corrosion is a new application of electrospun nanofibers. It has been proposed to replace traditional chromate coatings, which not only affects the environment but affects human health as well. Essaheb et al. [
53] fabricated electrospun PVC nanofiber coating on the surfaces of different metals such as aluminum, steel, and brass. The authors used cyclic potentiodynamic polarization and electrochemical impedance spectroscopy measurements to evaluate the protective effect of the coating in NaCl 3.5 wt%. The results showed that the nanofiber-coated samples had much lower corrosion rates, more passivated surfaces, and higher polarization resistance compared to the non-coated nanofiber samples.
Iribarren et al. [
83] studied coatings from PVC/zinc oxide (ZnO) nanofibers, and it was used as a corrosion inhibitor for aluminum substrates. The average nanofiber diameter was around 720 nm. The contact angle values of the nanofiber mats were in the range of 145°–155°. The results of anti-corrosion research showed that the aluminum surface significantly improved the resistance to pitting corrosion after being coated with PVC/ZnO nanofiber coating. In addition, Rivero et al. [
73] have proven that PVC nanofiber increases corrosion resistance through the Tafel polarization test.
4.7. Reinforcement in Composite
Composite polymer materials usually have two main components, one is the reinforcement, and the other is the matrix. When combined, they produce higher-quality materials such as high strength and high modulus, high flexibility, economic efficiency that one of the two components cannot achieve. Recently, nano-scale materials have been used as a reinforcement ingredient that has attracted the attention of scientists. Electrospun nanofiber material has many outstanding properties such as thin and light structure, high porosity, and higher mechanical properties than those of the same material in its bulk state [
114]. Therefore, nanofiber polymer materials are considered as a potential material used as composite reinforcement [
115,
116,
117].
Al-Dabbagh et al. [
118] used PVC microfiber (2–4 µm) and nanofiber (~100 nm) for reinforcement unsaturated polyester. The results showed that changing the volume fraction of microfibers in composite led to an increase in the density of the composite, whereas a change in the volume fraction of nanofiber resulted in a decrease in the density of the composite. Increasing the volume fraction of PVC fiber increases the shore D hardness of the composite. Impact strength and bending strength (flexural strength) of composite increase as the volume fraction of PVC fiber increases. In addition, the impact strength and bending strength of nanocomposite are higher than that of the micro composite.
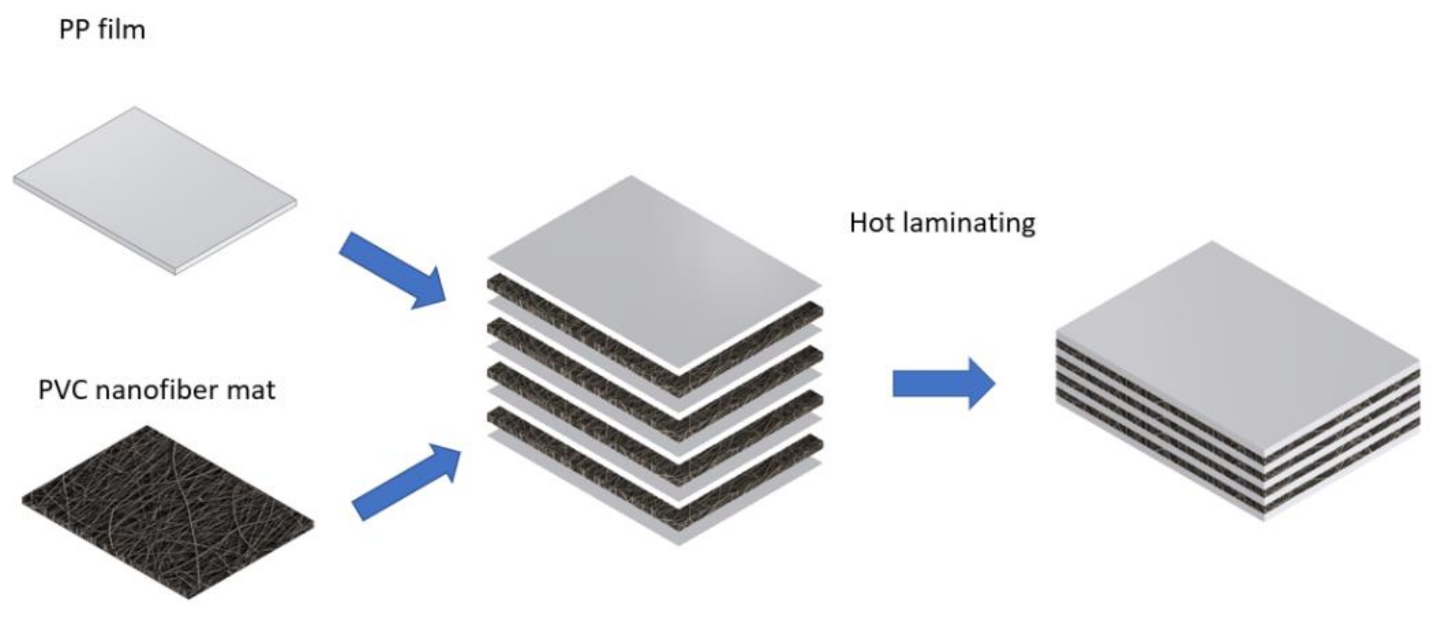
Zhang et al. [
119] have fabricated composites from PVC nanofiber mats and PP films (
Figure 10). The resulting material has a relatively constant index of refraction and has a low absorption coefficient; hence PVC/PP composite has the potential to be used as a material for terahertz optical components.
