Converging Electrospinning and 3D-Printing Technologies: From Innovative Design for Tissue Engineering to Global Patent Trends and Technology Transfer
Abstract
Highlights
- We propose a scoping review to demonstrate the innovative aspects of a new technological approach based on the integration of 3D-printing and electrospinning manufacturing techniques.
- The accurate description of relevant examples focused on 3D printing/electrospinning integration allowed us to remark on the distinctive aspects of different approaches, suggesting a prospective use in scientific research on tissue engineering and translational medicine.
Abstract
1. Introduction
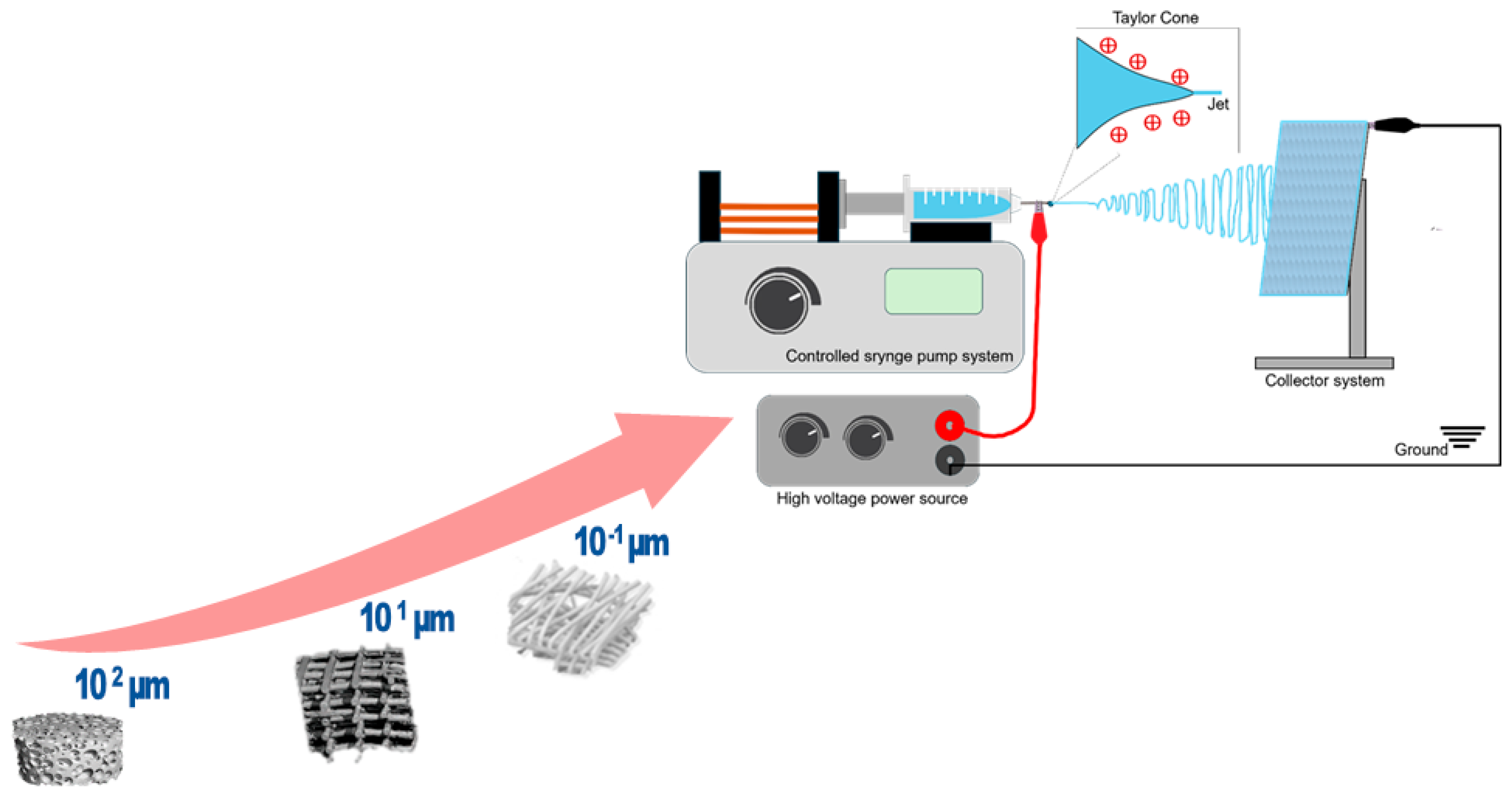
2. Fundamentals of Electrospinning
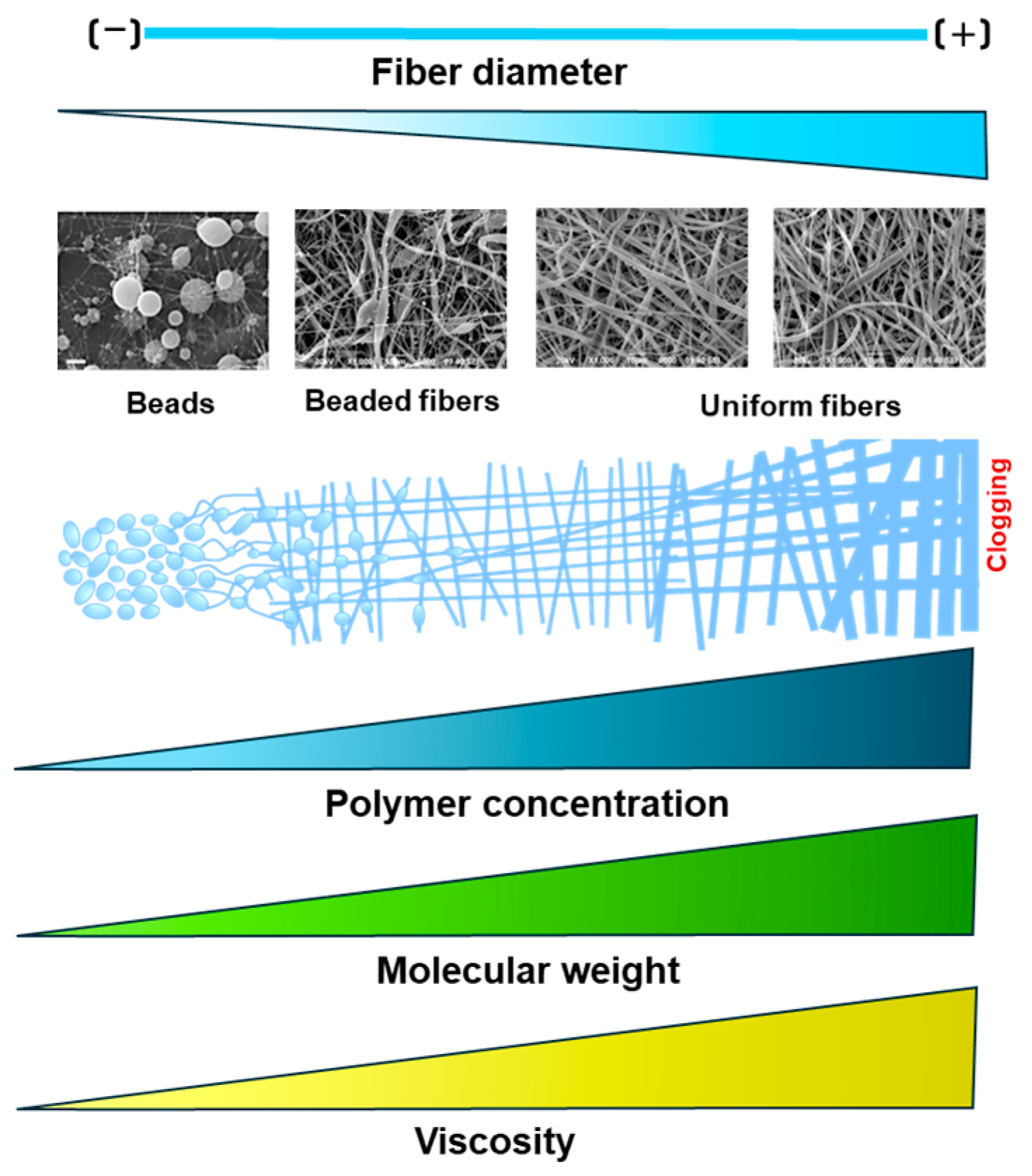
2.1. Solution and Polymer Parameters
2.2. Process Parameters
2.3. Environmental Parameters
3. Biomedical Applications for Electrospun Nanofibers
3.1. Electrospinning for Tissue Engineering
3.1.1. Bone
3.1.2. Skin
3.1.3. Cardiac and Vascular Tissues
3.1.4. Cartilage, Ligament, Muscle, and Tendons
3.1.5. Central and Peripheral Nerves
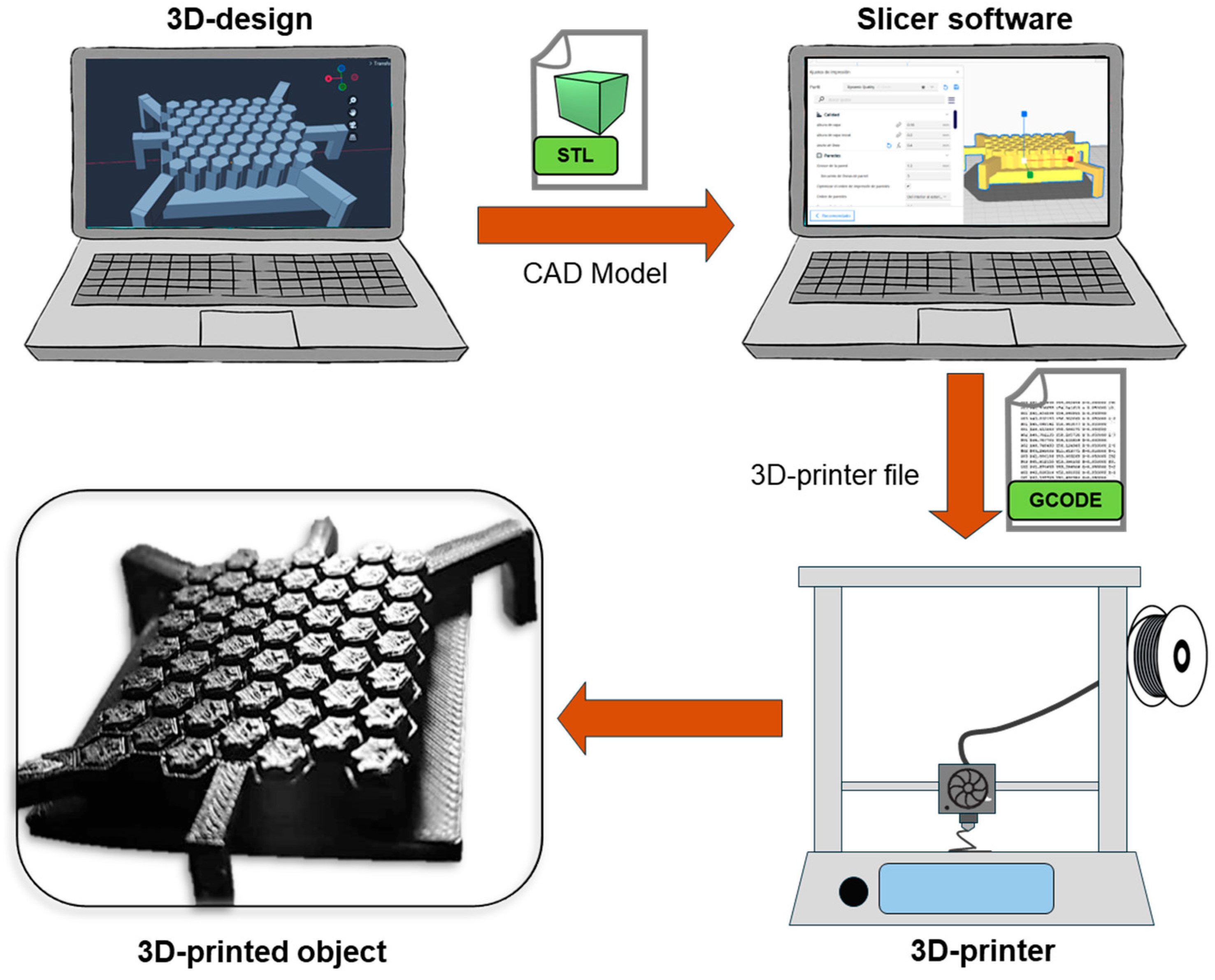
4. Basics of 3D-Printing Technology and Its Application in Tissue Engineering
4.1. Fused Deposition Modeling
4.2. Stereolithography
4.3. Selective Laser Sintering (SLS)
4.4. Digital Light Processing
4.5. Selective Laser Melting
4.6. Laminated Object Manufacturing
4.7. Melt Electrowriting
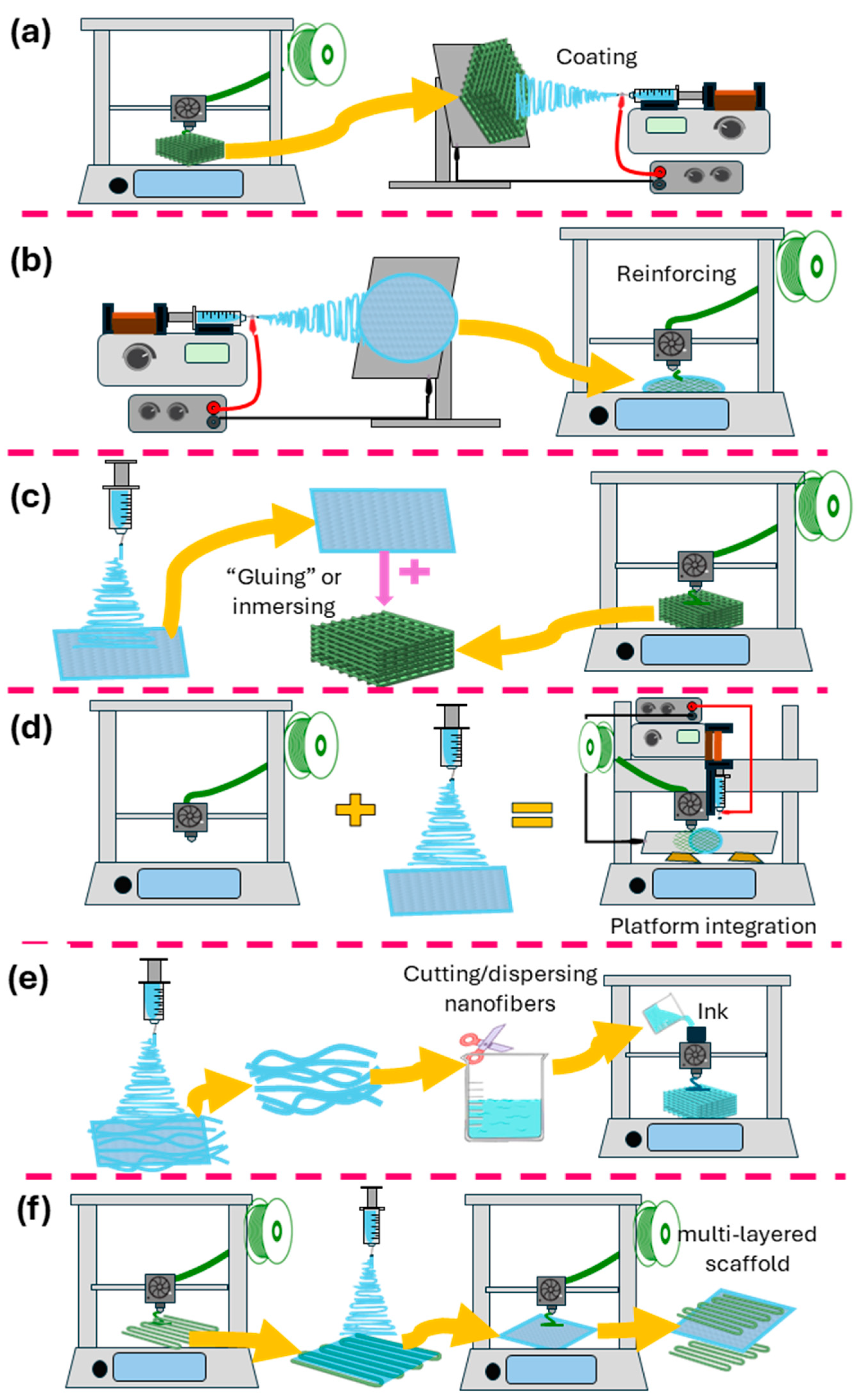
5. Combining Electrospinning and 3D Printing
5.1. Electrospinning onto 3D-Printed Scaffolds
5.2. Three-Dimensional Printing onto Electrospun Fibers
5.3. Alternate Use of 3D Printing and Electrospinning/Layer-by-Layer
5.4. Three-Dimensional-Printed Scaffolds Decorated/Infused with Electrospun Nanofibers
5.5. Electrospun Fibers as Inks for 3D Printing
5.6. Fabrication of Electrospun Scaffolds on 3D-Printed Collectors/Templates
5.7. Platforms Combining 3D-Printing and Electrospinning Techniques
6. Global Patent Landscape for Electrospinning and 3D Printing Scaffolds in TE
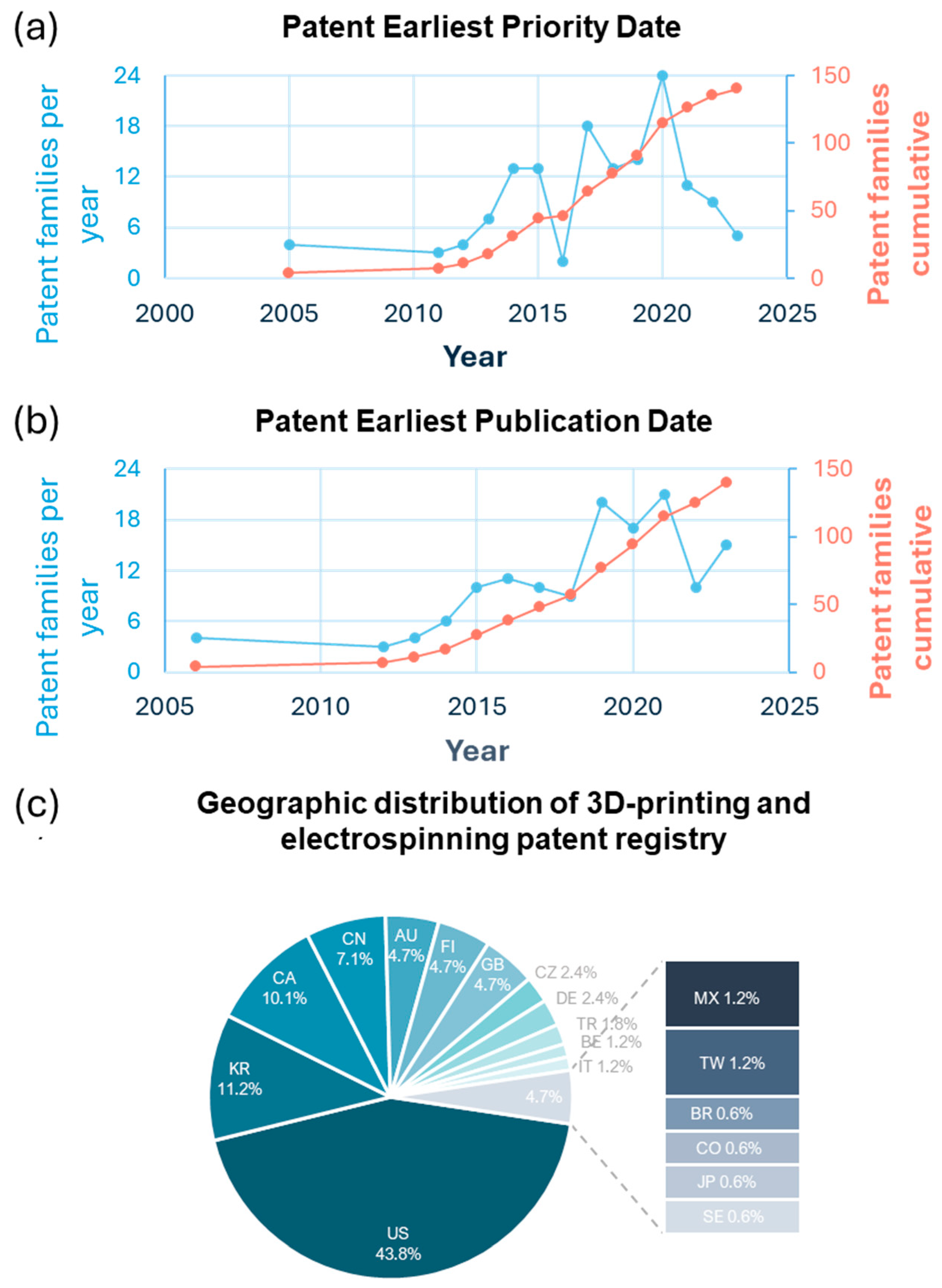
6.1. Analysis of Patent Distribution by Geographic Region and Trends over Time
6.2. Tissue-Specific Applications
6.3. Functional Enhancements
6.4. Challenges in Patent Development of TE Scaffolds
6.5. Emerging Innovations
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moysidou, C.M.; Barberio, C.; Owens, R.M. Advances in Engineering Human Tissue Models. Front. Bioeng. Biotechnol. 2021, 8, 620962. [Google Scholar] [CrossRef] [PubMed]
- Adel, I.M.; Elmeligy, M.F.; Elkasabgy, N.A. Conventional and Recent Trends of Scaffolds Fabrication: A Superior Mode for Tissue Engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S.; Scalia, A.C.; Cochis, A.; Rimondini, L.; Cruz-Maya, I.; Guarino, V.; Varesano, A.; Vineis, C. Topographical and Biomechanical Guidance of Electrospun Fibers for Biomedical Applications. Polymers 2020, 12, 2896. [Google Scholar] [CrossRef] [PubMed]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various Manufacturing Methods and Ideal Properties of Scaffolds for Tissue Engineering Applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Saracino, E.; Cirillo, V.; Marrese, M.; Guarino, V.; Benfenati, V.; Zamboni, R.; Ambrosio, L. Structural and Functional Properties of Astrocytes on PCL Based Electrospun Fibres. Mater. Sci. Eng. C 2021, 118, 111363. [Google Scholar] [CrossRef]
- Zulkifli, M.Z.A.; Nordin, D.; Shaari, N.; Kamarudin, S.K. Overview of Electrospinning for Tissue Engineering Applications. Polymers 2023, 15, 2418. [Google Scholar] [CrossRef]
- Guarino, V.; Cruz-Maya, I.; Altobelli, R.; Abdul Khodir, W.K.; Ambrosio, L.; Alvarez Pèrez, M.A.; Flores, A.A. Electrospun Polycaprolactone Nanofibres Decorated by Drug Loaded Chitosan Nano-Reservoirs for Antibacterial Treatments. Nanotechnology 2017, 28, 505103. [Google Scholar] [CrossRef]
- Mu, J.; Luo, D.; Li, W.; Ding, Y. Multiscale Polymeric Fibers for Drug Delivery and Tissue Engineering. Biomed. Technol. 2024, 5, 60–72. [Google Scholar] [CrossRef]
- Ghosh, A.; Orasugh, J.T.; Ray, S.S.; Chattopadhyay, D. Integration of 3D Printing-Coelectrospinning: Concept Shifting in Biomedical Applications. ACS Omega 2023, 8, 28002–28025. [Google Scholar] [CrossRef]
- Yang, D.L.; Faraz, F.; Wang, J.X.; Radacsi, N. Combination of 3D Printing and Electrospinning Techniques for Biofabrication. Adv. Mater. Technol. 2022, 7, 2101309. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific Publishing: Singapore, 2005. [Google Scholar]
- Neves, N.M. Electrospinning for Advanced Biomedical Applications and Therapies; Smithers Rapra Technology, Ltd.: Shropshire, UK, 2012; ISBN 9781847356000. [Google Scholar]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.; Daraei, A.; Lee, H.; Guthold, M. The Effect of Molecular Weight and Fiber Diameter on the Mechanical Properties of Single, Electrospun PCL Nanofibers. Mater. Today Commun. 2023, 35, 105773. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Jirsak, O.; Gemci, R. Effect of Polymer Concentration on Electrospinning System. In Proceedings of the Fiber Society Spring 2010 International Conference, Bursa, Turkey, 12–14 May 2010. [Google Scholar]
- Liu, Z.; Ramakrishna, S.; Ahmed, I.; Rudd, C.; Liu, X. Rheological, Surface Tension and Conductivity Insights on the Electrospinnability of Poly(Lactic-Co-Glycolic Acid)-Hyaluronic Acid Solutions and Their Correlations with the Nanofiber Morphological Characteristics. Polymers 2022, 14, 4411. [Google Scholar] [CrossRef]
- Higashi, S.; Hirai, T.; Matsubara, M.; Yoshida, H.; Beniya, A. Dynamic Viscosity Recovery of Electrospinning Solution for Stabilizing Elongated Ultrafine Polymer Nanofiber by TEMPO-CNF. Sci. Rep. 2020, 10, 13427. [Google Scholar] [CrossRef]
- Asran, A.S.; Salama, M.; Popescu, C.; Michler, G.H. Solvent Influences the Morphology and Mechanical Properties of Electrospun Poly(L-Lactic Acid) Scaffold for Tissue Engineering Applications. Macromol. Symp. 2010, 294, 153–161. [Google Scholar] [CrossRef]
- Luo, C.J.; Nangrejo, M.; Edirisinghe, M. A Novel Method of Selecting Solvents for Polymer Electrospinning. Polymer 2010, 51, 1654–1662. [Google Scholar] [CrossRef]
- Uyar, T.; Besenbacher, F. Electrospinning of Uniform Polystyrene Fibers: The Effect of Solvent Conductivity. Polymer 2008, 49, 5336–5343. [Google Scholar] [CrossRef]
- Abdulhussain, R.; Adebisi, A.; Conway, B.R.; Asare-Addo, K. Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery. J. Drug Deliv. Sci. Technol. 2023, 90, 105156. [Google Scholar] [CrossRef]
- Samatham, R.; Kim, K.J. Electric Current as a Control Variable in the Electrospinning Process. Polym. Eng. Sci. 2006, 46, 954–959. [Google Scholar] [CrossRef]
- Elveren, B.; Kurečič, M.; Maver, T.; Maver, U. Cell Electrospinning: A Mini-Review of the Critical Processing Parameters and Its Use in Biomedical Applications. Adv. Biol. 2023, 7, 2300057. [Google Scholar] [CrossRef]
- Matabola, K.P.; Moutloali, R.M. The Influence of Electrospinning Parameters on the Morphology and Diameter of Poly(Vinyledene Fluoride) Nanofibers—Effect of Sodium Chloride. J. Mater. Sci. 2013, 48, 5475–5482. [Google Scholar] [CrossRef]
- Utkarsh; Hegab, H.; Tariq, M.; Syed, N.A.; Rizvi, G.; Pop-Iliev, R. Towards Analysis and Optimization of Electrospun PVP (Polyvinylpyrrolidone) Nanofibers. Adv. Polym. Technol. 2020, 2020, 4090747. [Google Scholar] [CrossRef]
