Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of Starch
2.2. Preparation of Bioplastics Film
3. Characterization
3.1. Tensile Test
3.2. Thickness Measurement
3.3. Test for Moisture Content
3.4. Water Solubility Test
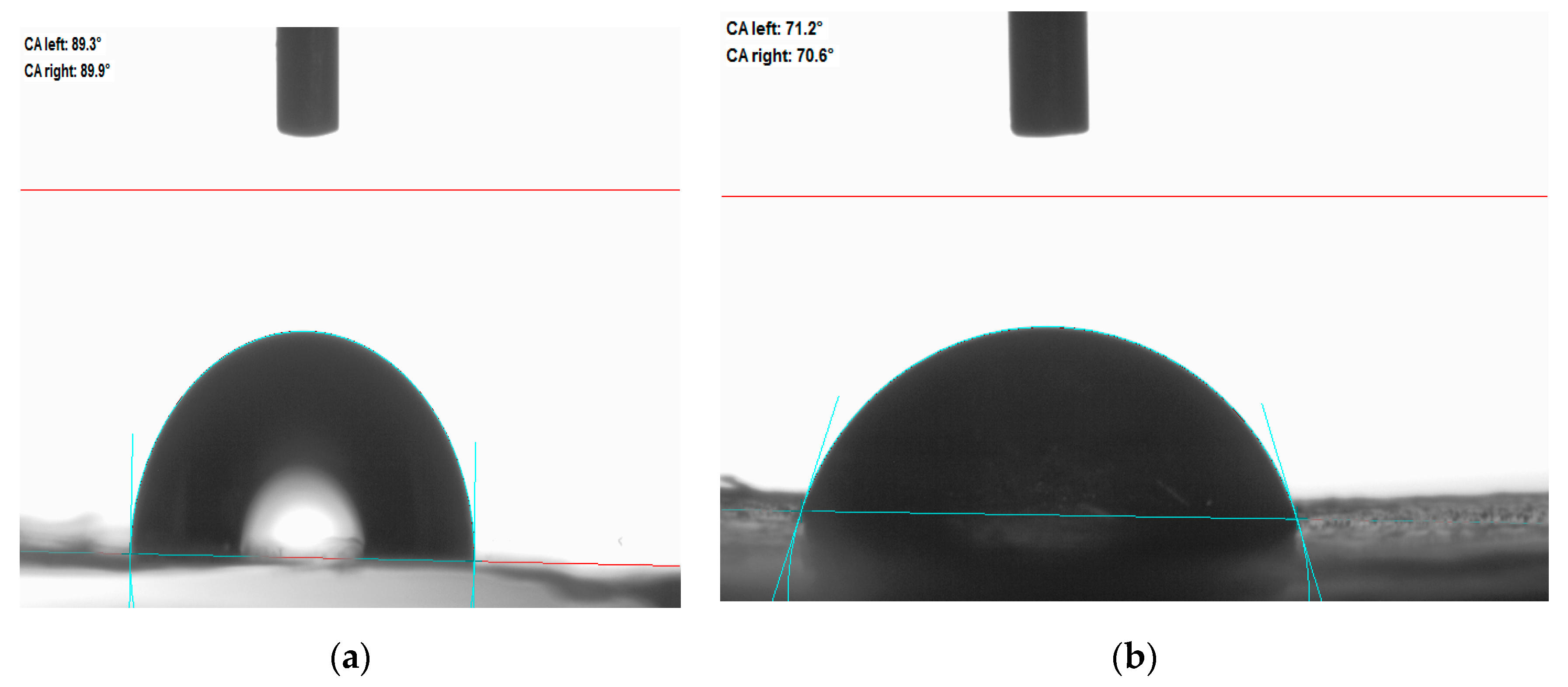
3.5. Water Contact Angle Measurement
3.6. Biodegradability Test
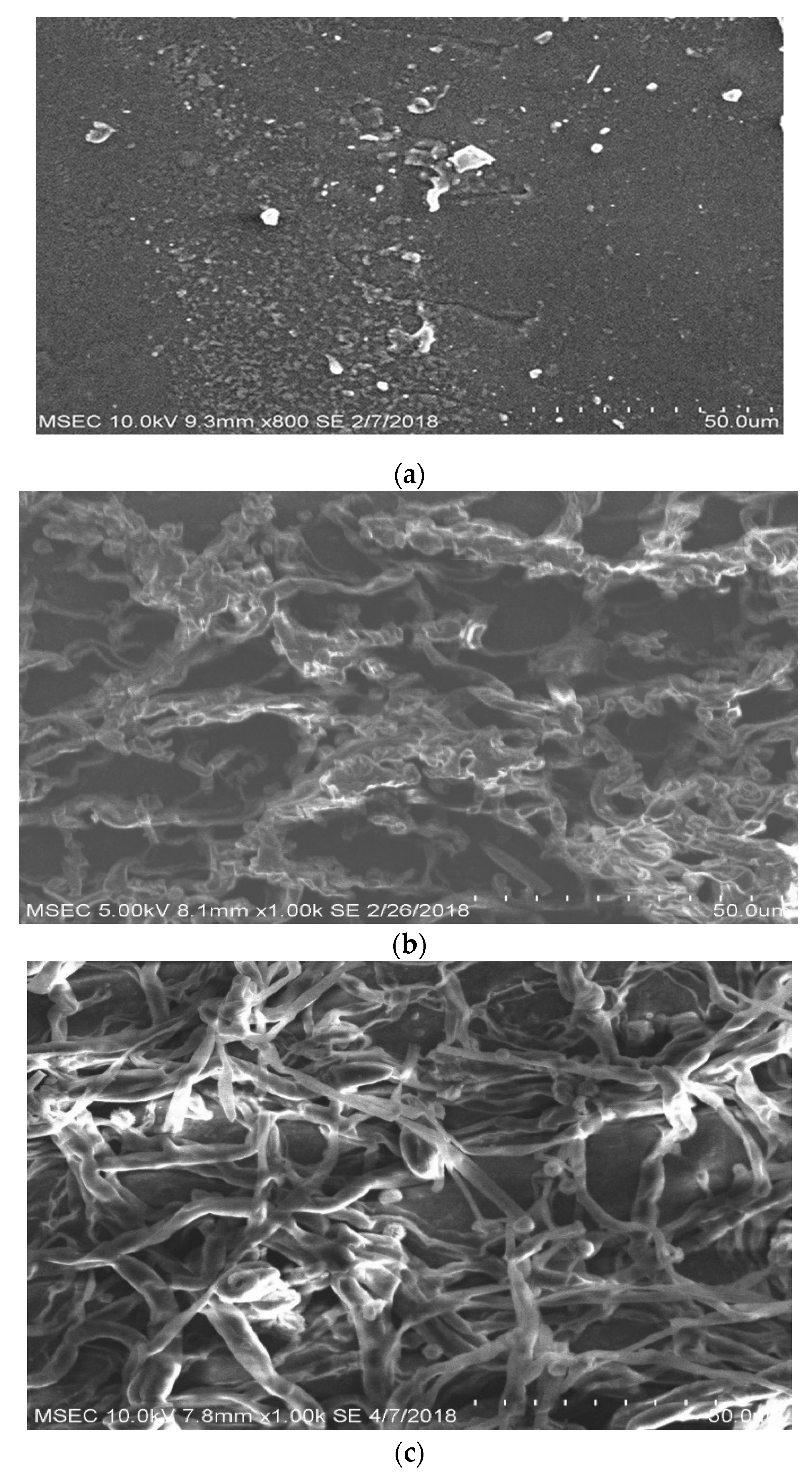
3.7. Scanning Electron Microscopy (SEM)
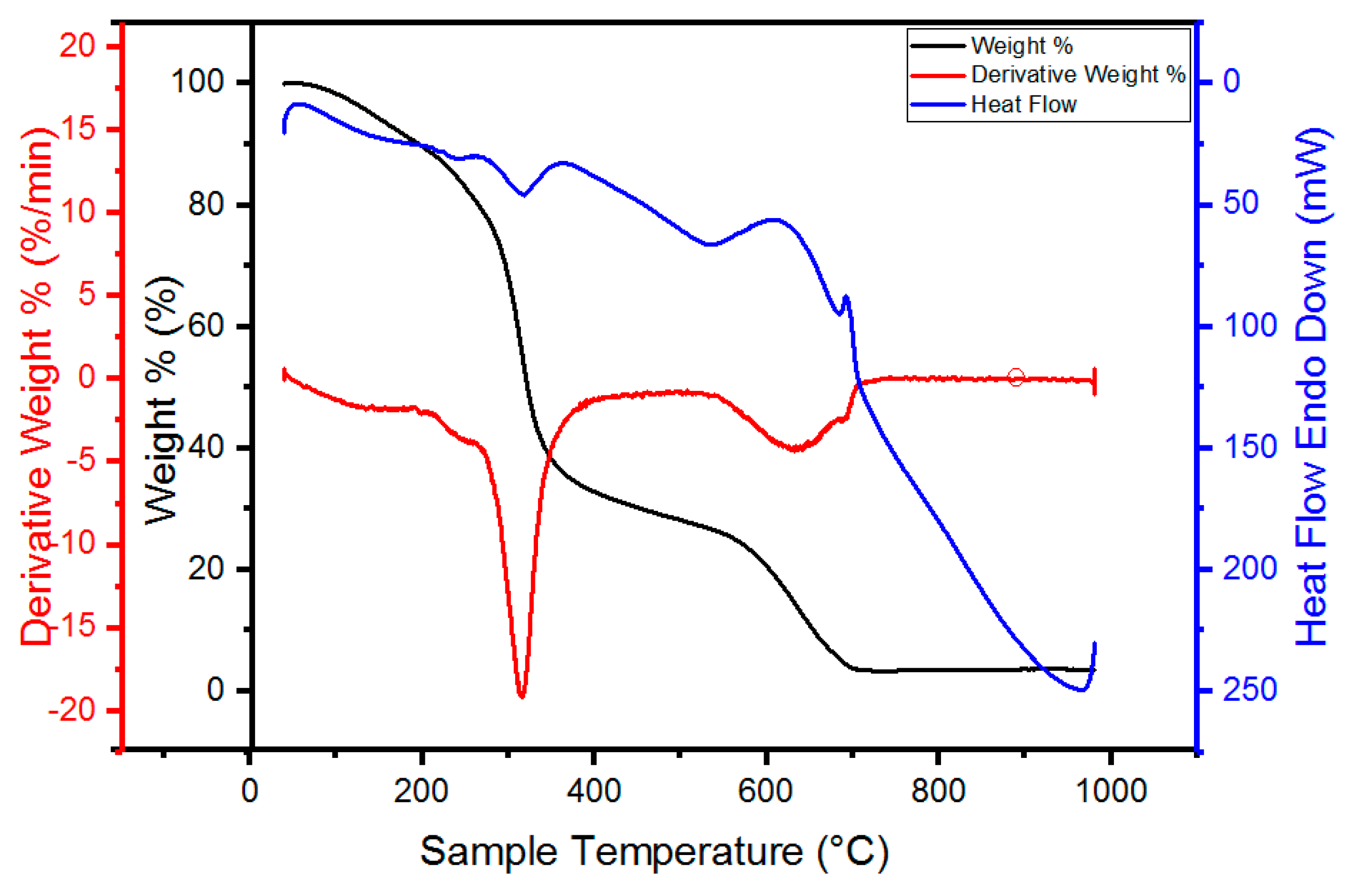
3.8. Thermogravimetric Analysis
3.9. Sealing Properties of Bioplastics
4. Results and Discussions
4.1. Tensile Properties
4.2. Bioplastic Thickness
4.3. Moisture Content
4.4. Water Solubility
4.5. Water Contact Angle
4.6. Biodegradability Properties
4.7. Thermogravimetry Analysis
4.8. Sealing Properties of Bioplastics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayer, I.S.; Guzman-Puyol, S.; Heredia-Guerrero, J.A.; Ceseracciu, L.; Pignatelli, F.; Ruffilli, R.; Cingolani, R.; Athanassiou, A. Direct transformation of edible vegetable waste into bioplastics. Macromolecules 2014, 47, 5135–5143. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Jain, R.; Tiwari, A. Biosynthesis of planet friendly bioplastics using renewable carbon source. J. Environ. Health Sci. Eng. 2015, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Azahari, N.; Othman, N.; Ismail, H. Biodegradation studies of polyvinyl alcohol/corn starch blend films in solid and solution media. J. Phys. Sci. 2011, 22, 15–31. [Google Scholar]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.; Asim, M.; Saba, N. Natural fiber reinforced polylactic acid composites: A review. Polym. Compos. 2019, 40, 446–463. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Simon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552. [Google Scholar] [CrossRef]
- Abidin, M.Z.A.Z.; Julkapli, N.M.; Juahir, H.; Azaman, F.; Sulaiman, N.H.; Abidin, I.Z. Fabrication and properties of chitosan with starch for packaging application. Malays. J. Anal. Sci. 2015, 19, 1032–1042. [Google Scholar]