4.8. Other Application
PVC nanofibers are also used for microwave frequency absorption [
62,
120]. Metal powders and metal oxides, such as Fe
2O
3, Fe
3O
4, and CoFe
2O
4/CoO, can be added to the polymer solution before electrospinning. The resulting material exhibits a wave absorption in a frequency domain range from 8–12 GHz. Özkan et al. [
121] prepared a composite made of PVC nanofiber with glass fiber reinforced-epoxy laminates for radar absorption investigations. The results showed that the composite has a reception capacity of up to 33.82% at a constant frequency. Akgül et al. [
122] obtained electrospun PVC/zinc nitrate nanofiber aiming to cover draped fabrics where controlled light transmission is required. Zhu et al. [
61] used PVC nanofiber mats as effective adsorbents for cationic dye removal from an aqueous medium.
Seewoogolam et al. [
60] used PVC nanofiber mats to develop insect-like flapping wings with a beat frequency between 4 and 10 Hz for small portable flying vehicles. The nanofiber mats were applied to heat treatment at different temperatures of 60 °C, 80 °C, 100 °C. After that, the material strengths and hydrophobicity were increased. In addition, wings made from nanofiber mats have a low weight. Moreover, PVC nanofiber mats can also be used to produce catalyst materials [
123].
Altinkok et al. [
124] fabricated PVC nanofibers with bile acid for biomedical application. Bile acids such as lithocholic acid and chenodeoxycholic acid bearing PVC by employing copper (I) -catalyzed azide-alkyne cycloaddition ‘click’ reaction. The obtained mixture was used to fabricate nanofibers by electrospinning process. PVC nanofibers are characterized by morphology, spectrum, wettability, and thermal properties. The presence of bile acids increases the hydrophilicity, the glass transition temperature, and particularly the electrospinnability of the nanofibers.
5. Conclusions
PVC is an inexpensive and popular polymer, and it has many advantages, such as low density, insolubility in water, acids, and bases, and many other organic solvents. Electrospinning is a simple process that allows us to produce nanofibers at a low cost. Currently, large-scale industrial fabrication is feasible. PVC is a non-conductive material, so a sufficiently thick coating on the collector surface will hinder the process of fiber generation. Therefore, this represents a challenge to produce large quantities of nanofiber, and high thickness nanofiber mats, during electrospinning. As a result, the time to produce large amounts of a nanofiber can become a quite long process.
The issue of durability of the nanofiber mats will also be of concern, although recently, there have been reported that the addition of natural polymers can increase the strength of the nanofiber mats. The durability of individual nanofibers showed to be higher than nanofiber mats, which showed lower durability due to the random arrangement of fibers. This characteristic opens the interest for further studies, aiming to improve the strength of nanofiber mats. A diameter smaller than 100 nm leads to a significant increase in the modulus and strength of nanofiber. Therefore, researching and manufacturing nanofibers with a low diameter has caught the attention of scientists.
One of the perspective applications for PVC nanofiber materials is environmental treatment, such as air purification, oil–water separation, and corrosion-resistant materials. Using PVC nanofiber to make protective clothing for medical and military uses have the potential to fight infections and protect from poisonous agents. Studies on the use of PVC nanofiber as a reinforcing component for composite fabrication are still limited. Nanocomposite research and development promises to bring good results in the future.
PVC nanofibers have the potential to be used in traditional fields such as in the construction industry. PVC nanofiber can be used to fabricate materials with high porosity and excellent thermal properties. Window panels for air filtration, bacteria filtration, moisture protection, and radiation protection also use PVC nanofiber. Moreover, with the excellent PVC nanofiber properties, such as high corrosion resistance and water repellency, they can be applied to the fabrication of waterproof materials. In addition, research into PVC nanofiber as a corrosion-resistant material for pipes may also be of interest.
PVC nanofibers and other polymer nanofibers offer many outstanding properties, such as increased mechanical strength, increased porosity, and tunable hydrophobicity characteristics. Therefore, the fabrication of PVC nanofibers blended with different polymers represents a paramount duty that must be addressed. In particular, the expansion of less toxic solvents that can co-dissolve PVC with other polymers will also be of interest for further investigations. In addition, in combination with other functional metals or other chemical compounds, PVC nanofibers present amazing characteristics that can be implemented in many different applications, which make them of research interest.
Author Contributions
Conceptualization, M.V.U.; formal analysis, Q.P.L and R.O.O.; investigation, Q.P.L., R.O.O., and R.A.O.B.; resources M.V.U.; writing—original draft preparation, writing—review and editing, Q.P.L., R.O.O., and R.A.O.B.; supervision, M.V.U.; funding acquisition, M.V.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Government of Russian Federation (Grant 08-08).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Conflicts of Interest
The authors declared that they have no conflicts of interest to this work.
