Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers
Abstract
:1. Introduction
2. Experimental
2.1. Materials
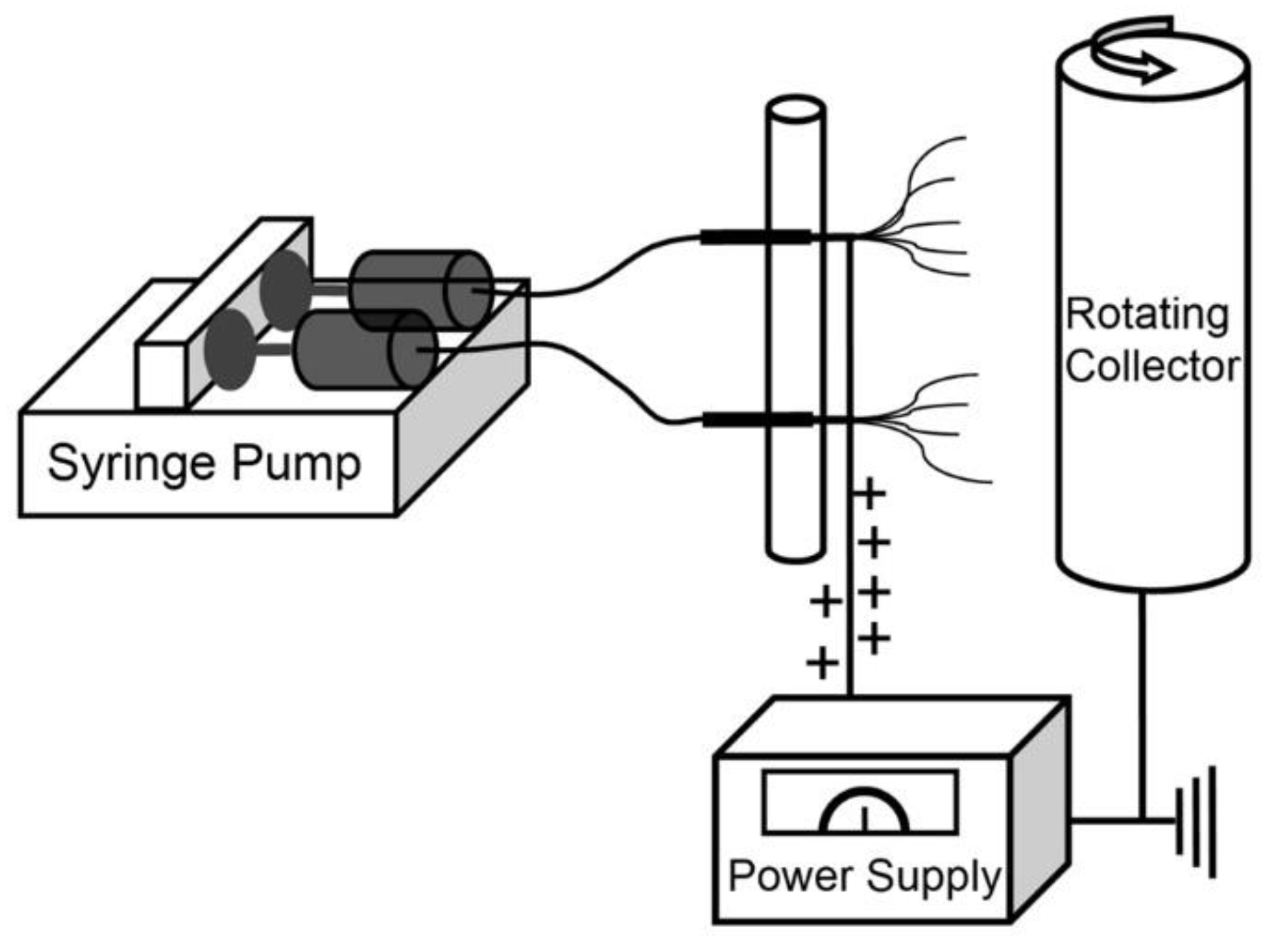
2.2. Fabrication of PdCl2 Doped Aluminum Acetate/PVP Composite Submicron Fibers
2.3. Thermal Treatment of Electrospun PdO Doped Alumina Submicron Fibers
2.4. Preparation of Catalytic Fiber Media
2.5. Reduction
2.6. Catalytic Reaction Set up for NO Decomposition
2.7. Characterization
3. Results and Discussion
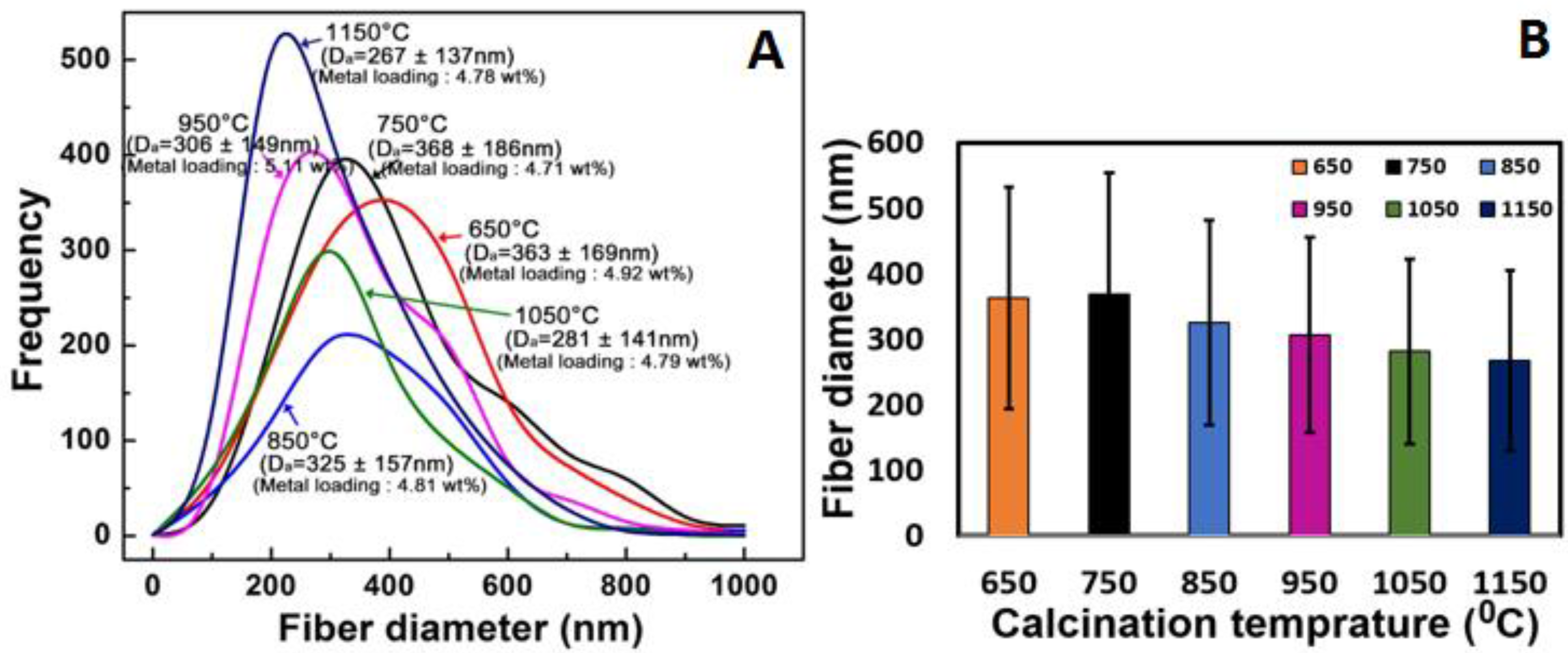
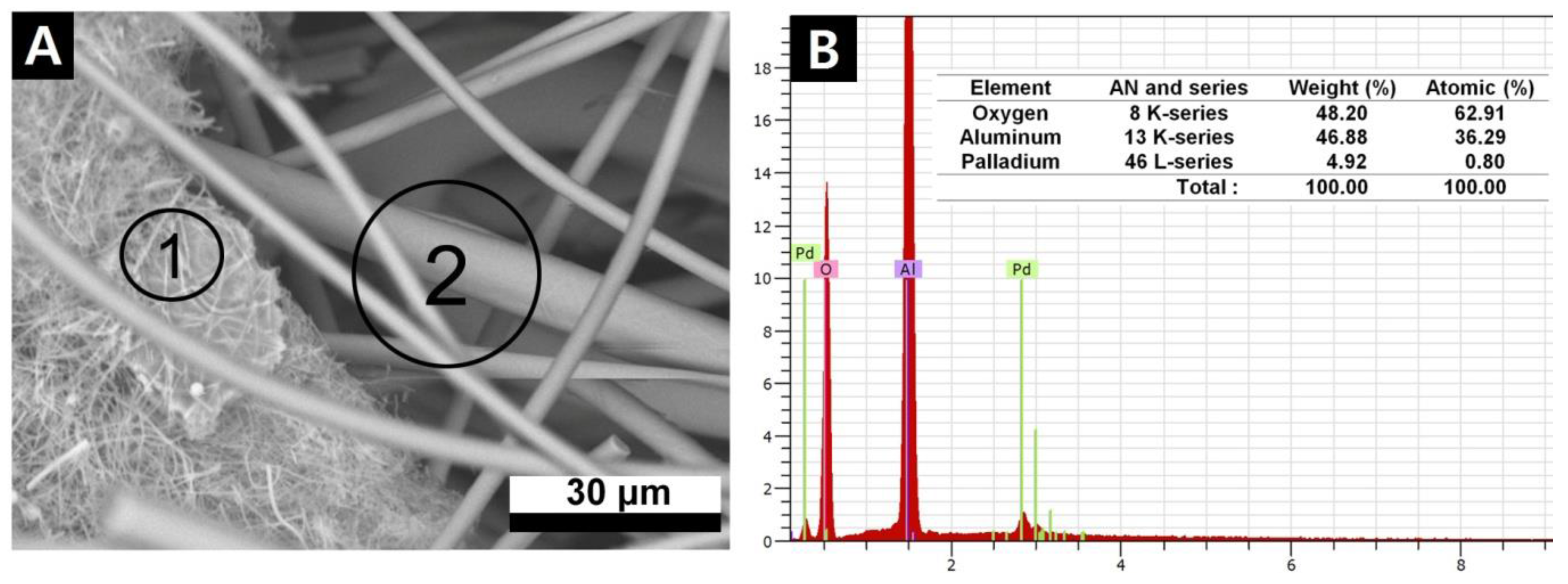
3.1. Scanning Electron Microscopy Study
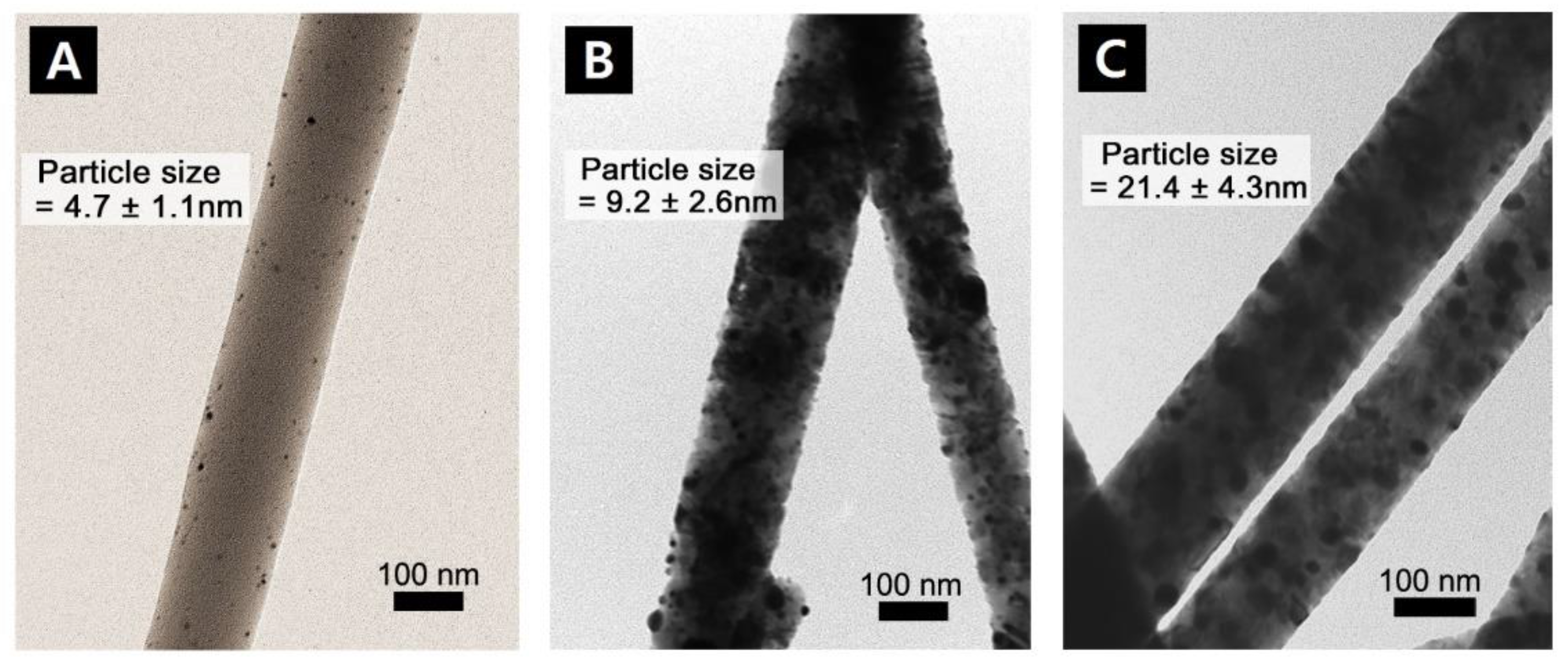
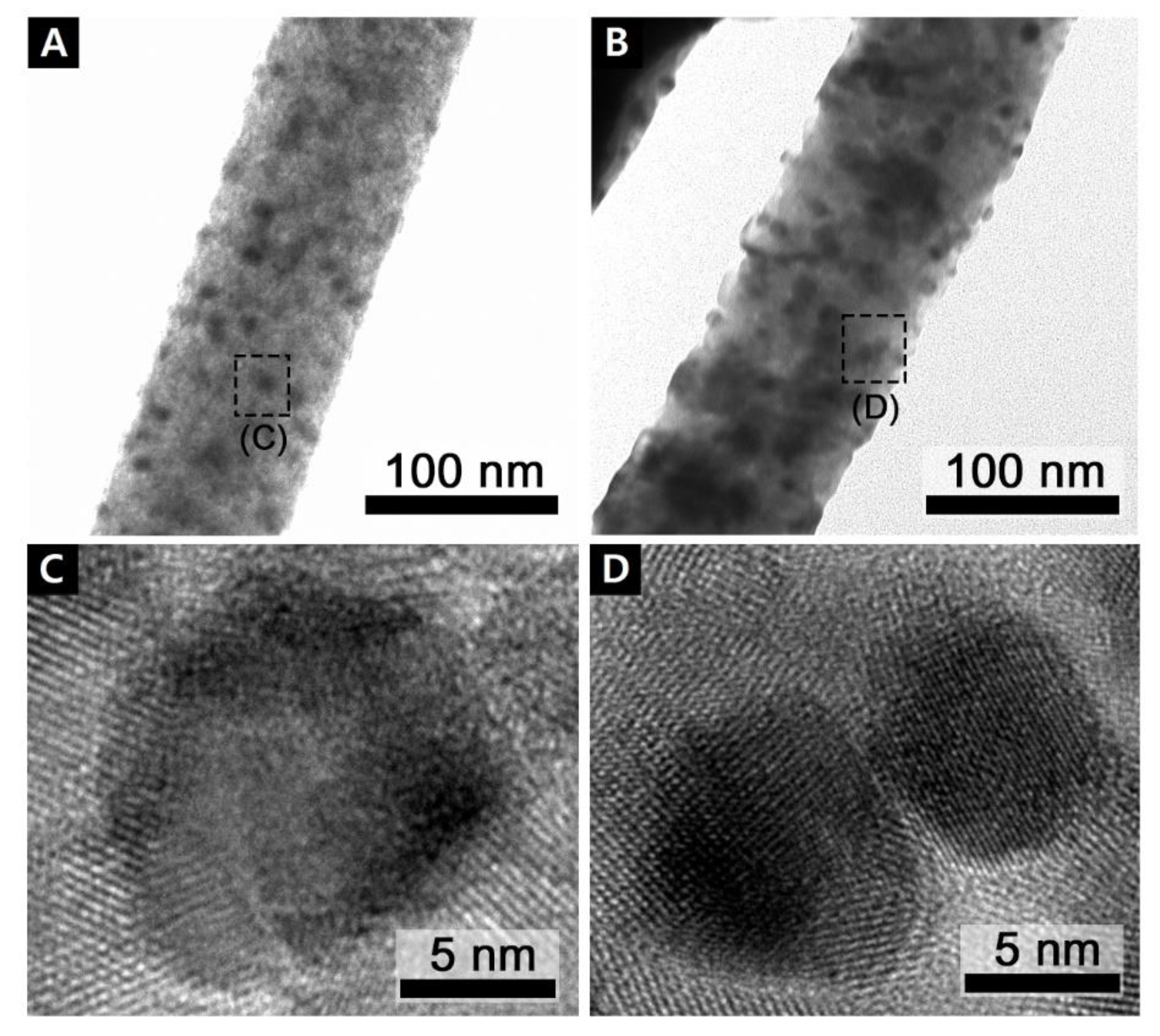
3.2. Transmission Electron Microscopy Study (TEM)
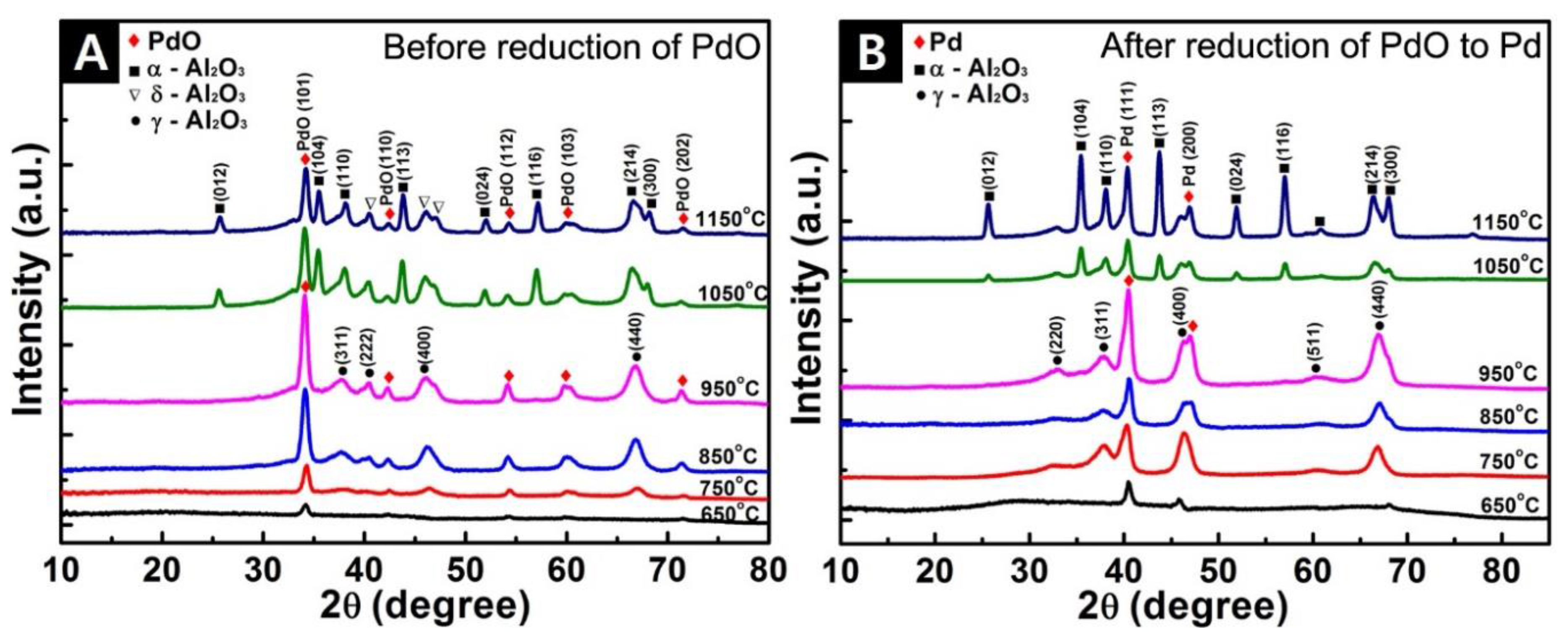
3.3. X-ray Diffraction (XRD) Analysis
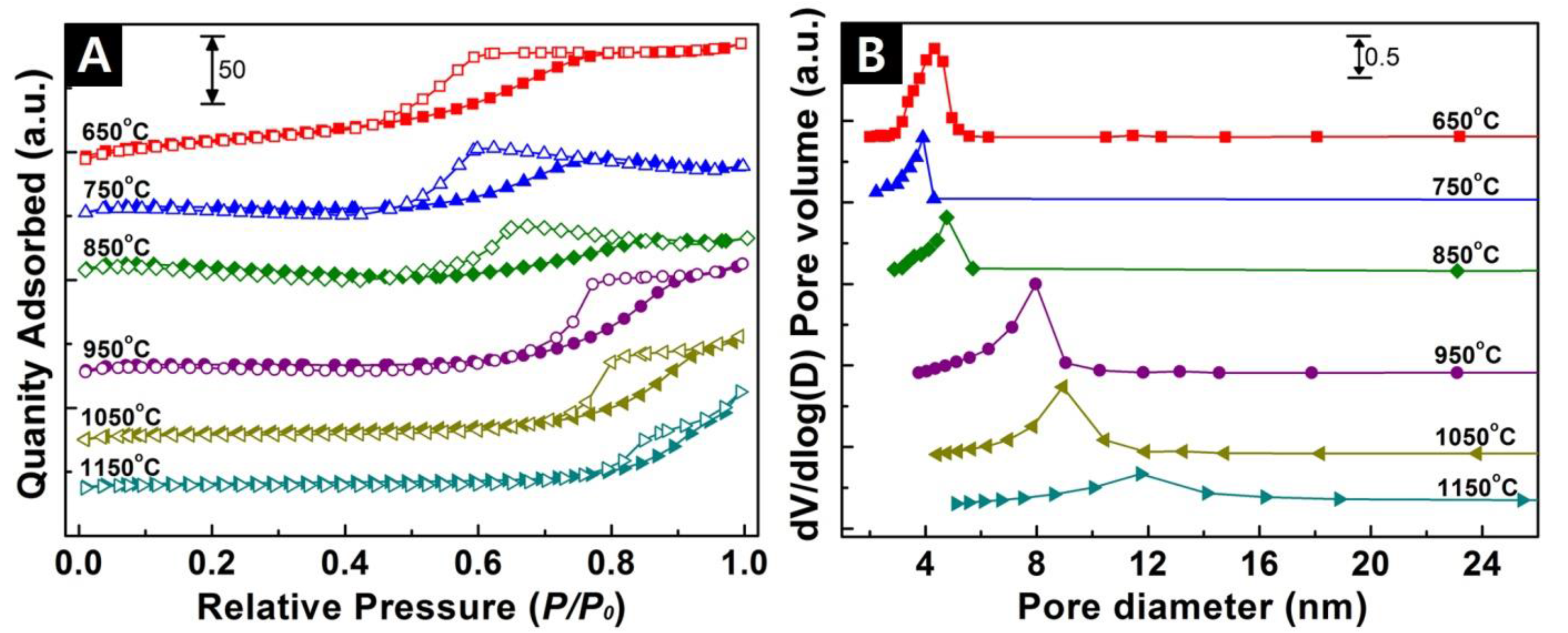
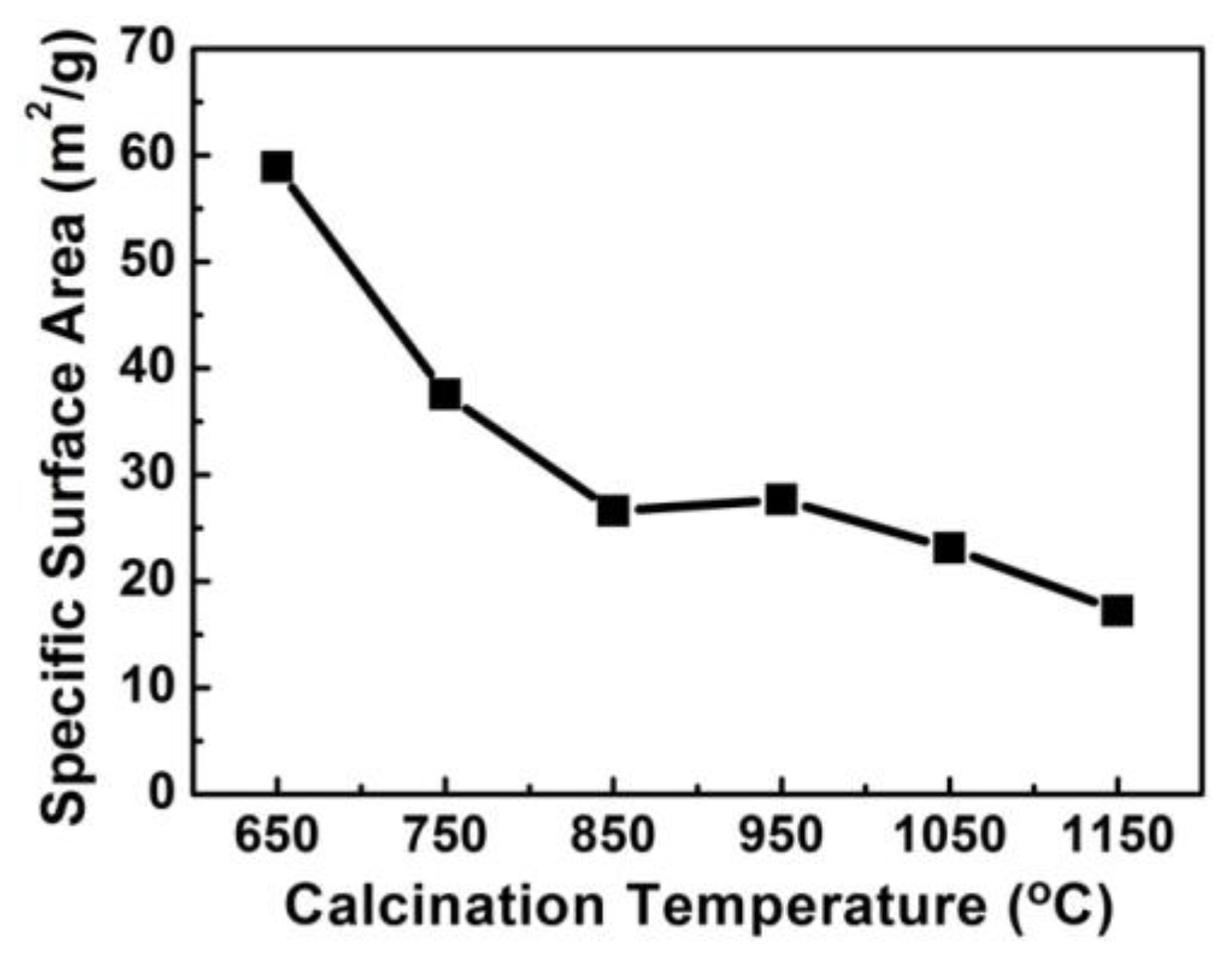
3.4. BET Characterization
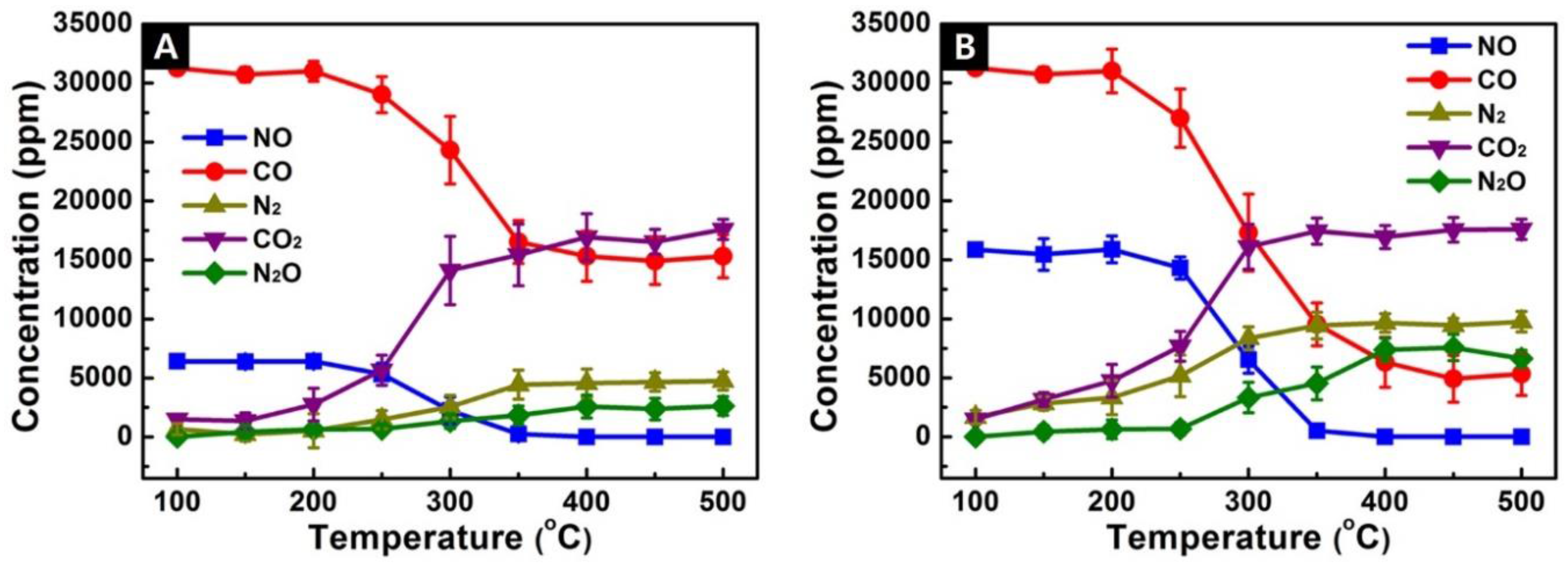
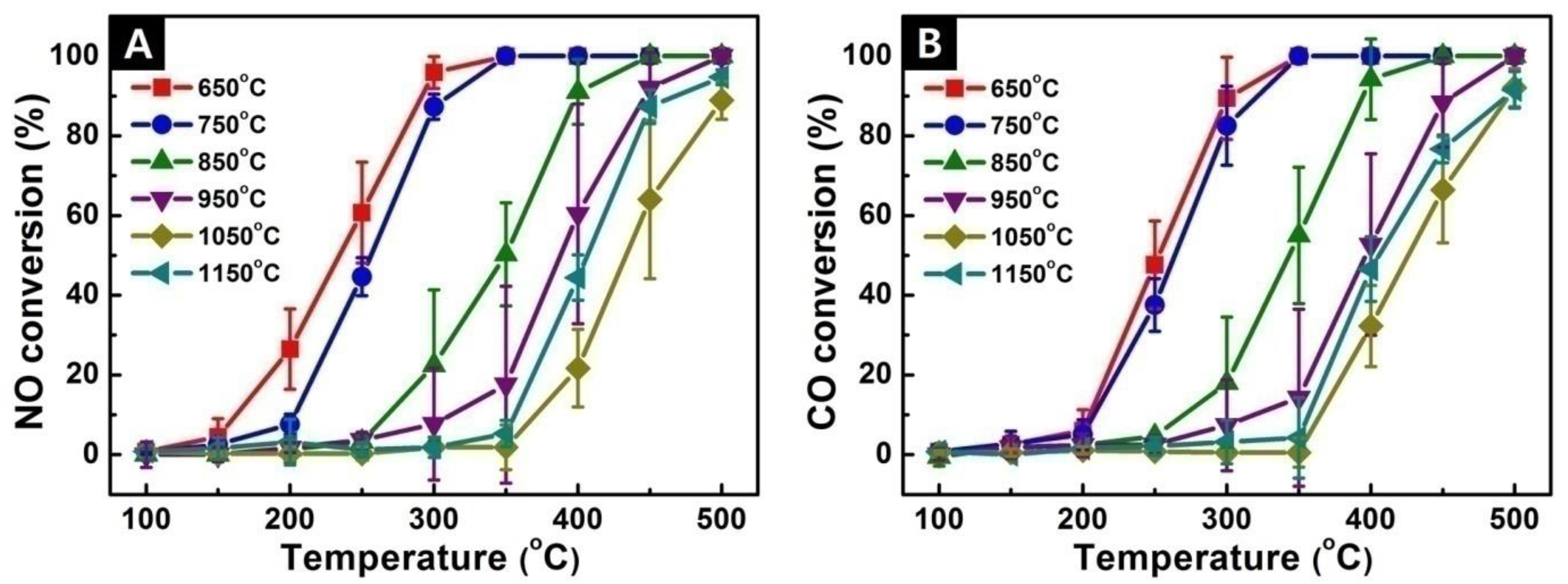
3.5. Catalytic Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhattacharyya, S.; Das, R.K. Catalytic control of automotive NOx: A review. Int. J. Energy Res. 1999, 23, 351–369. [Google Scholar] [CrossRef]
- Mantri, D.; Aghalayam, P. Detailed surface reaction mechanism for reduction of NO by CO. Catal. Today 2007, 119, 88–93. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, Y.; Lin, P. A low-temperature ir spectroscopic study of selective adsorption of NO and CO on CuO/γ-Al2O3. J. Catal. 1991, 132, 85–91. [Google Scholar] [CrossRef]
- Kašpar, J.; Fornasiero, P.; Hickey, N. Automotive catalytic converters: Current status and some perspectives. Catal. Today 2003, 77, 419–449. [Google Scholar] [CrossRef]