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch Based Bio-Plastics: The Future of Sustainable Packaging. Open J. Polym. Chem. 2018, 8, 21–33. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.; Jawaid, M.; Zahari, N.I. Cassava/sugar palm fiber reinforced cassava starch hybrid composites: Physical, thermal and structural properties. Int. J. Biol. Macromol. 2017, 101, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Cassava: Its polymer, fiber, composite, and application. Polym. Compos. 2017, 38, 555–570. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.M.; Jawaid, M.; Zahari, N.I. Effect of various plasticizers and concentration on the physical, thermal, mechanical, and structural properties of cassava-starch-based films. Starch-Stärke 2017, 69, 1500366. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.; Jawaid, M.; Zahari, N.I. Preparation and characterization of cassava bagasse reinforced thermoplastic cassava starch. Fibers Polym. 2017, 18, 162–171. [Google Scholar] [CrossRef]
- Edhirej, A.; Sapuan, S.; Jawaid, M.; Zahari, N.I. Tensile, Barrier, Dynamic Mechanical, and Biodegradation Properties of Cassava/Sugar Palm Fiber Reinforced Cassava Starch Hybrid Composites. BioResources 2017, 12, 7145–7160. [Google Scholar]
- Edhirej, A.; Sapuan, S.; Jawaid, M.; Ismarrubie Zahari, N. Preparation and Characterization of Cassava Starch/Peel Composite Film. Polym. Compos. 2018, 39, 1704–1715. [Google Scholar] [CrossRef]
- Sanyang, M.; Sapuan, S.; Jawaid, M.; Ishak, M.; Sahari, J. Development and characterization of sugar palm starch and poly (lactic acid) bilayer films. Carbohydr. Polym. 2016, 146, 36–45. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of sugar palm-derived cellulose reinforcement on the mechanical and water barrier properties of sugar palm starch biocomposite films. BioResources 2016, 11, 4134–4145. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Muniandy, Y.; Sapuan, S.M.; Sahari, J. Tea Tree (Melaleuca alternifolia) Fiber as Novel Reinforcement Material for Sugar Palm Biopolymer Based Composite Films. BioResources 2017, 12, 3751–3765. [Google Scholar] [CrossRef][Green Version]