Abbreviations
| PVC | Polyvinyl chloride |
| PS | Polystyrene |
| PP | Polypropylene |
| PU | Polyurethane |
| PA6 | Polyamide 6 |
| PVP | Polyvinylpyrrolidone |
| MWCNT | Multiwalled carbon nanotubes |
| PVDF | Poly (vinylidene fluoride) |
| PE | Polyester |
| PVA | Polyvinyl alcohol |
| PAN | Polyacrylonitrile |
| PEG | Poly (ethylene glycol) |
| CA | Cellulose acetate |
| ENR | Epoxidized natural rubber |
| PEDOT | Poly (3,4-ethylenedioxythiophene) |
| PVDF-HFP | Poly (vinylidene fluoride- hexafluoropropylene) |
| DMF | N,N-dimethyl formamide |
| THF | Tetrahydrofuran |
| MEK | Methyl ethyl ketone |
| DMA | N,N-Dimethylacetamide |
| HEPA | High efficiency particulate air |
| FT-IR | Fourier transform infrared spectroscopy |
| DSC | Differential scanning calorimetry |
| TGA | Thermogravimetric analysis |
| DTA | Differential thermal analysis |
| SEM | Scanning electron microscopy |
| RH | Relative humidity |
| TDS | Total dissolved solids |
References
- Doworkin, R.D. PVC Stabilizers of the past, present, and future. J. Vinyl Technol. 1989, 11, 15–22. [Google Scholar] [CrossRef]
- Daniels, P.H. A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J. Vinyl Addit. Technol. 2009, 15, 219–223. [Google Scholar] [CrossRef]
- Garside, M. Global PVC Production Volume 2018 & 2025. Available online: https://www.statista.com/statistics/720296/global-polyvinyl-chloride-market-size-in-tons/ (accessed on 30 October 2020).
- Wilkes, C.E.; Summers, J.W.; Daniels, C.A.; Berard, M.T. PVC Handbook; Hanser: Munich, Germany, 2005; Volume 184. [Google Scholar]
- Kolahalam, L.A.; Kasi Viswanath, I.V.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, W.; Formo, E.; Sun, Y.; Xia, Y. Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Polym. Adv. Technol. 2011, 22, 326–338. [Google Scholar] [CrossRef]
- Li, M.; Wang, D.; Xiao, R.; Sun, G.; Zhao, Q.; Li, H. A novel high flux poly(trimethylene terephthalate) nanofiber membrane for microfiltration media. Sep. Purif. Technol. 2013, 116, 199–205. [Google Scholar] [CrossRef]
- Ondarçuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. Europhys. Lett. 1998, 42, 215–220. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, H.; Zhai, J.; Song, Y.; Jiang, L.; Zhu, D. Super-hydrophobic surface of aligned polyacrylonitrile nanofibers. Angew. Chem. Int. Ed. 2002, 41, 1221–1223. [Google Scholar] [CrossRef]
- Qiu, P.; Mao, C. Biomimetic branched hollow fibers templated by self-assembled fibrous polyvinylpyrrolidone structures in aqueous solution. ACS Nano 2010, 4, 1573–1579. [Google Scholar] [CrossRef][Green Version]
- Stupp, S.I.; Zha, R.H.; Palmer, L.C.; Cui, H.; Bitton, R. Self-assembly of biomolecular soft matter. Faraday Discuss. 2013, 166, 9–30. [Google Scholar] [CrossRef]
- Parviz, B.A.; Ryan, D.; Whitesides, G.M. Using Self-Assembly for the Fabrication of Nano-Scale Electronic and Photonic Devices. IEEE Trans. Adv. Packag. 2003, 26, 233–241. [Google Scholar] [CrossRef]
- Ellison, C.J.; Phatak, A.; Giles, D.W.; Macosko, C.W.; Bates, F.S. Corrigendum to “Melt blown nanofibers: Fiber diameter distributions and onset of fiber breakup”. Polymer 2007, 48, 6180. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano-composite scaffold of PLLA/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Chen, J.; Chen, F.; Hu, P.; Wu, X.-F.; Reneker, D.H.; Hou, H. High-strength and high-toughness polyimide nanofibers: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 116, 1581–1586. [Google Scholar] [CrossRef]
- Haghi, A.K. Preparation and structures of electrospun PAN nanofibers. Electrospinn. Process Nanofiber Res. 2011, 155, 173–184. [Google Scholar]
- Al-Attabi, R.; Dumée, L.F.; Kong, L.; Schütz, J.A.; Morsi, Y. High Efficiency Poly(acrylonitrile) Electrospun Nanofiber Membranes for Airborne Nanomaterials Filtration. Adv. Eng. Mater. 2018, 20, 1700572. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, T.; Liu, Q.; Zhang, L.; Zhou, J.; Li, B.; Peng, Q. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2019, 62, 423–436. [Google Scholar] [CrossRef]