- Syed Bakar, S.S.; Foong, K.M.; Abdul Halif, N.; Yahud, S. Effect of Solution Concentration and Applied Voltage on Electrospun Polyacrylonitrile Fibers. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing Ltd.: Bristol, UK, 2019; Volume 701. [Google Scholar]
- Liu, Z.; Ju, K.; Wang, Z.; Li, W.; Ke, H.; He, J. Electrospun Jets Number and Nanofiber Morphology Effected by Voltage Value: Numerical Simulation and Experimental Verification. Nanoscale Res. Lett. 2019, 14, 310. [Google Scholar] [CrossRef]
- Jacobs, V.; Anandjiwala, R.D.; Maaza, M. The Influence of Electrospinning Parameters on the Structural Morphology and Diameter of Electrospun Nanofibers. J. Appl. Polym. Sci. 2010, 115, 3130–3136. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Moghadam, B.H.; Abatari, M.H.M.; Haghi, A.K. On the Production Optimization of Polyacrylonitrile Electrospun Nanofiber. Bulg. Chem. Commun. 2013, 45, 178–190. [Google Scholar]
- Duan, Y.; Kalluri, L.; Satpathy, M.; Duan, Y. Effect of Electrospinning Parameters on the Fiber Diameter and Morphology of PLGA Nanofibers. Dent. Oral. Biol. Craniofacial Res. 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Ranjbar-Mohammadi, M.; Bahrami, S.H.; Joghataei, M.T. Fabrication of Novel Nanofiber Scaffolds from Gum Tragacanth/Poly(Vinyl Alcohol) for Wound Dressing Application: In Vitro Evaluation and Antibacterial Properties. Mater. Sci. Eng. C 2013, 33, 4935–4943. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Gómez-Tejedor, J.A.; Vallés-Lluch, A. PLA/PCL Electrospun Membranes of Tailored Fibres Diameter as Drug Delivery Systems. Eur. Polym. J. 2018, 99, 445–455. [Google Scholar] [CrossRef]
- He, H.; Kara, Y.; Molnar, K. Effect of Needle Characteristic on Fibrous PEO Produced by Electrospinning. Resolut. Discov. 2019, 4, 7–11. [Google Scholar] [CrossRef]
- Barua, B.; Saha, M.C. Influence of Humidity, Temperature, and Annealing on Microstructure and Tensile Properties of Electrospun Polyacrylonitrile Nanofibers. Polym. Eng. Sci. 2018, 58, 998–1009. [Google Scholar] [CrossRef]
- Raksa, A.; Numpaisal, P.O.; Ruksakulpiwat, Y. The Effect of Humidity during Electrospinning on Morphology and Mechanical Properties of SF/PVA Nanofibers. Mater. Today Proc. 2021, 47, 3458–3461. [Google Scholar] [CrossRef]
- Park, B.K.; Um, I.C. Effect of Relative Humidity on the Electrospinning Performance of Regenerated Silk Solution. Polymers 2021, 13, 2479. [Google Scholar] [CrossRef]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J. Advances in Tissue Engineering. J. Pediatr. Surg. 2016, 51, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef]
- Flores-Rojas, G.G.; Gómez-Lazaro, B.; López-Saucedo, F.; Vera-Graziano, R.; Bucio, E.; Mendizábal, E. Electrospun Scaffolds for Tissue Engineering: A Review. Macromol 2023, 3, 524–553. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Guarino, V.; Ambrosio, L. Electrofluidodynamics: Exploring a New Toolbox to Design Biomaterials for Tissue Regeneration and Degeneration. Nanomedicine 2016, 11, 1515–1518. [Google Scholar] [CrossRef]
- Meng, C.; Liu, X.; Li, R.; Malekmohammadi, S.; Feng, Y.; Song, J.; Gong, R.H.; Li, J. 3D Poly (L-Lactic Acid) Fibrous Sponge with Interconnected Porous Structure for Bone Tissue Scaffold. Int. J. Biol. Macromol. 2024, 268, 131688. [Google Scholar] [CrossRef]
- Kalidas, S.; Sumathi, S. Copper Substituted Hydroxyapatite Reinforced Gelatin/Polyvinyl Alcohol/Silk Fibre-Based Scaffold for Bone Tissue Engineering Application. Mater. Chem. Phys. 2024, 320, 129410. [Google Scholar] [CrossRef]
- García, G.; Moreno-Serna, V.; Saavedra, M.; Cordoba, A.; Canales, D.; Alfaro, A.; Guzmán-Soria, A.; Orihuela, P.; Zapata, S.; Grande-Tovar, C.D.; et al. Electrospun Scaffolds Based on a PCL/Starch Blend Reinforced with CaO Nanoparticles for Bone Tissue Engineering. Int. J. Biol. Macromol. 2024, 273, 132891. [Google Scholar] [CrossRef]
- Canales, D.A.; Reyes, F.; Saavedra, M.; Peponi, L.; Leonés, A.; Palza, H.; Boccaccini, A.R.; Grünewald, A.; Zapata, P.A. Electrospun Fibers of Poly (Lactic Acid) Containing Bioactive Glass and Magnesium Oxide Nanoparticles for Bone Tissue Regeneration. Int. J. Biol. Macromol. 2022, 210, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Eren Boncu, T.; Uskudar Guclu, A.; Catma, M.F.; Savaser, A.; Gokce, A.; Ozdemir, N. In Vitro and in Vivo Evaluation of Linezolid Loaded Electrospun PLGA and PLGA/PCL Fiber Mats for Prophylaxis and Treatment of MRSA Induced Prosthetic Infections. Int. J. Pharm. 2020, 573, 118758. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Sun, X.; Li, D.; Wang, M.; Song, S.; Liu, C.; Ma, N.; Yin, X.; Wang, C. UCNPs-Labeled Electrospun Scaffolds Used to Monitor in Vivo Degradation and Bone Tissue Regeneration. Colloids Surf. B Biointerfaces 2024, 237, 113860. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.; Li, T.; Arya, D.K.; Ali, D.; Alarifi, S.; Yulin, W.; Hengtong, Z.; Rajinikanth, P.S.; Ao, Q. Biomimetic Electrospun Nanofibrous Scaffold for Tissue Engineering: Preparation, Optimization by Design of Experiments (DOE), in-Vitro and in-Vivo Characterization. Front. Bioeng. Biotechnol. 2023, 11, 1288539. [Google Scholar] [CrossRef] [PubMed]
- Shadman-Manesh, V.; Gholipour-Kanani, A.; Najmoddin, N.; Rabbani, S. Preclinical Evaluation of the Polycaprolactone-Polyethylene Glycol Electrospun Nanofibers Containing Egg-Yolk Oil for Acceleration of Full Thickness Burns Healing. Sci. Rep. 2023, 13, 919. [Google Scholar] [CrossRef]
- Lizarazo-Fonseca, L.; Correa-Araujo, L.; Prieto-Abello, L.; Camacho-Rodríguez, B.; Silva-Cote, I. In Vitro and in Vivo Evaluation of Electrospun Poly (ε-Caprolactone)/Collagen Scaffolds and Wharton’s Jelly Mesenchymal Stromal Cells (HWJ-MSCs) Constructs as Potential Alternative for Skin Tissue Engineering. Regen. Ther. 2023, 24, 11–24. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Cruz-Maya, I.; Vineis, C.; Tonetti, C.; Varesano, A.; Guarino, V. Design of Asymmetric Nanofibers-Membranes Based on Polyvinyl Alcohol and Wool-Keratin for Wound Healing Applications. J. Funct. Biomater. 2021, 12, 76. [Google Scholar] [CrossRef]
- Shahghasempour, L.; Fattahi, R.; Hosseinzadeh, S.; Haddadi, A.; Shams, F. In Vivo Assessment of Lactobacillus Plantarum and Co-Cultured Cells on a Polyurethane/PRGF/Gelatin/Polyurethane Scaffold in Skin Wound Healing. SPE Polym. 2024, 5, 637–662. [Google Scholar] [CrossRef]
- Du, P.; Chen, X.; Chen, Y.; Li, J.; Lu, Y.; Li, X.; Hu, K.; Chen, J.; Lv, G. In Vivo and in Vitro Studies of a Propolis-Enriched Silk Fibroin-Gelatin Composite Nanofiber Wound Dressing. Heliyon 2023, 9, e13506. [Google Scholar] [CrossRef]
- Petrova, V.A.; Poshina, D.N.; Golovkin, A.S.; Mishanin, A.I.; Zhuravskii, S.G.; Yukina, G.Y.; Naumenko, M.Y.; Sukhorukova, E.G.; Savin, N.A.; Erofeev, A.S.; et al. Electrospun Composites of Chitosan with Cerium Oxide Nanoparticles for Wound Healing Applications: Characterization and Biocompatibility Evaluation In Vitro and In Vivo. Polymers 2024, 16, 1787. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.H.; Khalil, I.A.; El-Sherbiny, I.M. Design, Development, in-Vitro and in-Vivo Evaluation of Polylactic Acid-Based Multifunctional Nanofibrous Patches for Efficient Healing of Diabetic Wounds. Sci. Rep. 2023, 13, 3215. [Google Scholar] [CrossRef] [PubMed]
