Abstract
Two high-performance bacterial cellulose (BC) nanofiber-based hybrid structures were produced using an in situ self-assembly approach, one with microfibrillated cellulose (MFC) and another with sisal fiber, by incorporating them in the fermentation media. The fabricated BC-MFC hybrid and BC-sisal hybrid fibers showed enhanced mechanical properties compared to pure BC and sisal fibers, respectively. Tensile tests indicated BC-MFC hybrid and their nanocomposites fabricated with soy protein isolate (SPI) resin had better tensile properties than corresponding BC and BC-SPI nanocomposites. This was because of the uniform distribution of MFC within the BC nanofiber network structure which reduced the defects such as pores and voids or intersections of the BC nanofibers. BC-sisal hybrid fibrous structures were obtained after BC nanofibers self-assembled on the surface of the sisal fibers during the fermentation. The results of the microbond tests indicated that the BC-sisal hybrid fiber/SPI resin bond strength was higher than the control sisal fiber/SPI resin bond with p value of 0.02 at the significance level of 0.05. Higher bond strength is preferred since it can potentially lead to better tensile properties of the composites. The presented work suggests a novel route to fabricate hybrid nanocomposites with higher functional properties.
1. Introduction
Bacterial cellulose (BC) secreted by Acetobacter xylinum is considered to be a sustainable and promising nanofibrous extracellular material. BC, with an identical polysaccharide structure to plant derived cellulose, consisting of a linear chain of β(1→4)-linked d-glucose units, is also fully biodegradable [1]. However, BC fibers have diameters that range between 55 and 60 nanometers and display many unique characteristics, such as high purity and high water-holding capacity [1,2]. Due to their high crystallinity, high degree of polymerization, and higher molecular orientation they also have high strength and stiffness (Young′s modulus), as well as higher thermal stability compared to conventional plant-derived cellulose fibers [1,3,4,5]. BC membranes obtained through static fermentation tend to have a highly porous structure combined with strong biocompatibility [1,6,7,8,9] and excellent environmental biodegradability [3,4,5,10] and, importantly, their size, shape, and thickness can be easily controlled [1,11,12]. BC materials in various forms have already been used in many applications including binding agents for fibers and other materials [1,13], cosmetics [2], high-quality paper [1], foods (e.g., nata-de-coco) [2], loud speaker diaphragms [13], textiles and apparel [13], artificial skin and blood vessels [14,15], nanocomposite membranes [1,3,11,12,13], and others [16,17]. Due to their excellent tensile properties, several studies on BC-based hybrids and nanocomposites using various fabrication methods [1,4,18] for different applications have been conducted in the past couple of decades [1].
In situ self-assembly fabrication approach is a novel and facile way to produce BC-based fibrous hybrid structures and nanocomposites. The Acetobacter xylinum bacterial strain has been found to grow preferably on the surfaces of some polymers and natural fibers when placed in the culture medium, rather than freely in pure medium [13,19,20,21,22]. The natural fibers and some natural polymers (e.g., starch) can provide ideal substrates for the bacteria to grow on. As a result, BC-based hybrid structures and nanocomposites can be formed through the fermentation process in the presence of natural fibers or polymers. The plentiful hydroxyl groups present on the surfaces of cellulosic fibers and polymeric substrates and the BC help to promote strong interaction between the two through hydrogen bonding [13,19,20,21,22].
Microfibrillated cellulose (MFC) has been commonly produced by mechanical treatment, such as shearing, of pulp, e.g., kraft pulp. Cellulose fibers have been further sheared to submicrometer and nanometer size (diameter) fibrils by combining the processes of high-pressure homogenization and refining [10,23,24,25,26]. Since the diameters or widths of most fibrils in MFC are in submicrometer range, many smaller than 100 nm, they are considered as nanofibrils but can have high aspect ratio [10,23,24,26,27,28,29]. Due to their high molecular orientation and high crystallinity, the nanofibrils in the MFC have high tensile properties. The modulus of such nanofibrils is estimated to be around 140 GPa and tensile strength between 2 GPa and 6 GPa [30]. Both of these properties are comparable to the high strength aramid fibers such as Kevlar®. Sisal fiber is a plant derived natural fiber which possesses high tensile properties and good biodegradability. Sisal fibers have been used in fabricating environmentally friendly and biodegradable ‘green’ composites by combining them with biodegradable resins [13,31].
