Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. Qualitative and Quantitative Determination of CS
2.3. Surface Characterization
2.4. Degradation In Vitro
2.5. Tensile Strength
3. Results and Discussion
3.1. Preparation of CS–PLA

| Treatment Processes | P-1 | P-2 | P-3 | P-4 | P-5 |
|---|---|---|---|---|---|
| alkali treatment (sec) a | 10 | 300 | 10 | 300 | 60 |
| first wash process b | W | W | W | W | S |
| CS treatment (min) c | – | – | 1 | 1 | 1 |
| second wash process b | – | – | W | W | S & W |
| heat treatment (h) d | 1 | 1 | 1 | 1 | 1 |
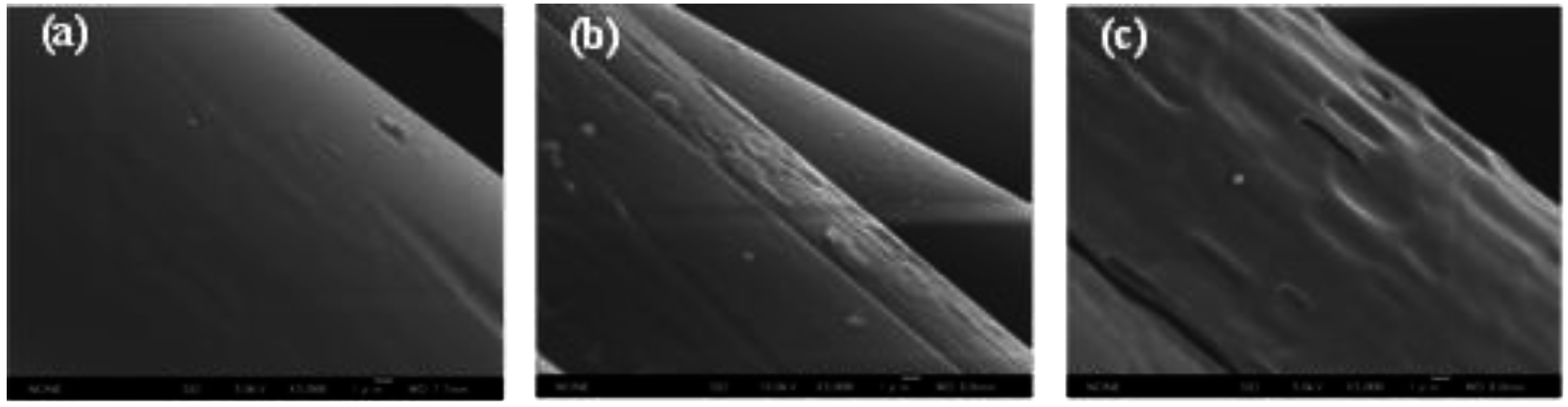
3.2. Surface Morphology
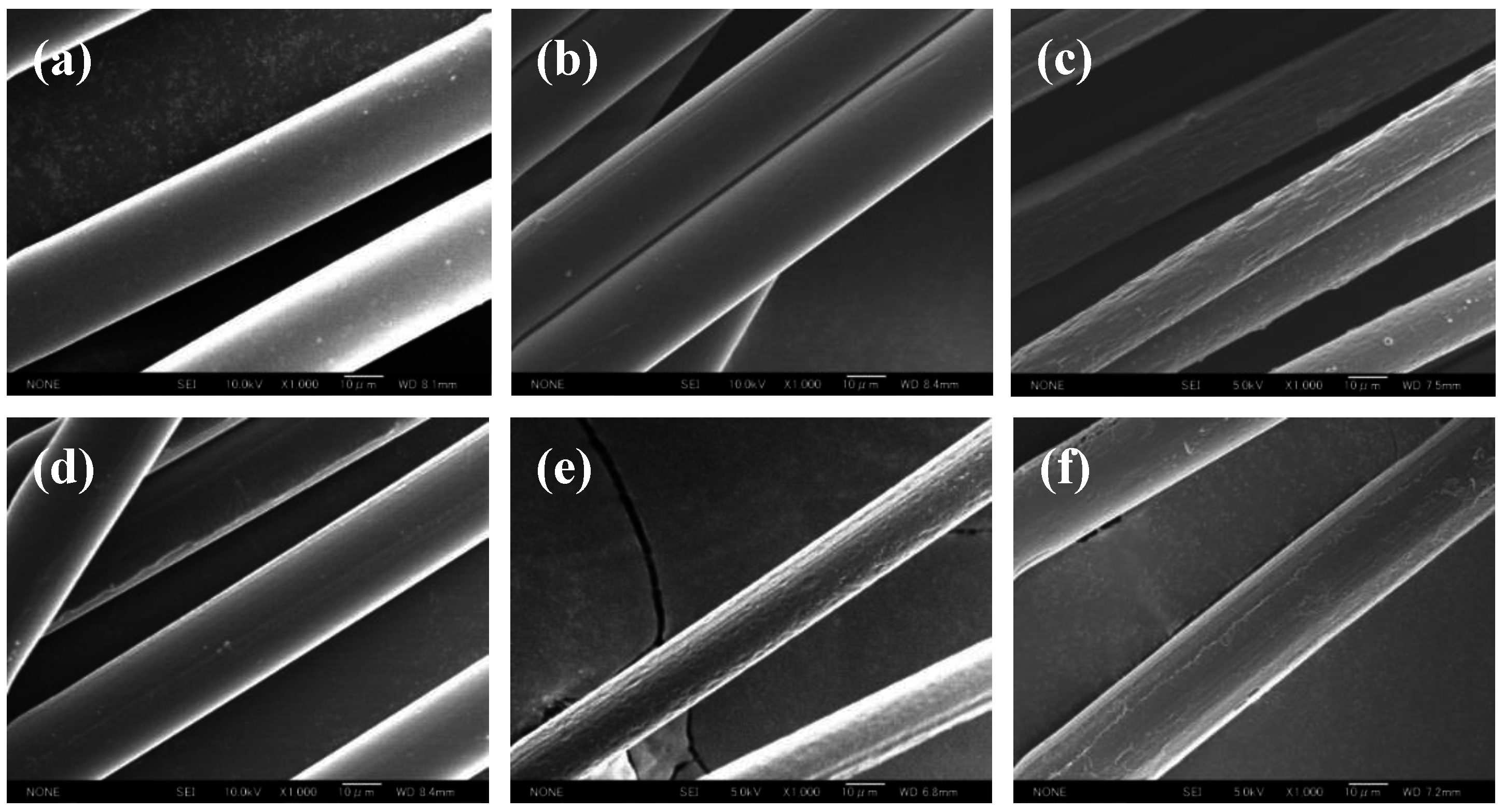
3.3. Qualitative and Quantitative Determination of CS
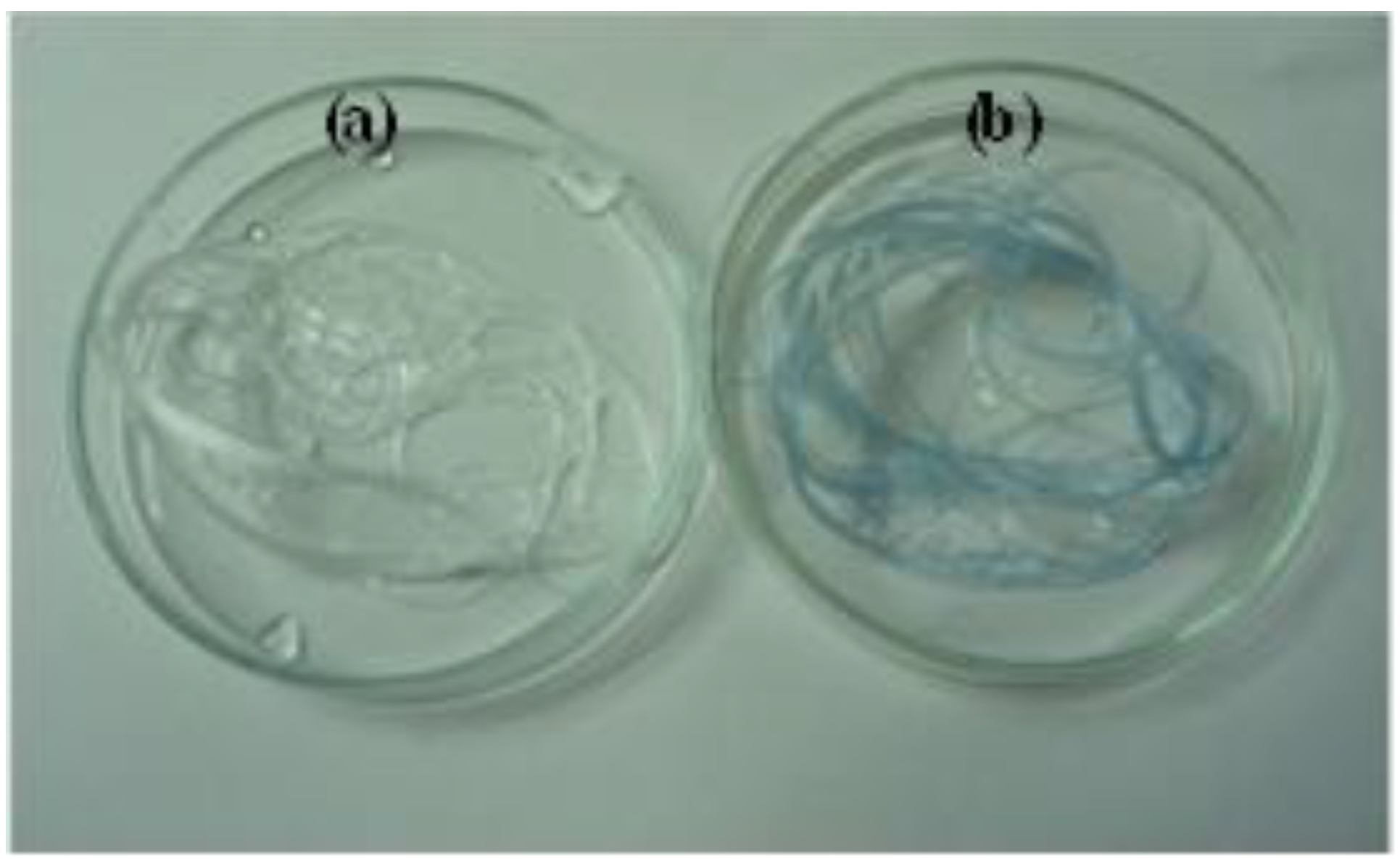
| Measurement method | CS (g)/CS–PLA (g) a |
|---|---|
| Kjeldahl nitrogen analysis | 4.69 × 10−3 |
| elemental analysis | 4.62 × 10−3 |
3.4. Surface Characterization
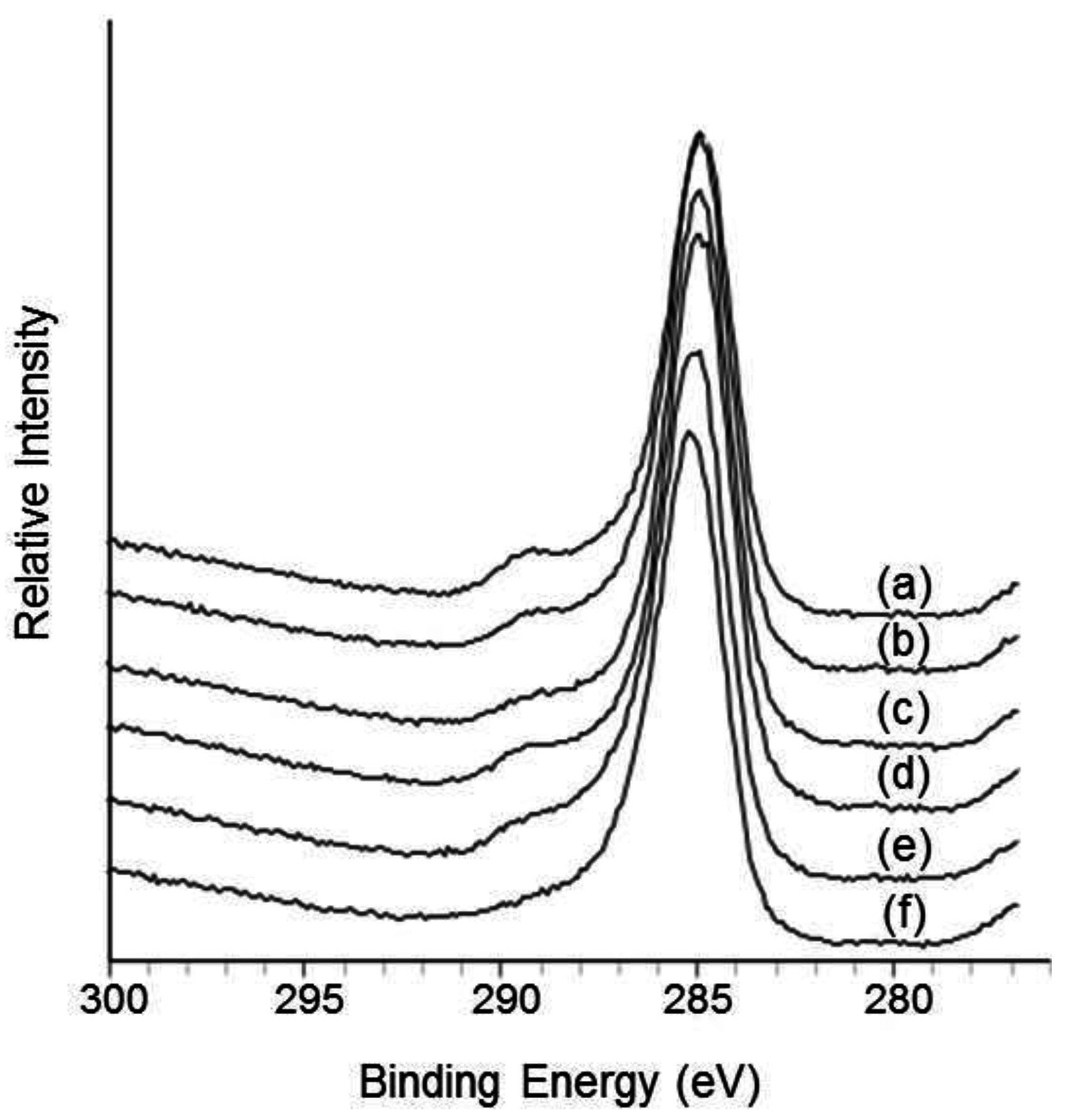