- Hu, Z.; Wan, C.Z.; Lui, Y.K.; Dettling, J.; Steger, J.J. Design of a novel Pd three-way catalyst: Integration of catalytic functions in three dimensions. Catal. Today 1996, 30, 83–89. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, A.; Zhang, Y.; Au, C.T.; Yang, X.; Shi, C. Catalytic reduction of NO by CO over NiO/CeO2 catalyst in stoichiometric NO/CO and NO/CO/O2 reaction. Appl. Catal. B 2008, 81, 141–149. [Google Scholar] [CrossRef]
- Yao, X.; Tang, C.; Ji, Z.; Dai, Y.; Cao, Y.; Gao, F.; Dong, L.; Chen, Y. Investigation of the physicochemical properties and catalytic activities of Ce0.67 m0.33 O2 (m = zr4+, Ti4+, Sn4+) solid solutions for NO removal by CO. Catal. Sci. Technol. 2013, 3, 688–698. [Google Scholar] [CrossRef]
- Almusaiteer, K.; Chuang, S.S.C. Isolation of active adsorbates for the NO–CO reaction on Pd/Al2O3 by selective enhancement and selective poisoning. J. Catal. 1998, 180, 161–170. [Google Scholar] [CrossRef]
- Shahreen, L.; Chase, G.G.; Turinske, A.J.; Nelson, S.A.; Stojilovic, N. NO decomposition by CO over Pd catalyst supported on TiO2 nanofibers. Chem. Eng. J. 2013, 225, 340–349. [Google Scholar] [CrossRef]
- Tsou, J.; Magnoux, P.; Guisnet, M.; Órfão, J.J.M.; Figueiredo, J.L. Catalytic oxidation of volatile organic compounds: Oxidation of methyl-isobutyl-ketone over Pt/zeolite catalysts. Appl. Catal. B 2005, 57, 117–123. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Y.; Yang, M.; Wu, Y. Effects of pretreatment atmospheres on the catalytic performance of Pd/γ-Al2O3 catalyst in benzene degradation. Catal. Commun. 2014, 46, 11–16. [Google Scholar] [CrossRef]
