Synthesis and Characterization of SiO2@CNTs Microparticles: Evaluation of Microwave-Induced Heat Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SiO2@CNTs
2.3. Characterization
3. Results and Discussion
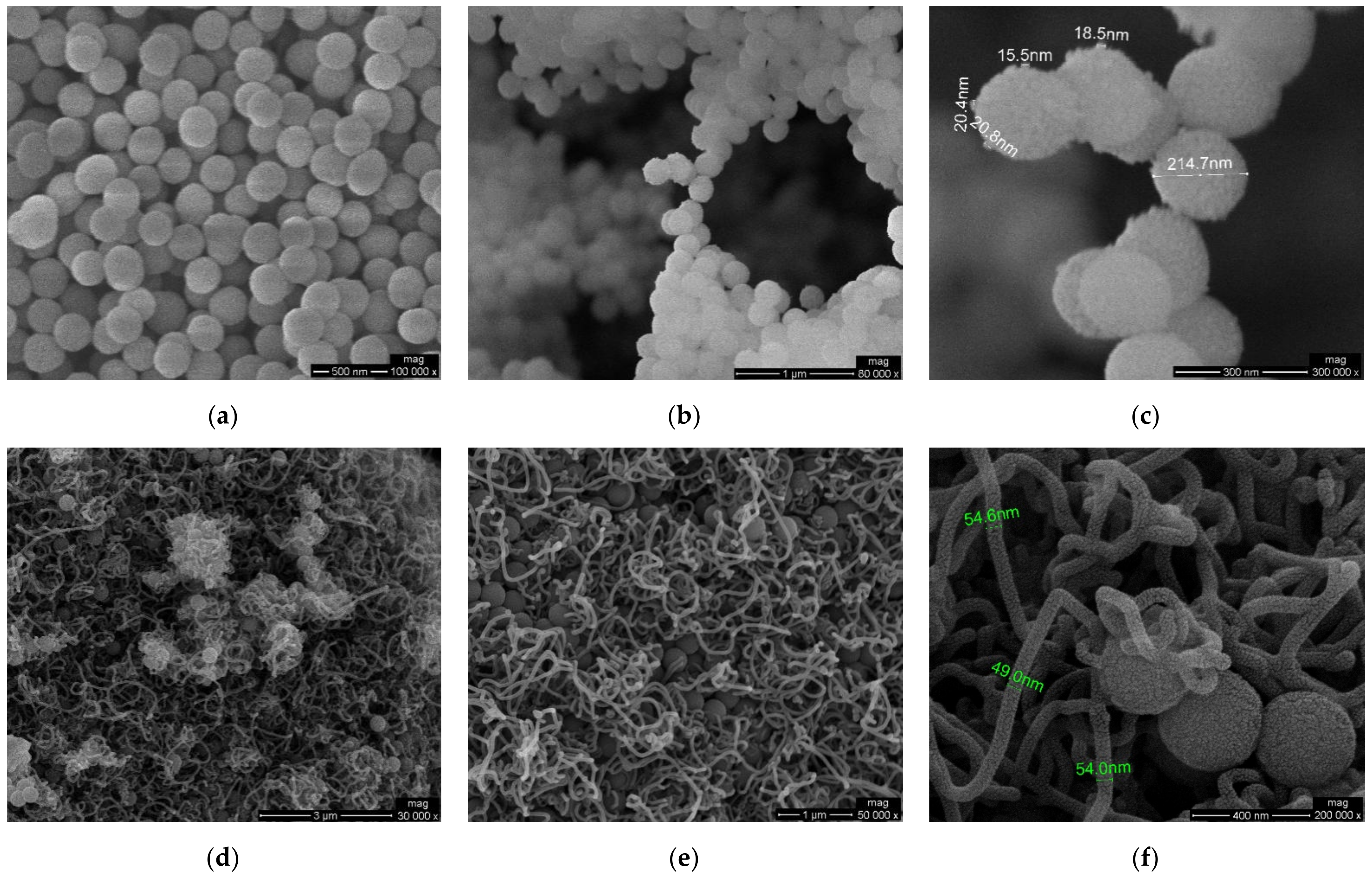
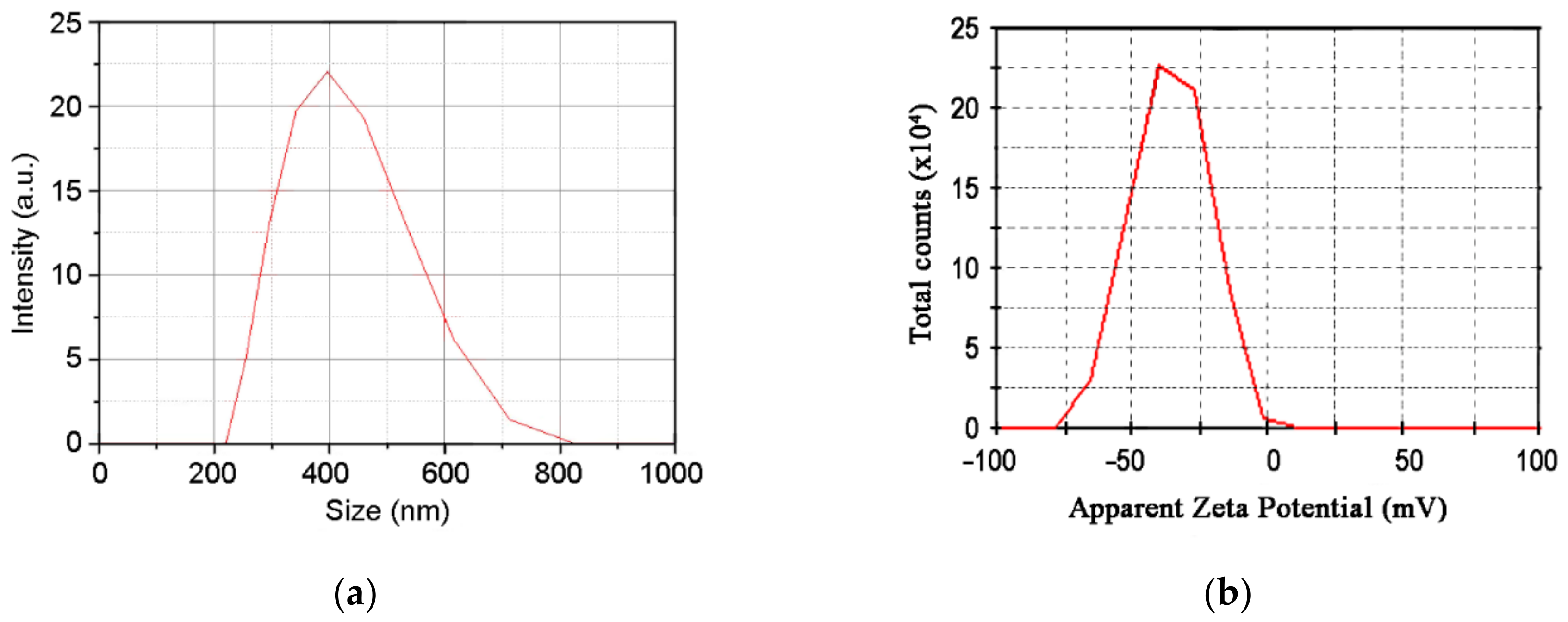
3.1. Morphological Characterization
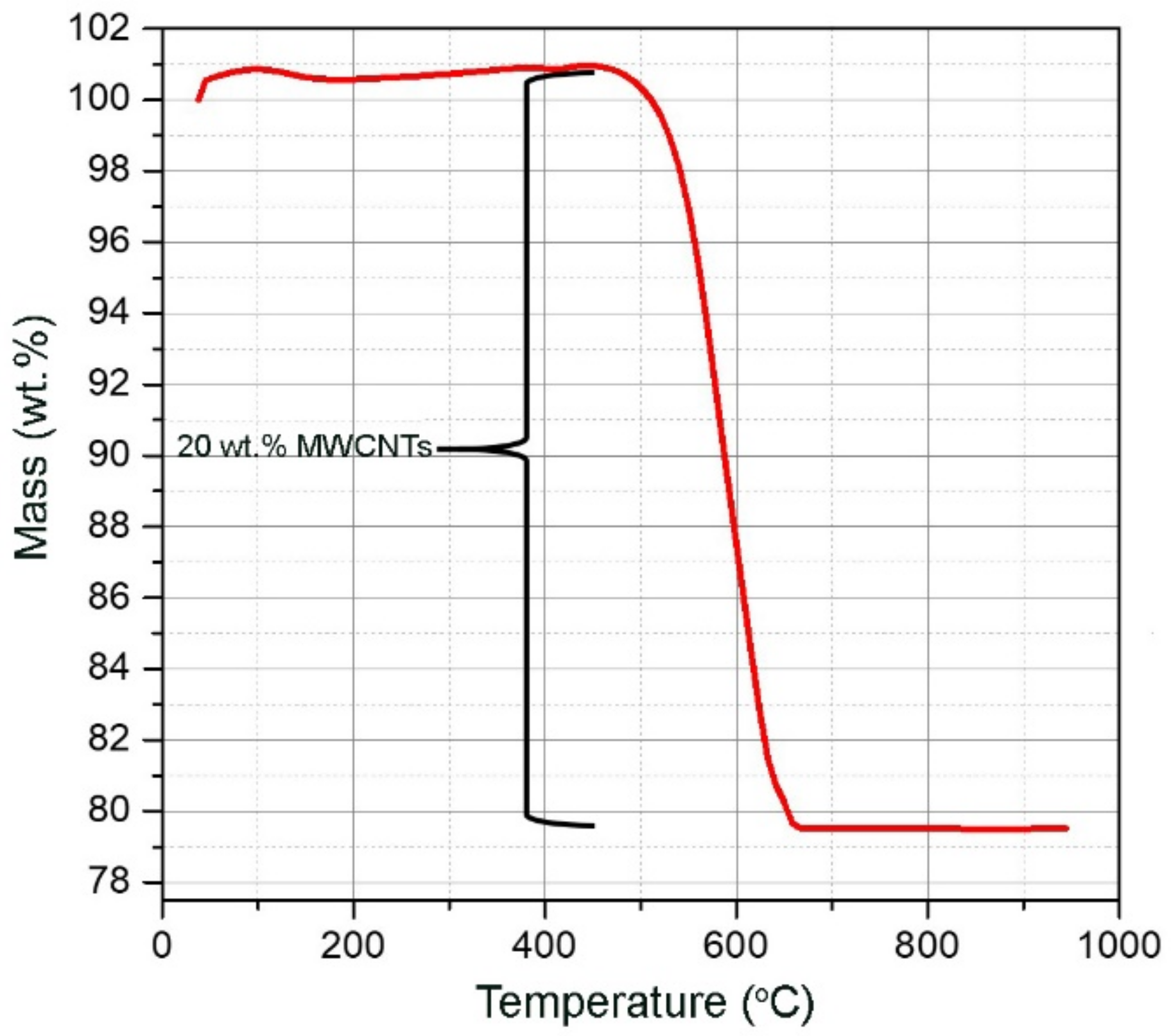
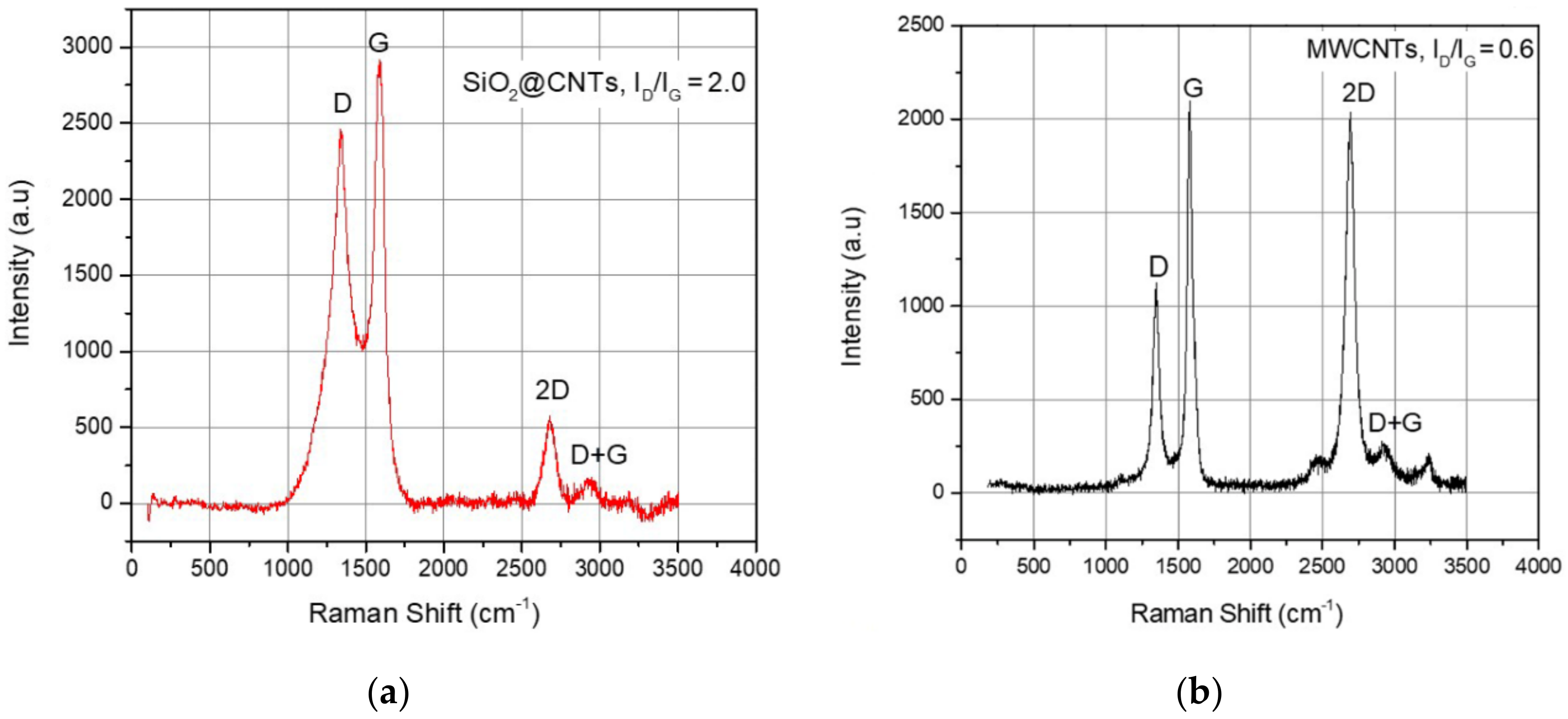
3.2. Structural and Thermal Analyses
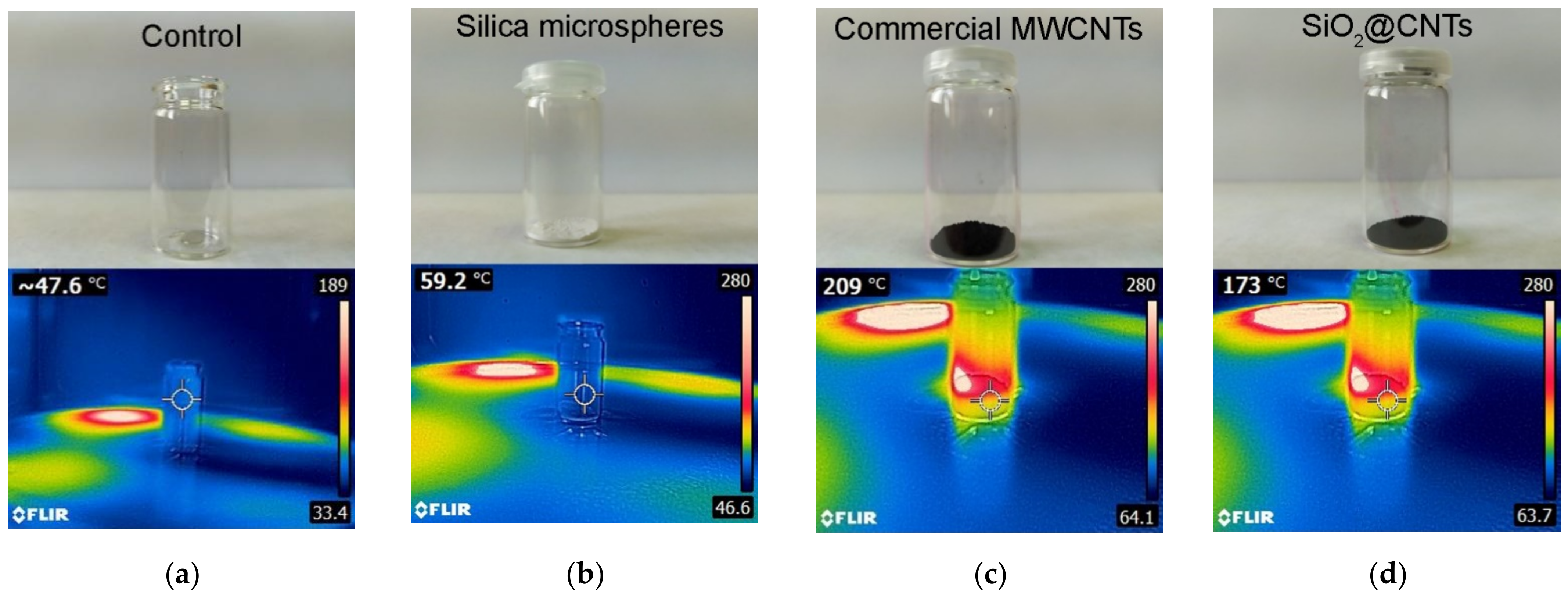
3.3. Microwave Absorption Evaluation
3.4. The Outcome Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rhein, S.; Sträter, K.F. Corporate self-commitments to mitigate the global plastic crisis: Recycling rather than reduction and reuse. J. Clean. Prod. 2021, 296, 126571. [Google Scholar] [CrossRef]
- Wypych, G. 3-mechanisms of adhesion loss. In Handbook of Adhesion Promoters; Wypych, G., Ed.; ChemTec Publishing: Scarborough, ON, Canada, 2018; pp. 45–53. [Google Scholar] [CrossRef]
- Bandl, C.; Kern, W.; Schlögl, S. Adhesives for “debonding-on-demand”: Triggered release mechanisms and typical applications. Int. J. Adhes. Adhes. 2020, 99, 102585. [Google Scholar] [CrossRef]
- Banea, M. Debonding on Demand of Adhesively Bonded Joints: A Critical Review. Rev. Adhes. Adhes. 2019, 7, 33–50. [Google Scholar] [CrossRef]