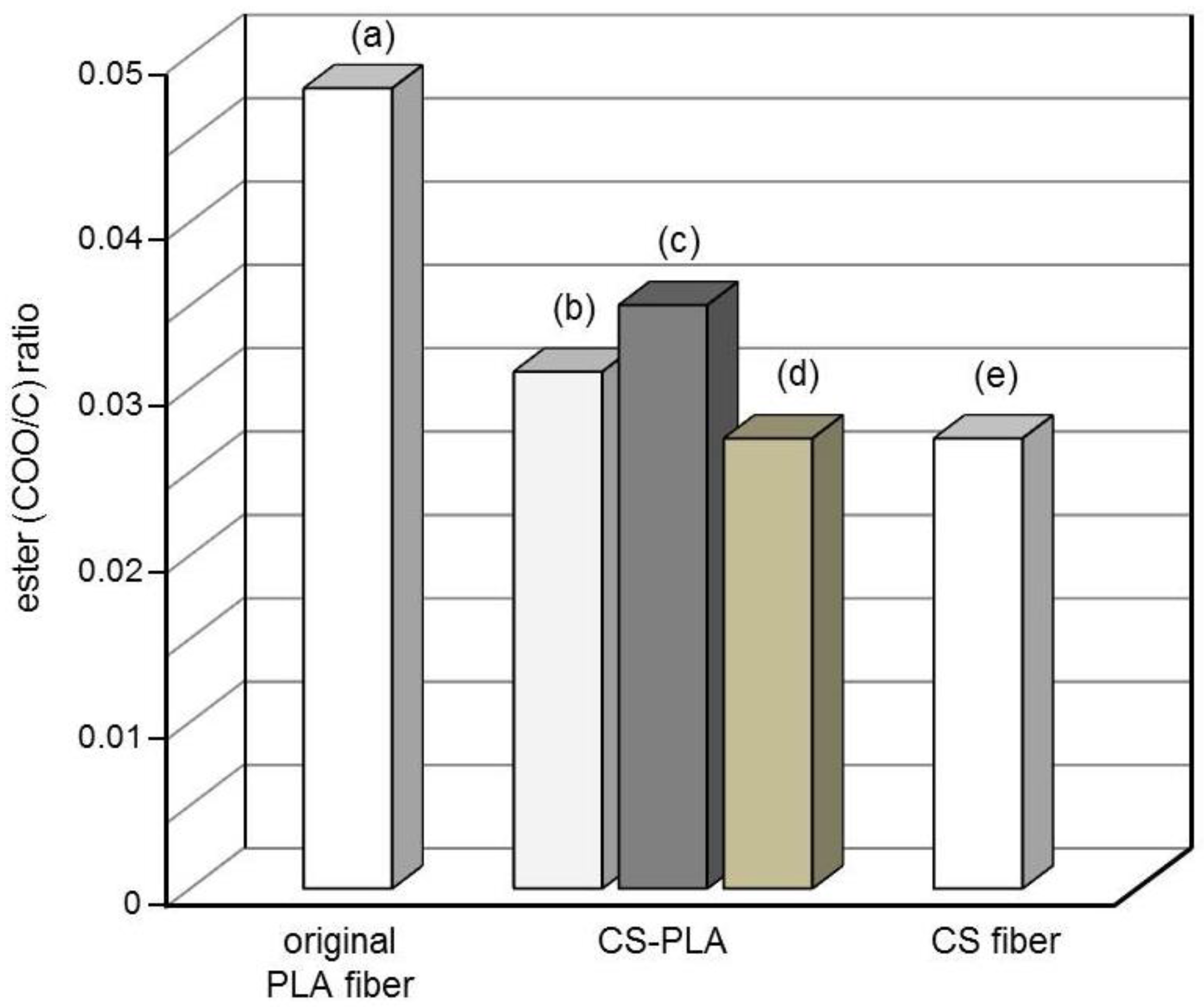
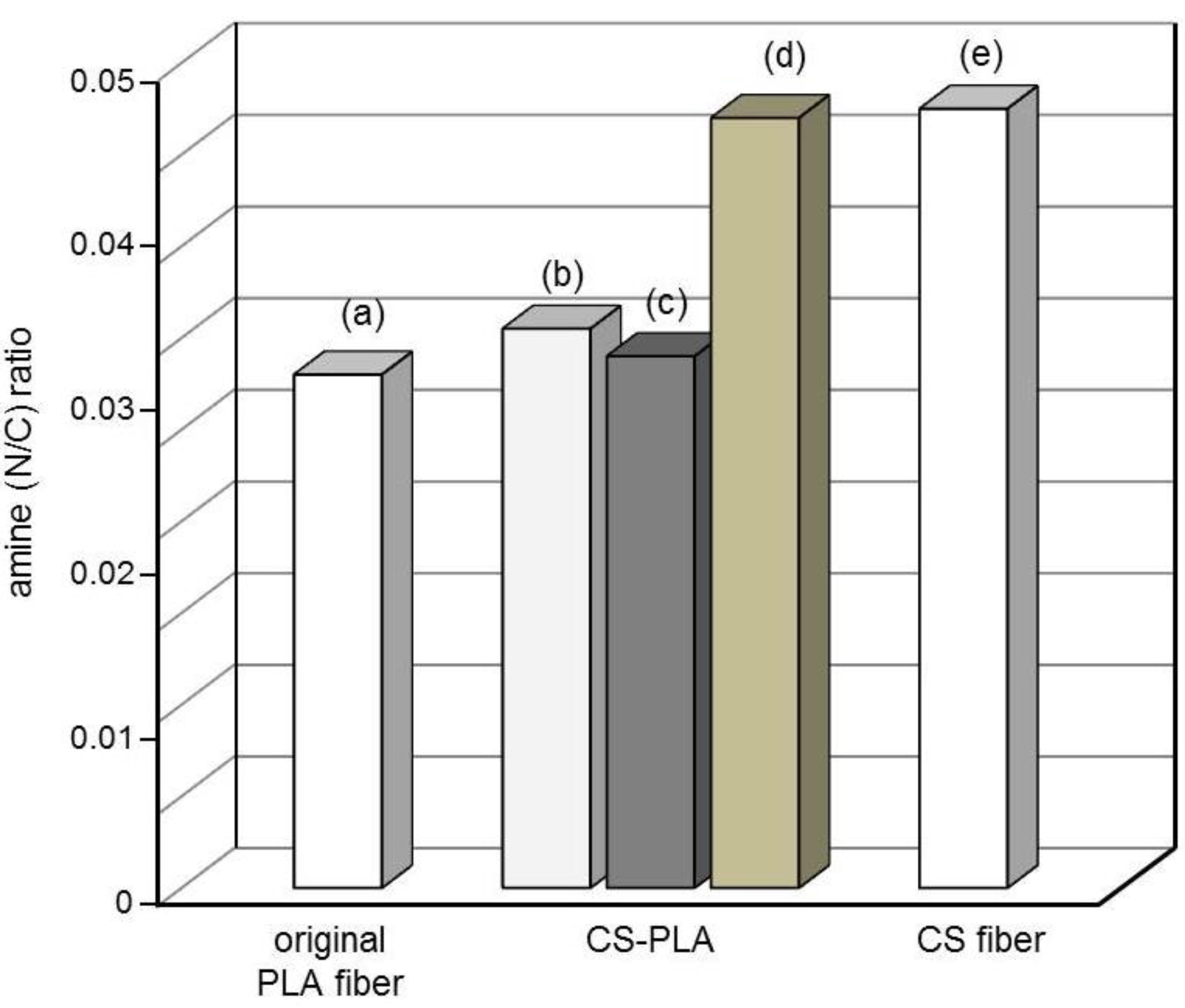
3.5. Degradation Properties

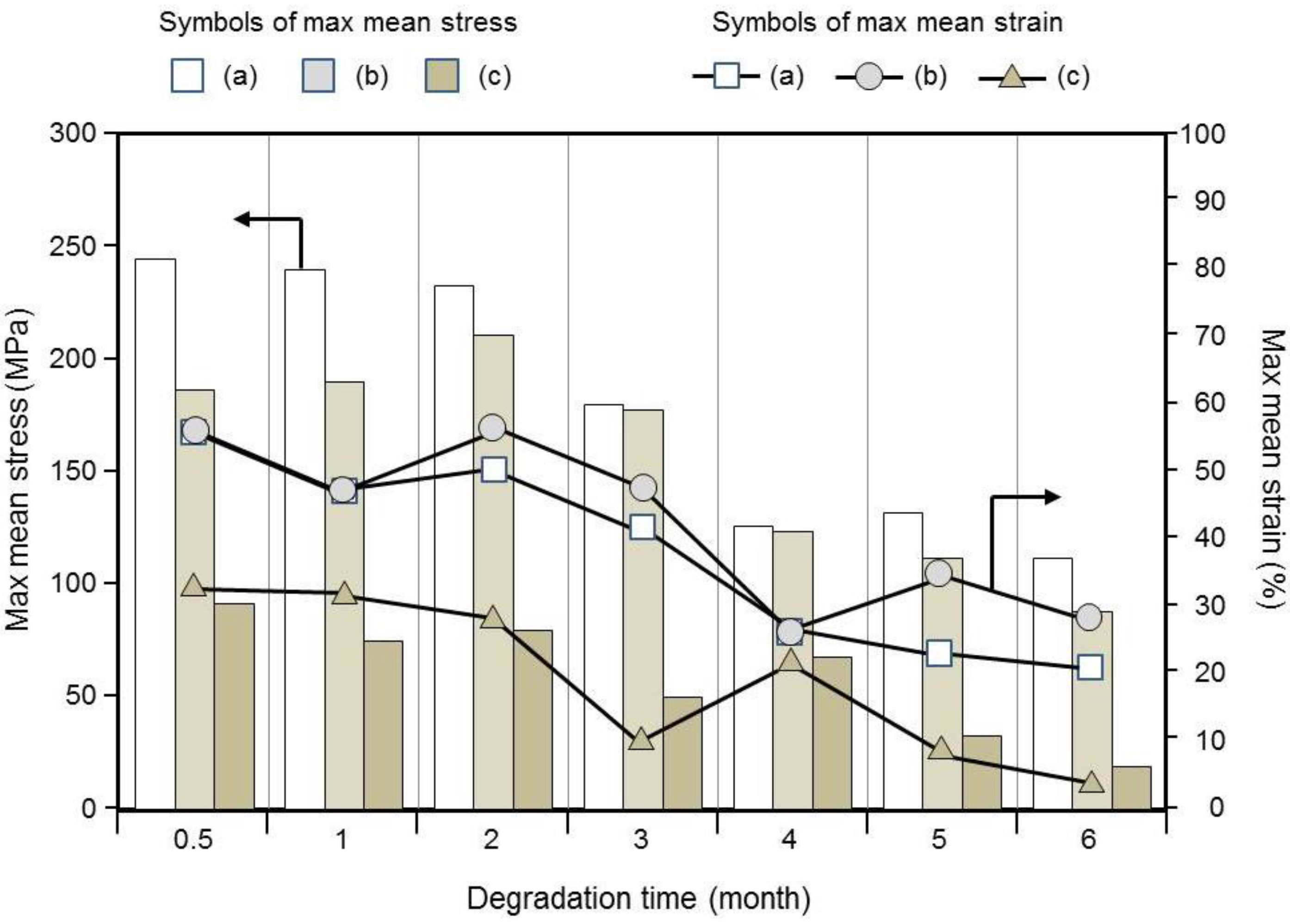
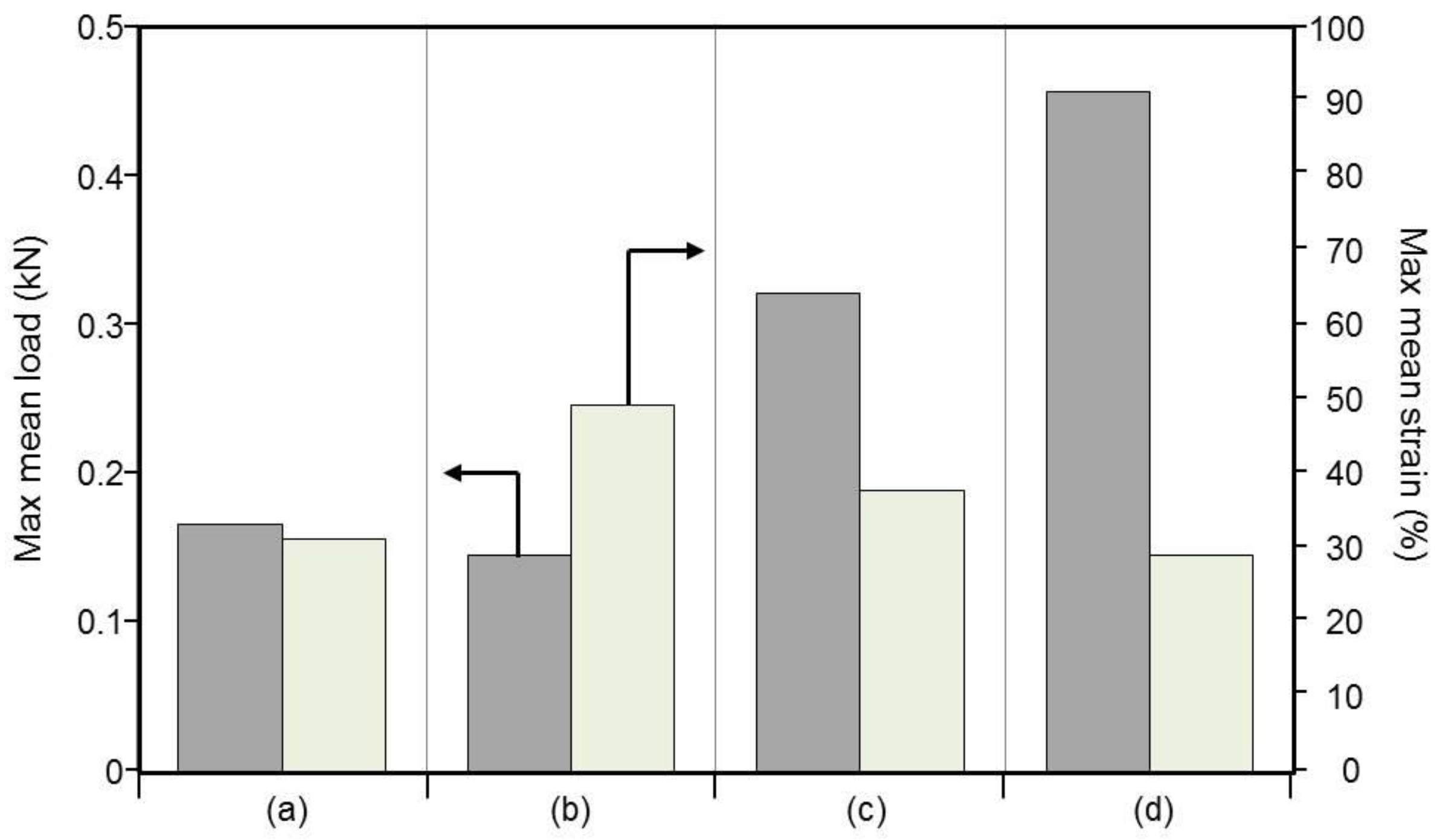
3.6. Mechanical Properties of Braided Ropes
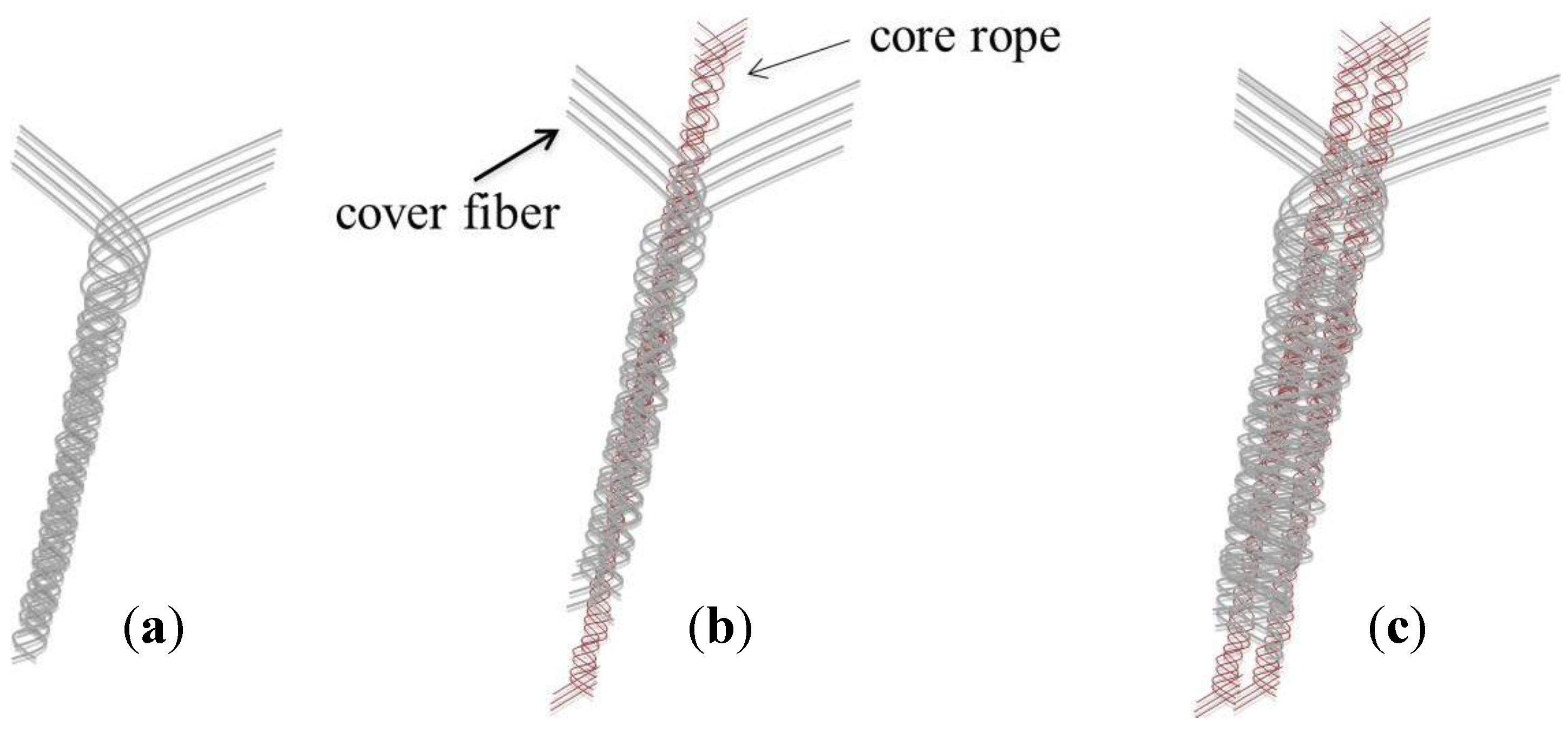
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikada, Y.; Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 2000, 21, 117–132. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Blends of aliphatic polyesters. II. Hydrolysis of solution-cast blends from poly(l-lactide) and poly(ε-caprolactone) in phosphate-buffered solution. J. Appl. Polym. Sci. 1998, 67, 405–415. [Google Scholar] [CrossRef]
- Saravanan1, M.; Domb, A.J. A contemporary review on—Polymer stereocomplexes and its biomedical application. Eur. J. Nanomed. 2013, 5, 81–96. [Google Scholar] [CrossRef]
- Razak, S.I.A.; Sharif, N.F.A.; Rahman, W.A.W.A. Biodegradable polymers and their bone applications: A review. Int. J. Basic Appl. Sci. 2012, 12, 31–49. [Google Scholar]