- Grbic, B.; Radic, N.; Terlecki-Baricevic, A. Kinetics of deep oxidation of n-hexane and toluene over Pt/Al2O3 catalysts: Oxidation of mixture. Appl. Catal. B 2004, 50, 161–166. [Google Scholar] [CrossRef]
- Eberhardt, A.M.; Benvenutti, E.V.; Moro, C.C.; Tonetto, G.M.; Damiani, D.E. NO decomposition on PdMo/γ-Al2O3 catalysts. J. Mol. Catal. A 2003, 201, 247–261. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. Drifts study of photo-assisted catalytic CO + NO redox reaction over CuO/CeO2-TiO2. Catal. Today 2015, 258, 139–147. [Google Scholar] [CrossRef]
- Gamarra, D.; Belver, C.; Fernández-García, M.; Martínez-Arias, A. Selective CO oxidation in excess H2 over copper−ceria catalysts: Identification of active entities/species. J. Am. Chem. Soc. 2007, 129, 12064–12065. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Liu, L.; Zhang, L.; Li, L.; Cao, Y.; Wang, X.; Tang, C.; Gao, F.; Dong, L. Crystal-plane effects on surface and catalytic properties of Cu2O nanocrystals for NO reduction by CO. Appl. Catal. A 2015, 505, 334–343. [Google Scholar] [CrossRef]
- Yao, X.; Xiong, Y.; Zou, W.; Zhang, L.; Wu, S.; Dong, X.; Gao, F.; Deng, Y.; Tang, C.; Chen, Z.; et al. Correlation between the physicochemical properties and catalytic performances of CexSn1–xO2 mixed oxides for NO reduction by CO. Appl. Catal. B 2014, 144, 152–165. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, G.; Li, J.; Liu, H.; Wang, Q.; Chen, Y. Catalytic removal of benzene over CeO2–mNOx composite oxides prepared by hydrothermal method. Appl. Catal. B 2013, 138–139, 253–259. [Google Scholar] [CrossRef]
- Ilieva, L.; Pantaleo, G.; Velinov, N.; Tabakova, T.; Petrova, P.; Ivanov, I.; Avdeev, G.; Paneva, D.; Venezia, A.M. NO reduction by CO over gold catalysts supported on Fe-loaded ceria. Appl. Catal. B 2015, 174–175, 176–184. [Google Scholar] [CrossRef]