- Husain, K.; Malik, A.; Kirchens, J.; Choi, G. A Prospective, Blinded, Randomized Controlled Clinical Trial Evaluating the Effect of the Synthetic Electrospun Fiber Matrix in the Treatment of Chronic Diabetic Foot Ulcers. Foot Ankle Surg. Tech. Rep. Cases 2024, 4, 100362. [Google Scholar] [CrossRef]
- Carrabba, M.; Fagnano, M.; Ghorbel, M.T.; Rapetto, F.; Su, B.; De Maria, C.; Vozzi, G.; Biglino, G.; Perriman, A.W.; Caputo, M.; et al. Development of a Novel Hierarchically Biofabricated Blood Vessel Mimic Decorated with Three Vascular Cell Populations for the Reconstruction of Small-Diameter Arteries. Adv. Funct. Mater. 2024, 34, 2300621. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Cai, Z.; Xing, Y.; Fang, Z.; Ye, L.; Geng, X.; Zhang, A.-Y.; Gu, Y.; Feng, Z.-G. Fabrication of Small-Diameter in Situ Tissue Engineered Vascular Grafts with Core/Shell Fibrous Structure and a One-Year Evaluation via Rat Abdominal Vessel Replacement Model. Biomater. Adv. 2024, 165, 214018. [Google Scholar] [CrossRef]
- Wei, X.; Wang, L.; Duan, C.; Chen, K.; Li, X.; Guo, X.; Chen, P.; Liu, H.; Fan, Y. Cardiac Patches Made of Brown Adipose-Derived Stem Cell Sheets and Conductive Electrospun Nanofibers Restore Infarcted Heart for Ischemic Myocardial Infarction. Bioact. Mater. 2023, 27, 271–287. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, Z.; Kang, Y.; Chen, T.; Xu, C.; Zhu, T. Durable Immunomodulatory Nanofiber Niche for the Functional Remodeling of Cardiovascular Tissue. ACS Nano 2024, 18, 951–971. [Google Scholar] [CrossRef]
- Handley, E.L.; Callanan, A. Effects of Electrospun Fibers Containing Ascorbic Acid on Oxidative Stress Reduction for Cardiac Tissue Engineering. J. Appl. Polym. Sci. 2023, 140, e54242. [Google Scholar] [CrossRef]
- Liu, S.; Yao, L.; Wang, Y.; Li, Y.; Jia, Y.; Yang, Y.; Li, N.; Hu, Y.; Kong, D.; Dong, X.; et al. Immunomodulatory Hybrid Micro-Nanofiber Scaffolds Enhance Vascular Regeneration. Bioact. Mater. 2023, 21, 464–482. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Osipova, O.; Nuankai, Z.; Shundrina, I.; Murashov, I.S.; Larichev, Y.; Karpenko, A.A.; Laktionov, P.P. Evaluation of Properties for CarbothaneTM 3575A–Based Electrospun Vascular Grafts in Vitro and in Vivo. Biomed. Mater. 2024, 19, 065012. [Google Scholar] [CrossRef]
- Xie, B.; Ma, H.; Yang, F.; Chen, H.; Guo, Y.; Zhang, H.; Li, T.; Huang, X.; Zhao, Y.; Li, X.; et al. Development and Evaluation of 3D Composite Scaffolds with Piezoelectricity and Biofactor Synergy for Enhanced Articular Cartilage Regeneration. J. Mater. Chem. B 2024, 12, 10416–10433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, R.; Li, J.; Wu, X. The Immunosuppressive Niche Established with a Curcumin-Loaded Electrospun Nanofibrous Membrane Promotes Cartilage Regeneration in Immunocompetent Animals. Membranes 2023, 13, 335. [Google Scholar] [CrossRef]
- Huo, Y.; Bai, B.; Zheng, R.; Sun, Y.; Yu, Y.; Wang, X.; Chen, H.; Hua, Y.; Zhang, Y.; Zhou, G.; et al. In Vivo Stable Allogenic Cartilage Regeneration in a Goat Model Based on Immunoisolation Strategy Using Electrospun Semipermeable Membranes. Adv. Healthc. Mater. 2023, 12, e2203084. [Google Scholar] [CrossRef]
- Fasolino, I.; Guarino, V.; Cirillo, V.; Ambrosio, L. 5-Azacytidine-Mediated HMSC Behavior on Electrospun Scaffolds for Skeletal Muscle Regeneration. J. Biomed. Mater. Res. A 2017, 105, 2551–2561. [Google Scholar] [CrossRef]
- Abdulmalik, S.; Gallo, J.; Nip, J.; Katebifar, S.; Arul, M.; Lebaschi, A.; Munch, L.N.; Bartly, J.M.; Choudhary, S.; Kalajzic, I.; et al. Nanofiber Matrix Formulations for the Delivery of Exendin-4 for Tendon Regeneration: In Vitro and in Vivo Assessment. Bioact. Mater. 2023, 25, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Iorio, F.; El Khatib, M.; Wöltinger, N.; Turriani, M.; Di Giacinto, O.; Mauro, A.; Russo, V.; Barboni, B.; Boccaccini, A.R. Electrospun Poly(ε-Caprolactone)/Poly(Glycerol Sebacate) Aligned Fibers Fabricated with Benign Solvents for Tendon Tissue Engineering. J. Biomed. Mater. Res. A 2025, 113, e37794. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Sun, Z.; Tao, Z.; Pavel, V.; Li, Y.; Wang, F.; Cui, W.; Liu, S. Unidirectional Gene Delivery Electrospun Fibrous Membrane via Charge Repulsion for Tendon Repair. Bioact. Mater. 2024, 37, 191–205. [Google Scholar] [CrossRef]
- Xiong, F.; Wei, S.; Wu, S.; Jiang, W.; Li, B.; Xuan, H.; Xue, Y.; Yuan, H. Aligned Electroactive Electrospun Fibrous Scaffolds for Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2023, 15, 41385–41402. [Google Scholar] [CrossRef]
- Wei, S.; Xiong, F.; Gu, H.; Zhang, Z.; Xuan, H.; Jin, Y.; Xue, Y.; Li, B.; Feng, W.; Yuan, H. Highly Aligned Electroactive Ultrafine Fibers Promote the Differentiation of Mesenchymal Stem Cells into Schwann-like Cells for Nerve Regeneration. Int. J. Biol. Macromol. 2024, 279, 135388. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, R.; Mao, X.; Li, X.; Li, T.; Liang, F.; He, J.; Wen, L.; Wang, W.; Li, X.; et al. Preparation of PLCL/ECM Nerve Conduits by Electrostatic Spinning Technique and Evaluation in Vitro and in Vivo. J. Neural Eng. 2024, 21, 026028. [Google Scholar] [CrossRef]
- Puhl, D.L.; Funnell, J.L.; Fink, T.D.; Swaminathan, A.; Oudega, M.; Zha, R.H.; Gilbert, R.J. Electrospun Fiber-Mediated Delivery of Neurotrophin-3 MRNA for Neural Tissue Engineering Applications. Acta Biomater. 2023, 155, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.R.; Guarino, V.; Oliveira, M.J.; Ribeiro, C.C.; Barbosa, M.A.; Ambrosio, L.; Pêgo, A.P. Ibuprofen-Loaded Poly(Trimethylene Carbonate-Co-ε-Caprolactone) Electrospun Fibres for Nerve Regeneration. J. Tissue Eng. Regen. Med. 2016, 10, E154–E166. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Li, T.; Cheng, J.; Tao, M.; Li, Z.; Ma, Y.; Javed, R.; Bao, J.; Liang, F.; Guo, W.; et al. Nerve ECM and PLA-PCL Based Electrospun Bilayer Nerve Conduit for Nerve Regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1103435. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.F.; Mishra, N.C. Gelatin-Polycaprolactone-Nanohydroxyapatite Electrospun Nanocomposite Scaffold for Bone Tissue Engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Asefnejad, A.; Rafienia, M.; Khorasani, M.T. Potential of Novel Electrospun Core-Shell Structured Polyurethane/Starch (Hyaluronic Acid) Nanofibers for Skin Tissue Engineering: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2020, 146, 627–637. [Google Scholar] [CrossRef]
- Xie, B.; Yang, F.; Chen, H.; Zhang, H.; Ma, H.; Li, T.; Chen, Z.; Li, J.; Li, X.; Du, J. Electrospun Polycaprolactone/Silk Fibroin Nanofiber Scaffold with Aligned Fiber Orientation for Articular Chondrocyte Regeneration. Front. Mater. 2023, 10, 1292098. [Google Scholar] [CrossRef]
- Yang, L.; Wang, X.; Xiong, M.; Liu, X.; Luo, S.; Luo, J.; Wang, Y. Electrospun Silk Fibroin/Fibrin Vascular Scaffold with Superior Mechanical Properties and Biocompatibility for Applications in Tissue Engineering. Sci. Rep. 2024, 14, 3942. [Google Scholar] [CrossRef]
- Rezvova, M.A.; Ovcharenko, E.A.; Klyshnikov, K.Y.; Glushkova, T.V.; Kostyunin, A.E.; Shishkova, D.K.; Matveeva, V.G.; Velikanova, E.A.; Shabaev, A.R.; Kudryavtseva, Y.A. Electrospun Bioresorbable Polymer Membranes for Coronary Artery Stents. Front. Bioeng. Biotechnol. 2024, 12, 1440181. [Google Scholar] [CrossRef]
- Kyriakou, S.; Acosta, S.; El Maachi, I.; Rütten, S.; Jockenhoevel, S. A Dexamethasone-Loaded Polymeric Electrospun Construct as a Tubular Cardiovascular Implant. Polymers 2023, 15, 4332. [Google Scholar] [CrossRef]