Soy protein isolate (SPI) is an abundant and inexpensive natural resource with protein content (polypeptide chains) of over 90% that contain several reactive amino acid residues [32]. It has been used as a ‘green’ resin of choice in many applications such as adhesives, binders, composites, and many other products [33]. SPI based thermoset resins also have good strength and biodegradability. Their properties can be further enhanced by nanoadditives, such as nanoclay, nanofibrils, etc. As a result, SPI based resin has been regarded as excellent for fabricating fiber-reinforced ‘green’ composites [32,34].
‘Green’ composites are defined as those that are fabricated by combining natural plant-based fibers with non-petroleum based biodegradable resins that are environment-friendly, fully biodegradable, sustainable, and will not harm nature [33]. These composites have attracted significant attention since they can be easily composted at the end of their service life as compared to conventional composites which mostly end up in landfills [4,32,33,34]. The composites fabricated using BC nanofibers, MFC, sisal fibers, and SPI are considered fully ‘green’ composites since both fibers and resin are biodegradable and derived from sustainable sources.
In the present study, both of BC-MFC and BC-sisal hybrid fibrous structures were prepared using in situ self-assembly approach in fermentation media. Tensile tests were performed to characterize the nanocomposites prepared using these hybrid structures with SPI resin. In addition, microbond tests were performed to investigate the BC/sisal hybrid fibrous structure/SPI resin interfacial shear stress (IFSS) to get a deeper understanding of the effect of incorporating BC layer around the sisal fibers. Higher fiber/resin IFSS can be expected to result in improved tensile properties of the nanocomposites.
The present study also describes how MFC and sisal fibers can be utilized as substrates to self-assemble high-performance BC nanofibers onto their surfaces to form hybrid fibrous structures via a novel one-pot fermentation route. The two hybrids can be utilized to fabricate green nanocomposites using plant protein/starch based or other biodegradable resins, with better mechanical properties. Once fully developed, these green composites could be suitable for many applications where conventional, petroleum-based composites are currently used, including high-end racket frames, ski poles, circuit boards, automobile interiors, etc.
2. Experimental
2.1. Design of Samples and Tests
BC-MFC hybrid (membrane) was designed to be fabricated using the in situ self-assembly approach in MFC containing culture medium in a static incubator. The corresponding BC-MFC-SPI nanocomposite (membrane) was designed to be fabricated via immersing the BC-MFC hybrid fibrous structure into the SPI resin to obtain the best possible resin impregnation prior to curing it. BC, SPI resin, and BC-SPI (composite membranes) were fabricated to be used as test samples and for comparing the results. After fabrication, tensile tests were performed on all membrane samples to evaluate their mechanical properties.
BC-sisal hybrid (fibrous structure) was designed to be fabricated through in-situ self-assembly approach by adding sisal fibers to the culture medium kept in a rotary shaker. A layer of BC nanofibers was expected to self-assemble onto the sisal fiber surfaces forming the BC-sisal hybrid fibrous structure. The mechanical properties (interfacial bond) of BC-sisal hybrid (fiber) with resin (SPI) were evaluated using the microbond method for interfacial shear strength (IFSS). The interfacial bond of sisal fiber with resin, as control, were also tested for comparison.
2.2. Microorganism and Culture Media
Acetobacter xylinum, ATCC 23769, was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and used as the model BC producing strain and was kept on agar plates. The agar in the plates contained 5 g/L tryptone, 5 g/L yeast extract, 20 g/L agar, and 25 g/L d-mannitol. All chemicals used for preparation of agar plates and culture medium were obtained from Fisher Scientific (Fair Lawn, NJ, USA) and were used as received.