- Sanyang, M.; Ilyas, R.; Sapuan, S.; Jumaidin, R. Sugar Palm Starch-Based Composites for Packaging Applications. In Bionanocomposites for Packaging Applications; Springer: Berlin, Germany, 2018; pp. 125–147. [Google Scholar]
- Lii, C.; Shao, Y.-Y.; Tseng, K.-H. Gelation mechanism and rheological properties of rice starch. Cereal Chem. 1995, 72, 393–400. [Google Scholar]
- Ratnayake, W.S.; Jackson, D.S. Gelatinization and solubility of corn starch during heating in excess water: new insights. J. Agric. Food Chem. 2006, 54, 3712–3716. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, X.; Seib, P. Mechanical properties of poly (lactic acid) and wheat starch blends with methylenediphenyl diisocyanate. J. Appl. Polym. Sci. 2002, 84, 1257–1262. [Google Scholar] [CrossRef]
- Young, A.H. Fractionation of starch. In Starch: Chemistry and Technology (Second Edition); Elsevier: Amsterdam, The Netherland, 1984; pp. 249–283. [Google Scholar]
- Ceseracciu, L.; Heredia-Guerrero, J.A.; Dante, S.; Athanassiou, A.; Bayer, I.S. Robust and biodegradable elastomers based on corn starch and polydimethylsiloxane (PDMS). ACS Appl. Mater. Interfaces 2015, 7, 3742–3753. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; De la Caba, K. Functional properties of chitosan-based films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Costa, D.; Yamashita, F.; Martelli, S.M.; Jesus, R.C.; Alganer, K.; Collares-Queiroz, F.P.; Innocentini-Mei, L.H. Comparative study of processing methods for starch/gelatin films. Carbohydr. Polym. 2013, 95, 681–689. [Google Scholar] [CrossRef]
- Schirmer, M.; Höchstötter, A.; Jekle, M.; Arendt, E.; Becker, T. Physicochemical and morphological characterization of different starches with variable amylose/amylopectin ratio. Food Hydrocoll. 2013, 32, 52–63. [Google Scholar] [CrossRef]