- Dolatyar, B.; Zeynali, B.; Shabani, I.; Tafreshi, A.P.; Karimi-Soflou, R. Enhanced Axonal Regeneration and Functional Recovery of the Injured Sciatic Nerve in a Rat Model by Lithium-Loaded Electrospun Nanofibrous Scaffolds. Biodes Manuf. 2024, 7, 701–720. [Google Scholar] [CrossRef]
- Ikegami, Y.; Shafiq, M.; Aishima, S.; Ijima, H. Heparin/Growth Factors-Immobilized Aligned Electrospun Nanofibers Promote Nerve Regeneration in Polycaprolactone/Gelatin-Based Nerve Guidance Conduits. Adv. Fiber Mater. 2023, 5, 554–573. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Gokhare, V.G.; Raut, D.N.; Shinde, D.K. A Review Paper on 3D-Printing Aspects and Various Processes Used in the 3D-Printing. Int. J. Eng. Res. Technol. 2017, 6, 953–958. [Google Scholar]
- Jandyal, A.; Chaturvedi, I.; Wazir, I.; Raina, A.; Ul Haq, M.I. 3D Printing—A Review of Processes, Materials and Applications in Industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar] [CrossRef]
- Sahmani, S.; Khandan, A.; Esmaeili, S.; Saber-Samandari, S.; Ghadiri Nejad, M.; Aghdam, M.M. Calcium Phosphate-PLA Scaffolds Fabricated by Fused Deposition Modeling Technique for Bone Tissue Applications: Fabrication, Characterization and Simulation. Ceram. Int. 2020, 46, 2447–2456. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High Strength Porous PLA Gyroid Scaffolds Manufactured via Fused Deposition Modeling for Tissue-Engineering Applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D Printing of a Bladder Device for Intravesical Drug Delivery. Mater. Sci. Eng. C 2021, 120, 111773. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.A.; Sprecher, C.M.; Stadelmann, V.A.; Grijpma, D.W.; Tang, T.T.; Qin, L.; Lai, Y.; Alini, M.; de Bruijn, J.D.; et al. Surface-Enrichment with Hydroxyapatite Nanoparticles in Stereolithography-Fabricated Composite Polymer Scaffolds Promotes Bone Repair. Acta Biomater. 2017, 54, 386–398. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, C.; Chen, F.; Wang, X.; Lin, K. A Comparative Study of the Osteogenic Performance between the Hierarchical Micro/Submicro-Textured 3D-Printed Ti6Al4V Surface and the SLA Surface. Bioact. Mater. 2020, 5, 9–16. [Google Scholar] [CrossRef]
- Wiria, F.E.; Leong, K.F.; Chua, C.K.; Liu, Y. Poly-ε-Caprolactone/Hydroxyapatite for Tissue Engineering Scaffold Fabrication via Selective Laser Sintering. Acta Biomater. 2007, 3, 1–12. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Sudarmadji, N.; Yu, H.Y.; Chua, C.K.; Leong, K.F.; Venkatraman, S.S.; Boey, Y.C.F.; Tan, L.P. Porous Polycaprolactone Scaffold for Cardiac Tissue Engineering Fabricated by Selective Laser Sintering. Acta Biomater. 2010, 6, 2028–2034. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of Porous Polyvinyl Alcohol Scaffold for Bone Tissue Engineering via Selective Laser Sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, H.; Shi, T.; Xie, D.; Chen, R.; Han, X.; Shen, L.; Wang, C.; Tian, Z. Additive Manufacturing of Hydroxyapatite Bone Scaffolds via Digital Light Processing and in Vitro Compatibility. Ceram. Int. 2019, 45, 11079–11086. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, T.; Jiao, C.; Liang, H.; Chen, R.; Tian, Z.; Zou, A.; Yang, Y.; Wei, Z.; Wang, C.; et al. Fabrication and Properties of Zirconia/Hydroxyapatite Composite Scaffold Based on Digital Light Processing. Ceram. Int. 2020, 46, 2300–2308. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Cao, Q.; Wu, Y.; Wang, Y.; Yuan, B.; Li, X.; Zhou, Y.; Chen, X.; Zhu, X.; et al. Fabrication and Biological Evaluation of 3D-Printed Calcium Phosphate Ceramic Scaffolds with Distinct Macroporous Geometries through Digital Light Processing Technology. Regen. Biomater. 2022, 9, rbac005. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Brandt, M.; Wen, C. Ultrahigh-Strength Titanium Gyroid Scaffolds Manufactured by Selective Laser Melting (SLM) for Bone Implant Applications. Acta Mater. 2018, 158, 354–368. [Google Scholar] [CrossRef]
- Kelly, C.N.; Francovich, J.; Julmi, S.; Safranski, D.; Guldberg, R.E.; Maier, H.J.; Gall, K. Fatigue Behavior of As-Built Selective Laser Melted Titanium Scaffolds with Sheet-Based Gyroid Microarchitecture for Bone Tissue Engineering. Acta Biomater. 2019, 94, 610–626. [Google Scholar] [CrossRef]
- Attaeyan, A.; Shahgholi, M.; Khandan, A. Fabrication and Characterization of Novel 3D Porous Titanium-6Al-4V Scaffold for Orthopedic Application Using Selective Laser Melting Technique. Iran. J. Chem. Chem. Eng. (IJCCE) Res. Artic. 2024, 43, 66–82. [Google Scholar]
- Kumar, S.; Singh, I.; Kumar, D.; Yahya, M.Y.; Rahimian Koloor, S.S. Mechanical and Morphological Characterizations of Laminated Object Manufactured 3D Printed Biodegradable Poly(Lactic)Acid with Various Physical Configurations. J. Mar. Sci. Eng. 2022, 10, 1954. [Google Scholar] [CrossRef]
- Xu, C.; Yang, K.; Xu, Y.; Meng, X.; Zhou, Y.; Xu, Y.; Li, X.; Qiao, W.; Shi, J.; Zhang, D.; et al. Melt-Electrowriting-Enabled Anisotropic Scaffolds Loaded with Valve Interstitial Cells for Heart Valve Tissue Engineering. J. Nanobiotechnol. 2024, 22, 378. [Google Scholar] [CrossRef]
- Xie, J.; Gao, Q.; del Prado, Z.N.; Venkateswaran, N.; Mousa, H.M.; Salero, E.; Ye, J.; De Juan-Pardo, E.M.; Sabater, A.L.; Perez, V.L. Establishment of a Bi-Layered Tissue Engineered Conjunctiva Using a 3D-Printed Melt Electrowritten Poly-(ε-Caprolactone) Scaffold. Int. Ophthalmol. 2023, 43, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Huang, J.; Huang, S.; Wang, J.; Zheng, Y.; Luo, Y.; Tang, L.; Gao, B.; Tang, Y. Antibacterial and Osteogenic Dual-Functional Micronano Composite Scaffold Fabricated via Melt Electrowriting and Solution Electrospinning for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2024, 16, 37707–37721. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Kocak-Oztug, N.A.; Sheikh, F.A.; Han, P.; Anwar, S.; Fournier, B.P.J.; Ivanovski, S. Fabrication of 3D Bioactive Melt Electrowriting Composite Scaffold with High Osteogenic Potential. Colloids Surf. B Biointerfaces 2025, 245, 114270. [Google Scholar] [CrossRef]
- Chennakesava, P.; Narayan, Y.S. Fused Deposition Modeling-Insights. In Proceedings of the International Conference on Advances in Design & Manufacturing (ICAD&M’14), Tiruchirappalli, Tamil Nadu, India, 5–7 December 2014. [Google Scholar]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused Deposition Modelling: Current Status, Methodology, Applications and Future Prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Kristiawan, R.B.; Imaduddin, F.; Ariawan, D.; Ubaidillah; Arifin, Z. A Review on the Fused Deposition Modeling (FDM) 3D Printing: Filament Processing, Materials, and Printing Parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3d/4d Printing of Polymers: Fused Deposition Modelling (Fdm), Selective Laser Sintering (Sls), and Stereolithography (Sla). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef]
- Deshmane, S.; Kendre, P.; Mahajan, H.; Jain, S. Stereolithography 3D Printing Technology in Pharmaceuticals: A Review. Drug Dev. Ind. Pharm. 2021, 47, 1362–1372. [Google Scholar] [CrossRef]
- Maines, E.M.; Porwal, M.K.; Ellison, C.J.; Reineke, T.M. Sustainable Advances in SLA/DLP 3D Printing Materials and Processes. Green. Chem. 2021, 23, 6863–6897. [Google Scholar] [CrossRef]