- Ferahian, A.-C.; Hohl, D.K.; Weder, C.; Montero de Espinosa, L. Bonding and Debonding on Demand with Temperature and Light Responsive Supramolecular Polymers. Macromol. Mater. Eng. 2019, 304, 1900161. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Goulis, P.; Charitidis, C.A. Triggerable Super Absorbent Polymers for Coating Debonding Applications. Polymers 2021, 13, 1432. [Google Scholar] [CrossRef] [PubMed]
- Goulis, P.; Kartsonakis, I.A.; Charitidis, C.A. Synthesis and Characterization of a Core-Shell Copolymer with Different Glass Transition Temperatures. Fibers 2020, 8, 71. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, S.M.; Sharma, S.K. Carbon nanotubes: Synthesis, properties and engineering applications. Carbon Lett. 2019, 29, 419–447. [Google Scholar] [CrossRef]
- Imholt, T.J.; Dyke, C.A.; Hasslacher, B.; Perez, J.M.; Price, D.W.; Roberts, J.A.; Scott, J.B.; Wadhawan, A.; Ye, Z.; Tour, J.M. Nanotubes in Microwave Fields: Light Emission, Intense Heat, Outgassing, and Reconstruction. Chem. Mater. 2003, 15, 3969–3970. [Google Scholar] [CrossRef]
- Strozzi, M.; Pellicano, F. Linear vibrations of triple-walled carbon nanotubes. Math. Mech. Solids 2017, 23, 1456–1481. [Google Scholar] [CrossRef]
- He, X.Q.; Eisenberger, M.; Liew, K.M. The effect of van der Waals interaction modeling on the vibration characteristics of multiwalled carbon nanotubes. J. Appl. Phys. 2006, 100, 124317. [Google Scholar] [CrossRef]
- Pantano, A.; Parks, D.M.; Boyce, M.C. Mechanics of deformation of single- and multi-wall carbon nanotubes. J. Mech. Phys. Solids 2004, 52, 789–821. [Google Scholar] [CrossRef]
