Abstract
In the present review article immune responses induced by carbon nanotubes (CNTs) are addressed. As inhalation is considered to be the primary entry route, and concern has been raised by similar high aspect ratio materials, the main focus lies on immune responses upon pulmonary exposure. Inflammation-related findings from both in vivo studies and in vitro models are reviewed, and the major responsible characteristics, which may drive CNT-induced inflammation in the lung, are discussed. In a second part, responses upon intentional administration of CNTs via subcutaneous and intravenous application are addressed, including their potential benefits and drawbacks for immunotherapy. Finally, the gastrointestinal tract as an alternative exposure route is briefly discussed. While there are many studies identifying numerous other factors involved in CNT-driven toxicity, e.g., cytotoxicity, oxidative stress, and genotoxicity, the focus of this review was kept solely on CNT-induced inflammation. Overall the literature has shown that CNTs are able to induce inflammation, which in some cases was a particularly robust response coinciding with the development of pro-fibrotic conditions. In the majority of cases the greatest inflammatory responses were associated with CNTs of considerable length and a high aspect ratio, accompanied by other factors like dispersion and sample purity.
1. Introduction
According to The International Organization for Standardization (ISO), a carbon nanofiber (CNF) is defined as a (flexible or rigid) nano-object composed of carbon with two similar external dimensions in the nanoscale and the third dimension being significantly larger. Although this definition includes the group of carbon nanotubes (CNTs), classifying them as “hollow carbon nanofibers”, many studies use the terms CNFs and CNTs to describe two distinct groups, with the difference being based on the orientation of the graphene sheet. Principally, CNTs can be either multi-walled (MWCNT) or single-walled (SWCNT), and are continuous rolls of graphene sheets that form tubes along a common axis, while CNFs are derived from different stacking arrangements of graphene layers; e.g., perpendicular to the fiber axis, inclined, parallel (unrolled) and even spiral-shaped [1]. As they are relatively easy to produce and their production costs are significantly lower compared to CNTs, there is a growing interest in using CNFs instead of CNTs for certain applications, mainly for electronics, reinforcing materials, and in the biomedical field [2,3]. Several studies suggest that these materials are especially promising for use in orthopaedic and dental applications [4,5,6]. Carbon nanotubes (CNTs), however, have already reached high volumes of industrial production, and with numerous commercial applications [7], are arguably the most notable high aspect ratio nanoparticle (HARN) currently on the market. Therefore, the primarily focus here is upon CNTs, while some attention is also given to CNFs.
With biological responses to CNTs being linked to the pathogenicity of asbestos, there is great concern surrounding CNT exposure, and there is a need to fully understand biological responses to this material. A key defense mechanism of the immune system and a factor highlighted in particle-induced responses is inflammation. In terms of the pathogenicity induced by asbestos, and other fibers and nanoparticles (NPs), the development of chronic inflammation [8] and progression of pulmonary fibrosis [9,10,11] are also often discussed. Therefore, using an assessment of CNT mediated inflammation it may be possible to predict and determine possible health implications associated with CNT exposure.
Inflammation attributed to pathogenicity induced by particles and fibers is often epitomized by the secretion of specific regulatory mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-8, and monocyte chemoattractant protein (MCP)-1, by respiratory epithelial cells and alveolar macrophages [12]. These pro-inflammatory secreted proteins can be mediated by the activity of certain transcription factors and of intracellular signaling molecules, including nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), cyclic AMP (cAMP), and intracellular calcium, upon stimulation through oxidative and physical stress [13,14,15]. CNTs have also been shown to induce NF-κB and AP-1 activation, leading to oxidative stress-induced secretion of IL-8 [16,17,18]. In this review an insight into CNT-induced inflammatory responses is provided, with a main focus on pulmonary exposure, as inhalation is arguably the most relevant exposure route, through occupational exposure. In this case, inflammation-induced fibrosis is a key element to be considered. In addition to the pulmonary exposure route, subcutaneous, and intravenous administration, significant for nanomedical applications of CNTs, have gained increased attention and will, together with alternative exposure routes to CNTs, (namely the GIT), be discussed.
2. Immune Responses upon Pulmonary Exposure
2.1. Considerations for CNT Inhalation
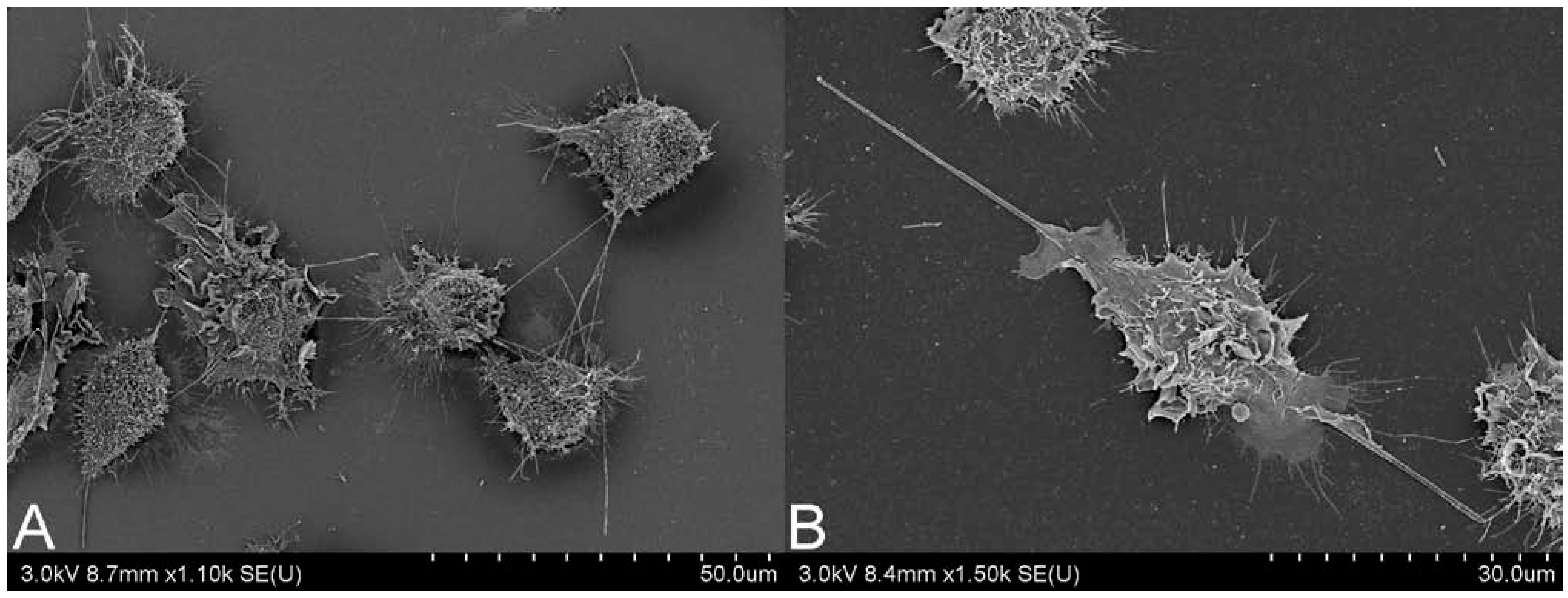
When assigning a similar pathogenicity to CNTs as has previously been observed in particularly aggressive materials, such as asbestos, there are two main issues to consider, biopersistence and respirability. The combination of both is essential for prolonged immune responses and for development of pulmonary fibrosis. With biopersistence, the clearance of inhaled material is hampered, through simple particle overload of immune cells, or potentially more importantly due to the physicochemical characteristics of the material. When fiber-like material, such as CNTs, have a length which exceeds 20 µm, a width under 3 µm and are durable, becoming biopersistent, they are considered particularly pathogenic [19]. Normal clearance of material reaching the alveoli relies on alveolar macrophages, however, successful phagocytosis by these cells is limited to 20 µm [20]. Fibers over this length can induce frustrated phagocytosis, which may lead to oxidative stress, enhanced cell proliferation, and chronic inflammation [21,22,23]. In Figure 1, frustrated phagocytosis can be observed in response to both (A) MWCNT and (B) long fiber amosite (LFA) asbestos. There have been some efforts to determine parameters for CNT biopersistence, such as deposition patterns and clearance rates.
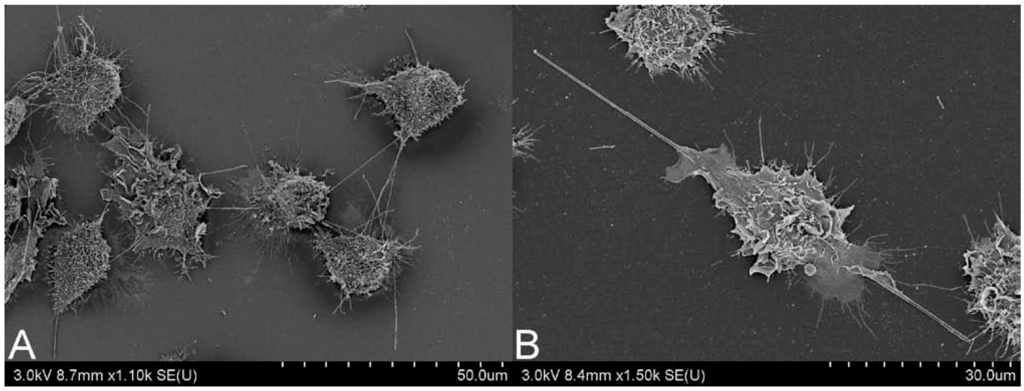
Figure 1.
Scanning electron microscopy image of J774.A1 cells undergoing frustrated phagocytosis, induced by (A) multi-walled carbon nanotubes and (B) long fiber amosite asbestos. Scale bar is on bottom right of each image. Images courtesy of Matthew Boyles, Lesley Young and Vicki Stone.
Figure 1.
Scanning electron microscopy image of J774.A1 cells undergoing frustrated phagocytosis, induced by (A) multi-walled carbon nanotubes and (B) long fiber amosite asbestos. Scale bar is on bottom right of each image. Images courtesy of Matthew Boyles, Lesley Young and Vicki Stone.
Biopersistence. Recent literature has indicated that in some cases CNTs can be degraded by biological defensive mechanisms found within the lung. For example, the degradation of SWCNTs has been shown in the presence of hypochlorite, human neutrophil enzyme myeloperoxidase catalyze, and upon direct exposure to neutrophils and macrophages, with the degraded material eliciting no inflammatory reactions [24]. This result has been confirmed by the observation of impaired clearance of CNT in myeloperoxidase-deficient mice [25], and eosinophil peroxidase has also been shown to degrade SWCNT [26]. This is very promising and might indicate that the accumulation of certain CNTs within the lungs would not be so dramatic. However, neither neutrophils nor eosinophils are expected to be the normal responders to CNTs. The macrophages, which would be the cell type expected to clear irritating materials in the various compartments of the lung, have far less expression of the enzymes that have been shown to degrade CNT [24], urging care when dealing with questions of persistence. In particular, the accumulation of macrophages has often been associated with asbestos-related pathologies [27,28]. In addition, structurally, CNTs are diverse and findings on biological degradation cannot be considered to apply for all CNT materials. In a phagolysosome simulant fluid, Liu et al. [29] demonstrated similar findings to Kagan et al. [24] when assessing the durability of carboxylated SWCNT. While, adversely, when SWCNT received no functionalization, underwent high temperature annealing, reducing surface functional groups, or were functionalized by ozonolysis or aryl-sulfonation, no degradation was found [29], indicating a potential for biopersistence of these CNTs.
Respirability. The respirability of particularly CNTs, but also CNFs, is an important consideration. Due to electrostatic properties of CNTs and resultant formation of large agglomerates, it has been considered that CNTs are not respirable to distal lung regions, however, a number of studies have shown otherwise. Sprague Dawley rats exposed to vapor grown CNFs for 90 days in a subchronic inhalation study showed slight inflammatory effects (sub-acute to chronic) and changes in lung morphology for administered doses of 2.5–25 mg/m3, some of which were still persistent (although reduced) after a 30-day recovery period. The observed changes included thickening of the interstitial walls and hypertrophy/hyperplasia of type II alveolar cells [30]. A dose-dependent deposition of MWCNTs throughout the lungs of exposed mice (via inhalation) was found by Mitchell et al. [31]. Alveolar macrophages were found laden with CNTs. No pulmonary toxicity was observed, however, systemically there was reduced immune function, in the form of decreased natural killer (NK) cell function, which may indicate further translocation from the lungs [31]. Ryman-Rasmussen et al. [32] have also shown inhalation of MWCNTs 0.3–50 μm in length. CNTs were observed throughout distal lung regions, within epithelial cells and alveolar macrophages. This study was conducted in naive and ovalbumin-sensitized mice. Combined, these treatments were shown to induce strong inflammatory responses with secretion of PDGF and MCP-1, and pro-fibrotic conditions with fibroblast proliferation, collagen deposition and secretion of IL-13, TGF-β1, and IL-5. No fibrosis was observed with individual exposures, although inflammatory mediators were still present, with IL-13 and TGF-β1 in response to ovalbumin, and PDGF and MCP-1 in response to MWCNTs. MWCNT inhalation of aggregates with an aerodynamic diameter of <3 μm also resulted in deposition within the alveolar region in a study conducted by Ma-Hock et al. [33]. Dose-dependent systemic and pulmonary inflammation was observed, as well as granuloma formation in the lungs and lymph nodes and the upper respiratory tract and immune cells were shown to accumulate. Uptake of MWCNTs was observed in both epithelial cells and alveolar macrophages with inhalation of 0.1, 0.5, or 2.5 mg/m3 MWCNTs. Although fibroblasts were present, no fibrosis was evident during the 13-week study. Systemic effects observed by Mitchell et al. [31], and Ma-Hock et al. [33] only infer the ability of MWCNT to translocate to neighboring tissues, while Ryman-Rasmussen et al. [34], and Mercer et al. [35,36] identified migration of CNTs to alveolar interstitium, subpleural tissue, and intrapleural spaces using inhalation or aspiration, respectively. As did Mercer et al. [37], with lung aspiration of relatively short (3.86 μm) MWCNTs into C57BL/6J mice. In as little as seven days, no MWCNTs were found within the airways, instead a particle lung burden was observed within alveolar macrophages, alveolar tissue, granulomatous lesions and subpleural tissues. The presence of MWCNTs within alveolar tissue was associated with an increase in alveolar connective tissue thickness. With CNT inhalation, the translocation observed by Ryman-Rasmussen et al. [34] was associated with subpleural fibrogenesis. CNT translocation upon inhalation has been further confirmed in recent publications. When C57BL/6 J mice exposed to MWCNTs, with post-exposure periods of one day and almost one year, MWCNTs were found predominantly within the lymph nodes, but also within the diaphragm, chest wall, liver, kidney, heart and brain [38]. A study conducted under similar conditions highlighted inflammatory responses generated under these conditions, and the progression of fibrosis. During the 336-day post-exposure period the lung burden pattern indicated an early uptake within alveolar macrophages and gradual decline in this location, with an increasing burden within alveolar tissue as the 336 days progressed. This was accompanied by a rapid inflammatory response in the form on polymorphonuclear leukocyte (PMN) accumulation, which declined over time, and a time dependent deposition of collagen within the alveolar region [39]. In an acute inhalation study, Porter et al. [40] reported substantial MWCNT lung burden within the alveolar region, predominantly within macrophages, and a significant increase in PMNs, which was associated with mild fibrosis. Pleural penetration was also observed during this acute study. Each of these studies adds great weight to the similarities suggested for CNT-induced responses to those of asbestos.