- Qin, X.H.; Wang, S.Y. Electrospun nanofibers from crosslinked poly(vinyl alcohol) and its filtration efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, C. Applications of Electrospun Fibers in Sensors. Recent Pat. Nanotechnol. 2017, 2, 169–182. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Qing, X. A flexible capacitive sensor based on the electrospun PVDF nanofiber membrane with carbon nanotubes. Sens. Actuators A Phys. 2019, 299, 111579. [Google Scholar] [CrossRef]
- Unal, B.; Yalcinkaya, E.E.; Demirkol, D.O.; Timur, S. An electrospun nanofiber matrix based on organo-clay for biosensors: PVA/PAMAM-Montmorillonite. Appl. Surf. Sci. 2018, 444, 542–551. [Google Scholar] [CrossRef]
- Li, B.; Pan, S.; Yuan, H.; Zhang, Y. Optical and mechanical anisotropies of aligned electrospun nanofibers reinforced transparent PMMA nanocomposites. Compos. Part A Appl. Sci. Manuf. 2016, 90, 380–389. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef] [PubMed]
- Ponnamma, D.; Aljarod, O.; Parangusan, H.; Al-Maadeed, M.A.A. Electrospun nanofibers of PVDF-HFP composites containing magnetic nickel ferrite for energy harvesting application. Mater. Chem. Phys. 2020, 239, 122257. [Google Scholar] [CrossRef]
- Sang, Q.; Williams, G.R.; Wu, H.; Liu, K.; Li, H.; Zhu, L.M. Electrospun gelatin/sodium bicarbonate and poly(lactide-co-ε-caprolactone)/sodium bicarbonate nanofibers as drug delivery systems. Mater. Sci. Eng. C 2017, 81, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Coşkuner Filiz, B.; Kantürk Figen, A. Fabrication of electrospun nanofiber catalysts and ammonia borane hydrogen release efficiency. Int. J. Hydrogen Energy 2016, 41, 15433–15442. [Google Scholar] [CrossRef]
- Bae, J.; Baek, I.; Choi, H. Efficacy of piezoelectric electrospun nanofiber membrane for water treatment. Chem. Eng. J. 2017, 307, 670–678. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; La, Y.M.; Lee, D.R.; Sung, N.H. Influence of a mixing solvent with tetrahydrofuran and N,N-dimethylformamide on electrospun poly(vinyl chloride) nonwoven mats. J. Polym. Sci. Part B Polym. Phys. 2002, 40, 2259–2268. [Google Scholar] [CrossRef]
- Alarifi, I.M.; Alharbi, A.R.; Khan, M.; Khan, W.S.; Usta, A.; Asmatulu, R. Water Treatment Using Electrospun PVC/PVP NANOFIBERS as Filter Medium. Int. J. Mater. Sci. Res. 2018, 2, 43–49. [Google Scholar] [CrossRef]
- Asmatulu, R.; Muppalla, H.; Veisi, Z.; Khan, W.S.W.A.W.S.; Asaduzzaman, A.; Nuraje, N.; Ramazan, A.; Harish, M.; Zeinab, V.; Waseem, S.K.; et al. Study of hydrophilic electrospun nanofiber membranes for filtration of micro and nanosize suspended particles. Membranes 2013, 3, 375–388. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef]
- He, J.H.; Wan, Y.Q. Allometric scaling for voltage and current in electrospinning. Polymer 2004, 45, 6731–6734. [Google Scholar] [CrossRef]
- Freier, G. The coalescence of water drops in an electric field. J. Geophys. Res. 1960, 65, 3979–3985. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; Du Toit, L.C.; Ndesendo, V.M.K. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Liu, J.P.; Fu, J.Q.; Feng, R.H.; Zhu, G.H. Effects of working parameters on gasoline engine exergy balance. J. Cent. South Univ. 2013, 20, 1938–1946. [Google Scholar] [CrossRef]
- Yang, Y.; Jia, Z.; Li, Q.; Guan, Z. Experimental investigation of the governing parameters in the electrospinning of polyethylene oxide solution. IEEE Trans. Dielectr. Electr. Insul. 2006, 13, 580–584. [Google Scholar] [CrossRef]
- Lee, J.S.; Choi, K.H.; Ghim, H.D.; Kim, S.S.; Chun, D.H.; Kim, H.Y.; Lyoo, W.S. Role of molecular weight of atactic poly(vinyl alcohol) (PVA) in the structure and properties of PVA nanofabric prepared by electrospinning. J. Appl. Polym. Sci. 2004, 93, 1638–1646. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. High-flux microfiltration filters based on electrospun polyvinylalcohol nanofibrous membranes. Polymer 2013, 54, 548–556. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, I.H.; Bea, G.N. Optimization of the electrospinning conditions for preparation of nanofibers from polyvinylacetate (PVAc) in ethanol solvent. J. Ind. Eng. Chem. 2008, 14, 707–713. [Google Scholar] [CrossRef]
- Jeun, J.P.; Lim, Y.M.; Nho, Y.C. Study on morphology of electrospun poly(caprolactone) nanofiber. J. Ind. Eng. Chem. 2005, 11, 573–578. [Google Scholar]
- Wang, T.; Kumar, S. Electrospinning of polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2006, 102, 1023–1029. [Google Scholar] [CrossRef]