- Borges, J.; Romani, V.; Cortez-Vega, W.; Martins, V. Influence of different starch sources and plasticizers on properties of biodegradable films. Int. Food Res. J. 2015, 22, 2346–2351. [Google Scholar]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/potato starch edible biocomposite films: Correlation between morphology and physical properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef]
- Gómez-Heincke, D.; Martínez, I.; Stading, M.; Gallegos, C.; Partal, P. Improvement of mechanical and water absorption properties of plant protein based bioplastics. Food Hydrocoll. 2017, 73, 21–29. [Google Scholar] [CrossRef]
- Kulshreshtha, Y.; Schlangen, E.; Jonkers, H.; Vardon, P.; Van Paassen, L. Corncrete: A corn starch based building material. Constr. Build. Mater. 2017, 154, 411–423. [Google Scholar] [CrossRef]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; de la Caba, K. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biol. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- Zakaria, N.; Muhammad, N.; Sandu, A.; Abdullah, M. Effect of Mixing Temperature on Characteristics of Thermoplastic Potato Starch Film. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Brisol, UK, 2018; p. 012083. [Google Scholar]
- Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable polymers for food packaging: a review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and food packaging: A review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Gaspar, M.; Benkő, Z.; Dogossy, G.; Reczey, K.; Czigany, T. Reducing water absorption in compostable starch-based plastics. Polym. Degrad. Stab. 2005, 90, 563–569. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Ghasemlou, M.; Shojaee-Aliabadi, S.; Noghabi, M.S.; Khaksar, R. Characterization of soluble soybean polysaccharide film incorporated essential oil intended for food packaging. Carbohydr. Polym. 2013, 98, 1127–1136. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jane, J.-L.; Lamsal, B. Hydroxypropylation improves film properties of high amylose corn starch. Ind. Crops Prod. 2017, 95, 175–183. [Google Scholar] [CrossRef]