Deposition. The deposition pattern of CNTs can be quite diverse, with subsequent variation to induced pulmonary responses. Deposition of CNTs throughout the pulmonary system is evident in many animal models, using administration by aspiration, inspiration, but also inhalation. With aerosolisation of MWCNT aggregates of aerodynamic diameter 714 ± 328 nm Ryman-Rasmussen et al. [32] observed a homogenous distribution throughout the mouse lung, including alveoli, with particle aggregates and single MWCNTs within alveolar macrophages and epithelial cells. A size-dependent deposition was shown by Muller et al. [41] with intratracheal instillation of intact and ground MWCNT into rat lungs. Agglomerates of intact MWCNT (individual length 5.9 μm) would remain within the upper airways, while grinding to a length of 0.7 μm increased dispersion throughout the lung tissue. Both samples were persistent within the lungs of rats for the duration of the study, however, the clearance of ground MWCNTs was faster than that of the pristine, with 81% remaining of the pristine after 60 days, and only 36% of the ground. Both were associated with an influx of neutrophils and eosinophils, granuloma formation, collagen deposition, and fibrogenesis. The inflammatory response was greater to the intact MWCNT, however, the granuloma formation to these CNTs was limited to the sites of CNT accumulation, mainly in the bronchi. The dispersed, ground CNTs induced lesions predominantly within alveolar spaces and interstitial tissue, and also stimulated an acute and prolonged TNF-α release, while TNF-α release in response to intact MWCNTs only appeared to be acute.
2.2. CNT Instillation and Aspiration
The use of these more common methods of in vivo pulmonary exposure to CNTs, but also direct injection into peritoneal and pleural cavities, provide valuable information regarding pulmonary responses to CNTs and CNFs, and determination of clearance mechanisms. An increase in immune cells and pro-inflammatory cytokines can be found in response to numerous CNT samples [22,35,41,42,43], and an induction of inflammation-related complications has often been shown, including granuloma formation, alveolar wall thickening [35,43,44,45], collagen deposition [46], and the development of fibrotic lesions [41,43].
An increase in alveolar macrophages, PMNs, pro-inflammatory cytokines and fibrosis, as well as oxidative stress, have been reported upon exposure of C57BL/6 mice to SWCNTs, CNFs, and asbestos via pharyngeal aspiration. The most severe effects and earliest onset were observed in treatments of SWCNTs, followed by CNFs, and then asbestos [47]. A significant neutrophil recruitment was also observed with 18-hour post-instillation of MWCNT into the rat lung, which was considered CNT length-dependent, as similar results were not evident with short or entangled samples [42]. An acute post-exposure period of six hours has also been shown to elicit significant immune responses to 100 μm double-walled CNTs (DWCNTs). Certain pro-inflammatory markers, such as leptin and IL-6, were found elevated in the mouse lung, but not TNF-α or IL-1 [48]. In addition, in mice, an acute inflammatory response to SWCNT was found three hours after particle instillation, with measurements of MIP-2, MCP-1, and IL-6. This inflammatory response to SWCNT lasted throughout the 24-h exposure period [49].
The progress of fibrosis in response to CNT exposure is considered a response to prolonged inflammation and the development of pro-fibrotic conditions. The release of mediators such as PDGF and TGF-β1, and the increase in proliferation and cell recruitment in response to CNT [43,45,50] are considered to induce granuloma formation, alveolar wall thickening, and fibrosis [22,35,43,44,45]. The development of fibrosis may first rely on the resolution of the initial inflammatory response and removal of recruited immune cells [51], although the continued release of TNF-α and progression of fibrosis can coincide [41], and a fibrotic response can be found in the absence of any obvious acute inflammatory response, but in the presence of pro-fibrotic growth factors, such as platelet-derived growth factor –A, –B, and –C [50,52]. Additionally, the progression of fibrosis may occur during instances of CNT exposure to already inflamed environments, such as in allergic disorders. Such a synergistic response of CNTs was observed in ovalbumin-sensitized mice [32]. In a similar study intranasal exposure of CNTs and CNFs to ovalbumin-sensitized mice induced an increase in immune cells in both treatments, compared to ovalbumin alone [53]. Total cell counts, and those of neutrophils and macrophages were elevated in BAL fluid in response to CNFs, however, eosinophil influx was not observed, indicating a less significant allergic airway inflammation. In contrast, all these cell phenotypes were found increased in response to CNTs. Both carbon forms were also shown to promote allergen-specific IgE and IgG production; however, generally the effects of CNFs were less potent than for CNTs, presumably due to the higher aspect ratio and biopersistence of the latter.
With many exposure models using either CNT instillation or aspiration it is important to assess the differences between these methods and CNT inhalation. Using repeated inhalations or aspiration short SWCNTs (<1 μm), with considerably high Fe content (17 wt%), were shown to induce a strong inflammatory response, with influx of immune cells, increased pro-inflammatory cytokine release, and also the progression of fibrosis. However, rats exposed via inhalation were considered more susceptible than those exposed via aspiration [54].
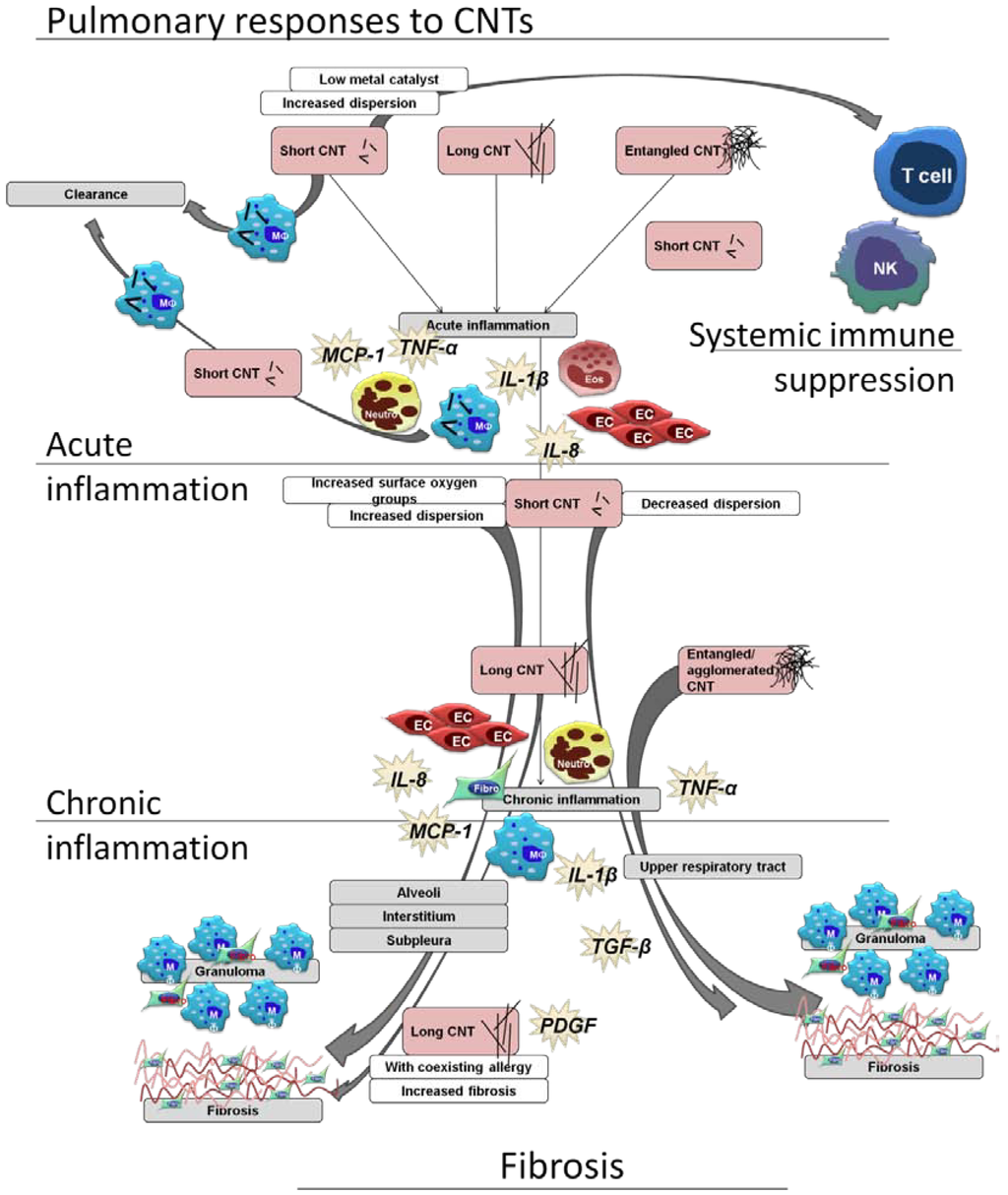
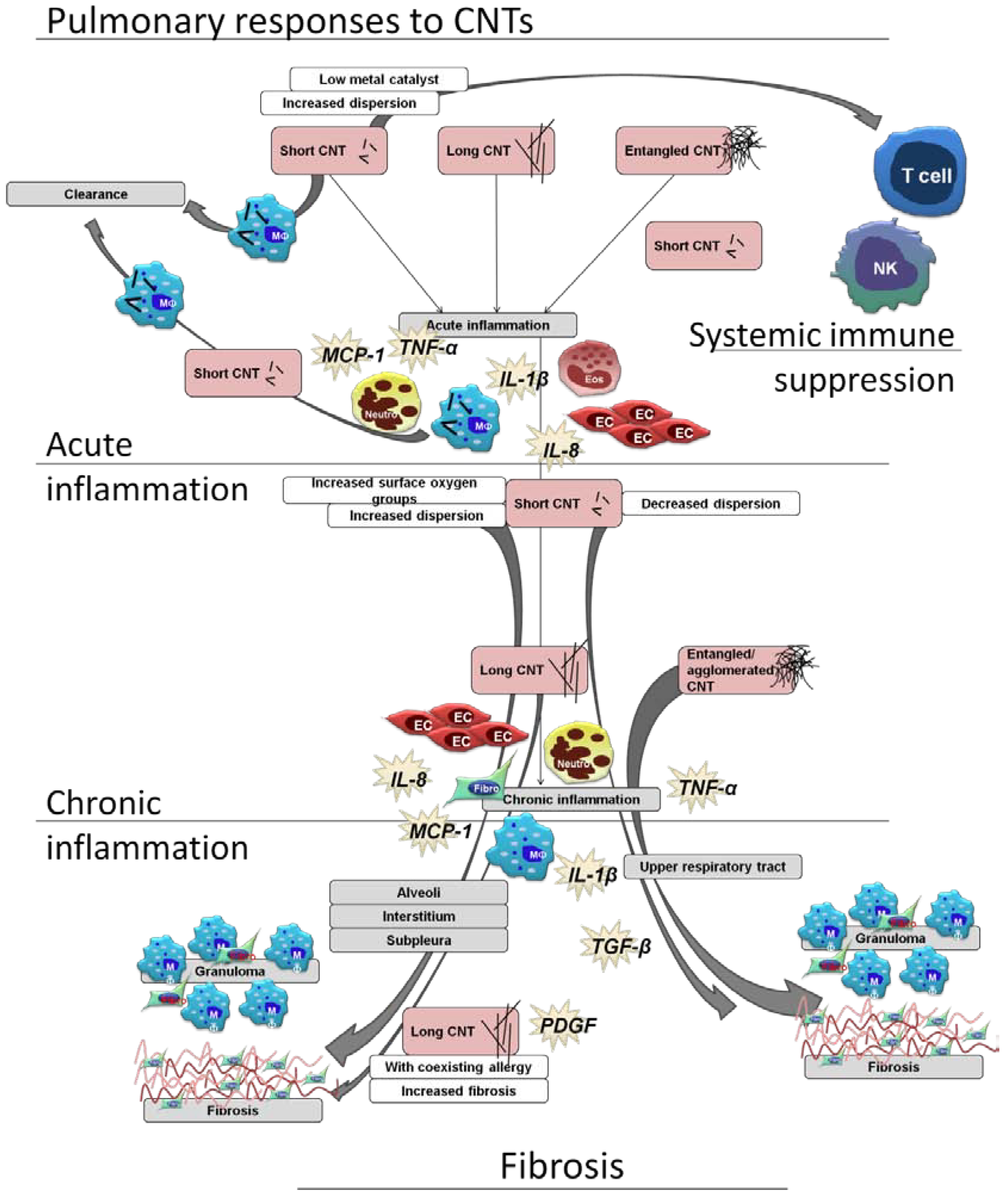
Figure 2.