2.3. In Situ Fabrication of BC
The BC producing strain was extracted from the agar plate using an inoculation loop and was then inoculated into a conical flask containing mannitol culture medium as the seed culture period of about 10 days. The pH value of the medium was initially kept at 5.0 and was not regulated thereafter for the entire culture period. The seed culture was incubated on a rotary shaker (MAXQ 4450, Thermo Scientific, Thermo Fisher Scientific Inc., Hudson, NH, USA) at 130 rpm for two days at 30 °C, and 9 mL of this seed culture was inoculated into a 150-mL culture medium in 1000-mL conical flask for BC production. Initial pH of 5.0 was used for the cultivation in a static incubator for 10 days. Additionally, a temperature of 30 °C was maintained throughout the cultivations. The produced BC pellicles were harvested from the surface of the mannitol culture medium after the 10-day incubation period. They were then rinsed successively with water and 1% (w/v) aqueous NaOH at 90 °C for 15 min, and then further rinsed with deionized water to remove all remaining microbial contaminants and obtain purified pellicles.
2.4. In Situ Fabrication of BC-MFC Hybrid Fibrous Structure
MFC, as water based slurry (KY-100G) was received from Daicel Chemical Industries, Kita-Ku, Osaka, Japan. Homogeneous MFC containing culture medium was prepared by adding MFC (5% by weight of the culture media) into the mannitol culture medium (described above) to produce BC-MFC hybrid fibrous structure. The seed liquid (6 mL) was then inoculated into a 100-mL MFC-containing culture medium (600-mL conical flask) after two days in seed culture (as described above). Conditions of initial pH of 5.0 and 30 °C were applied for BC cultivation in a static incubator for 10 days. The BC-MFC fibrous hybrid structure produced on the surface of the MFC-containing mannitol culture medium was harvested after the 10-day incubation period. The BC-MFC hybrid fibrous structure was then rinsed successively with water and 1% (w/v) aqueous NaOH at 90 °C for 15 min and then further rinsed with deionized water to remove all remaining microbial contaminants. During the multi-step rinsing process, the BC-MFC fibrous hybrid structure was treated with extreme care so as to avoid any separation of the MFC from the BC pellicle.
2.5. In Situ Fabrication of BC-Sisal Hybrid Fibrous Structure
The BC-producing strain, extracted from the agar plate, was used to inoculate the mannitol culture medium as the seed culture, kept in a conical flask. During the culture, the initial pH value of the medium was adjusted to 5.0 and was not regulated afterwards throughout the fermentation process. The seed culture was incubated at 30 °C and at 130 rpm on the same rotary shaker for two days. After that, 9 mL of the culture medium was inoculated into a 150-mL sisal-mannitol culture medium containing 20 sisal fibers of 12 cm length with weight of 0.1 g in a 1000-mL conical bottle to produce the BC-sisal fibrous hybrid structure. The cultivation was conducted at the initial pH of 5.0, at 30 °C while on a rotary shaker (105 rpm) for three days. After incubation, a layer of BC nanofibers was seen to have self-assembled onto the surface of sisal fibers forming the BC-sisal hybrid fibrous structure. The BC-modified sisal fibers were then harvested and purified by rinsing with water and 1% (w/v) aqueous NaOH at 90 °C for 15 min successively and then further rinsed by deionized water to remove contaminants from the microbial product.
2.6. Fabrication of BC-MFC-SPI Nanocomposites and BC-Sisal-SPI Microbond Specimens
Pre-cured SPI resin was prepared prior to fabricating BC-MFC-SPI nanocomposites and BC-sisal-SPI microbond specimens. SPI powder and deionized water were initially mixed in a ratio of 1:15 (w/w). Glycerol (plasticizer) was added (15% by weight of SPI) and pH value of the solution was adjusted to 10 by adding required amount of sodium hydroxide [22]. To obtain ′precured′ SPI resin, the solution was maintained at 75 °C while stirring continuously for 30 min. The precuring process helps to denature the globular protein through opening of the molecules.