- Larotonda, F.D.; Matsui, K.N.; Soldi, V.; Laurindo, J.B. Biodegradable films made from raw and acetylated cassava starch. Braz. Arch. Biol. Technol. 2004, 47, 477–484. [Google Scholar] [CrossRef]
- Woggum, T.; Sirivongpaisal, P.; Wittaya, T. Properties and characteristics of dual-modified rice starch based biodegradable films. Int. J. Biol. Macromol. 2014, 67, 490–502. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Fontes, L.C.B.; Gonçalves, P.V.d.M.; Milanez, C.R.; Steel, C.J.; Collares-Queiroz, F.P. Films and edible coatings based on native starches and gelatin in the conservation and sensory acceptance of Crimson gra. Food Sci. Technol. 2007, 27, 369–375. [Google Scholar] [CrossRef]
- Kavoosi, G.; Dadfar, S.M.M.; Purfard, A.M. Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci. 2013, 78, E244–E250. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible films and coatings based on starch/gelatin: Film properties and effect of coatings on quality of refrigerated Red Crimson grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Martelli, S.M.; Laurindo, J.B. Chicken feather keratin films plasticized with polyethylene glycol. Int. J. Polym. Mater. 2012, 61, 17–29. [Google Scholar] [CrossRef]
- Li, Y.; Ceylan, M.; Shrestha, B.; Wang, H.; Lu, Q.R.; Asmatulu, R.; Yao, L. Nanofibers support oligodendrocyte precursor cell growth and function as a neuron-free model for myelination study. Biomacromolecules 2013, 15, 319–326. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S. Thermal properties of coir and pineapple leaf fibre reinforced polylactic acid hybrid composites. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Brisol, UK, 2018; p. 012019. [Google Scholar]


| Source | Amylose (in %) | Amylopectin (in %) |
|---|---|---|
| Arrowroot | 20.5 | 79.5 |
| Banana | 17 | 83 |
| Cassava | 18.6 | 81.4 |
| Corn | 28 | 72 |
| Potato | 17.8 | 82.2 |
| Rice | 35 | 65 |
| Tapioca | 16.7 | 83.3 |
| Wheat | 20 | 80 |
| Properties | Corn Starch | Rice Starch |