Schematic view of pulmonary immune cell responses related to CNT characteristics, using a selection of the literature reviewed in this article. A range of responses can be seen, with the secretion of both pro-inflammatory and pro-fibrotic mediators, and effects upon epithelial cells (EC), T cells, monocytes (MO), macrophages, natural killer cells (NK), eosinophils (Eos), and neutrophils (neutro). Short, long, and entangled CNTs all are able to induce acute and chronic inflammation, and all able to induce granulomas and fibrosis. Fibrotic lesions induced by aggregated and entangled CNTs are restricted to the upper airways, while well-dispersed short and long CNTs are found within the alveoli and interstitium, with associated fibrosis. Allergen sensitization is shown to enhance inflammation and fibrosis induced by CNTs, as is CNT length. At times, a systemic immune effect can be observed without obvious pulmonary inflammation. [31,32,34,41,42,52,55].
Figure 2.
Schematic view of pulmonary immune cell responses related to CNT characteristics, using a selection of the literature reviewed in this article. A range of responses can be seen, with the secretion of both pro-inflammatory and pro-fibrotic mediators, and effects upon epithelial cells (EC), T cells, monocytes (MO), macrophages, natural killer cells (NK), eosinophils (Eos), and neutrophils (neutro). Short, long, and entangled CNTs all are able to induce acute and chronic inflammation, and all able to induce granulomas and fibrosis. Fibrotic lesions induced by aggregated and entangled CNTs are restricted to the upper airways, while well-dispersed short and long CNTs are found within the alveoli and interstitium, with associated fibrosis. Allergen sensitization is shown to enhance inflammation and fibrosis induced by CNTs, as is CNT length. At times, a systemic immune effect can be observed without obvious pulmonary inflammation. [31,32,34,41,42,52,55].
2.3. In vivo Determination of Responsible Characteristics and Significance of in Vitro Models
There are many characteristics proposed that may contribute to CNT immunotoxicity and enhance pathogenicity, including metal contaminants and their bioavailability, high aspect ratio, aerodynamic diameter, as well as structural defects, pertaining to sample purity, surface chemistry, and sample dispersion. For hazard assessment and to allow safe usage and production it is important to establish which components are less or more influential, and possibly which biological environments are less or more susceptible. Here, we want to provide a few examples of how each characteristic has been shown to play a role in vivo; and furthermore, draw attention to in vitro systems which have attributed toxicity to these same CNT characteristics and, therefore, can be used as fast, high throughput methods to assess these characteristics. There is extensive literature that has elucidated upon which CNT characteristics are most responsible for the resultant toxicity, and to cover them all is beyond the scope of this review. To provide an overview of pulmonary responses to CNTs, Figure 2 offers a schematic view of immune cell responses related to CNT exposure characteristics.
Involvement of oxidative stress and metal impurities. Much of the particle toxicology performed in the past has placed an emphasis upon the induction of oxidative stress. This may also be relevant for CNT exposures, primarily due to the activity of metal contaminants found within many CNT samples, as the redox activity of metals, such as iron, is known to induce activation of key inflammatory instigators, such as NF-κB, AP-1, and p53 [56]. There are numerous studies that can shed some light on this subject. With exposure to material of fiber-like dimensions [57,58], including MWCNT [59], reactive oxygen species (ROS) production during phagocytosis is intensified; with associated inflammatory responses [21], through ROS-mediated NF-κB activation [16]. In vitro, this has been attributed to the initiation of respiratory burst [21,59] and, at times, to fiber-induced frustrated phagocytosis [20,59]. When pro-inflammatory markers, such as leptin and IL-6, were found in vivo, in the absence of ROS, the lack of redox activity was attributed to either a low presence of metal contaminants or to the ability of relatively pure CNT samples to appropriate ROS [48].
Carbon structure, metal impurities and sample dispersion. An acute inflammatory response has been observed in vivo with exposure to iron-depleted SWCNTs with the systematic presentation of neutrophils, lymphocytes and macrophages, respectively, as the post-exposure period progressed. An early elevation of TNF-α and IL-1β was observed, at later stages progressing to elevated TGF-β1, granuloma formation, collagen deposition, and fibrosis [43]. A parallel in vitro study was found to be both complementary and contrary. In RAW 264.7 macrophages the same SWCNTs did not induce the secretion of TNF-α, nor IL-1β. However, the purified (iron depleted) SWCNTs were found to induce TGF-β1 after six-hour exposure to 100 μg/mL [43], confirming that the progression of a pro-fibrotic environment may be instigated by CNTs, and highlighting that iron content has a lesser importance in these responses. The above mentioned in vivo events were often associated with SWCNT aggregates, but observations were made of additional collagen deposition and alveolar wall thickening independent of SWCNT aggregates, and possibly due to undetectable, dispersed SWCNTs [43]. As previously mentioned, Muller et al. [41] identified inflammatory responses and patterns of fibrosis in vivo that were dependent of CNT dispersion within the lung. Ground MWCNTs provided a better dispersion, a greater capacity for reaching lower lung regions, and an enhanced inflammatory response when compared to intact MWCNT; this enhanced inflammatory response was corroborated in vitro with the exposure of peritoneal macrophages, when ground MWCNTs were shown to induce significant TNF-α expression whereas intact MWCNTs did not. This was later confirmed by Mercer et al. [35], with pharyngeal aspiration of well-dispersed SWCNTs similar to those used by Shvedova et al. [43]. With administration of the same SWCNT weight, a similar acute inflammatory response was observed in C57BL/6 mice; an acute influx of inflammatory cells was observed which subsided over time, and was not associated with the appearance of granulomatous lesions when SWCNTs were well-dispersed. SWCNTs were found within the interstitial spaces, in close proximity to the pleura, with the associated time-dependent alveolar wall thickening [35] that had also been observed by Shvedova et al. [43].
In a study controlling CNT properties such as metal contamination and oxygenated functional groups it was shown that biological responses can be influenced by CNT structural imperfections, and not metal contamination [60]. In vivo, immune cell influx, elevated IL-1β, and TNF-α were all found to be enhanced in response to CNTs with high levels of surface oxygen groups, as was granuloma formation [60]. This dependency on structural defects was confirmed in vitro in the form of CNT-induced micronucleated binucleated cells [60], which provides a good corroboration between in vivo and in vitro systems. Although the endpoints differed, the causative factor was confirmed. Lam et al. [44] have also shown the significance of sample impurities, with an emphasis on metal contamination, with instillation of short CNTs. Substantial granuloma formation was observed irrespective of iron content, with considerable granuloma and lethality observed in exposure of mice to CNTs with high nickel content. Although it has been shown, by Kobayashi et al. [55], that short MWCNTs induce no marked inflammatory responses, it must be noted that the “low dose” used by Lam et al. [44] was considerably greater than the highest dose administered by Kobayashi et al. [55]. However, similar dosing with carbon black particles induced no such responses during the full exposure period used by Lam et al. [44]. When considering dose, in assessment of normal workplace exposure it may be prudent to remain reserved in respect to particle dosimetry. However, when considering an accident scenario exposure dose really has few limits. The main limiting factor may be respirability, and although Lam et al. [44] used instillation as their method of exposure the CNT dust samples were evaluated as a generated aerosol and were found to be predominantly of respirable size. In consideration of normal workplace exposure, it was reported by Maynard et al. [61] that with SWCNT dust handling there is potential exposure to 53 μg/m3, which would lead to 106.8 μg SWCNT deposition upon lung epithelium per day. The dose used by Mercer et al. [35] of 10 μg per mouse was a calculated representation of approximately 200 days normal workplace exposure in humans. With 0.5 mg CNTs/kg body weight, and the average body weight of a mouse being 20 grams, the dose used by Mercer et al. [35] lies within the dose range used by Kobayashi et al. [55] of 0.04, 0.2, and 1 mg/kg body weight of MWCNTs instilled into Sprague–Dawley rats; the dose used by Lam et al. [44] was certainly significantly higher at (again using average mouse weight of 20 grams) 5 and 25 mg/kg body weight, and can only be used in reference to an accident scenario, while the two studies by Mercer et al. [35] and Kobayashi et al. [55] provide a suitable determination of potential occupational exposure.
CNT length. An emerging pattern within current literature on in vivo and in vitro studies is the increased immunogenicity of particularly high aspect ratio CNTs, and, thus, it is tentatively proposed that this characteristic plays a pivotal role in inducing pulmonary inflammatory responses, and may be equally important in the proposed disease progression. The pathogenicity of any fibre can often be attributed to length and durability, both of which allow evasion of host abilities to remove fibres from distal lung regions. When a fiber is over 10–20 μm in length the ability of alveolar macrophages to remove these fibers is impaired, induced by frustrated phagocytosis, which allows for further interaction with epithelial cells and the opportunity for fibers to translocate to pleural spaces [20,62]. In previous fiber toxicology research, such studies looking into asbestos, length has been shown to be instrumental in inflammation-mediated disease progression. This is therefore a key aspect that has been considered for CNT hazard assessment.
MWCNT length- and shape-dependent responses have also been confirmed in vitro. In primary monocytes, Brown et al. [21] demonstrated that long, straight, well-dispersed CNTs can induce significant TNF-α release. The authors attributed these effects primarily to frustrated phagocytosis induced during these exposures, as responses to other material that were easily engulfed by phagocytes, such as short (a few microns) carbon nanofibers, small carbon nanofiber bundles and nanoparticle carbon black (NPCB), were not shown to be as strong, incidentally nor were responses to LFA [21]. This does raise a question to which other CNT components may play a role in CNT immunogenicity, as LFA would undoubtedly also cause frustrated phagocytosis. The role of oxidative stress was proposed in a later study by Brown et al. [63] when THP-1 cells were again found to secrete significant quantities of pro-inflammatory mediators, such as IL-1β, in parallel with enhanced HO-1 and Nrf2 gene expression in response to long straight MWCNT. These responses were not matched by short MWCNTs or small MWCNT bundles, neither by amorphous carbon particles or LFA [63]. LFA-induced frustrated phagocytosis was observed with exposure of J774A.1 murine macrophage cells in a study by Boyles et al. [59], which coincided with significant inflammation. However, the secretion of pro-inflammatory mediators in response to long straight MWCNTs was significantly higher than that of LFA-treated cells [59]. This study was designed to investigate many of the CNT properties proposed as potentially pathogenic: CNT length, iron content, and crystallinity. It was shown that long MWCNTs could induce considerable pro-inflammatory, pro-fibrotic, and pro-angiogenic conditions, with exposure of mouse and human phagocytes. MWCNTs of lengths greater than 30 μm were shown to elicit significantly greater release of MCP-1, TGF-β1, and TNF-α, when compared to shorter MWCNTs (<20 μm) but also to known pathogenic material, such as LFA. The iron content and crystal structure was found to have less impact on MWCNT immunogenicity [59]. A length-dependent immune response was further confirmed in A549 epithelial cells when, through oxidant dependent NF-κB activation, MWCNTs of up to 30 μm in length were shown to induce significant IL-8 secretion, which was not found with exposure of carbon particles [16].
The conclusions of these in vitro studies complement numerous in vivo studies. Fibrogenic and inflammatory responses to MWCNTs of a particularly high aspect ratio have been shown within the lung [42,52] and within peritoneal and pleural cavities, often greater than those of shorter CNTs, other particles or known pathogenic fibers [22,64]. At times the exposure to short CNTs and small CNT agglomerates, in vivo, has induced little or no prolonged inflammation or fibrosis, and it appears that lung defense mechanisms can effectively control exposures of short MWCNTs [55]. Injection of high aspect ratio CNTs into the peritoneal cavity of mice was shown to induce an enhanced inflammatory response and encourage considerable granuloma formation, this was in comparison to negative controls but also when compared to short CNTs and long fiber amosite [22]. Long MWCNTs have been shown to induce a significant cell recruitment upon instillation into the rat lung, not observed in treatments of short nor entangled CNTs [42]. In a chronic exposure study, with post-exposure periods of three days to six months, intratracheal instillation of well-dispersed short MWCNTs (<20 μm), Kobayashi et al. [55] observed no significant long-term inflammation within the rat lung. Neutrophil and eosinophil recruitment was observed during an acute period, which was not evident after chronic exposure, while it was maintained with treatments of crystalline silica. Observations of alveolar macrophages laden with MWCNTs and the lack of inflammation [55] indicated the ability of rats to safely control MWCNTs of this length.
2.4. The Role of in Vitro Systems
The use of in vitro systems, of course, does not truly mimic the in vivo situation. However, they may be used as relatively fast and cheap assays for determining biological responses to CNTs, as well as to other nanomaterials (NMs), and can provide medium-to-high throughput screening methods for dealing with large sample numbers, e.g., for monitoring, or toxicity profiling. This method of in vitro toxicity profiling has recently been reported by Snyder-Talkington et al. [65] using the exposure of mice and small airway epithelial cells (SAEC) to MWCNTs, and subsequent correlation of MWCNT induced lung pathology data with tissue and SAEC mRNA expression. Many markers perceived as responsible for lung inflammation and fibrosis were identified during the analysis of tissue mRNA, and a good correlation of VEGF and CCL2 levels were shown in the coinciding in vitro study, confirming the usefulness of in vitro toxicity profiling. In vitro systems can also provide determination of acute pro-inflammatory responses, they can be used to infer the initiation of pro-fibrotic environments upon particle exposure, and to determine the initiation of fibrosis more directly. For example the collagen formation by fibroblast cells in vitro was shown in response to SWCNTs dispersed using both natural lung surfactant and acetone, but not in the absence of a dispersion aid; this was substantiated in vivo when the same materials were found to induce collagen deposition upon aspiration by C57BL/6J mice [46].