- Matsui, T.; Harada, M.; Ichihashi, Y.; Bando, K.K.; Matsubayashi, N.; Toba, M.; Yoshimura, Y. Effect of noble metal particle size on the sulfur tolerance of monometallic Pd and Pt catalysts supported on high-silica USY zeolite. Appl. Catal. A 2005, 286, 249–257. [Google Scholar] [CrossRef]
- Smorygo, O.; Marukovich, A.; Mikutski, V.; Sadykov, V. Evaluation of SiC-porcelain ceramics as the material for monolithic catalyst supports. J. Adv. Ceram. 2014, 3, 230–239. [Google Scholar] [CrossRef]
- Van Gulijk, C.; Linders, M.J.G.; Valdes-Solis, T.; Kapteijn, F. Intrinsic channel maldistribution in monolithic catalyst support structures. Chem. Eng. J. Amst. Neth. 2005, 109, 89–96. [Google Scholar] [CrossRef]
- Leonov, A.N.; Smorygo, O.L.; Sheleg, V.K. Monolithic catalyst supports with foam structure. Reac. Kinet. Catal. Lett. 1997, 60, 259–267. [Google Scholar] [CrossRef]
- DeLuca, J.P.; Campbell, L.E. Monolithic catalyst supports. Adv. Mater. Catal. 1977, 35, 293–324. [Google Scholar]
- Sebastian, D.; Lazaro, M.J.; Moliner, R.; Suelves, I.; Arico, A.S.; Baglio, V. Oxidized carbon nanofibers supporting PtRu nanoparticles for direct methanol fuel cells. Int. J. Hydrog. Energy 2014, 39, 5414–5423. [Google Scholar] [CrossRef]
- Park, J.-H.; Ju, Y.-W.; Park, S.-H.; Jung, H.-R.; Yang, K.-S.; Lee, W.-J. Effects of electrospun polyacrylonitrile-based carbon nanofibers as catalyst support in PEMFC. J. Appl. Electrochem. 2009, 39, 1229–1236. [Google Scholar] [CrossRef]