- Vázquez, E.; Prato, M. Carbon Nanotubes and Microwaves: Interactions, Responses, and Applications. ACS Nano 2009, 3, 3819–3824. [Google Scholar] [CrossRef]
- Wadhawan, A.; Garrett, D.; Perez, J.M. Nanoparticle-assisted microwave absorption by single-wall carbon nanotubes. Appl. Phys. Lett. 2003, 83, 2683–2685. [Google Scholar] [CrossRef]
- Tobias, G.; Mendoza, E.; Ballesteros, B. Functionalization of Carbon Nanotubes. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 911–919. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J.; Feng, H.; Li, J.; Pu, Z.; Yin, X. Facile preparation of the dendritic Fe3O4 with a core-shell microstructure in SiO2-B2O3-Al2O3-CaO-Fe2O3 glass-ceramic system for enhanced microwave absorbing performance. J. Alloys Compd. 2021, 877, 160147. [Google Scholar] [CrossRef]
- Green, M.; Liu, Z.; Xiang, P.; Liu, Y.; Zhou, M.; Tan, X.; Huang, F.; Liu, L.; Chen, X. Doped, conductive SiO2 nanoparticles for large microwave absorption. Light Sci. Appl. 2018, 7, 87. [Google Scholar] [CrossRef]
- Kainourgios, P.; Tziveleka, L.-A.; Kartsonakis, I.A.; Ioannou, E.; Roussis, V.; Charitidis, C.A. Silver Nanoparticles Grown on Cross-Linked Poly (Methacrylic Acid) Microspheres: Synthesis, Characterization, and Antifungal Activity Evaluation. Chemosensors 2021, 9, 152. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, D.; Tøtdal, B.; Holmen, A. Effect of catalyst preparation on the carbon nanotube growth rate. Catal. Today 2005, 100, 261–267. [Google Scholar] [CrossRef]