The studies presented in this section have demonstrated that the outcome of in vitro studies can complement those of in vivo systems. In vitro it must, however, be communicated that in vitro studies can be used as standalone investigations, particularly with the development of complex multi-cellular systems and, additionally, when addressing questions concerning cellular mechanisms, in vitro will often be the only appropriate method. A good example of this concerns the secretion of IL-1β. Neither Boyles et al. [59], nor Hirano et al. [17] observed any secretion of IL-1β, although Hirano et al. [17] reported the expression of IL-1 genes were enhanced, and both studies identified numerous other pro-inflammatory cytokine secretions. The induction of IL-1βrelease in response to CNT exposure may be slightly more complicated than for most other cytokines and may be due to the nature of IL-1β formation, as the transcription, translation, and eventual release of mature IL-1β usually require more than one stimulus. For the initial expression and synthesis of pro-IL-1, TLR binding can provide the first stimulus. Formation of mature IL-1β occurs through the action of inflammasome-associated caspase-1 enzyme [66]. The involvement of inflammasomes in many inflammatory disorders is evident, in cases of allergic asthma [67], in relation to known carcinogens, such as silica, associated with fiber-mediated respiratory disorders such asbestosis [11], and also in inflammatory responses to CNTs [66]. DWCNTs have been shown to induce IL-1 maturation, attributed to NLRP3 inflammasome activation, which was thought to occur during binding and internalisation of CNTs when potassium was released through increased cell membrane permeability [66]. The ROS generated during CNT-induced membrane impairment, through frustrated phagocytosis, has also been implicated in inflammasome activation by Dostert et al. [11]. Palomäki et al. [68], who attributed NLRP3 inflammasome activation-induced IL-1β secretion to the fiber properties of long CNTs and asbestos. These studies highlight the importance of in vitro models, and incidentally, both place an importance on CNT aspect ratio in immune regulation.
The emerging field of in vitro co-culture techniques has been used for the assessment of numerous NMs, and is advancing in vitro methods towards a more realistic view of particle interactions and biological responses, as they allow for the complex interaction between phenotypically distinct cells. A comparison between a complex triple cell co-culture model, consisting of monocyte-derived macrophages (MDM), monocyte-derived dendritic cells (MDDC), and 16HBE14o- epithelial cells, with each of these cells grown under traditional single cell culture conditions, has shown no difference in CNT induced cytotoxicity or inflammation, when assessing the different cell culture conditions [69]. A finding also reported by Müller et al. [70], who also observed no differences between these systems when assessing the oxidative potential of CNTs. Here, Clift et al. [69] differ, as they did demonstrate a difference between culture methods for oxidative potential, which is in line with a study performed by Gasser et al. [71] who also determined that co-culture and mono-culture exposures provide different responses for oxidative stress, and interestingly also for immune responses. The development of these techniques has been well reported, however, as yet conclusions regarding whether these systems provide different responses to NMs are undetermined, they do, however, add another dimension to in vitro toxicity testing.
This review has, thus far, focused on CNT immunogenicity within the pulmonary system, inflammation-related disease progression and in vitro systems related to pulmonary exposures, as this is still considered the primary exposure route. However, numerous studies have been performed concerning alternative exposure routes, such as subcutaneous implantation and intravenous injection, or, ingestion. All of which warrant consideration, and in terms of subcutaneous implantation and intravenous injection, often relate to intentional administration with proposed nanomedical applications. These aspects will be covered in the subsequent sections.
3. Intentional Administration of CNTs—Nanomedical Applications
As CNTs have been shown to easily penetrate the plasma membrane [72] and enter cells, they are therefore proposed in promising biomedical applications, such as nanovehicles in drug delivery systems [73], and for the transport of proteins [74], peptides [75], and DNA [76] into cells. Moreover, CNTs may exert an excellent adjuvant function when used as a carrier in immunization protocols, which has been shown for macromolecules [77,78,79] as well as for peptides [80] and small haptens. This also bears the potential to use CNTs as adjuvants in the production of antibodies for medical treatment, or for the development of immunoassays for pesticides, drugs of abuse, environmental contaminants, food additives, hormones, and toxins [81]. Due to their hydrophobic and, often, water-insoluble nature, CNTs have been subjected to an array of functionalization with various compounds resulting in different biological effects [82]. However, water-dispersible CNT biodistribution studies in mice, using the intravenous administration, have revealed that CNTs localize primarily within the liver and lung, and to a lesser extent the kidney and spleen. This form of CNT was considered biocompatible as TNF-α expression in hepatocytes cultured with CNTs was comparable to those of controls [83].
As holds true for any NM, the potential use of CNTs in nanomedicine relies upon a balance between desirable and undesirable immune responses (Figure 3). This can be evaluated with observations of the immunotoxicological profile of systemically administered CNTs [84]. The list of desirable immune effects can include increased vaccine efficacy, or improved treatments of cancer, and various inflammatory, autoimmune and infectious disorders; both immune stimulation as well as immune suppression can be the desired outcome. The impact of CNTs on the balance between the desirable and undesirable effects in potential therapeutic applications is summarized in Figure 3. Adverse reactions to CNTs can comprise of immunostimulatory responses such as hypersensitivity reactions, anaphylaxis, coagulopathy, but also immunosuppression, lowering the body’s response against infections with pathogens or against malignant cells [85]. Apart from direct immune activation, adverse effects may include hemolysis and platelet aggregation leading to coagulopathy, both of which cause hepato- and nephrotoxicity and, furthermore, secondary immune effects. Moderate platelet aggregation by CNT-driven GPIIb/IIIa activation has been observed in vitro using human platelets (independent of protein kinase C), which was corroborated in vivo by vascular thrombosis upon CNT infusion in rats [86]. The authors could not exclude CNT metal contamination as the source of this response. Depending on surface charge CNTs induce ROS, and, thus, activate platelets. Whereas bridging of platelets via their GPIIb/IIIa receptor appears reliant on CNT morphology, as spheres of fullerene did not display such effects. Meanwhile, platelet-aggregating activity in response to CNTs has been reported to act via a mechanism involving calcium influx in human thrombocytes [87].
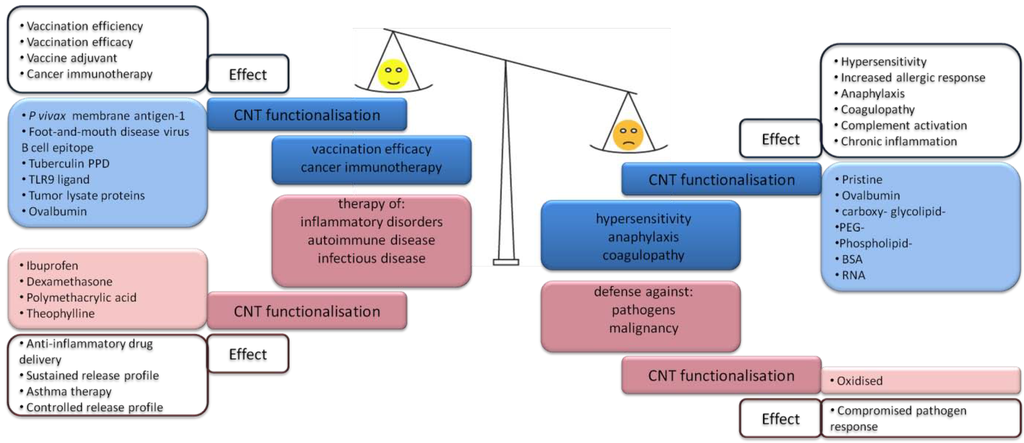
Figure 3.
Desirable versus adverse effects may be due to immunostimulatory (blue) as well as immunosuppressive (red) functions of CNTs. In nanomedical applications of the desirable effects of intentionally administered CNT-based drugs have to outbalance adverse effects [77,79,80,86,88,89,90,91,92,93,94,95,96,97].
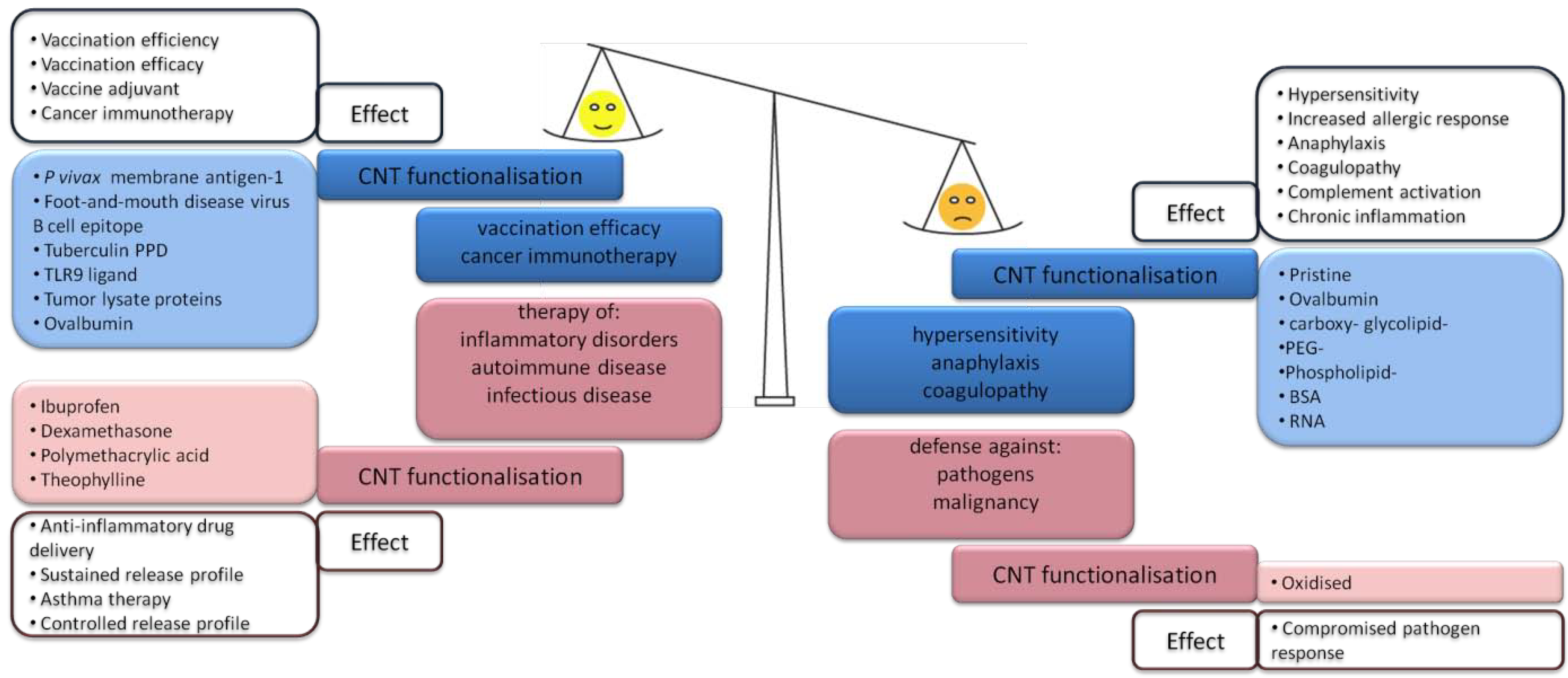
Figure 3.
Desirable versus adverse effects may be due to immunostimulatory (blue) as well as immunosuppressive (red) functions of CNTs. In nanomedical applications of the desirable effects of intentionally administered CNT-based drugs have to outbalance adverse effects [77,79,80,86,88,89,90,91,92,93,94,95,96,97].
3.1. CNT-Induced Immune Responses upon Subcutaneous Administration
Subcutaneous injection is a common and often the most effective way to administer drugs, as the tissue readily absorbs the administered substance due to its high vascularity. CNTs are being considered as drug delivery agents, and as such, administration of drugs carried by CNTs might affect the subcutaneous tissue homeostasis. The subcutaneous injection of MWCNTs has been shown in BALB/c mice to induce short-term immune responses. CNTs were observed within macrophages at the site of administration, accompanied by low levels of lymphocyte recruitment, collagen deposition and complement activation. Many inflammation-related cytokines were up-regulated during acute exposure to MWCNTs, including IL-17, IL-1β, IFN-γ, IL-1α, IL-2, IL-3, IL-6, IL-10, IL-13, G-CSF, GM-CSF, and TNF-a; all of which subsided over time [98]. Furthermore, the subcutaneous injection of MWCNTs had previously been shown by Meng et al. [99] to induce similar cytokine release and macrophage and complement activation in tumor-bearing mice. With the exception of the lymph nodes, no obvious accumulation of the CNTs was found in liver, spleen, kidney, or heart. The group of Kazuyuki Tohji has focused several studies on the effects of CNTs on the subcutaneous tissue of rats. When hat-stacked carbon nanofibers (H-CNFs) were implanted into the subcutaneous layer of rats, macrophages were recruited and foreign body giant cells were formed at the affected tissues. Additionally, H-CNFs were found within the phagocyte lysosomal vacuoles [100]. In a more recent long-term exposure study, the same group examined biopersistence of powdered tangled oxidized MWCNTs (t-ox-MWCNTs) after implantation into the subcutaneous tissue of rats. Segments of the tissue were examined one week, one year and two years after implantation. At all three time points, large agglomerates had formed which were found in close proximity with fibroblasts and foreign body giant cells; smaller agglomerates were engulfed by phagocytes. With one and two years post-exposure periods, t-ox-MWCNTs were shown to migrate into the connective adipose tissue [101]. There are currently only few studies investigating the response of CNTs on subcutaneous tissue and additional research is needed in these areas if CNTs conjugates are to be used as nanomedicines in this fashion.