- Huang, J.; Qin, Q.; Wang, J. A Review of Stereolithography: Processes and Systems. Processes 2020, 8, 1138. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (Sls), a New Chapter in the Production of Solid Oral Forms (Sofs) by 3d Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Lekurwale, S.; Karanwad, T.; Banerjee, S. Selective Laser Sintering (SLS) of 3D Printlets Using a 3D Printer Comprised of IR/Red-Diode Laser. Ann. 3D Print. Med. 2022, 6, 100054. [Google Scholar] [CrossRef]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective Laser Sintering 3D Printing–an Overview of the Technology and Pharmaceutical Applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, X.; Song, X. Engineering Materials with Light: Recent Progress in Digital Light Processing Based 3D Printing. J. Mater. Chem. C Mater. 2020, 8, 13896–13917. [Google Scholar] [CrossRef]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive Manufacturing by Digital Light Processing: A Review. Progress. Addit. Manuf. 2023, 8, 331–351. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, S.; Wang, R.; Ge, Q. Digital Light Processing Based Multimaterial 3D Printing: Challenges, Solutions and Perspectives. Int. J. Extrem. Manuf. 2024, 6, 042006. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A Review of Research Progress in Selective Laser Melting (SLM). Micromachines 2023, 14, 57. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of Selective Laser Melting: Materials and Applications. Appl. Phys. Rev. 2015, 2, 041101. [Google Scholar] [CrossRef]
- Valino, A.D.; Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Messman, J.; Advincula, R.C. Advances in 3D Printing of Thermoplastic Polymer Composites and Nanocomposites. Prog. Polym. Sci. 2019, 98, 101162. [Google Scholar] [CrossRef]
- Park, J.; Tari, M.J.; Hahn, H.T. Characterization of the Laminated Object Manufacturing (LOM) Process. Rapid Prototyp. J. 2000, 6, 36–50. [Google Scholar] [CrossRef]
- Dermeik, B.; Travitzky, N. Laminated Object Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2020, 22, 2000256. [Google Scholar] [CrossRef]
- Asad, H.; Ihsanullah, K. A Review of Laminated Object Manufacturing (LOM) Aspects and Various Processes Used in It. Int. J. Adv. Eng. Res. Sci. 2023, 10, 46–54. [Google Scholar] [CrossRef]
- Saiz, P.G.; Reizabal, A.; Vilas-Vilela, J.L.; Dalton, P.D.; Lanceros-Mendez, S. Materials and Strategies to Enhance Melt Electrowriting Potential. Adv. Mater. 2024, 36, 2312084. [Google Scholar] [CrossRef]
- Dalton, P.D. Melt Electrowriting with Additive Manufacturing Principles. Curr. Opin. Biomed. Eng. 2017, 2, 49–57. [Google Scholar] [CrossRef]
- Daghrery, A.; de Souza Araújo, I.J.; Castilho, M.; Malda, J.; Bottino, M.C. Unveiling the Potential of Melt Electrowriting in Regenerative Dental Medicine. Acta Biomater. 2023, 156, 88–109. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.L.; Dalton, P.D. A Decade of Melt Electrowriting. Small Methods 2023, 7, e2201589. [Google Scholar] [CrossRef] [PubMed]
- Loewner, S.; Heene, S.; Baroth, T.; Heymann, H.; Cholewa, F.; Blume, H.; Blume, C. Recent Advances in Melt Electro Writing for Tissue Engineering for 3D Printing of Microporous Scaffolds for Tissue Engineering. Front. Bioeng. Biotechnol. 2022, 10, 896719. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Farsi, M.; Asefnejad, A.; Baharifar, H. A Hyaluronic Acid/PVA Electrospun Coating on 3D Printed PLA Scaffold for Orthopedic Application. Prog. Biomater. 2022, 11, 67–77. [Google Scholar] [CrossRef]
- Kurowska, A.; Nikodem, A.; Jabłoński, A.; Janusz, J.; Szczygieł, P.; Ziąbka, M.; Menaszek, E.; Dziadek, M.; Zagrajczuk, B.; Kobielarz, M.; et al. Layered PCL Scaffolds Modified with Bioactive Additives Fabricated by Electrospinning and 3D-Printing for the Nasal Bone and Cartilage Defects. Mater. Des. 2023, 233, 112255. [Google Scholar] [CrossRef]
- Romero-Araya, P.; Pino, V.; Nenen, A.; Cárdenas, V.; Pavicic, F.; Ehrenfeld, P.; Serandour, G.; Lisoni, J.G.; Moreno-Villoslada, I.; Flores, M.E. Combining Materials Obtained by 3D-Printing and Electrospinning from Commercial Polylactide Filament to Produce Biocompatible Composites. Polymers 2021, 13, 3806. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Chellappan, V.; Neisiany, R.E.; Dubey, N.; Amuthavalli, K.; Verma, N.K.; Lakshminarayanan, R.; Ramakrishna, S. An Innovative Tunable Bimodal Porous PCL/Gelatin Dressing Fabricated by Electrospinning and 3D Printing for Efficient Wound Healing and Scalable Production. Compos. Sci. Technol. 2024, 247, 110402. [Google Scholar] [CrossRef]
- Lou, L.; Rubfiaro, A.S.; Deng, V.; He, J.; Thomas, T.; Roy, M.; Dickerson, D.; Agarwal, A. Harnessing 3D Printing and Electrospinning for Multiscale Hybrid Patches Mimicking the Native Myocardium. ACS Appl. Mater. Interfaces 2024, 16, 37596–37612. [Google Scholar] [CrossRef]
- Fazal, F.; Melchels, F.P.W.; McCormack, A.; Silva, A.F.; Handley, E.L.; Mazlan, N.A.; Callanan, A.; Koutsos, V.; Radacsi, N. Fabrication of a Compliant Vascular Graft Using Extrusion Printing and Electrospinning Technique. Adv. Mater. Technol. 2024, 9, 2400224. [Google Scholar] [CrossRef]
- Rezaei, E.S.; Poursamar, S.A.; Naeimi, M.; Taheri, M.M.; Rafienia, M. An in Vitro and in Vivo Study of Electrospun Polyvinyl Alcohol/Chitosan/Sildenafil Citrate Mat on 3D-Printed Polycaprolactone Membrane as a Double Layer Wound Dressing. Int. J. Biol. Macromol. 2024, 269, 131859. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zou, Q.; Wang, C.; Lin, M.; Li, Y.; Zhang, R.; Li, Y. Electrospinning and 3D Printed Hybrid Bi-Layer Scaffold for Guided Bone Regeneration. Mater. Des. 2021, 210, 110047. [Google Scholar] [CrossRef]
- Song, Y.; Hu, Q.; Liu, S.; Wang, Y.; Zhang, H.; Chen, J.; Yao, G. Electrospinning/3D Printing Drug-Loaded Antibacterial Polycaprolactone Nanofiber/Sodium Alginate-Gelatin Hydrogel Bilayer Scaffold for Skin Wound Repair. Int. J. Biol. Macromol. 2024, 275, 129705. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, E.; Ghitman, J.; Pircalabioru, G.G.; Zaharia, A.; Iovu, H.; Sarbu, A. Electrospun/3D-Printed Bicomponent Scaffold Co-Loaded with a Prodrug and a Drug with Antibacterial and Immunomodulatory Properties. Polymers 2023, 15, 2854. [Google Scholar] [CrossRef]
- Pensa, N.W.; Curry, A.S.; Bonvallet, P.P.; Bellis, N.F.; Rettig, K.M.; Reddy, M.S.; Eberhardt, A.W.; Bellis, S.L. 3D Printed Mesh Reinforcements Enhance the Mechanical Properties of Electrospun Scaffolds. Biomater. Res. 2019, 23, 22. [Google Scholar] [CrossRef]
- Atari, M.; Saroukhani, A.; Manshaei, M.; Bateni, P.; Zargar kharazi, A.; Vatankhah, E.; Haghjooy Javanmard, S. Preclinical in Vivo Assessment of a Cell-Free Multi-Layered Scaffold Prepared by 3D Printing and Electrospinning for Small-Diameter Blood Vessel Tissue Engineering in a Canine Model. Biomater. Sci. 2023, 11, 6871–6880. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, L.; Wang, M. 4D Bioprinted Tri-Layer Scaffolds with Biomimetic and Hierarchical Structure for Uterine Tissue Regeneration. In Proceedings of the Society For Biomaterials 2023 Annual Meeting & Exposition, San Diego, CA, USA, 19–22 April 2023. [Google Scholar]
- Mirhaj, M.; Varshosaz, J.; Labbaf, S.; Emadi, R.; Marcus Seifalian, A.; Sharifianjazi, F. An Antibacterial Multi-Layered Scaffold Fabricated by 3D Printing and Electrospinning Methodologies for Skin Tissue Regeneration. Int. J. Pharm. 2023, 645, 123357. [Google Scholar] [CrossRef]