- Yue, R.; Chen, Q.; Li, S.; Zhang, X.; Huang, Y.; Feng, P. One-step synthesis of 1,6-hexanediamine modified magnetic chitosan microspheres for fast and efficient removal of toxic hexavalent chromium. Sci. Rep. 2018, 8, 11024. [Google Scholar] [CrossRef]
- Chiani, E.; Azizi, S.N.; Ghasemi, S. PdCu bimetallic nanoparticles decorated on ordered mesoporous silica (SBA-15)/MWCNTs as superior electrocatalyst for hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 25468–25485. [Google Scholar] [CrossRef]
- Mansfield, E.; Kar, A.; Hooker, S.A. Applications of TGA in quality control of SWCNTs. Anal. Bioanal. Chem. 2010, 396, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Abbas, S.M.; Hussain, S.; Siddiq, M.; Han, D.; Niu, L. Amino-functionalized silica anchored to multiwall carbon nanotubes as hybrid electrode material for supercapacitors. Mater. Sci. Energy Technol. 2018, 1, 70–76. [Google Scholar] [CrossRef]
- Yudianti, R.; Indrarti, L.; Onggo, H. Thermal Behavior of Purified Multi Walled Carbon Nanotube. J. Appl. Sci. 2010, 10, 1978–1982. [Google Scholar] [CrossRef][Green Version]
- Silva, M.A.; Felgueiras, H.P.; de Amorim, M.T.P. Carbon based membranes with modified properties: Thermal, morphological, mechanical and antimicrobial. Cellulose 2019, 27, 1497–1516. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim. Acta 2019, 672, 142–149. [Google Scholar] [CrossRef]