- Gu, X.; Song, X.; Shao, C.; Zeng, P.; Lu, X.; Shen, X.; Yang, Q. Electrospinning of poly(butylene-carbonate): Effect of solvents on the properties of the nanofibers film. Int. J. Electrochem. Sci. 2014, 9, 8045–8056. [Google Scholar]
- Wannatong, L.; Sirivat, A.; Supaphol, P. Effects of solvents on electrospun polymeric fibers: Preliminary study on polystyrene. Polym. Int. 2004, 53, 1851–1859. [Google Scholar] [CrossRef]
- Grause, G.; Hirahashi, S.; Toyoda, H.; Kameda, T.; Yoshioka, T. Solubility parameters for determining optimal solvents for separating PVC from PVC-coated PET fibers. J. Mater. Cycles Waste Manag. 2017, 19, 612–622. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Ryu, Y.J.; Kim, K.W.; Choi, S.W. Mechanical behavior of electrospun fiber mats of poly(vinyl chloride)/polyurethane polyblends. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1256–1262. [Google Scholar] [CrossRef]
- ElMessiry, M.; Fadel, N. The tensile properties of electrospun Poly Vinyl Chloride and Cellulose Acetate (PVC/CA) bi-component polymers nanofibers. Alex. Eng. J. 2019, 58, 885–890. [Google Scholar] [CrossRef]
- Hussain, F.S.J.; Yusoff, M.B.M. Fabrication and characterization of electrospun silver nanofibers with unmatched porosity. In Proceedings of the 12th IEEE International Conference on Nanotechnology, Birmingham, UK, 20–23 August 2012; pp. 1–4. [Google Scholar]
- Taweepreda, W. Fabrication and Properties of Rubber Nanofiber from Electrospinning. Elastomers 2017, 121. [Google Scholar] [CrossRef]
- Phatcharasit, K.; Taweepreda, W.; Boonkerd, K.; Kim, J.K. Preparation and properties of electrospun PVC nanofiber. Adv. Mater. Res. 2013, 770, 193–196. [Google Scholar] [CrossRef]
- Chiscan, O.; Dumitru, I.; Tura, V.; Stancu, A. PVC/Fe electrospun nanofibers for high frequency applications. J. Mater. Sci. 2012, 47, 2322–2327. [Google Scholar] [CrossRef]
- Zulfi, A.; Rezeki, Y.A.; Edikresnha, D.; Munir, M.M.; Khairurrijal, K. Synthesis of Fibers and Particles from Polyvinyl Chloride (PVC) Waste Using Electrospinning. Mater. Sci. Eng. 2018, 367, 12014. [Google Scholar] [CrossRef]
- Es-saheb, M.; Elzatahry, A.A.; Sherif, E.S.M.; Alkaraki, A.S.; Kenawy, E.R. A novel electrospinning application for polyvinyl chloride nanofiber coating deposition as a corrosion inhibitor for aluminum, steel, and brass in chloride solutions. Int. J. Electrochem. Sci. 2012, 7, 5962–5976. [Google Scholar]
- Pham, L.Q.; Uspenskaya, M.V. Morphology pvc nanofiber, produced by electrospinning method. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, Bulgaria, 28 June–7 July 2019; Volume 19, pp. 289–295. [Google Scholar]
- Medeiros, E.S.; Mattoso, L.H.C.; Offeman, R.D.; Wood, D.F.; Orts, W.J. Effect of relative humidity on the morphology of electrospun polymer fibers. Can. J. Chem. 2008, 86, 590–599. [Google Scholar] [CrossRef]
- Alarifi, I. Fig 4. SEM Images of PVC Nanofibers Incorporated. 2018. Available online: https://figshare.com/articles/figure/Fig_4_SEM_images_of_PVC_nanofibers_incorporated/7150931 (accessed on 21 December 2020). [CrossRef]
- Carrizales, C.; Pelfrey, S.; Rincon, R.; Eubanks, T.M.; Kuang, A.; McClure, M.J.; Bowlin, G.L.; Macossay, J. Thermal and mechanical properties of electrospun PMMA, PVC, Nylon 6, and Nylon 6,6. Polym. Adv. Technol. 2008, 19, 124–130. [Google Scholar] [CrossRef]
- Jaworek, A.; Krupa, A.; Lackowski, M.; Sobczyk, A.T.; Czech, T.; Ramakrishna, S.; Sundarrajan, S.; Pliszka, D. Nanocomposite fabric formation by electrospinning and electrospraying technologies. J. Electrostat. 2009, 67, 435–438. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Seewoogolam, V.; Alarifi, I.; Asmatulu, R. Highly robust electrospun nanofiber films for the fabrication of MAV wings. In Proceedings of the CAMX 2016—Composites and Advanced Materials Expo, Anaheim, CA, USA, 26–29 September 2016. [Google Scholar]
- Zhu, X.; Jiang, X.; Cheng, S.; Wang, K.; Mao, S.; Fan, L.J. Preparation of high strength ultrafine polyvinyl chloride fibrous membrane and its adsorption of cationic dye. J. Polym. Res. 2010, 17, 769–777. [Google Scholar] [CrossRef]