- Simotwo, S.K.; Kalra, V. Study of co-electrospun nafion and polyaniline nanofibers as potential catalyst support for fuel cell electrodes. Electrochim. Acta 2016, 198, 156–164. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Zhong, L.; Wu, L.; Cao, X.; Sun, R.C. Electrospun cellulose acetate supported Ag@AgCl composites with facet-dependent photocatalytic properties on degradation of organic dyes under visible-light irradiation. Carbohydr. Polym. 2016, 136, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Savva, I.; Kalogirou, A.S.; Chatzinicolaou, A.; Papaphilippou, P.; Pantelidou, A.; Vasile, E.; Vasile, E.; Koutentis, P.A.; Krasia-Christoforou, T. PVP-crosslinked electrospun membranes with embedded Pd and Cu2O nanoparticles as effective heterogeneous catalytic supports. RSC Adv. 2014, 4, 44911–44921. [Google Scholar] [CrossRef]
- Park, S.-J.; Bhargava, S.; Chase, G.G. Fitting of kinetic parameters of NO reduction by CO in fibrous media using a genetic algorithm. Comput. Chem. Eng. 2010, 34, 485–490. [Google Scholar] [CrossRef]
- Shin, H.U.; Lolla, D.; Nikolov, Z.; Chase, G.G. Pd–Au nanoparticles supported by TiO2 fibers for catalytic NO decomposition by CO. J. Ind. Eng. Chem. 2016, 33, 91–98. [Google Scholar] [CrossRef]
- Patel, A.C.; Li, S.; Wang, C.; Zhang, W.; Wei, Y. Electrospinning of porous silica nanofibers containing silver nanoparticles for catalytic applications. Chem. Mater. 2007, 19, 1231–1238. [Google Scholar] [CrossRef]
- Park, S.J.; Bhargava, S.; Bender, E.T.; Chase, G.G.; Ramsier, R.D. Palladium nanoparticles supported by alumina nanofibers synthesized by electrospinning. J. Mater. Res. 2008, 23, 1193–1196. [Google Scholar] [CrossRef]