- Li, Z.Q.; Lu, C.J.; Xia, Z.P.; Zhou, Y.; Luo, Z. X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 2007, 45, 1686–1695. [Google Scholar] [CrossRef]
- Das, R.; Hamid, S.; Ali, M.; Ramakrishna, S.; Yongzhi, W. Carbon Nanotubes Characterization by X-ray Powder Diffraction—A Review. Curr. Nanosci. 2014, 11, 23–35. [Google Scholar] [CrossRef]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjöström, K. Elemental Iron as a Tar Breakdown Catalyst in Conjunction with Atmospheric Fluidized Bed Gasification of Biomass: A Thermodynamic Study. Energy Fuels 2006, 20, 890–895. [Google Scholar] [CrossRef]
- Azarshin, S.; Moghadasi, J.; Aboosadi, Z.A. Surface functionalization of silica nanoparticles to improve the performance of water flooding in oil wet reservoirs. Energy Explor. Exploit. 2017, 35, 685–697. [Google Scholar] [CrossRef]
- Ahangaran, F.; Hassanzadeh, A.; Nouri, S. Surface modification of Fe3O4@SiO2 microsphere by silane coupling agent. Int. Nano Lett. 2013, 3, 1–5. [Google Scholar] [CrossRef]
- Savi, P.; Giorcelli, M.; Quaranta, S. Multi-Walled Carbon Nanotubes Composites for Microwave Absorbing Applications. Appl. Sci. 2019, 9, 851. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, A.; Frederick, M.J.; Ramanath, G. Hybrid Microstructures from Aligned Carbon Nanotubes and Silica Particles. Small 2005, 1, 823–826. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, Y.; Li, X.; Yuan, S.; Jin, Z.; Xu, J.; Li, Y. Preferential Growth of Single-Walled Carbon Nanotubes on Silica Spheres by Chemical Vapor Deposition. J. Phys. Chem. B 2005, 109, 6963–6967. [Google Scholar] [CrossRef]