- Wachsen, O.; Platkowski, K.; Reichert, K.-H. Thermal degradation of poly-l-lactide-studies on kinetics, modelling and melt stabilisation. Polym. Degrad. Stab. 1997, 57, 87–94. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Yamashita, K.; Doi, Y. Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone], and poly[(S)-lactide]. Polym. Degrad. Stab. 2002, 76, 53–59. [Google Scholar] [CrossRef]
- Lunt, J. Large-scale production, properties and commercial applications of polylactic acid polymers. Polym. Degrad. Stab. 1998, 59, 145–152. [Google Scholar] [CrossRef]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Mikos, A.G.; Lyman, M.D.; Freed, L.E.; Langer, R. Wetting of poly(l-lactic acid) and poly(dl-lactic-co-glycolic acid) foams for tissue culture. Biomaterials 1994, 15, 55–58. [Google Scholar] [CrossRef]
- Mi, F.-L.; Syu, S.-S.; Lin, Y.-M.; Wu, Y.-B.; Peng, C.-K.; Tsai, Y.-H. Chitin/PLGA blend microspheres as a biodegradable drug delivery system: A new delivery system for protein. Biomaterials 2003, 24, 5023–5036. [Google Scholar] [CrossRef]
- Nagahama, K.; Mori, Y.; Ohya, Y.; Ouchi, T. Biodegradable nanogel formation of polylactide-grafted dextran copolymer in dilute aqueous solution and enhancement of its stability by stereocomplexation. Biomacromolecules 2007, 8, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Sasmal, A.; Nayak, P.L. Preparation and characterization of chitosan–polylactide composites blended with Cloisite 30B for control release of the anticancer drug paclitaxel. Carbohydr. Polym. 2011, 83, 988–994. [Google Scholar] [CrossRef]
- Preechawong, D.; Peesan, M.; Supaphol, P.; Rujiravanit, R. Preparation and characterization of starch/poly(l-lactic acid) hybrid foams. Carbohydr. Polym. 2005, 59, 329–337. [Google Scholar] [CrossRef]
- Chen, C.; Dong, L.; Cheung, M.K. Preparation and characterization of biodegradable poly(l-lactide)/chitosan blends. Eur. Polym. J. 2005, 41, 958–966. [Google Scholar] [CrossRef]