- Chambers, A.; Nemes, T.; Rodriguez, N.M.; Baker, R.T.K. Catalytic behavior of graphite nanofiber supported nickel particles. 1. Comparison with other support media. J. Phys. Chem. B 1998, 102, 2251–2258. [Google Scholar] [CrossRef]
- Guceri, S.; Gogotsi, Y.G.; Kuznetsov, V. Nanoengineered Nanofibrous Materials; Springer: Berlin, Germany, 2004. [Google Scholar]
- Hou, W.; Zhou, J.; Yu, C.; You, S.; Gao, X.; Luo, Z. Pd/Al2O3 sorbents for elemental mercury capture at high temperatures in syngas. Ind. Eng. Chem. Res. 2014, 53, 9909–9914. [Google Scholar] [CrossRef]
- Shin, H.U.; Ramsier, R.D.; Chase, G.G. Influence of calcination temperature on the surface area of submicron-sized Al2O3 electrospun fibers. Appl. Phys. A 2016, 122, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Jiao, X.; Chen, D. Electrospinning preparation and adsorption properties of mesoporous alumina fibers. J. Mater. Chem. A 2013, 1, 10720–10726. [Google Scholar] [CrossRef]
- Swaminathan, S.; Chase, G. Electrospinning of Metal Doped Alumina Nanofibers for Catalyst Applications; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Ayral, A.; Droguet, J.C. Alumina powders via a controlled precipitation of aluminum acetate. J. Mater. Res. 1989, 4, 967–971. [Google Scholar] [CrossRef]
- McCabe, R.W.; Usmen, R.K.; Ober, K. The effect of alumina phase structure on the dispersion of rhodium/alumina catalysts. J. Catal. 1995, 151, 385–393. [Google Scholar] [CrossRef]
- Komeili, S.; Ravanchi, M.T.; Taeb, A. The influence of alumina phases on the performance of Pd-Ag/Al2O3 catalyst in tail-end selective hydrogenation of acetylene. Appl. Catal. A 2015, 502, 287–296. [Google Scholar] [CrossRef]