3.2. CNT Induced Immune Responses within the Venous System—Pro-Inflammatory Effects
Unspecific immune activation mechanisms by CNTs can operate via ROS production [102] or secretion of acute phase proteins like TNF-α, IFN-γ, IL-8, IL-1β, or IL-6 from CNT-targeted cells [103]. The source of these pro-inflammatory molecules in case of systemically administered CNTs ranges from endothelial cells (upon vascular endothelium damage) to fibroblasts and immune cells within the blood and liver. There has been some debate in regard to whether the observed effects were due to the CNTs themselves or to present impurities, which also demands considerations of the pro-inflammatory impact due to coating procedures or composites [104,105]. Several studies emphasized the importance of CNT functionalization, where surface coating by polyethylene glycol (PEGylation) seemed to increase CNT-mediated activation of neutrophils and hence stimulate biodegradation [106]. While at high concentrations being cytotoxic, low doses of CNTs administered systemically mediated the NF-κB-dependent production of TNF-α and IFN-γ in human lymphocytes, resulting in indirect toxicity [107].
MWCNTs functionalized with single-stranded DNA were administered intraperitoneally into rats and a pro-inflammatory profile was induced in the liver by a combination of oxidative stress and production of NF-κB-driven cytokines [108]. Using a proteomic approach, based on isobaric tagged relative and absolute quantification (iTRAQ)-coupled 2D liquid chromatography-tandem mass spectrometry, 30 differentially expressed proteins involved in cell cycle arrest, DNA repair, stress-induced apoptosis, as well as NF-κB-driven inflammation were found in human HepG2 liver cells upon exposure to oxidized SWCNTs [109]. For the investigation of genotoxicity and pro-inflammatory responses in the liver, the eventual terminus of CNTs with intravenous, intraperitoneal, or subcutaneous administration, but also when inhaled or ingested, the hepatoblastoma cell line C3A was proposed as a well-suited model [110], and just a very limited pro-inflammatory response to CNTs was observed, including a dose dependent increase in IL-8 secretion [111].
As previously discussed, induction of an innate immune response can be driven by various NM via the activation of the NLRP3 inflammasome, and this may even represent a desired effect for optimising the efficacy of a vaccine [112]. Nanomaterial-driven activation of innate immune responses includes specific interaction of NM with a large array of pattern-recognition receptors including Toll-like receptors (TLRs), C-type lectine receptors (CLRs), and scavenger receptors expressed on the plasma membrane, or different classes of NOD-like receptors (NLRs) and RIG-I-like receptors inside the cell [113]. In one study, the surface chemistry of CNTs was changed in order to target them rather to scavenger receptors and to a lesser extent to the usually preferred mannose receptors followed by uptake. This was shown to reduce NF-κB-mediated immunotoxicity in vitro in THP-1 human monocytic cells, as well as in vivo [114].
Upon systemic administration of NM, including CNTs, the complement system also represents an important candidate for innate immune activation [115,116]. The importance of CNT functionalization in regard to complement activation has been reviewed extensively [94,117]. In addition to the classical pathway via C1 and C-reactive protein (CRP), the involvement of the mannose-binding lectin (MBL)/ficolin pathway and the alternative pathway via C3 has been discussed for macrophage uptake and activation in dependent on the type of CNT functionalization. Excessive or inappropriate complement activation can cause tissue damage and autoimmune disease [118]. During complement activation the anaphylatoxins C3a, C4a, and the most potent C5a, accumulate, which are responsible for smooth muscle contractions (bronchospasm), histamine release from mast cells and enhanced vascular permeability, and, as such, represent typical signs of severe anaphylaxis. Complement activation in vitro associated with binding of C1 to CNTs was found to be impaired by protein adsorption in vivo [119]; and, also in vitro, coating of SWCNT with human serum albumin has been shown to induce complement activation through C1q-mediated classical and alternative pathways, while amphiphile functionalization of the SWCNTs, resulted in complement activation of the lectin pathway [120]. The impact of these findings in vivo still remains to be clarified. Selective protein binding in serum was found in psychosine-functionalized CNTs. A novel glycolipid coating was shown to mediate stable suspensions in biological fluids, however, complement activation via C1 and CRP was also reported [94,121].
3.3. CNT-Induced Immune Responses within the Venous System—Immunomodulatory Profile
The specific, adaptive immune mechanisms rely on highly variable receptors such as the major histocompatibility complex and the T cell receptor, as well as on antibodies. All have the capacity to recognize a large array of non-self compounds including engineered NM. In particular, the functionalization of CNTs with peptides and proteins, which represents a major playground for nanomedicine applications, bears a strong potential to activate adaptive immunity. Therefore, adverse reactions against CNTs themselves have to be distinguished from functionalization-derived immune effects. As such, dendritic cell (DC) activation resulting in a marked T helper (Th)1- and Th17-polarizing capability was achieved by functionalizing CNTs with a TLR-7 agonist [122]. Notably, the same concentration of TLR7 agonist alone or even in combination with CNT, but not covalently linked, did not reach the same effect underlining the potential of CNTs to be used as immunogenic carriers [123]. Immunomodulatory effects may, however, also arise from the spontaneous formation of a protein corona (PC) [124,125], which holds true also for other NMs. Recently, hydrogen bonds and charged interactions have been shown, by label-free mass spectrometry-based proteomics, to be the main driving forces for PC formation of unmodified, carboxylated, and polyvinylpyrrolidone-coated SW- and MWCNTs under cell culture conditions [126]. The authors concluded a low degree of selectivity for PC formation of CNTs, as no relationship between the protein corona composition and isoelectric point, aliphatic index or hydropathy was found. Selective PC formation would be attributed to differences in the amino acid content of the formed PC, and, hence, hydrophobic interactions and pi-stacking of the different involved proteins would represent the main driving forces. However, the pathophysiology of systemically administered NM may still be affected by various kinetic impacts during human plasma PC formation and two scenarios are possible: pristine toxic NM may be encapsulated within a protective bio-shell or pristine non-toxic NM become toxic due to PC formation [127]. Thus, selective cytotoxicity of CNTs may have an impact on immune deviation, as DCs, T cells, or B cells may be targeted specifically and an alteration in subsets of these cellular players may cause severe effects, in an immunological context. A pivotal role of MWCNTs was reported recently from healthy and mite-allergic patients, as CNTs were found to promote and suppress immune effects depending on which type of peripheral blood mononuclear cells they interacted with [128].
To date, there is still very limited information available on DC-targeting effects exerted by CNTs. Using mouse bone marrow-derived DCs, CNTs were identified as not specifically affecting antigen-presenting cells in comparison to other NM, such as ZnO [129]. However, recently CNTs have been shown in vitro to induce a lower immunogenic profile of human differentiated and activated DCs, based on transcriptional analysis [130]. Another study reported apoptosis of T cells using the Jurkat cell line and human peripheral blood-derived T cells, however, dispersion, aggregation, or high effective concentration may have caused the observed cytotoxicity [95]. At low concentrations no T cell receptor-dependent effect could be detected using pristine CNTs [131]. In mice, immunosuppression by acid-functionalized CNTs was observed in vivo and in vitro, and these effects were shown to be mediated by down-regulation of the cytotoxic T cell response [132].
Direct binding of pristine CNTs to an antibody generated against fullerene has been described, so cross-reactivity seems to be also an issue for engineered NM [133]. However, even at very high concentrations no CNT-specific antibodies could be determined upon application of hapten carrier CNTs in mice [134]. In general, functionalized CNTs were reported as non-cytotoxic for primary B and T lymphocytes. Moreover, up-regulation of B and T cell-specific activation markers was not observed, while with less water-soluble functionalization a secretion of pro-inflammatory cytokines from human macrophages, NK cells and monocytes was present [135,136,137]. In contrast, in mice a decrease of NK cell function upon inhalation of MWCNTs had been observed, as described above [31]. A new term of “monocyte-activating CNTs” has been coined for a panel of oxidized MWCNTs for responses induced in THP-1 (monocytic) cells that were not shown in Jurkat (T cells) [138]. As far as it can be presently stated, adaptive immunity thus seems to play a minor role in CNT-mediated immune effects, which contrasts the above-described situation in innate immunity, where CNTs have been shown to mediate considerate immunogenicity. However, the close interconnection between innate and adaptive immunity promises potential applications of CNTs as (Th1-promoting) immunostimulatory carriers (adjuvants) for vaccines [79,89,139], while on the other hand it poses the risk of side-effects, such as hypersensitivity [93].
In summary, it has been extensively shown that intentionally administered CNTs interact with the immune system, and this can have desirable or adverse effects. Physical parameters including length, purity, surface chemistry, and aggregation state represent decisive factors for CNT-mediated immunotoxicity. For the potential use as drug carriers or vaccines it is evident that CNTs should be synthesized with the following properties: short, functionalized, water-soluble or easily dispersible, and biodegradable.
4. Other Routes of Exposure—Gastrointestinal Tract
There is a clear potential risk of occupational exposure to CNTs. Therefore the focus of this review, and much of the current literature, has been the pulmonary system; and with the proposed use of CNTs in nanomedicines, intentionally administered CNTs, via subcutaneous and intravenous routes, have also been deemed prominent exposure routes. However, with an estimated surface area of 200 m2, the mucosal barrier of the gastrointestinal tract (GIT) provides a large exposure area for NMs [140] and is also one of the main routes for many NMs to enter the human body. In general, possible ingestion routes for nanoparticles include uptake via food (intentionally added, leaked from NM-containing food packaging or kitchen utensils, contamination of water, animals or plants after distribution of NM in the environment) or secondary ingestion following inhalation due to mucociliary clearance of inhaled particles. Applications of CNTs, which may allow ingestion, are steadily increasing, and can include their implementation as adsorbents in water purification systems [141], or as antimicrobial agents or stabilizing materials in food packaging [142]. Recently, Prajapati et al. [143] investigated the use of CNTs as carriers for orally administered anti-parasite treatment in rats, with possible application in humans, and Kou et al. [144] identified functionalized MWCNTs as a promising coating material of dental implants for better osseointegration [144]. There have been observations that residual hydrophobic compounds, which are often adsorbed to CNTs during their synthesis, were released more rapidly in the presence of gastric enzymes and increased salt concentration in simulated GI fluids. This would make these compounds more accessible and possibly more toxic upon ingestion [145], and with proposed applications that could easily lead to the exposure of CNTs to the GIT, there is a clear need for research into this exposure route. To the best of our knowledge, there are only a few studies that have examined possible adverse effects of CNTs on the GIT, of which few have addressed immunogenicity. The genotoxicity of CNTs has been investigated by Szendi and Varga [146] and Folkmann et al. [147]; oxidative stress-induced DNA damage was found in the liver of mice when CNTs were transported there after entry to the bloodstream via the GIT [147]. With administration of CNTs via oral gavage Lim et al. [148], and Philbrook et al. [149] have investigated foetal development. In response to 14 days exposure of MWCNTs to pregnant rats, no clear responses were observed, excluding a particularly high dose (1000 mg/kg/day) inducing an increase in thymus size. Additionally, no abnormal foetal developments occurred, implicating that MWCNTs were unable to cross the blood-placenta barrier [148]. However, Philbrook et al. [149] reported an increase in foetal skeletal and ocular anomalies when mice were exposed to 10 mg/kg of SWCNTs (1–2 nm diameter) after nine days. An increase in dose, to 100 mg/kg, was not shown to affect any markers of foetal development [149]. The authors proposed that this was due to an increase in agglomeration with an increase in CNT concentration, resulting in a reduced adsorption rate by the GIT. Also using repeated oral gavage of both SW- and MWCNTs, Matsumoto et al. [150] found no obvious toxicological responses, including signs of inflammation with differential leukocyte counts, and also concluded that it was large CNTs agglomerates that reached the GIT which, due to their size, were not absorbed. If, however, CNTs were to be absorbed by the GIT, as postulated by Philbrook et al. [149] in respect to small single CNTs, their accumulation within the liver may be expected. The effects of CNTs upon ingestion are clearly an avenue that critically needs further investigation.
5. Conclusions
The reported and proposed use of CNTs clearly demonstrates the potential and the worth of this material. With a high tensile strength, optical and semi-conductive properties, and easy functionalization CNTs can be applied within many industrial and medical fields. With an increased commercial focus on CNTs hazard assessment was inevitable and soon the biological response to CNTs was being likened to that of asbestos. This has mainly focused on assumptions of inhalation within the workplace, where it was proposed that particularly high aspect ratio CNTs would follow the toxicity pattern that is outlined in the “fiber paradigm”, where fiber pathogenicity is associated with biopersistence, mainly governed by durability, length and diameter. Hence, CNTs have been shown to induce a state of frustrated phagocytosis in phagocytes in vitro, but also in recruited immune cells in vivo. If durable, CNTs would remain at the site of inflammation, causing the progression of chronic inflammatory disorders, such as fibrosis; which had been a key marker in asbestos-induced pathology. This attributed factor in CNT immunogenicity has been repeatedly reported, while other characteristics suggested to play a role in nanoparticle and pathogenic fiber immunogenicity, such as metal contamination, sample dispersion and structural defects, have been reported with inconsistent outcomes.
The area of intravenous administration is relatively well represented in CNT immunogenicity studies, and as with subcutaneous injection, intravenous injection is often focused on CNTs with a medical application. Here, it has been shown that CNTs can elicit varied responses; with extensive distribution, functionalized CNTs are shown to induce both low and high levels of inflammation, at times accompanied by other innate immune responses such as complement activation. It is often only the innate immune system that is affected, which is particularly relevant e.g., in vaccination. However, f-CNTs have been shown to stimulate T cell responses through the activation of DCs and can, thus, affect adaptive immunity as well. Physical parameters including length, purity, surface characteristics, and aggregation state represent decisive factors for CNT-mediated immunotoxicity systemically, as has been shown within the pulmonary system. For a potential use as drug carriers or vaccines, short, functionalized, water-soluble or easily dispersible, and biodegradable CNTs may appear the most appropriate option for future nanomedical research. With the promising use of CNTs in methods of immunotherapy it will be important to determine when these immune responses are induced by the intended CNT functional group, and when it is CNT themselves initiating these responses, particularly when CNTs have been shown to enhance existing allergic conditions. It is clear that intentionally administered CNTs can interact with the immune system. More research is required for a better understanding of these complex interactions, to provide suitable risk-to-benefit assessment and to allow the safe use of CNTs with nanomedical applications.