- Wei, B.Q.; Vajtai, R.; Jung, Y.; Ward, J.; Zhang, R.; Ramanath, G.; Ajayan, P.M. Organized assembly of carbon nanotubes. Nature 2002, 416, 495–496. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.; Kukovecz, Á.; Konya, Z.; Vilarinho, P.M. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Hekmatara, H.; Seifi, M.; Forooraghi, K.; Mirzaee, S. Synthesis and microwave absorption characterization of SiO2 coated Fe3O4-MWCNT composites. Phys. Chem. Chem. Phys. PCCP 2014, 16, 24069–24075. [Google Scholar] [CrossRef]
- Xiang, C.; Pan, Y.; Liu, X.; Sun, X.; Shi, X.; Guo, J. Microwave attenuation of multiwalled carbon nanotube-fused silica composites. Appl. Phys. Lett. 2005, 87, 123103. [Google Scholar] [CrossRef]
- Gomez, V.; Dunnill, C.W.; Barron, A.R. A microwave cured flux for the adhesion of ceramic particles using silica coated carbon nanotubes. Carbon 2015, 93, 774–781. [Google Scholar] [CrossRef]
- Wen, B.; Cao, M.-S.; Hou, Z.-L.; Song, W.-L.; Zhang, L.; Lu, M.-M.; Jin, H.-B.; Fang, X.-Y.; Wang, W.-Z.; Yuan, J. Temperature dependent microwave attenuation behavior for carbon-nanotube/silica composites. Carbon 2013, 65, 124–139. [Google Scholar] [CrossRef]



| Commercial MWCNTs | SiO2 | SiO2@CNTs | |