To prepare BC-MFC-SPI nanocomposite specimens, a wet BC-MFC hybrid fibrous structure was initially immersed into pre-cured SPI resin. The best possible resin impregnation into the BC-MFC hybrid fibrous structure was obtained by applying 12 h of ultrasonication. The wet BC-MFC-SPI nanocomposite sheet was dried at 35 °C in an air-circulating oven (8 h) to obtain the prepreg (i.e., pre-impregnated, and/or partially cured composite sheets). The prepreg was further cured by hot pressing using a Carver Hydraulic hot press (model 3981-4PROA00, Wabash, IN, USA). The curing was conducted at 120 °C for 25 min under a pressure of 7 MPa to obtain the BC-MFC-SPI nanocomposite. The BC-MFC (BC:MFC (w/w) = 1:1) fiber content in all BC-MFC-SPI nanocomposites was around 50% and the thickness of the nanocomposites was in the range of 0.2 mm. The cured BC-MFC-SPI nanocomposites were conditioned at ASTM conditions of 21 °C and 65% relative humidity (RH) for three days prior to characterizing their tensile properties. To compare properties, BC-SPI (BC:SPI (w/w) = 1:1) nanocomposites (control) were also prepared using the identical process.
To prepare a BC-Sisal-SPI microbond specimen, a single BC-sisal hybrid fiber was mounted on a paper tab and glued at both ends using cyanoacrylate glue. A small microdrop (microbead) of the pre-cured SPI resin was placed on the BC-sisal hybrid fiber. At least eight fibers with acceptable microbeads were prepared and tested. The fibers with microbeads were kept at room temperature for at least 4 h to allow the water from the resin to partially evaporate after which they were heated at 120 °C for 60 min to fully cure the resin in an air circulating oven. This process has been proven to cross-link the soy protein [29,30]. All specimens were conditioned at the American Society for Testing and Materials (ASTM) conditions of 21 °C and 65% RH for 24 h prior to carrying out the microbond tests and obtain the interfacial shear strength (IFSS) values [31,35]. Sisal fiber-SPI microbond specimens (control) were also prepared using the same method, for comparison.
2.7. Characterization
2.7.1. Tensile Test
All specimens were tensile tested using an Instron universal tensile testing machine (Instron, model 5566, Canton, MA, USA). A precise cutter was used to cut the specimens of dried BC-MFC hybrid composites, BC-SPI nanocomposites, BC-MFC-SPI nancomposites, and their corresponding components (BC and SPI sheets), into 10 mm wide and 60 mm long strips. According to ASTM D-882-02, the Young′s modulus values of all specimens were determined from the tensile test results. A gauge length of 30 mm and a strain rate of 0.02/min was maintained for all specimens. Eight successful tensile tests were conducted for each kind of specimens. All specimens were conditioned at the ASTM conditions of 21 °C and 65% RH for three days before carrying out the tensile tests.
2.7.2. Interfacial Shear Strength (IFSS) Test
IFSS tests were carried out using microbond method to investigate the sisal fiber/SPI resin and BC-sisal hybrid fiber/SPI resin interfacial bond and to understand the difference made by the self-assembled BC on sisal fibers [31,32,33,34,35,36].
Figure 1 shows a schematic of the microbond method for acquiring the IFSS value. The embedded length (L) and fiber diameter (d) were measured using a calibrated optical microscope (Model BX51, Olympus Optical Co., Tokyo, Japan), prior to conducting the IFSS test. To ensure accurate measurements, d and L were measured again after the IFSS tests. The microbeads were observed to shrink in diameter and become smaller and smaller in diameter with water evaporation. However, in the lengthwise direction no shrinkage was observed, as discussed later [31,35]. The microbond test was carried out on the same Instron universal tester (Instron, model 5566, Canton, MA, USA), using a microvise. The microvise plates were placed just above the microbead and brought closer until they almost touched the fiber. After that the fiber was pulled out from the resin until the microbead debonded. The following equation was used to calculate the IFSS, τ [31,36]:
where F is the force needed to debond the microbead. The shear strength was assumed to be uniform along the entire fiber/microbead interface. Eight successful tests were conducted to obtain average IFSS values [31,35].
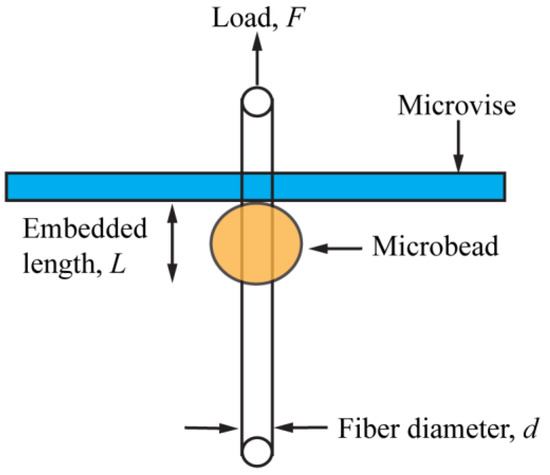
Figure 1.