The unspecific innate immune responses to CNTs, which have been shown to progress to the establishment of pro-fibrotic environments within the lungs in many animal models, with specific inflammatory mediators also identified in vitro, has not been observed to the same degree upon subcutaneous injection or with GIT exposure. These two exposure routes, however, are areas of CNT immunogenicity that are severely lacking, simply in volume of research studies that have been conducted. This is a gap that should be filled, as immunotherapy may often use the subcutaneous route, and there are considerable instances of how CNTs may end up within the GIT and continue to the venous system and the liver.
In terms of CNFs, a general observation is that CNFs do show a tendency towards immune reactivity, but however, there are many inconsistencies in the studies available which make it hard to evaluate the findings appropriately. It is also not clear whether CNFs are less potent or more potent than CNTs. Some studies suggest CNFs to be less reactive due to their smaller surface compared to CNTs, as well as their smaller aspect ratios. However, agglomeration of CNTs was often observed, which again changes the specific surface areas to smaller values (effective surface area). Further research is necessary to fully understand the mechanisms that control the responses of the immune system to CNTs and CNFs. There have been clear characteristics identified as detrimental, therefore, to reduce the potential hazard of this material these classifications should be observed in future developments of applications and production techniques, to allow a reduced risk to those who may be exposed to CNT material.
Note added in proof: An extensive review series edited by Ali-Boucetta, Bussy and Kostarelos (Advanced Drug Delivery Reviews (Volume 65, Issue 15, Pages 1897–2134—December 2013)) concerns issues of safety and toxicity of CNTs in biology and medicine, also with implications for therapy and diagnostics, and covers numerous issues dealt within the present review in extensive detail.
Acknowledgments
This work was supported by the EU 7th framework program, Marie Curie Actions, Network for Initial Training NanoTOES (PITN-GA-2010-264506), the NanoValid project—Development of reference methods for hazard identification, risk assessment and LCA of engineered nanomaterials (grant agreement No: 263147), and by the Research Cluster Immunity in Cancer and Allergy (ICA) at PLUS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martin-Gullon, I.; Vera, J.; Conesa, J.A.; Gonzalez, J.L.; Merino, C. Differences between carbon nanofibers produced using Fe and Ni catalysts in a floating catalyst reactor. Carbon 2006, 44, 1572–1580. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Sargent, L.; Lowry, D.; Chirila, M.; Siegrist, K.J.; Schwegler-Berry, D.; Leonard, S.; Castranova, V.; Fadeel, B.; et al. Genotoxicity of carbon nanofibers: Are they potentially more or less dangerous than carbon nanotubes or asbestos? Toxicol. Appl. Pharmacol. 2011, 252, 1–10. [Google Scholar] [CrossRef]
- Sanchez, V.C.; Pietruska, J.R.; Miselis, N.R.; Hurt, R.H.; Kane, A.B. Biopersistence and potential adverse health impacts of fibrous nanomaterials: What have we learned from asbestos? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 511–529. [Google Scholar] [CrossRef]
- Price, R.L.; Waid, M.C.; Haberstroh, K.M.; Webster, T.J. Selective bone cell adhesion on formulations containing carbon nanofibers. Biomaterials 2003, 24, 1877–1887. [Google Scholar] [CrossRef]
- Price, R.L.; Haberstroh, K.M.; Webster, T.J. Improved osteoblast viability in the presence of smaller nanometre dimensioned carbon fibres. Nanotechnology 2004, 15, 892–900. [Google Scholar] [CrossRef]
- Elias, K.L.; Price, R.L.; Webster, T.J. Enhanced functions of osteoblasts on nanometer diameter carbon fibers. Biomaterials 2002, 23, 3279–3287. [Google Scholar] [CrossRef]
- Tran, C.L.; Hankin, S.M.; Ross, B.; Aitken, R.J.; Jones, A.D.; Donaldson, K.; Stone, V.; Trantra, R. An Outline Scoping Study to Determine whether High Aspect Ratio Nanoparticles (HARN) should Raise the Same Concerns as do Asbestos Fibres. Report on Project CB0406 2008. Available online: http://www.safenano.org/Portals/3/SN_Content/Documents/HARN.pdf (accessed on 1 November 2013).
- Coker, R.K.; Laurent, G.J. Pulmonary fibrosis: Cytokines in the balance. Eur. Respir. J. 1998, 11, 1218–1221. [Google Scholar] [CrossRef]
- Mossman, B.T.; Churg, A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am. J. Respir. Crit. Care Med. 1998, 157, 1666–1680. [Google Scholar] [CrossRef]
- Cassel, S.L.; Eisenbarth, S.C.; Iyer, S.S.; Sadler, J.J.; Colegio, O.R.; Tephly, L.A.; Carter, A.B.; Rothman, P.B.; Flavell, R.A.; Sutterwala, F.S. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 2008, 105, 9035–9040. [Google Scholar] [CrossRef]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef]
- Driscoll, K.E.; Carter, J.; Hassenbein, D.; Howard, B. Cytokines and particle-induced inflammatory cell recruitment. Environ. Health Perspect. 1997, 105, 1159–1164. [Google Scholar]
- Brown, D.M.; Hutchison, L.; Donaldson, K.; MacKenzie, S.J.; Dick, C.A.J.; Stone, V. The effect of oxidative stress on macrophages and lung epithelial cells: The role of phosphodiesterases 1 and 4. Toxicol. Lett. 2007, 168, 1–6. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Packer, L. Involvement of intracellular Ca2+ in oxidant-induced NF-[kappa]B activation. FEBS Lett. 1996, 385, 58–62. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Han, M.; Ye, S.; Wen, W.; Zhang, Q. Oxidative stress-mediated pro-inflammatory responses in lung epithelial cells exposed to multi-walled carbon nanotubes. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering (iCBBE), Chengdu, China, 18–20 June 2010; pp. 1–4.
- Hirano, S.; Fujitani, Y.; Furuyama, A.; Kanno, S. Uptake and cytotoxic effects of multi-walled carbon nanotubes in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2010, 249, 8–15. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; Vallyathan, V. Raw single-wall carbon nanotubes induce oxidative stress and activate MAPKs, AP-1, NF-kappaB, and Akt in normal and malignant human mesothelial cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, C.L. An introduction to the short-term toxicology of respirable industrial fibres. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 553, 5–9. [Google Scholar] [CrossRef]
- Dörger, M.; Münzing, S.; Allmeling, A.-M.; Messmer, K.; Krombach, F. Differential responses of rat alveolar and peritoneal macrophages to man-made vitreous fibers in vitro. Environ. Res. 2001, 85, 207–214. [Google Scholar] [CrossRef]
- Brown, D.M.; Kinloch, I.A.; Bangert, U.; Windle, A.H.; Walter, D.M.; Walker, G.S.; Scotchford, C.A.; Donaldson, K.; Stone, V. An in vitro study of the potential of carbon nanotubes and nanofibres to induce inflammatory mediators and frustrated phagocytosis. Carbon 2007, 45, 1743–1756. [Google Scholar] [CrossRef]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Ye, J.; Shi, X.; Jones, W.; Rojanasakul, Y.; Cheng, N.; Schwegler-Berry, D.; Baron, P.; Deye, G.J.; Li, C.; Castranova, V. Critical role of glass fiber length in TNF-alpha production and transcription factor activation in macrophages. Am. J. Physiol. 1999, 276, L426–L434. [Google Scholar]
- Kagan, V.E.; Konduru, N.V.; Feng, W.; Allen, B.L.; Conroy, J.; Volkov, Y.; Vlasova, I.I.; Belikova, N.A.; Yanamala, N.; Kapralov, A.; et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010, 5, 354–359. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kapralov, A.A.; Feng, W.H.; Kisin, E.R.; Murray, A.R.; Mercer, R.R.; St. Croix, C.M.; Lang, M.A.; Watkins, S.C.; Konduru, N.V.; et al. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One 2012, 7, e30923. [Google Scholar] [CrossRef]
- Andón, F.T.; Kapralov, A.A.; Yanamala, N.; Feng, W.; Baygan, A.; Chambers, B.J.; Hultenby, K.; Ye, F.; Toprak, M.S.; Brandner, B.D.; et al. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small 2013, 9, 2721–2729. [Google Scholar] [CrossRef]
- Spurzem, J.R.; Saltini, C.; Rom, W.; Winchester, R.J.; Crystal, R.G. Mechanisms of macrophage accumulation in the lungs of asbestos-exposed subjects. Am. Rev. Respir. Dis. 1987, 136, 276–280. [Google Scholar] [CrossRef]
- Choe, N.; Tanaka, S.; Xia, W.; Hemenway, D.R.; Roggli, V.L.; Kagan, E. Pleural macrophage recruitment and activation in asbestos-induced pleural injury. Environ. Health Perspect. 1997, 105, 1257–1260. [Google Scholar]
- Liu, X.; Hurt, R.H.; Kane, A.B. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon 2010, 48, 1961–1969. [Google Scholar] [CrossRef]
- DeLorme, M.P.; Muro, Y.; Arai, T.; Banas, D.A.; Frame, S.R.; Reed, K.L.; Warheit, D.B. Ninety-day inhalation toxicity study with a vapor grown carbon nanofiber in rats. Toxicol. Sci. 2012, 128, 449–460. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Gao, J.; Vander Wal, R.; Gigliotti, A.; Burchiel, S.W.; McDonald, J.D. Pulmonary and systemic immune response to inhaled multiwalled carbon nanotubes. Toxicol. Sci. 2007, 100, 203–214. [Google Scholar]
- Ryman-Rasmussen, J.P.; Tewksbury, E.W.; Moss, O.R.; Cesta, M.F.; Wong, B.A.; Bonner, J.C. Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am. J. Respir. Cell Mol. Biol. 2009, 40, 349–358. [Google Scholar] [CrossRef]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation toxicity of multiwall carbon nanotubes in rats exposed for 3 months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Cesta, M.F.; Brody, A.R.; Shipley-Phillips, J.K.; Everitt, J.I.; Tewksbury, E.W.; Moss, O.R.; Wong, B.A.; Dodd, D.E.; Andersen, M.E.; et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat. Nanotechnol. 2009, 4, 747–751. [Google Scholar] [CrossRef]
- Mercer, R.R.; Scabilloni, J.; Wang, L.; Kisin, E.; Murray, A.R.; Schwegler-Berry, D.; Shvedova, A.A.; Castranova, V. Alteration of deposition pattern and pulmonary response as a result of improved dispersion of aspirated single-walled carbon nanotubes in a mouse model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L87–L97. [Google Scholar]
- Mercer, R.; Hubbs, A.; Scabilloni, J.; Wang, L.; Battelli, L.; Schwegler-Berry, D.; Castranova, V.; Porter, D. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part. Fibre Toxicol. 2010, 7, 28. [Google Scholar] [CrossRef]
- Mercer, R.; Hubbs, A.; Scabilloni, J.; Wang, L.; Battelli, L.; Friend, S.; Castranova, V.; Porter, D. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part. Fibre Toxicol. 2011, 8, 21. [Google Scholar] [CrossRef]
- Mercer, R.; Scabilloni, J.; Hubbs, A.; Wang, L.; Battelli, L.; McKinney, W.; Castranova, V.; Porter, D. Extrapulmonary transport of MWCNT following inhalation exposure. Part. Fibre Toxicol. 2013, 10, 38. [Google Scholar] [CrossRef]
- Mercer, R.; Scabilloni, J.; Hubbs, A.; Battelli, L.; McKinney, W.; Friend, S.; Wolfarth, M.; Andrew, M.; Castranova, V.; Porter, D. Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol. 2013, 10, 33. [Google Scholar] [CrossRef]
- Porter, D.W.; Hubbs, A.F.; Chen, B.T.; McKinney, W.; Mercer, R.R.; Wolfarth, M.G.; Battelli, L.; Wu, N.; Sriram, K.; Leonard, S.; et al. Acute pulmonary dose–responses to inhaled multi-walled carbon nanotubes. Nanotoxicology 2013, 7, 1179–1194. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef]
- Rothen-Rutishauser, B.; Brown, D.M.; Piallier-Boyles, M.; Kinloch, I.A.; Windle, A.H.; Gehr, P.; Stone, V. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicology 2010, 4, 331–342. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef]
- Lam, C.W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar]
- Shvedova, A.A.; Kisin, E.R.; Murray, A.R.; Gorelik, O.; Arepalli, S.; Castranova, V.; Young, S.-H.; Gao, F.; Tyurina, Y.Y.; Oury, T.D.; et al. Vitamin E deficiency enhances pulmonary inflammatory response and oxidative stress induced by single-walled carbon nanotubes in C57BL/6 mice. Toxicol. Appl. Pharmacol. 2007, 221, 339–348. [Google Scholar] [CrossRef]
- Wang, L.; Castranova, V.; Mishra, A.; Chen, B.; Mercer, R.; Schwegler-Berry, D.; Rojanasakul, Y. Dispersion of single-walled carbon nanotubes by a natural lung surfactant for pulmonary in vitro and in vivo toxicity studies. Part. Fibre Toxicol. 2010, 7, 31. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.R.; Tkach, A.V.; Yanamala, N.; Mercer, R.; Young, S.H.; Fadeel, B.; Kagan, V.E.; Shvedova, A.A. Factoring-in agglomeration of carbon nanotubes and nanofibers for better prediction of their toxicity versus asbestos. Part. Fibre Toxicol. 2012, 9, 10. [Google Scholar] [CrossRef]
- Crouzier, D.; Follot, S.; Gentilhomme, E.; Flahaut, E.; Arnaud, R.; Dabouis, V.; Castellarin, C.; Debouzy, J.C. Carbon nanotubes induce inflammation but decrease the production of reactive oxygen species in lung. Toxicology 2010, 272, 39–45. [Google Scholar] [CrossRef]
- Jacobsen, N.; Moller, P.; Jensen, K.; Vogel, U.; Ladefoged, O.; Loft, S.; Wallin, H. Lung inflammation and genotoxicity following pulmonary exposure to nanoparticles in ApoE-/- mice. Part. Fibre Toxicol. 2009, 6, 2. [Google Scholar] [CrossRef]
- Mangum, J.; Turpin, E.; Antao-Menezes, A.; Cesta, M.; Bermudez, E.; Bonner, J. Single-Walled Carbon Nanotube (SWCNT)-induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part. Fibre Toxicol. 2006, 3, 15. [Google Scholar] [CrossRef]
- Shvedova, A.A.; Kisin, E.R.; Murray, A.R.; Kommineni, C.; Castranova, V.; Fadeel, B.; Kagan, V.E. Increased accumulation of neutrophils and decreased fibrosis in the lung of NADPH oxidase-deficient C57BL/6 mice exposed to carbon nanotubes. Toxicol. Appl. Pharmacol. 2008, 231, 235–240. [Google Scholar] [CrossRef]
- Cesta, M.F.; Ryman-Rasmussen, J.P.; Wallace, D.G.; Masinde, T.; Hurlburt, G.; Taylor, A.J.; Bonner, J.C. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am. J. Respir. Cell Mol. Biol. 2010, 43, 142–151. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Samuelsen, M.; Marioara, C.D.; Lovik, M. Carbon nanofibers have IgE adjuvant capacity but are less potent than nanotubes in promoting allergic airway responses. Biomed. Res. Int. 2013, 2013, 476010. [Google Scholar]
- Shvedova, A.A.; Kisin, E.; Murray, A.R.; Johnson, V.J.; Gorelik, O.; Arepalli, S.; Hubbs, A.F.; Mercer, R.R.; Keohavong, P.; Sussman, N.; et al. Inhalation vs. Aspiration of Single-Walled Carbon Nanotubes in C57BL/6 Mice: Inflammation, Fibrosis, Oxidative Stress, and Mutagenesis. Am J Physiol Lung Cell Mol Physiol. 2008, 295, L552–L565. [Google Scholar] [CrossRef]
- Kobayashi, N.; Naya, M.; Ema, M.; Endoh, S.; Maru, J.; Mizuno, K.; Nakanishi, J. Biological response and morphological assessment of individually dispersed multi-wall carbon nanotubes in the lung after intratracheal instillation in rats. Toxicology 2010, 276, 143–153. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Hansen, K.; Mossman, B. Generation of superoxide (O2-.) from alveolar macrophages exposed to asbestiform and nonfibrous particles. Cancer Res. 1987, 47, 1681–1686. [Google Scholar]
- Hill, I.M.; Beswick, P.H.; Donaldson, K. Differential release of superoxide anions by macrophages treated with long and short fibre amosite asbestos is a consequence of differential affinity for opsonin. Occup. Environ. Med. 1995, 52, 92–96. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Young, L.; Brown, D.M.; MacCalman, L.; Cowie, H.; Moisala, A.; Smail, F.; Smith, P.J.W.; Proudfoot, L.; Windle, A.H.; et al. Length Dependent Effects of Multi-Walled Carbon Nanotubes on Macrophage Mediated Inflammation, Phagocytosis and Cytotoxicity—A Comparison with Asbestos and Nanoparticle Carbon Black, submitted.