|---|---|---|---|
| Physical Properties | |||
| Size (nm) | 25–35 (tube diameter) | 350 (sphere) | - |
| Zeta potential (mV) | - | −40 | - |
| Thermal degradation (°C) | 450–600 [39] | stable | 463–670 |
| Id/Ig | 0.6 | - | 2.0 |
| Heat production (°C) | 209.0 | 59.2 | 173.0 |
| Chemical Properties | |||
| Surface functionalization | inert | Fe; –OH | –OH |
| Composition (wt.%) | 94.1–C; 5.9–O | 49.2–O; 48.0–Si; 2.8–Fe | 37.0–C; 31.5–O; 30.3–Si; 1.2–Fe |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kainourgios, P.; Kartsonakis, I.A.; Charitidis, C.A. Synthesis and Characterization of SiO2@CNTs Microparticles: Evaluation of Microwave-Induced Heat Production. Fibers 2021, 9, 81. https://doi.org/10.3390/fib9120081
Kainourgios P, Kartsonakis IA, Charitidis CA. Synthesis and Characterization of SiO2@CNTs Microparticles: Evaluation of Microwave-Induced Heat Production. Fibers. 2021; 9(12):81. https://doi.org/10.3390/fib9120081
Chicago/Turabian StyleKainourgios, Panagiotis, Ioannis A. Kartsonakis, and Costas A. Charitidis. 2021. "Synthesis and Characterization of SiO2@CNTs Microparticles: Evaluation of Microwave-Induced Heat Production" Fibers 9, no. 12: 81. https://doi.org/10.3390/fib9120081
APA StyleKainourgios, P., Kartsonakis, I. A., & Charitidis, C. A. (2021). Synthesis and Characterization of SiO2@CNTs Microparticles: Evaluation of Microwave-Induced Heat Production. Fibers, 9(12), 81. https://doi.org/10.3390/fib9120081