- Peesan, M.; Supahol, P.; Rujiravanit, R. Preparation and characterization of hexanoly chitosan/polylactide blend films. Carbohydr. Polym. 2005, 60, 343–350. [Google Scholar] [CrossRef]
- Cai, K.; Yao, K.; Lin, S.; Yang, Z.; Li, X.; Xie, H.; Qing, T.; Gao, L. Poly(d,l-lactic acid) surfaces modified by silk fibroin: Effects on the culture of osteoblast in vitro. Baiomaterials 2002, 23, 1153–1160. [Google Scholar] [CrossRef]
- Chu, C.F.L.; Lu, A.; Liszkowski, M.; Sipehia, R. Enhanced growth of animal and human endothelial cells on biodegradable polymers. Biochim. Biophys. Acta 1999, 1472, 479–485. [Google Scholar] [CrossRef]
- Kito, H.; Matsuda, T. Biocompatible coatings for luminal and outer surfaces of small-caliber artificial grafts. J. Biomed. Mater. Res. 1996, 30, 321–330. [Google Scholar] [CrossRef]
- Zhu, A.; Zhang, M.; Wu, J.; Shen, J. Covalent immobilization of chitosan/heparin complex with a photosensitive hetero-bifunctional crosslinking reagent on PLA surface. Biomaterials 2002, 23, 4657–4665. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, J.; Gao, S.; Chang, J.; Zhang, J.; Kang, E.T. Immobilization of chitosan onto poly-l-lactic acid film surface by plasma graft polymerization to control the morphology of fibroblast and liver cells. Biomaterials 2004, 25, 1059–1067. [Google Scholar] [CrossRef]
- Tsuji, H.; Ishida, T. Poly(l-lactide). X. Enhanced surface hydrophilicity and chain-scission mechanisms of poly(l-lactide) film in enzymatic, alkali, and phosphate-buffered solutions. J. Appl. Polym. Sci. 2003, 87, 1628–1633. [Google Scholar] [CrossRef]
- Park, G.E.; Pattison, M.A.; Park, K.; Webster, T.J. Accelerated chondrocyte functions on NaOH-treated PLGA scaffolds. Biomaterials 2005, 26, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Tokura, S.; Ueno, K.; Miyazaki, S.; Nishi, N. Molecular weight dependent antimicrobial activity by chitosan. Macromol. Symp. 1997, 120, 1–9. [Google Scholar] [CrossRef]