- Belgheisi, G.; Haghbin Nazarpak, M.; Solati-Hashjin, M. Fabrication and Evaluation of Combined 3D Printed/Pamidronate-Layered Double Hydroxides Enriched Electrospun Scaffolds for Bone Tissue Engineering Applications. Appl. Clay Sci. 2022, 225, 106538. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, X.; Zhang, K.; EL-Newehy, M.; Abdulhameed, M.M.; Mo, X.; Cao, L.; Wang, Y. Application of Electrospinning and 3D-Printing Based Bilayer Composite Scaffold in the Skull Base Reconstruction during Transnasal Surgery. Colloids Surf. B Biointerfaces 2025, 245, 114337. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Bedir, T.; Gursoy, S.; Kaya, E.; Senel, I.; Tinaz, G.B.; Gunduz, O.; Ustundag, C.B. Development of Bilayer Tissue-Engineered Scaffolds: Combination of 3D Printing and Electrospinning Methodologies. Biomed. Mater. 2024, 19, 045029. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced Fabrication for Electrospun Three-Dimensional Nanofiber Aerogels and Scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef]
- He, W.; Li, C.; Zhao, S.; Li, Z.; Wu, J.; Li, J.; Zhou, H.; Yang, Y.; Xu, Y.; Xia, H. Integrating Coaxial Electrospinning and 3D Printing Technologies for the Development of Biphasic Porous Scaffolds Enabling Spatiotemporal Control in Tumor Ablation and Osteochondral Regeneration. Bioact. Mater. 2024, 34, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, Y.; Liu, Y.; Wang, Z.; Li, Y.; Jiang, G.; Mo, X.; Zhou, G. Three-Dimensional Printed Electrospun Fiber-Based Scaffold for Cartilage Regeneration. Mater. Des. 2019, 179, 107886. [Google Scholar] [CrossRef]
- Cai, P.; Li, C.; Ding, Y.; Lu, H.; Yu, X.; Cui, J.; Yu, F.; Wang, H.; Wu, J.; EL-Newehy, M.; et al. Elastic 3D-Printed Nanofibers Composite Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2023, 15, 54280–54293. [Google Scholar] [CrossRef]
- Zarei, M.; Żwir, M.J.; Michalkiewicz, B.; Gorący, J.; El Fray, M. Template-Assisted Electrospinning and 3D Printing of Multilayered Hierarchical Vascular Grafts. J. Biomed. Mater. Res. Part B Appl. Biomater. 2025, 113, e35525. [Google Scholar] [CrossRef]
- Song, J.Y.; Ryu, H.I.; Lee, J.M.; Bae, S.H.; Lee, J.W.; Yi, C.C.; Park, S.M. Conformal Fabrication of an Electrospun Nanofiber Mat on a 3D Ear Cartilage-Shaped Hydrogel Collector Based on Hydrogel-Assisted Electrospinning. Nanoscale Res. Lett. 2021, 16, 116. [Google Scholar] [CrossRef]
- Holjevac Grgurić, T.; Mijović, B.; Zdraveva, E.; Govorčin Bajsić, E.; Slivac, I.; Ujčić, M.; Dekaris, I.; Tominac Trcin, M.; Vuković, A.; Kuzmić, S.; et al. Electrospinning of PCL/CEFUROXIM® Fibrous Scaffolds on 3D Printed Collectors. J. Text. Inst. 2020, 111, 1288–1299. [Google Scholar] [CrossRef]
- Kasoju, N.; George, J.; Ye, H.; Cui, Z. Sacrificial Core-Based Electrospinning: A Facile and Versatile Approach to Fabricate Devices for Potential Cell and Tissue Encapsulation Applications. Nanomaterials 2018, 8, 863. [Google Scholar] [CrossRef] [PubMed]
- Doan, M.A.; Tobos, C.I.; Creighton, R.L.; Guo, T.; Faber, K.A.; Han, Y.; Chiew, C.; Hull, I.T.; Malakooti, M.H.; Woodrow, K.A. High-Resolution 3D Deposition of Electrospun Fibers on Patterned Dielectric Elastomers. Adv. Eng. Mater. 2023, 25, 2300670. [Google Scholar] [CrossRef]
- Brooks-Richards, T.L.; Paxton, N.C.; Allenby, M.C.; Woodruff, M.A. Dissolvable 3D Printed PVA Moulds for Melt Electrowriting Tubular Scaffolds with Patient-Specific Geometry. Mater. Des. 2022, 215, 110466. [Google Scholar] [CrossRef]
- Carranza, T.; Uranga, J.; Irastorza, A.; Izeta, A.; Guerrero, P.; de la Caba, K. Combination of 3D Printing and Electrospinning to Develop Chitin/Gelatin/PVA Scaffolds. Int. J. Bioprint. 2023, 9, 701. [Google Scholar] [CrossRef]
- Jiang, S.; Kang, Z.; Liu, F.; Fan, J. 2D and 3D Electrospinning of Nanofibrous Structures by Far-Field Jet Writing. ACS Appl. Mater. Interfaces 2023, 15, 23777–23782. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Carranza, T.; Santos-Vizcaino, E.; Igartua, M.; Guerrero, P.; Hernandez, R.M.; de la Caba, K. Hybrid 3D Printed and Electrospun Multi-Scale Hierarchical Polycaprolactone Scaffolds to Induce Bone Differentiation. Pharmaceutics 2022, 14, 2843. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ma, S.; Zheng, S.; Li, D.; Hu, W. Near-Field Direct-Writing Electrostatic Spinning 3D Bionic Tendon-Bone Repair Scaffold and Preparation Method Thereof. China Patent CN115845136A, 28 March 2023. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN115845136A (accessed on 19 May 2025).
- Liu, L.; Zhou, L. Composite 3D-Printing Forming System, Forming Method, and INTRAVascular Stent. China Patent CN106584836A, 26 April 2017. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN106584836A (accessed on 19 May 2025).
- Cárdenas, A.W.A.; Lara, B.A.L.; Silva, C.I.Z. Method of Manufacturing a Medical Device Using 3D Printing and Electrospinning. WO2023170557A1, 14 September 2023. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DWO2023170557A1 (accessed on 19 May 2025).
- Noh, I.S.; Roh, G.S.; Tran, H.N.; Janarthanan, G. A Biodegradable Meniscus Scaffold and Its Manufacturing Method. Korea Patent KR20220040773A, 31 March 2022. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DKR20220040773A (accessed on 19 May 2025).
- Wang, T.; Zhu, Q.; Quan, D.; Liu, X.; Wu, Z.; Yan, L. Multi-Channel Peripheral Nerve Conduit and Preparation Method Thereof. China Patent CN109172036A, 11 January 2019. Available online: https://worldwide.espacenet.com/patent/search/family/064948466/publication/CN109172036A?q=pn%3DCN109172036A (accessed on 19 May 2025).
- Qiao, Z.; Sun, B.; Chen, W.; Dai, K.; Wang, Y. Method for Preparing Tendon Scaffold by Using Three-Dimensional Printing and Electrostatic Spinning Technologies. China Patent CN108404213A, 17 August 2018. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN108404213A (accessed on 19 May 2025).
- Sun, D.; Wu, H.; Xu, F.; Jin, H.; Xue, M.; Zhang, Z.; Chen, S.; He, G. Method for Promoting Myocardial Tissue Morphological Induction via Bioprinting with 3D Nanofiber Constraints and Its Application. China Patent CN118048298A, 5 February 2024. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN118048298A (accessed on 19 May 2025).
- Zhang, G.; Huang, H.; Lan, H.; Li, W.; Song, D.; Wang, Z.; Peng, Z.; Zhao, J. Method for Preparing Conductive Biological Scaffold Based on Self-Excited Electrostatic Field Driven Melt-Jet Three-Dimensional (3D) Printing. China Patent CN112157906A, 1 January 2021. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN112157906A (accessed on 19 May 2025).
- Breuer, C.; Best, C.; Strouse, R.; Hibino, N.; Ung-Lee, Y. Systems and Methods for Optimized Patient Specific Tissue Engineering Vascular Grafts. U.S. Patent US11541149B2, 3 January 2023. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DUS11541149B2 (accessed on 19 May 2025).
- Hu, Q.; Li, D.; Liu, L.; Jiang, C.; Liu, Y.; Li, S.; Liu, Y. Micro-Nano Composite Dual-Layer Skin Framework and Manufacturing Method Thereof. China Patent CN106110401A, 16 November 2016. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN106110401A (accessed on 19 May 2025).
- Feng, J.; Zhao, J. Preparation Method of Multilayer Composite Nano-Micron Fiber Topological Morphology Scaffold Imitating ECM Structure. China Patent CN112121232A, 25 December 2020. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN112121232A (accessed on 19 May 2025).