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: Toxicological aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Maynard, A.D.; Baron, P.A.; Foley, M.; Shvedova, A.A.; Kisin, E.R.; Castranova, V. Exposure to carbon nanotube material: Aerosol release during the handling of unrefined single-walled carbon nanotube material. J. Toxicol. Environ. Health Part A 2004, 67, 87–107. [Google Scholar] [CrossRef]
- Bernstein, D.; Castranova, V.; Donaldson, K.; Fubini, B.; Hadley, J.; Hesterberg, T.; Kane, A.; Lai, D.; McConnell, E.E.; Muhle, H.; et al. Testing of fibrous particles: Short-term assays and strategies. Inhal. Toxicol. 2005, 17, 497–537. [Google Scholar] [CrossRef]
- Brown, D.; Donaldson, K.; Stone, V. Nuclear translocation of Nrf2 and expression of antioxidant defence genes in THP-1 cells exposed to carbon nanotubes. J. Biomed. Nanotechnol. 2010, 6, 224–233. [Google Scholar] [CrossRef]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef]
- Snyder-Talkington, B.N.; Dymacek, J.; Porter, D.W.; Wolfarth, M.G.; Mercer, R.R.; Pacurari, M.; Denvir, J.; Castranova, V.; Qian, Y.; Guo, N.L. System-based identification of toxicity pathways associated with multi-walled carbon nanotube-induced pathological responses. Toxicol. Appl. Pharmacol. 2013, 272, 476–489. [Google Scholar] [CrossRef]
- Meunier, E.; Coste, A.; Olagnier, D.; Authier, H.; Lefèvre, L.; Dardenne, C.; Bernad, J.; Béraud, M.; Flahaut, E.; Pipy, B. Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation. Nanomedicine 2012, 8, 987–995. [Google Scholar] [CrossRef]
- Besnard, A.-G.; Togbe, D.; Couillin, I.; Tan, Z.; Zheng, S.G.; Erard, F.; le Bert, M.; Quesniaux, V.; Ryffel, B. Inflammasome–IL-1–Th17 response in allergic lung inflammation. J. Mol. Cell Biol. 2012, 4, 3–10. [Google Scholar] [CrossRef]
- Palomäki, J.; Välimäki, E.; Sund, J.; Vippola, M.; Clausen, P.A.; Jensen, K.A.; Savolainen, K.; Matikainen, S.; Alenius, H. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano 2011, 5, 6861–6870. [Google Scholar] [CrossRef]
- Clift, M.J.; Endes, C.; Vanhecke, D.; Wick, P.; Gehr, P.; Schins, R.P.; Petri-Fink, A.; Rothen-Rutishauser, B. A comparative study of different in vitro lung cell culture systems to assess the most beneficial tool for screening the potential adverse effects of carbon nanotubes. Toxicol. Sci. 2014, 137, 55–64. [Google Scholar] [CrossRef]
- Müller, L.; Riediker, M.; Wick, P.; Mohr, M.; Gehr, P.; Rothen-Rutishauser, B. Oxidative stress and inflammation response after nanoparticle exposure: Differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways. J. R. Soc. Interface 2010, 7, S27–S40. [Google Scholar] [CrossRef]
- Gasser, M.; Wick, P.; Clift, M.; Blank, F.; Diener, L.; Yan, B.; Gehr, P.; Krug, H.; Rothen-Rutishauser, B. Pulmonary surfactant coating of multi-walled carbon nanotubes (MWCNTs) influences their oxidative and pro-inflammatory potential in vitro. Part. Fibre Toxicol. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, X.; Kis, A.; Zettl, A.; Bertozzi, C.R. A cell nanoinjector based on carbon nanotubes. Proc. Natl. Acad. Sci. USA 2007, 104, 8218–8222. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Da Ros, T.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. (Camb) 2012, 48, 3911–3926. [Google Scholar]
- Kam, N.W.; Dai, H. Carbon nanotubes as intracellular protein transporters: Generality and biological functionality. J. Am. Chem. Soc. 2005, 127, 6021–6026. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem. Commun. (Camb) 2004, 1, 16–17. [Google Scholar] [CrossRef]
- Singh, R.; Pantarotto, D.; McCarthy, D.; Chaloin, O.; Hoebeke, J.; Partidos, C.D.; Briand, J.P.; Prato, M.; Bianco, A.; Kostarelos, K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: Toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005, 127, 4388–4396. [Google Scholar] [CrossRef]
- Meng, J.; Meng, J.; Duan, J.; Kong, H.; Li, L.; Wang, C.; Xie, S.; Chen, S.; Gu, N.; Xu, H.; et al. Carbon nanotubes conjugated to tumor lysate protein enhance the efficacy of an antitumor immunotherapy. Small 2008, 4, 1364–1370. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Korontsvit, T.; Zakhaleva, V.; Batt, C.A.; Philips, M.R.; et al. Single-walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Zeinali, M.; Jammalan, M.; Ardestani, S.K.; Mosaveri, N. Immunological and cytotoxicological characterization of tuberculin purified protein derivative (PPD) conjugated to single-walled carbon nanotubes. Immunol. Lett. 2009, 126, 48–53. [Google Scholar] [CrossRef]
- Yandar, N.; Pastorin, G.; Prato, M.; Bianco, A.; Patarroyo, M.E.; Lozano, J.M. Immunological profile of a Plasmodium vivax AMA-1 N-terminus peptide-carbon nanotube conjugate in an infected Plasmodium berghei mouse model. Vaccine 2008, 26, 5864–5873. [Google Scholar] [CrossRef]
- Parra, J.; Abad-Somovilla, A.; Mercader, J.V.; Taton, T.A.; Abad-Fuentes, A. Carbon nanotube-protein carriers enhance size-dependent self-adjuvant antibody response to haptens. J. Control. Release 2013, 170, 242–251. [Google Scholar] [CrossRef]
- Klumpp, C.; Kostarelos, K.; Prato, M.; Bianco, A. Functionalized carbon nanotubes as emerging nanovectors for the delivery of therapeutics. Biochim. Biophys. Acta 2006, 1758, 404–412. [Google Scholar]
- Abe, S.; Itoh, S.; Hayashi, D.; Kobayashi, T.; Kiba, T.; Akasaka, T.; Uo, M.; Yawaka, Y.; Sato, S.; Watari, F.; et al. Biodistribution of aqueous suspensions of carbon nanotubes in mice and their biocompatibility. J. Nanosci. Nanotechnol. 2012, 12, 700–706. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; McNeil, S.E. Handbook of Immunological Properties of Engineered Nanomaterials; World Scientific: Singapore, Singapore, 2013; p. 692 S. [Google Scholar]
- Zolnik, B.S.; Gonzalez-Fernandez, A.; Sadrieh, N.; Dobrovolskaia, M.A. Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef]
- Radomski, A.; Jurasz, P.; Alonso-Escolano, D.; Drews, M.; Morandi, M.; Malinski, T.; Radomski, M.W. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br. J. Pharmacol. 2005, 146, 882–893. [Google Scholar] [CrossRef]
- Lacerda, S.H.; Semberova, J.; Holada, K.; Simakova, O.; Hudson, S.D.; Simak, J. Carbon nanotubes activate store-operated calcium entry in human blood platelets. ACS Nano 2011, 5, 5808–5813. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Luo, Y.-L.; Chen, Y.-S.; Wei, Q.-B.; Fan, L.-H. Preparation and theophylline delivery applications of novel PMAA/MWCNT-COOH nanohybrid hydrogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 1119–1135. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Fujitani, T.; Ohyama, K.; Nakae, D.; Hirose, A.; Nishimura, T.; Ogata, A. Effects of sustained stimulation with multi-wall carbon nanotubes on immune and inflammatory responses in mice. J. Toxicol. Sci. 2012, 37, 177–189. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Gay, C.G. Development of vaccines toward the global control and eradication of foot-and-mouth disease. Expert Rev. Vaccines 2011, 10, 377–387. [Google Scholar] [CrossRef]
- Bianco, A.; Hoebeke, J.; Godefroy, S.; Chaloin, O.; Pantarotto, D.; Briand, J.-P.; Muller, S.; Prato, M.; Partidos, C.D. Cationic carbon nanotubes bind to CpG oligodeoxynucleotides and enhance their immunostimulatory properties. J. Am. Chem. Soc. 2005, 127, 58–59. [Google Scholar] [CrossRef]
- Zhao, D.; Alizadeh, D.; Zhang, L.; Liu, W.; Farrukh, O.; Manuel, E.; Diamond, D.J.; Badie, B. Carbon nanotubes enhance CpG uptake and potentiate antiglioma immunity. Clin. Cancer Res. 2011, 17, 771–782. [Google Scholar] [CrossRef]
- Park, E.J.; Cho, W.S.; Jeong, J.; Yi, J.; Choi, K.; Park, K. Pro-inflammatory and potential allergic responses resulting from B cell activation in mice treated with multi-walled carbon nanotubes by intratracheal instillation. Toxicology 2009, 259, 113–121. [Google Scholar] [CrossRef]
- Andersen, A.J.; Wibroe, P.P.; Moghimi, S.M. Perspectives on carbon nanotube-mediated adverse immune effects. Adv. Drug Deliv. Rev. 2012, 64, 1700–1705. [Google Scholar] [CrossRef]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef]
- Inoue, K.; Koike, E.; Yanagisawa, R.; Hirano, S.; Nishikawa, M.; Takano, H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol. Appl. Pharmacol. 2009, 237, 306–316. [Google Scholar] [CrossRef]
- Nygaard, U.C.; Hansen, J.S.; Samuelsen, M.; Alberg, T.; Marioara, C.D.; Løvik, M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol. Sci. 2009, 109, 113–123. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Immune responses of BALB/c mice to subcutaneously injected multi-walled carbon nanotubes. Nanotoxicology 2011, 5, 583–591. [Google Scholar] [CrossRef]
- Meng, J.; Yang, M.; Jia, F.; Kong, H.; Zhang, W.; Wang, C.; Xing, J.; Xie, S.; Xu, H. Subcutaneous injection of water-soluble multi-walled carbon nanotubes in tumor-bearing mice boosts the host immune activity. Nanotechnology 2010, 21, 145104. [Google Scholar] [CrossRef]
- Yokoyama, A.; Sato, Y.; Nodasaka, Y.; Yamamoto, S.; Kawasaki, T.; Shindoh, M.; Kohgo, T.; Akasaka, T.; Uo, M.; Watari, F.; et al. Biological behavior of hat-stacked carbon nanofibers in the subcutaneous tissue in rats. Nano Lett. 2005, 5, 157–161. [Google Scholar] [CrossRef]
- Sato, Y.; Yokoyama, A.; Nodasaka, Y.; Kohgo, T.; Motomiya, K.; Matsumoto, H.; Nakazawa, E.; Numata, T.; Zhang, M.; Yudasaka, M.; et al. Long-term biopersistence of tangled oxidized carbon nanotubes inside and outside macrophages in rat subcutaneous tissue. Sci. Rep. 2013, 3, 2516. [Google Scholar]
- Cheng, W.W.; Lin, Z.Q.; Wei, B.F.; Zeng, Q.; Han, B.; Wei, C.X.; Fan, X.J.; Hu, C.L.; Liu, L.H.; Huang, J.H.; et al. Single-walled carbon nanotube induction of rat aortic endothelial cell apoptosis: Reactive oxygen species are involved in the mitochondrial pathway. Int. J. Biochem. Cell Biol. 2011, 43, 564–572. [Google Scholar] [CrossRef]
- Guo, X.; Jagannath, C.; Espitia, M.G.; Zhou, X. Uptake of silica and carbon nanotubes by human macrophages/monocytes induces activation of fibroblasts in vitro—Potential implication for pathogenesis of inflammation and fibrotic diseases. Int. J. Immunopathol. Pharmacol. 2012, 25, 713–719. [Google Scholar]