- Chiscan, O.; Dumitru, I.; Postolache, P.; Tura, V.; Stancu, A. Electrospun PVC/Fe3O4 composite nanofibers for microwave absorption applications. Mater. Lett. 2012, 68, 251–254. [Google Scholar] [CrossRef]
- Tarus, B.; Fadel, N.; Al-Oufy, A.; El-Messiry, M. Effect of polymer concentration on the morphology and mechanical characteristics of electrospun cellulose acetate and poly(vinyl chloride) nanofiber mats. Alex. Eng. J. 2016, 55, 2975–2984. [Google Scholar] [CrossRef]
- Zhong, Z.; Cao, Q.; Jing, B.; Wang, X.; Li, X.; Deng, H. Electrospun PVdF-PVC nanofibrous polymer electrolytes for polymer lithium-ion batteries. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2012, 177, 86–91. [Google Scholar] [CrossRef]
- Namsaeng, J.; Punyodom, W.; Worajittiphon, P. Synergistic effect of welding electrospun fibers and MWCNT reinforcement on strength enhancement of PAN–PVC non-woven mats for water filtration. Chem. Eng. Sci. 2019, 193, 230–242. [Google Scholar] [CrossRef]
- Wei, W.; Wu, N.; Xu, J.; Chen, Q.; Qian, Q.; Luo, Y.; Liu, X.; Xiao, L.; Huang, B. Preparation and characterization of PVC-based carbon nanofibers with barrel-like graphite granules by electrospinning. Mater. Lett. 2014, 126, 48–51. [Google Scholar] [CrossRef]
- Wang, X.; Hsiao, B.S. Electrospun nanofiber membranes. Curr. Opin. Chem. Eng. 2016, 12, 62–81. [Google Scholar] [CrossRef]
- Sabetzadeh, N.; Gharehaghaji, A.A. How porous nanofibers have enhanced the engineering of advanced materials: A review. J. Text. Polym. 2017, 5, 3–21. [Google Scholar]
- Yuan, Z.; Chen, H.; Zhang, J. Facile method to prepare lotus-leaf-like super-hydrophobic poly(vinyl chloride) film. Appl. Surf. Sci. 2008, 254, 1593–1598. [Google Scholar] [CrossRef]
- Alamir, M.A.; Alarifi, I.M.; Khan, W.A.; Khan, W.S.; Asmatulu, R. Electrospun Nanofibers: Preparation, Characterization and Atmospheric Fog Capturing Capabilities. Fibers Polym. 2019, 20, 2090–2098. [Google Scholar] [CrossRef]
- Bhran, A.; Shoaib, A.; Elsadeq, D.; El-gendi, A.; Abdallah, H. Preparation of PVC/PVP composite polymer membranes via phase inversion process for water treatment purposes. Chin. J. Chem. Eng. 2018, 26, 715–722. [Google Scholar] [CrossRef]
- Gao, X.; Wen, S.; Yang, B.; Xue, J.; Wang, H. Enhanced air filtration performance under high-humidity condition through electrospun membranes with optimized structure. Chin. J. Chem. Eng. 2020, 28, 1788–1795. [Google Scholar] [CrossRef]
- Rivero, P.J.; Rosagaray, I.; Fuertes, J.P.; Palacio, J.F.; Rodríguez, R.J. Designing multifunctional protective PVC electrospun fibers with tunable properties. Polymers 2020, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- El Messiry, M.; Fadel, N. Study of poly(vinyl chloride) nanofiber structured assemblies as oil sorbents. J. Text. Inst. 2019, 110, 1114–1125. [Google Scholar] [CrossRef]
- Xu, H.; Liu, H.; Huang, Y.; Xiao, C. Three-dimensional structure design of tubular polyvinyl chloride hybrid nanofiber membranes for water-in-oil emulsion separation. J. Memb. Sci. 2020, 118905. [Google Scholar] [CrossRef]
- Gu, S.Y.; Wu, Q.L.; Ren, J.; Vancso, G.J. Mechanical properties of a single electrospun fiber and its structures. Macromol. Rapid Commun. 2005, 26, 716–720. [Google Scholar] [CrossRef]
- Fu, Q.; Jin, Y.; Song, X.; Gao, J.; Han, X.; Jiang, X.; Zhao, Q.; Yu, D. Size-dependent mechanical properties of PVA nanofibers reduced via air plasma treatment. Nanotechnology 2010, 21, 95703. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Kim, S.I.; Kim, S.J.; Kim, S.K.; Lee, H.; Spinks, G.M. Size-dependent elastic modulus of single electroactive polymer nanofibers. Appl. Phys. Lett. 2006, 89, 231929. [Google Scholar] [CrossRef]
- Yao, J.; Bastiaansen, C.W.M.; Peijs, T. High strength and high modulus electrospun nanofibers. Fibers 2014, 2, 158–187. [Google Scholar] [CrossRef]
- Pham, L.Q.; Uspenskaya, M.V.; Snetkov, P.P. Obtaining Nanofibers From Polyvinyl Chloride Solutions in Tetrahydrofuran and Dimethyl Formamide Using the Method of Electrospinning. Bull. St. Petersb. State Inst. Technol. (Tech. Univ.) 2019, 51, 42–46. [Google Scholar]
- Pham Le, Q.; Uspenskaya, M.V.; Olekhnovich, R.O.; Baranov, M.A. The Mechanical Properties of PVC Nanofiber Mats Obtained by Electrospinning. Fibers 2021, 9, 2. [Google Scholar] [CrossRef]