Schematic for the microbond test [31]. Reprinted with permission; 2010, VSP/Brill NV, Leiden.
3. Results and Discussion
3.1. Mechanisms of In Situ Formation of BC-MFC and BC-Sisal Hybrid Fibrous Structures
Both BC-MFC and BC-sisal hybrid fibrous structures were produced using an in situ self-assembly approach during fermentation by directly dispersing MFC and sisal fiber to the culture media, respectively, as described earlier.
The BC-MFC hybrid fibrous structure is shown in Figure 2 as a schematic (a) and an SEM (Leica model 440X, Wetzlar, Germany) image (b). Since the MFC is already uniformly dispersed in the culture medium, the BC nanofibers become intertwined with MFC as well as each other to form the network-like fibrous structure during the fermentation process as the bacteria move randomly around the dispersed MFC. As a result, the MFC fibers become trapped within the BC network structure via the self-assembly process. Due to the micro/nano-scale size of the fibrils in MFC it is easier for the bacteria to go around them. It is clear that the MFC fibers are capable of homogenously filling the pores present in the BC nanofiber network structure and, thus, eliminating many potential weak points (pores or voids) in the BC network. Earlier researchers have shown that the BC pellicles have large amounts of pores or voids that are somewhat uniformly distributed [5]. Such structures have made BC membranes suitable for ultrafiltration [1].
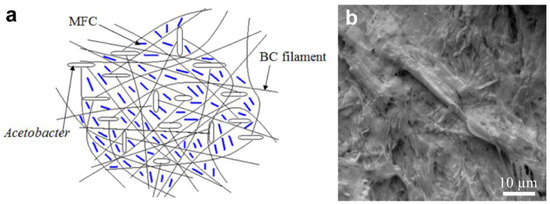
Figure 2.
BC-MFC hybrid fibrous structure. Schematic of the BC-MFC hybrid (a) and a SEM image of the BC-MFC hybrid (b).
Figure 3 presents the BC-sisal hybrid fibrous structures, including a schematic (a), a photograph of BC-sisal hybrid fibers in the culture medium (b) and an optical image showing the BC-sisal hybrid fiber (c). During the BC fermentation process, the BC nanofiber layers become self-assembled onto the sisal fibers placed in the culture medium as the bacteria move freely, but preferably, around these fibers to form a network-like structure that encompasses the fibers. Figure 3c clearly shows the increased surface roughness of the sisal fibers due to the presence of nanoscale BC in the BC-sisal hybrid. In addition, large number of hydrogen bonds formed between the sisal fibers and the BC because of their close proximity results in strong adhesion between the two. It can be expected that the BC nanofibers self-assembled on top of the sisal fibers get embedded in the resin during composite fabrication, enhancing the fiber-resin IFSS.
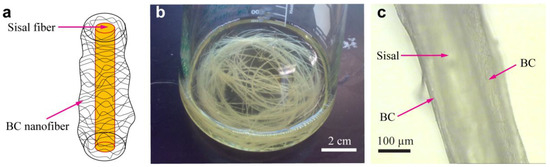
Figure 3.
BC-sisal hybrid fibrous structure. Schematic of the BC-sisal hybrid (a), a photograph of BC-sisal hybrid fibers in the culture medium (b) and an optical image showing a single BC-sisal hybrid fiber (c).