- Albini, A.; Mussi, V.; Parodi, A.; Ventura, A.; Principi, E.; Tegami, S.; Rocchia, M.; Francheschi, E.; Sogno, I.; Cammarota, R.; et al. Interactions of single-wall carbon nanotubes with endothelial cells. Nanomedicine 2010, 6, 277–288. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Vakhrusheva, T.V.; Sokolov, A.V.; Kostevich, V.A.; Gusev, A.A.; Gusev, S.A.; Melnikova, V.I.; Lobach, A.S. PEGylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012, 264, 131–142. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Meng, J.; Meng, J.; Duan, J.; Xie, S.; Lu, X.; Zhu, Z.; Wang, C.; Chen, S.; et al. Carbon nanotubes enhance cytotoxicity mediated by human lymphocytes in vitro. PLoS One 2011, 6, e21073. [Google Scholar]
- Clichici, S.; Biris, A.R.; Tabaran, F.; Filip, A. Transient oxidative stress and inflammation after intraperitoneal administration of multiwalled carbon nanotubes functionalized with single strand DNA in rats. Toxicol. Appl. Pharmacol. 2012, 259, 281–292. [Google Scholar] [CrossRef]
- Yuan, J.; Gao, H.; Sui, J.; Duan, H.; Chen, W.N.; Ching, C.B. Cytotoxicity evaluation of oxidized single-walled carbon nanotubes and graphene oxide on human hepatoma HepG2 cells: An iTRAQ-coupled 2D LC-MS/MS proteome analysis. Toxicol. Sci. 2012, 126, 149–161. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Gaiser, B.K.; Hutchison, G.R.; Stone, V. An in vitro liver model—Assessing oxidative stress and genotoxicity following exposure of hepatocytes to a panel of engineered nanomaterials. Part. Fibre Toxicol. 2012, 9, 28. [Google Scholar] [CrossRef]
- Kermanizadeh, A.; Pojana, G.; Gaiser, B.K.; Birkedal, R.; Bilanicová, D.; Wallin, H.; Jensen, K.A.; Sellergren, B.; Hutchison, G.R.; Marcomini, A.; et al. In vitro assessment of engineered nanomaterials using a hepatocyte cell line: Cytotoxicity, pro-inflammatory cytokines and functional markers. Nanotoxicology 2013, 7, 301–313. [Google Scholar] [CrossRef]
- Demento, S.L.; Eisenbarth, S.C.; Foellmer, H.G.; Platt, C.; Caplan, M.J.; Mark Saltzman, W.; Mellman, I.; Ledizet, M.; Fikrig, E.; Flavell, R.A.; et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine 2009, 27, 3013–3021. [Google Scholar] [CrossRef]
- Boraschi, D.; Duschl, A. Nanoparticles and the Immune System—Safety and Effects; Adademic Press: Oxford, UK, 2013. [Google Scholar]
- Gao, N.; Zhang, Q.; Mu, Q.; Bai, Y.; Li, L.; Zhou, H.; Butch, E.R.; Powell, T.B.; Snyder, S.E.; Jiang, G.; et al. Steering carbon nanotubes to scavenger receptor recognition by nanotube surface chemistry modification partially alleviates NFkappaB activation and reduces its immunotoxicity. ACS Nano 2011, 5, 4581–4591. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Andersen, A.J.; Ahmadvand, D.; Wibroe, P.P.; Andresen, T.L.; Hunter, A.C. Material properties in complement activation. Adv. Drug Deliv. Rev. 2011, 63, 1000–1007. [Google Scholar] [CrossRef]
- Salvador-Morales, C.; Flahaut, E.; Sim, E.; Sloan, J.; Green, M.L.; Sim, R.B. Complement activation and protein adsorption by carbon nanotubes. Mol. Immunol. 2006, 43, 193–201. [Google Scholar] [CrossRef]
- Rybak-Smith, M.J.; Sim, R.B. Complement activation by carbon nanotubes. Adv. Drug Deliv. Rev. 2011, 63, 1031–1041. [Google Scholar] [CrossRef]
- Carroll, M.V.; Sim, R.B. Complement in health and disease. Adv. Drug Deliv. Rev. 2011, 63, 965–975. [Google Scholar] [CrossRef]
- Ling, W.L.; Biro, A.; Bally, I.; Tacnet, P.; Deniaud, A.; Doris, E.; Frachet, P.; Schoehn, G.; Pebay-Peyroula, E.; Arlaud, G.J. Proteins of the innate immune system crystallize on carbon nanotubes but are not activated. ACS Nano 2011, 5, 730–737. [Google Scholar] [CrossRef]
- Andersen, A.J.; Robinson, J.T.; Dai, H.; Hunter, A.C.; Andresen, T.L.; Moghimi, S.M. Single-walled carbon nanotube surface control of complement recognition and activation. ACS Nano 2013, 7, 1108–1119. [Google Scholar] [CrossRef]
- Rybak-Smith, M.J.; Tripisciano, C.; Borowiak-Palen, E.; Lamprecht, C.; Sim, R.B. Effect of functionalization of carbon nanotubes with psychosine on complement activation and protein adsorption. J. Biomed. Nanotechnol. 2011, 7, 830–839. [Google Scholar] [CrossRef]
- Čolić, M.; Džopalić, T.; Tomić, S.; Rajković, J.; Rudolf, R.; Vuković, G.; Marinković, A.; Uskoković, P. Immunomodulatory effects of carbon nanotubes functionalized with a Toll-like receptor 7 agonist on human dendritic cells. Carbon 2014, 67, 273–287. [Google Scholar] [CrossRef]
- Gottardi, R.; Douradinha, B. Carbon nanotubes as a novel tool for vaccination against infectious diseases and cancer. J. Nanobiotechnology 2013, 11, 30. [Google Scholar] [CrossRef]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V. Time evolution of the nanoparticle protein corona. ACS Nano 2010, 4, 3623–3632. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Aberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Brown, J.M.; Chen, R.; Ke, P.C.; Lai, X.; Mitra, S.; Witzmann, F.A. Comparison of nanotube-protein corona composition in cell culture media. Small 2013, 9, 2171–2181. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Laverny, G.; Casset, A.; Purohit, A.; Schaeffer, E.; Spiegelhalter, C.; de Blay, F.; Pons, F. Immunomodulatory properties of multi-walled carbon nanotubes in peripheral blood mononuclear cells from healthy subjects and allergic patients. Toxicol. Lett. 2013, 217, 91–101. [Google Scholar] [CrossRef]
- Palomaki, J.; Karisola, P.; Pylkkanen, L.; Savolainen, K.; Alenius, H. Engineered nanomaterials cause cytotoxicity and activation on mouse antigen presenting cells. Toxicology 2010, 267, 125–131. [Google Scholar] [CrossRef]
- Aldinucci, A.; Turco, A.; Biagioli, T.; Toma, F.M.; Bani, D.; Guasti, D.; Manuelli, C.; Rizzetto, L.; Cavalieri, D.; Massacesi, L.; et al. Carbon nanotube scaffolds instruct human dendritic cells: Modulating immune responses by contacts at the nanoscale. Nano Lett. 2013, 13, 6098–6105. [Google Scholar] [CrossRef]
- Thurnherr, T.; Brandenberger, C.; Fischer, K.; Diener, L.; Manser, P.; Maeder-Althaus, X.; Kaiser, J.P.; Krug, H.F.; Rothen-Rutishauser, B.; Wick, P. A comparison of acute and long-term effects of industrial multiwalled carbon nanotubes on human lung and immune cells in vitro. Toxicol. Lett. 2011, 200, 176–186. [Google Scholar] [CrossRef]
- Alam, A.; Sachar, S.; Puri, N.; Saxena, R.K. Interactions of polydispersed single-walled carbon nanotubes with T cells resulting in downregulation of allogeneic CTL responses in vitro and in vivo. Nanotoxicology 2013, 7, 1351–1360. [Google Scholar] [CrossRef]
- Erlanger, B.F.; Chen, B.; Zhu, M.; Brus, L. Binding of an anti-fullerene IgG monoclonal antibody to single wall carbon nanotubes. Nano Lett. 2001, 1, 465–467. [Google Scholar] [CrossRef]
- Pantarotto, D.; Partidos, C.D.; Hoebeke, J.; Brown, F.; Kramer, E.; Briand, J.P.; Muller, S.; Prato, M.; Bianco, A. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem. Biol. 2003, 10, 961–966. [Google Scholar] [CrossRef]
- Delogu, L.G.; Venturelli, E.; Manetti, R.; Pinna, G.A.; Carru, C.; Madeddu, R.; Murgia, L.; Sgarrella, F.; Dumortier, H.; Bianco, A. Ex vivo impact of functionalized carbon nanotubes on human immune cells. Nanomedicine (Lond) 2012, 7, 231–243. [Google Scholar] [CrossRef]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.P.; Prato, M.; Muller, S.; Bianco, A. Functionalized carbon nanotubes are non-cytotoxic and preserve the functionality of primary immune cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef]
- Medepalli, K.; Alphenaar, B.; Raj, A.; Sethu, P. Evaluation of the direct and indirect response of blood leukocytes to carbon nanotubes (CNTs). Nanomedicine 2011, 7, 983–991. [Google Scholar] [CrossRef]
- Pescatori, M.; Bedognetti, D.; Venturelli, E.; Menard-Moyon, C.; Bernardini, C.; Muresu, E.; Piana, A.; Maida, G.; Manetti, R.; Sgarrella, F.; et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 2013, 34, 4395–4403. [Google Scholar] [CrossRef]
- Grecco, A.C.; Paula, R.F.; Mizutani, E.; Sartorelli, J.C.; Milani, A.M.; Longhini, A.L.; Oliveira, E.C.; Pradella, F.; Silva, V.D.; Moraes, A.S.; et al. Up-regulation of T lymphocyte and antibody production by inflammatory cytokines released by macrophage exposure to multi-walled carbon nanotubes. Nanotechnology 2011, 22, 265103. [Google Scholar] [CrossRef]
- Bergin, I.L.; Witzmann, F.A. Nanoparticle toxicity by the gastrointestinal route: Evidence and knowledge gaps. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.; Deng, S.G.; Mitchell, M.C.; Smith, G.B. Application of carbon nanotube technology for removal of contaminants in drinking water: A review. Sci. Total Environ. 2009, 408, 1–13. [Google Scholar] [CrossRef]
- Brody, A.L.; Bugusu, B.; Han, J.H.; Sand, C.K.; Mchugh, T.H. Innovative food packaging solutions. J. Food Sci. 2008, 73, R107–R116. [Google Scholar] [CrossRef]
- Prajapati, V.K.; Awasthi, K.; Yadav, T.P.; Rai, M.; Srivastava, O.N.; Sundar, S. An oral formulation of amphotericin b attached to functionalized carbon nanotubes is an effective treatment for experimental visceral leishmaniasis. J. Infect. Dis. 2012, 205, 333–336. [Google Scholar] [CrossRef]
- Kou, W.; Akasaka, T.; Watari, F.; Sjogren, G. An in vitro evaluation of the biological effects of carbon nanotube-coated dental zirconia. ISRN Dent. 2013, 2013, 296727. [Google Scholar]
- Wang, Z.Y.; Zhao, J.; Song, L.; Mashayekhi, H.; Chefetz, B.; Xing, B.S. Adsorption and desorption of phenanthrene on carbon nanotubes in simulated gastrointestinal fluids. Environ. Sci. Technol. 2011, 45, 6018–6024. [Google Scholar] [CrossRef]
- Szendi, K.; Varga, C. Lack of genotoxicity of carbon nanotubes in a pilot study. Anticancer Res. 2008, 28, 349–352. [Google Scholar]
- Folkmann, J.K.; Risom, L.; Jacobsen, N.R.; Wallin, H.; Loft, S.; Moller, P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ. Health Perspect. 2009, 117, 703–708. [Google Scholar]
- Lim, J.-H.; Kim, S.-H.; Shin, I.-S.; Park, N.-H.; Moon, C.; Kang, S.-S.; Kim, S.-H.; Park, S.-C.; Kim, J.-C. Maternal exposure to multi-wall carbon nanotubes does not induce embryo–fetal developmental toxicity in rats. Birth Defects Res. Part B Dev. Reprod. Toxicol. 2011, 92, 69–76. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.R.; Saleh, N.B.; Winn, L.M. Investigating the effects of functionalized carbon nanotubes on reproduction and development in Drosophila melanogaster and CD-1 mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar] [CrossRef]
- Matsumoto, M.; Serizawa, H.; Sunaga, M.; Kato, H.; Takahashi, M.; Hirata-Koizumi, M.; Ono, A.; Kamata, E.; Hirose, A. No toxicological effects on acute and repeated oral gavage doses of single-wall or multi-wall carbon nanotube in rats. J. Toxicol. Sci. 2012, 37, 463–474. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