- Chase, G.G.; Nartetamrongsutt, K.; Shin, H.U. Simple Device for Economically Producing Electrospun Fibers at Moderate Rates. U.S. Patent 20,150,158,230, 11 June 2015. [Google Scholar]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Lolla, D.; Abutaleb, M.; Shin, H.U.; Reneker, D.H.; Chase, G.G. Fabrication, Polarization of Electrospun Polyvinylidene Fluoride Electret Fibers and Effect on Capturing Nanoscale Solid Aerosols. Materials 2016, 9, 671. [Google Scholar] [CrossRef]
- Rajala, J.; Shin, H.U.; Lolla, D.; Chase, G. Core-Shell Electrospun Hollow Aluminum Oxide Ceramic Fibers. Fibers 2015, 3, 450–462. [Google Scholar] [CrossRef]
- Shin, H.U.; Li, Y.; Paynter, A.; Nartetamrongsutt, K.; Chase, G.G. Vertical rod method for electrospinning polymer fibers. Polymer 2015, 65, 26–33. [Google Scholar] [CrossRef]
- Lolla, D.; Gorse, J.; Kisielowski, C.; Miao, J.; Taylor, P.L.; Chase, G.G.; Reneker, D.H. Polyvinylidene fluoride molecules in nanofibers, imaged at atomic scale by aberration corrected electron microscopy. Nanoscale 2016, 8, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Demir, M.M.; Gulgun, M.A.; Menceloglu, Y.Z.; Erman, B.; Abramchuk, S.S.; Makhaeva, E.E.; Khokhlov, A.R.; Matveeva, V.G.; Sulman, M.G. Palladium nanoparticles by electrospinning from poly(acrylonitrile-co-acrylic acid)-PdCl2 solutions. Relations between preparation conditions, particle size, and catalytic activity. Macromolecules 2004, 37, 1787–1792. [Google Scholar] [CrossRef]
- Yu, P.-C.; Yang, R.-J.; Tsai, Y.-Y.; Sigmund, W.; Yen, F.-S. Growth mechanism of single-crystal α-Al2O3 nanofibers fabricated by electrospinning techniques. J. Eur. Ceram. Soc. 2011, 31, 723–731. [Google Scholar] [CrossRef]