3.2. Tensile Properties of BC-MFC Hybrid and BC-MFC-SPI Nanocomposite
The tensile properties of BC, pure SPI, BC-MFC hybrid, BC-SPI composites, and BC-MFC-SPI nanocomposites are presented in Table 1. The results clearly indicate that adding MFC to BC increased the fracture strain slightly, from 5.6% to 6.0%, while the Young′s modulus increased significantly from 2490 MPa to 2826 MPa (over 13% increase) and tensile strength increased from 79.1 MPa to 84.1 MPa (over 6% increase). The assumed homogenous distribution of MFC within BC-MFC hybrid structure seems to remove some of the defects in the BC network structure and perhaps acts as a crack bridging mechanism resulting in the increased fracture strain. However, the primary reason for adding MFC to BC was the excellent tensile properties of the MFC itself. The results for the BC-MFC-SPI composites indicate that the values for Young′s modulus and tensile strength of these nanocomposites were 1408 MPa and 44.0 MPa, respectively, which were higher than those of BC-SPI nanocomposites (1284 MPa and 41.8 MPa). The simple rule of mixture was applied to calculate the theoretical values for Young′s modulus and tensile strength of the BC-SPI nanocomposites [37]. These theoretical values are also presented in Table 1. The densities of cellulose and SPI were taken as 1.52 g/ml and 1.30 g/ml, respectively, and the weight ratio of BC and resin was 1:1 in the composites (volume ratio is about 0.86:1) [38]. As can be seen from Table 1, the theoretical and experimental values for both Young′s modulus and tensile strength are very close.

Table 1.
Tensile properties for BC, SPI, BC-MFC, and their based composites.
3.3. Comparison of IFSS for Sisal/SPI and BC-Sisal/SPI Microbond Specimens
Since the effect of BC on the MFC-SPI resin IFSS was impossible to characterize directly, separate experiments were carried out using sisal fibers. Microbond tests have proven to be useful in obtaining IFSS values for such fiber/resin combination [31,35]. In the present case the microbeads shrink and their diameters decrease as the water evaporates during drying and curing. However, in the lengthwise direction the resin grips the fiber preventing any shrinkage in that direction [31,35]. Figure 4 shows the comparison of sisal fiber/SPI resin and BC-sisal hybrid fiber/SPI resin IFSS values. For sisal/SPI and BC-sisal/SPI, the IFSS values were 3.0 MPa and 3.6 MPa, respectively, a 20% increase after self-assembling BC on sisal fibers. The ANOVA test showed the p value of 0.02 at the significance level of 0.05 for comparison of the IFSS values for sisal/SPI and BC-sisal/SPI, indicating that the result is significant. The reasons for the increased IFSS value can be summed up as follows: (1) increase in the surface roughness of the sisal fibers due to the presence of nanoscale BC; and (2) the potential for a large amount of hydrogen bonding between functional groups in the SPI resin and the hydroxyl groups present on the BC-sisal hybrid fiber surface [13,19,20,21]. However, since BC is very flexible because of its small diameter of 55 nm, there is a distinct possibility of some of it getting entrapped or incorporated in the resin and providing mechanical bonding or anchoring effect. The increased IFSS clearly indicate the benefits of using self-assembled BC to modify the fiber surfaces [31,36].
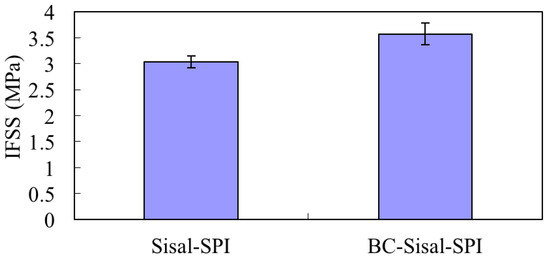
Figure 4.
Interfacial shear strength (IFSS) of BC/SPI and BC-sisal/SPI using microbond tests.