- Yeo, M.G.; Kim, M.S.; Lee, C.S.; Park, M.J.; Kim, S.M.; Jung, B.S.; Lee, J.W.; Son, K.H. Artificial Blood Vessel and the Manufacturing Method Thereof. Korea Patent KR102467263B1, 16 November 2022. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DKR102467263B1 (accessed on 19 May 2025).
- Zhang, T.; Li, Y.; Bai, H.; Liu, H.; Wang, L.; Zhang, Y.; Wang, Y.; Zhang, H.; Feng, L.; Sun, L. Preparation Method of Bionic Blood Vessel Graft Based on Electrostatic Spinning and 3D Printing. China Patent CN109701079A, 30 April 2019. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN109701079A (accessed on 19 May 2025).
- Liu, J.; Pang, Q.; Jiang, L.; Lin, J. Bionic Bone Tissue Engineering Scaffold Material and Preparation Method Thereof. China Patent CN115054728A, 16 September 2022. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN115054728A (accessed on 19 May 2025).
- Korean Intellectual Property Office. Bio Tubular Scaffold for Fabricating Artificial Vascular and the Fabricating Method thereof. Korea Patent KR101751986B1, 30 June 2017. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DKR101751986B1 (accessed on 19 May 2025).
- Jung, E.; Kim, I.; Cho, H. Artificial Esophagus Scaffold and Manufacturing Method Thereof. Korea Patent KR102347096B1, 5 January 2022. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DKR102347096B1 (accessed on 19 May 2025).
- Liu, Y.; Zhang, F.; Chen, W.; Yan, F.; Zheng, L.; Yu, Y.; Hu, Q. Regeneration Bone Scaffold Forming System and Method Based on Comprehensive 3D Printing Formation. China Patent CN103341989A, 9 October 2013. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN103341989A (accessed on 19 May 2025).
- Zhang, H.; Deng, K.; Yuan, Y. 3D Printing and Electrospinning Composite Scaffold for Cartilage Tissue Engineering and Preparation Method Thereof. China Patent CN106827496A, 13 June 2017. Available online: https://worldwide.espacenet.com/patent/search?q=pn%3DCN106827496A (accessed on 19 May 2025).
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D Bioprinting Technology for Tissue/Organ Regenerative Engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted Cancer Models: Revolutionizing Personalized Cancer Therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- De Mori, A.; Fernández, M.P.; Blunn, G.; Tozzi, G.; Roldo, M. 3D Printing and Electrospinning of Composite Hydrogels for Cartilage and Bone Tissue Engineering. Polymers 2018, 10, 285. [Google Scholar] [CrossRef]
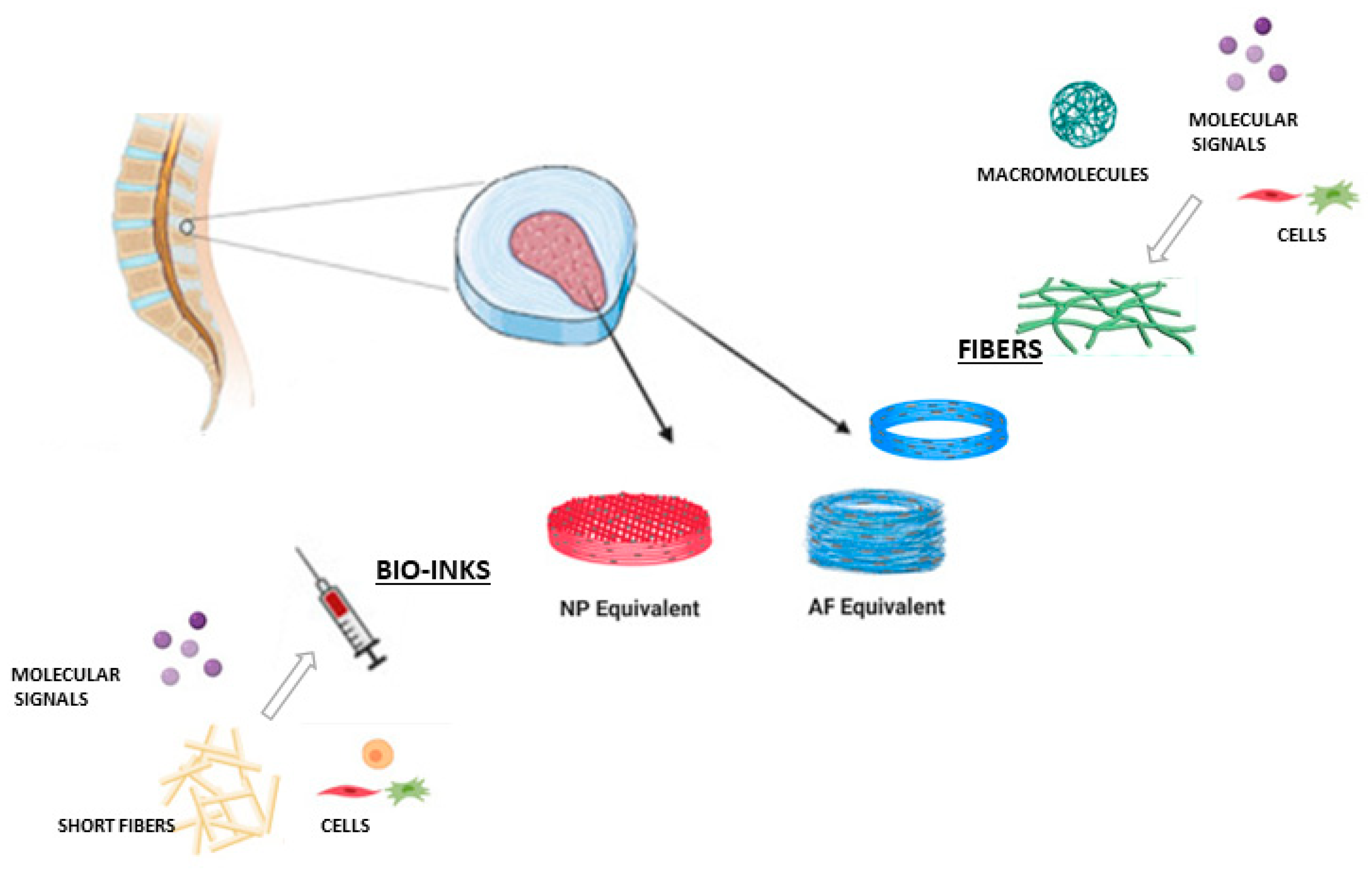
- De Pieri, A.; Byerley, A.M.; Musumeci, C.R.; Salemizadehparizi, F.; Vanderhorst, M.A.; Wuertz-Kozak, K. Electrospinning and 3D Bioprinting for Intervertebral Disc Tissue Engineering. JOR Spine 2020, 3, 901–915. [Google Scholar] [CrossRef]
- Urciuolo, A.; Poli, I.; Brandolino, L.; Raffa, P.; Scattolini, V.; Laterza, C.; Giobbe, G.G.; Zambaiti, E.; Selmin, G.; Magnussen, M.; et al. Intravital Three-Dimensional Bioprinting. Nat. Biomed. Eng. 2020, 4, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Neuhäusler, A.; Rogg, K.; Schröder, S.; Spiehl, D.; Zora, H.; Arefaine, E.; Schettler, J.; Hartmann, H.; Blaeser, A. Electrospun Microfibers to Enhance Nutrient Supply in Bioinks and 3D-Bioprinted Tissue Precursors. Biofabrication 2024, 17, 015038. [Google Scholar] [CrossRef] [PubMed]
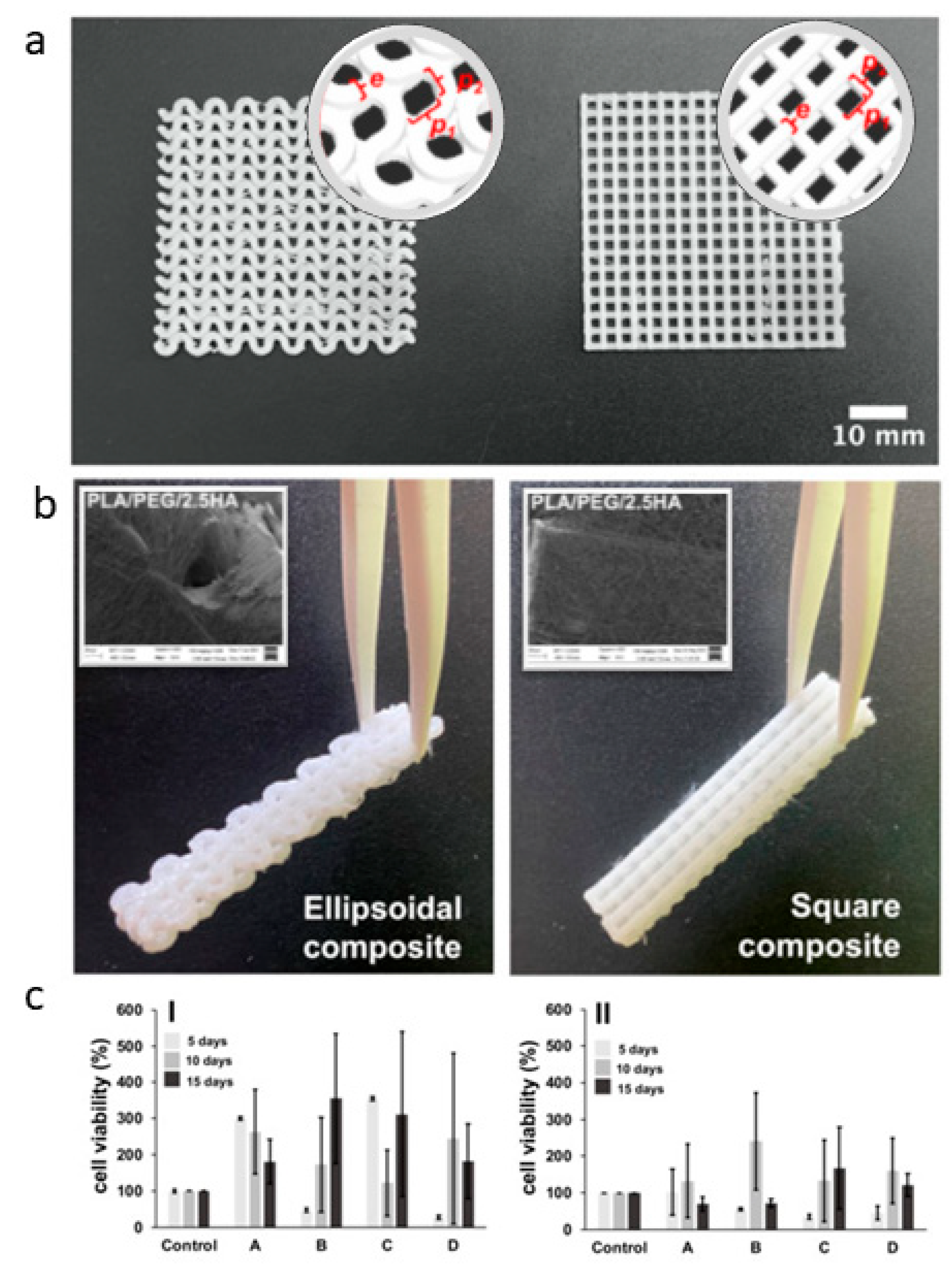
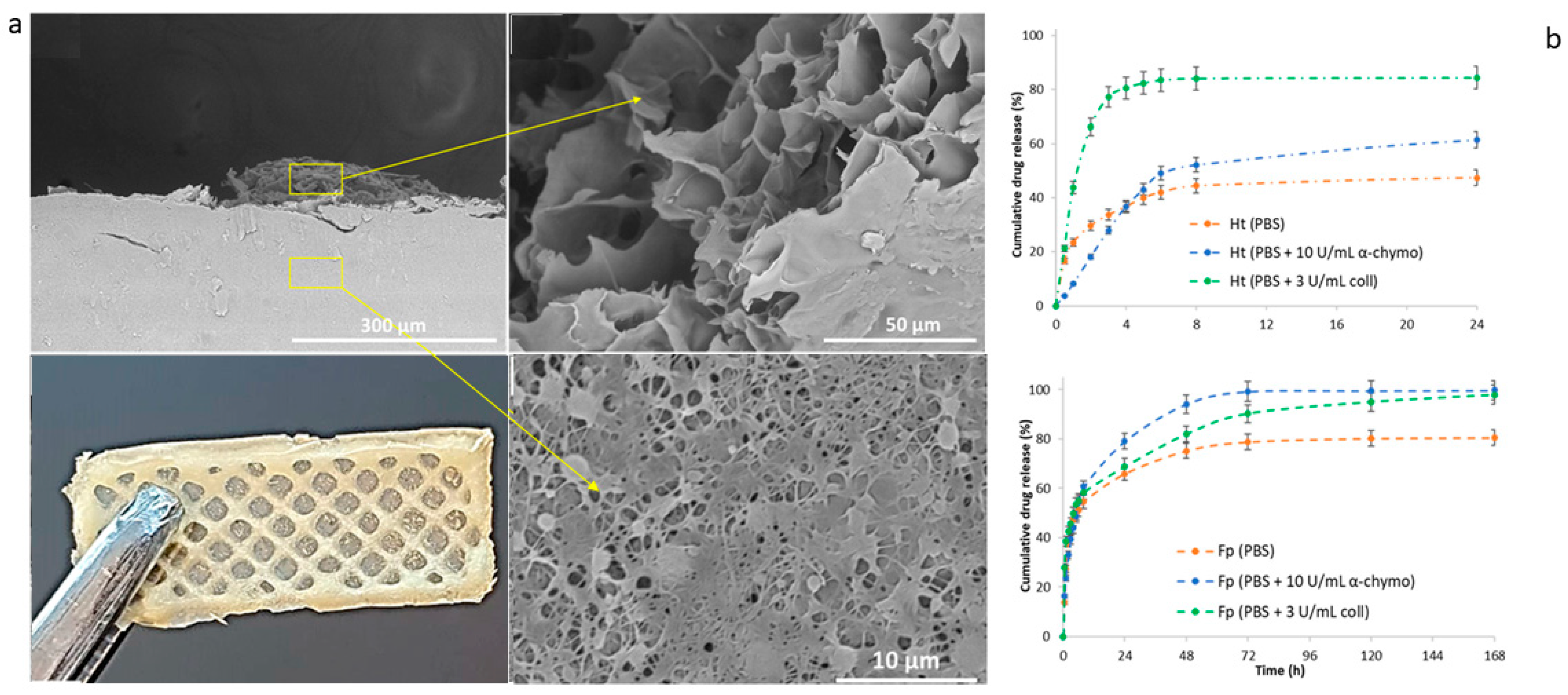
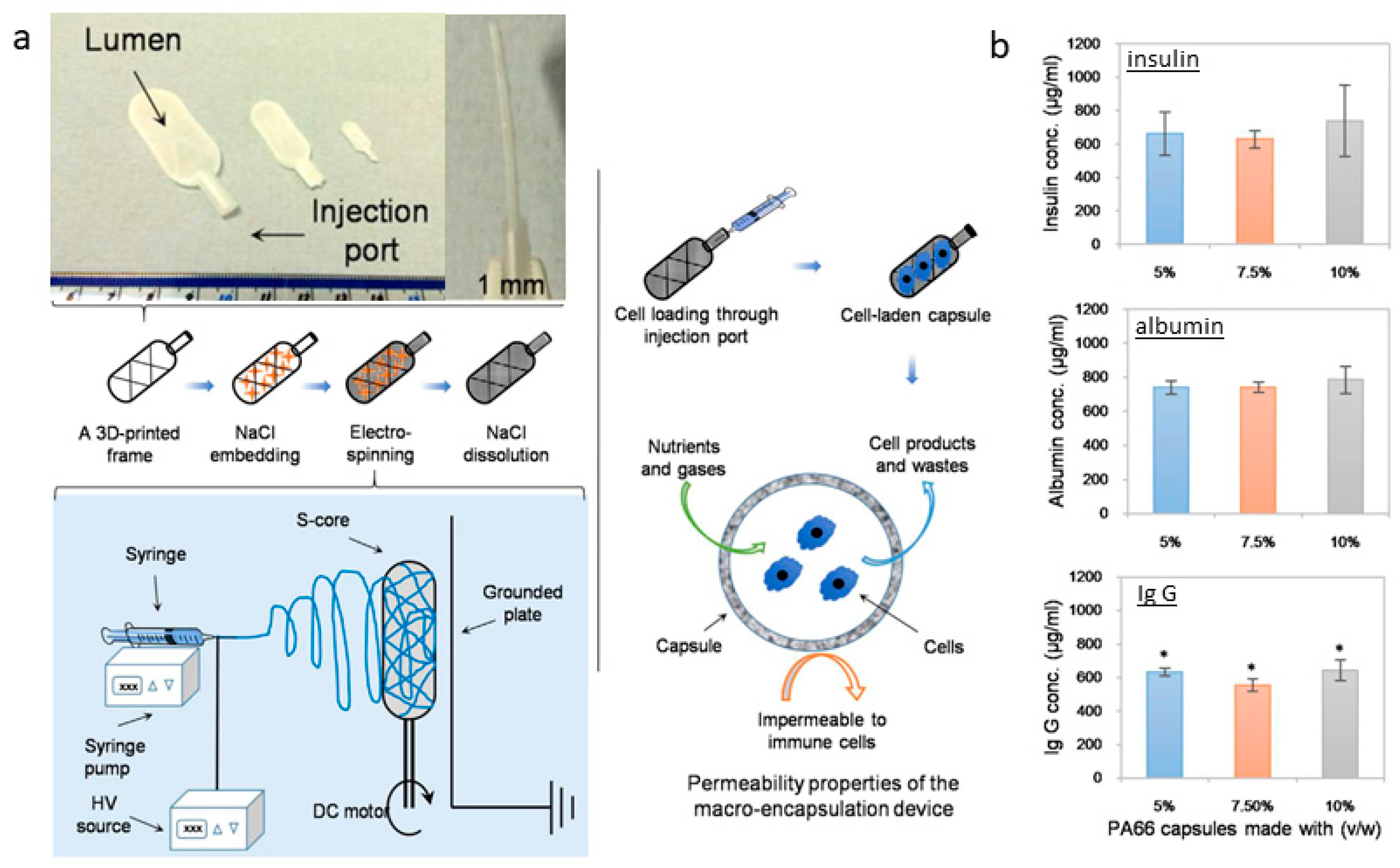
| Tissue | Composition/Solvents | Fiber Diameter | Key Findings | Ref. |
|---|---|---|---|---|
| Bone | -PLGA/PCL with linezolid -Solvent: HFIP | -PLGA: 930 ± 150 nm -PLGA/PCL with linezolid: varying diameters | -Effective methicillin-resistant S. aureus control in vitro and in vivo -Effective localized infection control for prosthetic applications | [47] |
| -PLA with bioactive glass (n-BG) and MgO -Solvent: DCM and DMF | -PLA-only: 1.7 ± 0.6 μm -PLA/n-BG/n-MgO: 2.8–3.1 μm | -Increased HA formation -Antimicrobial activity against S. aureus -Elevated ALP expression in osteoblast cells | [46] | |