- Jin, L.; Wang, T.; Feng, Z.Q.; Leach, M.K.; Wu, J.; Mo, S.; Jiang, Q. A facile approach for the fabrication of core-shell PEDOT nanofiber mats with superior mechanical properties and biocompatibility. J. Mater. Chem. B 2013, 1, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, A.; Rivero, P.J.; Berlanga, C.; Larumbe, S.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Multifunctional protective PVC-ZnO nanocomposite coatings deposited on aluminum alloys by electrospinning. Coatings 2019, 9, 216. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Yang, L.; Zhang, Q.; Fitzgerald, M.L.; Ueda, A.; Chen, Y.; Mu, R.; Li, D.; Bellan, L.M. Thermal transport in electrospun vinyl polymer nanofibers: Effects of molecular weight and side groups. Soft Matter 2018, 14, 9534–9541. [Google Scholar] [CrossRef]
- Ding, B.; Si, Y. Electrospun Nanofibers for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9787122120199. [Google Scholar]
- Abdel-Fatah, M.A. Nanofiltration systems and applications in wastewater treatment: Review article. Ain Shams Eng. J. 2018, 9, 3077–3092. [Google Scholar] [CrossRef]
- Yoon, K.; Hsiao, B.S.; Chu, B. Functional nanofibers for environmental applications. J. Mater. Chem. 2008, 18, 5326–5334. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Rushton, A.; Ward, A.S.; Holdich, R.G. Solid-Liquid Filtration and Separation Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 9783527614974. [Google Scholar]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2017, 302, 1600353. [Google Scholar] [CrossRef]
- Wang, N.; Raza, A.; Si, Y.; Yu, J.; Sun, G.; Ding, B. Tortuously structured polyvinyl chloride/polyurethane fibrous membranes for high-efficiency fine particulate filtration. J. Colloid Interface Sci. 2013, 398, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Lackowski, M.; Krupa, A.; Jaworek, A. Nanofabric nonwoven mat for fi ltration smoke and nanoparticles. Pol. J. Chem. Technol. 2013, 15, 48–52. [Google Scholar] [CrossRef]
- Shao, Z.; Jiang, J.; Wang, X.; Li, W.; Fang, L.; Zheng, G. Self-powered electrospun composite nanofiber membrane for highly efficient air filtration. Nanomaterials 2020, 10, 1706. [Google Scholar] [CrossRef]
- RESPILON®. Available online: https://www.respilon.com/ (accessed on 21 December 2020).
- Lin, J.; Shang, Y.; Ding, B.; Yang, J.; Yu, J.; Al-Deyab, S.S. Nanoporous polystyrene fibers for oil spill cleanup. Mar. Pollut. Bull. 2012, 64, 347–352. [Google Scholar] [CrossRef]
- Jiang, Z.; Tijing, L.D.; Amarjargal, A.; Park, C.H.; An, K.J.; Shon, H.K.; Kim, C.S. Removal of oil from water using magnetic bicomponent composite nanofibers fabricated by electrospinning. Compos. Part B Eng. 2015, 77, 311–318. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Radetic, M.; Ilic, V.; Radojevic, D.; Miladinovic, R.; Jocic, D.; Jovancic, P. Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525–530. [Google Scholar] [CrossRef]
- Wu, J.; An, A.K.; Guo, J.; Lee, E.J.; Farid, M.U.; Jeong, S. CNTs reinforced super-hydrophobic-oleophilic electrospun polystyrene oil sorbent for enhanced sorption capacity and reusability. Chem. Eng. J. 2017, 314, 526–536. [Google Scholar] [CrossRef]
- Lim, T.T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as a natural hollow hydrophobic-oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Cheng, Q.; Ye, D.; Chang, C.; Zhang, L. Facile fabrication of superhydrophilic membranes consisted of fibrous tunicate cellulose nanocrystals for highly efficient oil/water separation. J. Memb. Sci. 2017, 525, 1–8. [Google Scholar] [CrossRef]
- Gao, J.; Li, B.; Wang, L.; Huang, X.; Xue, H. Flexible membranes with a hierarchical nanofiber/microsphere structure for oil adsorption and oil/water separation. J. Ind. Eng. Chem. 2018, 68, 416–424. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, Z.; Xiao, Y.; Chen, Q. Stable highly hydrophobic and oleophilic meshes for oil-water separation. Appl. Surf. Sci. 2007, 253, 9054–9060. [Google Scholar] [CrossRef]
- Su, C. Highly hydrophobic and oleophilic foam for selective absorption. Appl. Surf. Sci. 2009, 256, 1413–1418. [Google Scholar] [CrossRef]
- Sun, G.; Sun, L.; Xie, H.; Liu, J. Electrospinning of nanofibers for energy applications. Nanomaterials 2016, 6, 129. [Google Scholar] [CrossRef] [PubMed]