4. Conclusions
By using microfibrillated cellulose (MFC) and sisal fibers as substrates, and using the in situ self-assembly approach, two bacterial cellulose (BC) nanofibers-based hybrid fibrous structures were prepared. The fabrication of hybrid structures were achieved by directly adding MFC and sisal fibers, separately, to the fermentation (culture) medium. The fabricated BC-MFC and BC-sisal hybrid fibrous structures had much better mechanical properties compared to control BC membrane and sisal fibers alone. Tensile tests used to evaluate the mechanical properties of BC-MFC hybrid fibrous structure and their nanocomposites made using soy protein isolate (SPI) resin, indicated better tensile properties than BC and BC-SPI nanocomposites, respectively. The uniform distribution of MFC incorporated within the BC nanofiber network leads to these improved results. MFC fibers, on their own, possess excellent mechanical properties for reinforcement purposes and their uniform distribution can help eliminate many potential weak points, e.g., pores or voids, within the BC network structure, possibly by filling them. Additionally, because the BC nanofibers have small diameters and a high aspect ratio, they can become incorporated within the resin and provide a mechanical anchoring effect and, thus, enhance the fiber/resin IFSS. BC-sisal hybrid fibers were acquired after BC nanofibers were deposited onto the surface of the sisal fibers during the fermentation process. The microbond test results exhibited the BC-sisal hybrid fiber/SPI resin IFSS to be about 20% stronger than that between sisal fiber/SPI resin, which can lead to higher tensile properties of the composites. The improved IFSS is a result of increased roughness related to the presence of nanoscale BC on the sisal fiber surface, and the high potential for hydrogen bonding between functional groups in the SPI resin and the large number of hydroxyl groups present on the BC-sisal hybrid fiber surface. Another factor for increased IFSS, as mentioned earlier, is that some of the BC nanofibers on the fiber surface can get embedded or incorporated into the resin providing mechanical contribution.
Acknowledgments
This work was partly funded by the National Textile Center (NTC) and the Wallace Foundation. The authors would like to thank Dan Luo, John March and Antje Baeumner of Cornell University for allowing the use of their laboratory facilities. The authors also thank the Cornell Center for Materials Research (CCMR) facilities supported by National Science Foundation (NSF) (award no. DMR-1120296).
Author Contributions
K.Q. and A.N.N. conceived and designed the experiments; K.Q. performed the experiments; K.Q. analyzed the data; K.Q. and A.N.N. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Qiu, K.; Netravali, A.N. A review of fabrication and applications of bacterial cellulose based nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Liebner, F.; Aigner, N.; Schimper, C.; Potthast, A.; Rosenau, T. Bacterial cellulose aerogels: From lightweight dietary food to functional materials. In Functional Materials from Renewable Sources; American Chemical Society: Washington, DC, USA, 2012; Volume 1107, pp. 57–74. [Google Scholar]
- Qiu, K.; Netravali, A.N. Polyvinyl alcohol based biodegradable polymer nanocomposites. In Biodegradable Polymers; Chu, C.-C., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2015; Volume 1, pp. 325–379. [Google Scholar]
- Qiu, K.; Netravali, A.N. ˝Green˝ composites based on bacterial cellulose produced using novel low-cost carbon source and soy protein resin. In Recent Advances in Adhesion Science and Technology in Honor of Dr. Kash Mittal; CRC Press: Boca Raton, FL, USA, 2014; pp. 193–208. [Google Scholar]
- Qiu, K.; Netravali, A.N. Bacterial cellulose-based membrane-like biodegradable composites using cross-linked and noncross-linked polyvinyl alcohol. J. Mater. Sci. 2012, 47, 6066–6075. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.K.; Hutter, J.L.; Milton, L.; Guhados, G. Bacterial cellulose and its nanocomposites for biomedical applications. In Cellulose Nanocomposites; American Chemical Society: Washingdon, DC, USA, 2006; Volume 938, pp. 221–241. [Google Scholar]
- Qiu, K.; Netravali, A.N. Fabrication and characterization of biodegradable composites based on microfibrillated cellulose and polyvinyl alcohol. Compos. Sci. Technol. 2012, 72, 1588–1594. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Hong, L.; Jia, S.R.; Huang, Y.; Zhu, Y.; Wang, Y.L.; Jiang, H.J. Synthesis and characterization of hydroxyapatite—Bacterial cellulose nanocomposites. Compos. Sci. Technol. 2006, 66, 1825–1832. [Google Scholar] [CrossRef]