|---|---|---|
| Moisture content (in %) | 10.82 | 11.24 |
| Ash content (in %) | 0.32 | 0.29 |
| Protein (in %) | 0.38 | 0.43 |
| Fat (in %) | 0.32 | 0.34 |
| Fiber (in %) | 0.10 | 0.12 |
| Amylose (in %) | 29.4 | 33.6 |
| Density (g/ml) | 1.356 | 1.282 |
| pH | 6.72 | 6.82 |
| Sample | Rice Starch | Corn Starch | Glycerol | Citric Acid | Gelatin | Water |
|---|---|---|---|---|---|---|
| Weight (in Grams) | ||||||
| S1 | 5 | 5 | 3 | 1 | 2 | 100 |
| S2 | 5.5 | 4.5 | 3 | 1 | 2 | 100 |
| S3 | 6 | 4 | 3 | 1 | 2 | 100 |
| S4 | 6.5 | 3.5 | 3 | 1 | 2 | 100 |
| S5 | 7 | 3 | 3 | 1 | 2 | 100 |
| Samples | Tensile Strength (MPa) | Young’s Modulus (GPa) | Elongation (in %) |
|---|---|---|---|
| S1 | 6.11 | 0.09 | 3.38 |
| S2 | 7.3 | 0.11 | 5.1 |
| S3 | 10.6 | 0.15 | 5.3 |
| S4 | 11.38 | 0.17 | 6.19 |
| S5 | 12.5 | 0.183 | 6.8 |
| Sample | Initial Weight Wi (in gram) | Final Weight Wf (in gram) | Moisture Content in Percentage (%) |
|---|---|---|---|
| S1 | 0.311 | 0.265 | 12.9 |
| S2 | 0.291 | 0.255 | 12.3 |
| S3 | 0.280 | 0.241 | 13.9 |
| S4 | 0.285 | 0.246 | 13.6 |
| S5 | 0.282 | 0.249 | 11.7 |
| Sample | Initial Weight Wo (in grams) | Final Weight Wf (in grams) | Water Solubility in (%) |
|---|---|---|---|
| S1 | 0.301 | 0.265 | 11.9 |
| S2 | 0.291 | 0.251 | 11.6 |
| S3 | 0.280 | 0.245 | 12.5 |
| S4 | 0.282 | 0.247 | 12.4 |
| S5 | 0.311 | 0.275 | 11.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers 2019, 7, 32. https://doi.org/10.3390/fib7040032
Marichelvam MK, Jawaid M, Asim M. Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers. 2019; 7(4):32. https://doi.org/10.3390/fib7040032
Chicago/Turabian StyleMarichelvam, M. K., Mohammad Jawaid, and Mohammad Asim. 2019. "Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials" Fibers 7, no. 4: 32. https://doi.org/10.3390/fib7040032
APA StyleMarichelvam, M. K., Jawaid, M., & Asim, M. (2019). Corn and Rice Starch-Based Bio-Plastics as Alternative Packaging Materials. Fibers, 7(4), 32. https://doi.org/10.3390/fib7040032