- Webb, P.A.; Orr, C. Analytical Methods in Fine Particle Technology; Micromeritics Instrument Corp.: Norcross, GA, USA, 1997. [Google Scholar]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Granger, P.; Dhainaut, F.; Pietrzik, S.; Malfoy, P.; Mamede, A.S.; Leclercq, L.; Leclercq, G. An overview: Comparative kinetic behaviour of Pt, Rh and Pd in the NO + CO and NO + H2 reactions. Top. Catal. 2006, 39, 65–76. [Google Scholar] [CrossRef]
- Yao, X.; Gao, F.; Cao, Y.; Tang, C.; Deng, Y.; Dong, L.; Chen, Y. Tailoring copper valence states in CuOδ/γ-Al2O3 catalysts by an in situ technique induced superior catalytic performance for simultaneous elimination of NO and CO. Phys. Chem. Chem. Phys. 2013, 15, 14945–14950. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Gao, F.; Yu, Q.; Qi, L.; Tang, C.; Dong, L.; Chen, Y. NO reduction by CO over CuO-CeO2 catalysts: Effect of preparation methods. Catal. Sci. Technol. 2013, 3, 1355–1366. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, H.U.; Abutaleb, A.; Lolla, D.; Chase, G.G. Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers. Fibers 2017, 5, 22. https://doi.org/10.3390/fib5020022
Shin HU, Abutaleb A, Lolla D, Chase GG. Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers. Fibers. 2017; 5(2):22. https://doi.org/10.3390/fib5020022
Chicago/Turabian StyleShin, Hyeon Ung, Ahmed Abutaleb, Dinesh Lolla, and George G. Chase. 2017. "Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers" Fibers 5, no. 2: 22. https://doi.org/10.3390/fib5020022
APA StyleShin, H. U., Abutaleb, A., Lolla, D., & Chase, G. G. (2017). Effect of Calcination Temperature on NO–CO Decomposition by Pd Catalyst Nanoparticles Supported on Alumina Nanofibers. Fibers, 5(2), 22. https://doi.org/10.3390/fib5020022