- Qiu, K. Biobased and Biodegrable Polymer Nanocomposites; Cornell University: Ithaca, NY, USA, 2012. [Google Scholar]
- Netravali, A.N.; Qiu, K. Bacterial Cellulose Based ‘Green’ Composites. US Patent No. 9,499,686 B2, 22 November 2016. [Google Scholar]
- Fontana, J.D.; De Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; De Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure formation of regenerated cellulose materials from nmmo-solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Shibazaki, H.; Kuga, S.; Onabe, F.; Usuda, M. Bacterial cellulose membrane as separation medium. J. Appl. Polym. Sci. 1993, 50, 965–969. [Google Scholar] [CrossRef]
- Hong, F.; Qiu, K. An alternative carbon source from konjac powder for enhancing production of bacterial cellulose in static cultures by a model strain acetobacter aceti subsp. Xylinus atcc 23770. Carbohydr. Polym. 2008, 72, 545–549. [Google Scholar] [CrossRef]
- Pommet, M.; Juntaro, J.; Heng, J.Y.Y.; Mantalaris, A.; Lee, A.F.; Wilson, K.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Surface modification of natural fibers using bacteria: Depositing bacterial cellulose onto natural fibers to create hierarchical fiber reinforced nanocomposites. Biomacromolecules 2008, 9, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose—Starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Juntaro, J.; Pommet, M.; Kalinka, G.; Mantalaris, A.; Shaffer, M.S.P.; Bismarck, A. Creating hierarchical structures in renewable composites by attaching bacterial cellulose onto sisal fibers. Adv. Mater. 2008, 20, 3122–3126. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Bharadia, P.; Blaker, J.J.; Bismarck, A. Short sisal fibre reinforced bacterial cellulose polylactide nanocomposites using hairy sisal fibres as reinforcement. Compos. Part A 2012, 43, 2065–2074. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of fiber content on the mechanical and thermal expansion properties of biocomposites based on microfibrillated cellulose. Cellulose 2008, 15, 555–559. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Fujimura, A.; Sakai, T.; Hama, Y.; Yano, H. Production of microfibrillated cellulose (mfc)-reinforced polylactic acid (pla) nanocomposites from sheets obtained by a papermaking-like process. Compos. Sci. Technol. 2009, 69, 1293–1297. [Google Scholar] [CrossRef]
- Huang, X.; Netravali, A. Biodegradable green composites made using bamboo micro/nano-fibrils and chemically modified soy protein resin. Compos. Sci. Technol. 2009, 69, 1009–1015. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. A composting study of membrane-like polyvinyl alcohol based resins and nanocomposites. J. Polym. Environ. 2013, 21, 658–674. [Google Scholar] [CrossRef]
- Stenstad, P.; Andresen, M.; Tanem, B.S.; Stenius, P. Chemical surface modifications of microfibrillated cellulose. Cellulose 2008, 15, 35–45. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Kim, J.T.; Netravali, A.N. Effect of protein content in soy protein resins on their interfacial shear strength with ramie fibers. J. Adhes. Sci. Technol. 2010, 24, 203–215. [Google Scholar] [CrossRef]
- Kim, J.R.; Netravali, A.N. Self-healing properties of protein resin with soy protein isolate-loaded poly(d,l-lactide-co-glycolide) microcapsules. Adv. Funct. Mater. 2016, 26, 4786–4796. [Google Scholar] [CrossRef]
- Netravali, A.N.; Chabba, S. Composites get greener. Mater. Today 2003, 6, 22–29. [Google Scholar] [CrossRef]
- Lodha, P.; Netravali, A.N. Characterization of stearic acid modified soy protein isolate resin and ramie fiber reinforced ‘green’ composites. Compos. Sci. Technol. 2005, 65, 1211–1225. [Google Scholar] [CrossRef]
- Nam, S.; Netravali, A.N. Characterization of ramie fiber/soy protein concentrate (spc) resin interface. J. Adhes. Sci. Technol. 2004, 18, 1063–1076. [Google Scholar] [CrossRef]
- Nam, S.; Netravali, A.N. Green composites. II. Environment-friendly, biodegradable composites using ramie fibers and soy protein concentrate (spc) resin. Fibers Polym. 2006, 7, 380–388. [Google Scholar] [CrossRef]
- Warner, S.B. Fiber Science; Prentice Hall: Upper Saddle River, NJ, USA, 1995. [Google Scholar]
- Lodha, P. Fundamental Approaches to Improving Performance of Soy Protein Isolate Based ‘Green’ Plastics and Composites; Cornell University: Ithaca, NY, USA, 2004. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).