- Okamoto, Y.; Minami, S.; Matsuhashi, A.; Sashiwa, H.; Saimoto, H.; Shigemasa, Y.; Tanigawa, T.; Tanaka, Y.; Tokura, S. Application of polymeric n-acetyl-d-glucosamine (chitin) to veterinary practice. J. Vet. Med. Sci. 1993, 55, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Tamura, H.; Tsuruta, Y.; Itoyama, I.; Worakitkanchanakul, W.; Rujiravanit, R.; Tokura, S. Preparation of chitosan filament applying new coagulation system. Carbohydr. Polym. 2004, 56, 205–211. [Google Scholar] [CrossRef]
- Cam, D.; Hyon, S.-H.; Ikada, Y. Degradation of high molecular weight poly(l-lactide) in alkaline medium. Biomaterials 1995, 16, 833–843. [Google Scholar] [CrossRef]
- Li, S.; Garreum, H.; Vert, M. Structure-property relationships in the case of the degradation of massive poly(α-hydroxy acids) in aqueous media. J. Mater. Sci. Mater. Med. 1990, 1, 198–206. [Google Scholar] [CrossRef]
- Tsuji, H.; Ikada, Y. Properties and morphology of poly (l-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly (l-lactide) in phosphate-buffered solution. Polym. Degrad. Stab. 2000, 67, 179–189. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuike, T.; Nagahama, H.; Chaochai, T.; Tamura, H. Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope. Fibers 2015, 3, 380-393. https://doi.org/10.3390/fib3040380
Furuike T, Nagahama H, Chaochai T, Tamura H. Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope. Fibers. 2015; 3(4):380-393. https://doi.org/10.3390/fib3040380
Chicago/Turabian StyleFuruike, Tetsuya, Hideaki Nagahama, Thitirat Chaochai, and Hiroshi Tamura. 2015. "Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope" Fibers 3, no. 4: 380-393. https://doi.org/10.3390/fib3040380
APA StyleFuruike, T., Nagahama, H., Chaochai, T., & Tamura, H. (2015). Preparation and Characterization of Chitosan-Coated Poly(l-Lactic Acid) Fibers and Their Braided Rope. Fibers, 3(4), 380-393. https://doi.org/10.3390/fib3040380