- Abbrent, S.; Plestil, J.; Hlavata, D.; Lindgren, J.; Tegenfeldt, J.; Wendsjö, Å. Crystallinity and morphology of PVdF-HFP-based gel electrolytes. Polymer 2001, 42, 1407–1416. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Zhu, Q.Y.; Mai, L.Q.; Chen, W. Electrochemical studies on PVC/PVdF blend-based polymer electrolytes. J. Solid State Electrochem. 2007, 11, 543–548. [Google Scholar] [CrossRef]
- Kim, D.K.; Park, S.H.; Kim, B.C.; Chin, B.D.; Jo, S.M.; Kim, D.Y. Electrospun polyacrylonitrile-based carbon nanofibers and their hydrogen storages. Macromol. Res. 2005, 13, 521–528. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Electrospun trilayer polymeric membranes as separator for lithium-ion batteries. Electrochim. Acta 2014, 127, 167–172. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Z.; Wu, C.; Xu, R.; Wu, F.; Liu, Y.; Li, H.; Li, Y.; Lu, J.; Amine, K. Hard carbon originated from polyvinyl chloride nanofibers as high-performance anode material for Na-ion battery. ACS Appl. Mater. Interfaces 2015, 7, 5598–5604. [Google Scholar] [CrossRef]
- Ryauzov, A.N.; Gruzdev, V.A.; Kostrov, Y.A.; Seagal, M.B. Chemical Fiber Production Technology; Chemistry: Moscow, Russian, 1965; p. 516. (In Russian) [Google Scholar]
- Sundarrajan, S.; Ramakrishna, S. Fabrication of nanocomposite membranes from nanofibers and nanoparticles for protection against chemical warfare stimulants. J. Mater. Sci. 2007, 42, 8400–8407. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S.; Liu, Y.; Barhate, R.S.; Lala, N.L.; Ramakrishna, S. Functionalized polymer nanofibre membranes for protection from chemical warfare stimulants. Nanotechnology 2006, 17, 2947–2953. [Google Scholar] [CrossRef]
- Palazzetti, R.; Zucchelli, A. Electrospun nanofibers as reinforcement for composite laminates materials—A review. Compos. Struct. 2017, 182, 711–727. [Google Scholar] [CrossRef]
- Alam, A.M.; Liu, Y.; Park, M.; Park, S.J.; Kim, H.Y. Preparation and characterization of optically transparent and photoluminescent electrospun nanofiber composed of carbon quantum dots and polyacrylonitrile blend with polyacrylic acid. Polymer 2015, 59, 35–41. [Google Scholar] [CrossRef]
- De Schoenmaker, B.; Van Der Heijden, S.; De Baere, I.; Van Paepegem, W.; De Clerck, K. Effect of electrospun polyamide 6 nanofibres on the mechanical properties of a glass fibre/epoxy composite. Polym. Test. 2013, 32, 1495–1501. [Google Scholar] [CrossRef]
- Phong, N.T.; Gabr, M.H.; Okubo, K.; Chuong, B.; Fujii, T. Improvement in the mechanical performances of carbon fiber/epoxy composite with addition of nano-(Polyvinyl alcohol) fibers. Compos. Struct. 2013, 99, 380–387. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.M.; AL-Shimary, H.J. Comparsion between Some Properties of Nano and Micro Pvc Fibers Reinforced Polyester Composites. Int. J. Metall. Mater. Sci. Eng. 2016, 6, 33–38. [Google Scholar]
- Zhang, T.; Nazarov, R.; Pham, L.Q.; Soboleva, V.; Demchenko, P.; Uspenskaya, M.; Olekhnovich, R.; Khodzitsky, M. Polymer composites based on polyvinyl chloride nanofibers and polypropylene films for terahertz photonics. Opt. Mater. Express 2020, 10, 2456. [Google Scholar] [CrossRef]
- Chiscan, O.; Dumitru, I.; Tura, V.; Chiriac, H.; Stancu, A. High frequency absorption of PVC/iron oxides and PVC/CoFe2O4/CoO nanofibers produced by electrospinning technique. IEEE Trans. Magn. 2011, 47, 4511–4516. [Google Scholar] [CrossRef]
- Özkan, V.; Yapici, A.; Karaaslan, M.; Akgöl, O. Electromagnetic Scattering Properties of MWCNTs/Graphene Doped Epoxy Layered with PVC Nanofiber/E-Glass Composites. J. Electron. Mater. 2020, 49, 2249–2256. [Google Scholar] [CrossRef]
- Akgül, Y.İ.; Aykut, Y. Partially Transformation of Zinc Nitrate to Zinc Compounds on PVC Nanofibers at. Tekstil ve Mühendis 2019, 26, 118–124. [Google Scholar] [CrossRef]
- Basheer, C. Nanofiber-membrane-supported TiO2 as a catalyst for oxidation of benzene to phenol. J. Chem. 2013, 2013, 562305. [Google Scholar] [CrossRef]
- Altinkok, C.; Karabulut, H.R.F.; Tasdelen, M.A.; Acik, G. Bile acid bearing poly(vinyl chloride) nanofibers by combination of CuAAC click chemistry and electrospinning process. Mater. Today Commun. 2020, 25, 101425. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).