| -PLLA/PCL with 58S bioactive glass -Solvent: DCM and DMF | 1.5 µm | -Enhanced cell proliferation (Saos-2 cell line) -Compressive modulus 6.1 ± 0.4 kPa -Pore size ~335.3 µm | [43] | |
| -GT/PVA/SF with Cu-HAP -Solvent: Double-distilled water and acetic acid | Not reported | -Antimicrobial activity -High porosity (99%); hemolysis <5% -ALP activity increases over 7–14 days | [44] | |
| -PCL, UCNPs, MgO -Solvent: HFIP | PCL: 1.78 µm, PCL-UCNPs-1%MgO: 1.65 µm | -Enhanced ALP activity -Bone regeneration in rat skull defects -Scaffold degradation tracked non-invasively | [48] | |
| -PCL blended with starch and 5% CaO -Solvent: Formic acid | -PCL/starch/CaO: 750 ± 120 nm | -Bioactivity and controlled scaffold -Promoted HA formation -Increased Young’s modulus | [45] | |
| -Gelatin–PCL blend with nHAp; -Solvents: Acetic acid and chloroform/methanol | -Gelatin-PCL-nHAp: 615 ± 269 nm | -Improved osteoblast adhesion and proliferation -Cells exhibited healthy morphology -Increased mechanical strength | [78] | |
| Skin | -PCL with collagen type I (19:1 ratio) -Solvent: TFE | -PCol: 390 ± 71 nm -PCL-only: 804 ± 186 nm | -Increased wettability and cell adhesion -Promoted organized skin regeneration in normal skin morphology in vivo -PCL/collagen/hWJ-MSC supported cell adhesion and regeneration | [51] |
| -PLA with phenytoin, sildenafil citrate, and simvastatin -Solvent: Acetone, acetic acid, and DCM | 709 ± 178 nm | -Sustained drug release and high biocompatibility -Promoted complete re-epithelialization, organized cell layers, and no scar tissue after 21 days in vivo | [56] | |
| -PU and starch/hyaluronic acid -Solvent: DMSO and DMF | 428 ± 78.32 nm | -Enhanced cell attachment and proliferation -Improved wound healing with organized tissue structure in vivo | [79] | |
| -SEFM is composed of polyglactin 910 and polydioxanone -Solvent: Not specified | Not specified; matrix designed to mimic human ECM | Clinical trial: 74% of diabetic foot ulcer patients achieved 100% re-epithelialization in 12 weeks, vs. 33% in standard care | [57] | |
| -PVP/PVA -Solvent: ethanol and 90% acetic acid | 150–400 nm | -Non-toxic to fibroblasts, low hemolysis -In vivo slow degradation and minimal inflammation in rats | [49] | |
| -Silk fibroin and gelatin with propolis (SF/GT-EP) -Solvent: formic acid | SF/GT-1%EP: 200–400 nm | -Enhanced fibroblast cell migration -Promoted wound healing and re-epithelialization in mice | [54] | |
| -PU, PRGF, and gelatin with L. plantarum -Solvent: THF, DMF | 516 ± 147 nm | -Supported cell proliferation -Antibacterial potential -Improved wound healing, angiogenesis, and reduced infection in a rat model | [53] | |
| -CS and polyethylene oxide with cerium oxide nanoparticles -Solvent: 70% acetic acid | 175 ± 76 nm | -High MSC compatibility, supported cell growth -Good tissue integration, slow degradation, minimal inflammation in vivo | [55] | |
| -PCL and PEG supplemented with egg yolk oil -Solvent: DCM: DMF (9:1) | 191 ± 61 nm | -Enhanced fibroblast cell viability and antibacterial properties -Promoted wound healing, re-epithelialization, and collagen synthesis | [50] | |
| Cartilage and tendon | -PLGA with curcumin -Solvent: Trichloromethane | Not specified; nanoscale structure confirmed by SEM | -Sustained curcumin release and reduced inflammation -Rat cartilage defect model showed immunosuppressive effects and cartilage preservation | [66] |
| -PCL and silk fibroin -Solvent: HFIP (R: Randomly oriented, A: Aligned) | -RPCL 552.4 ± 189.8 nm -RPCL/SF 423.6 ± 199.4 nm -APCL/SF 361.9 ± 151.3 nm | -APCL/Silk fibroin nanofibers promoted articular chondrocyte proliferation and type II collagen gene expression -Promoted the elongation of articular chondrocytes in the direction of parallel fiber alignment | [80] | |
| -PLLA with barium titanate (BT) infused with FGF-18 -Solvent: HFIP | Not specified; scaffold morphology assessed via SEM | -Enhanced chondrocyte proliferation and cartilage-related gene expression -Cartilage regeneration and ECM remodeling | [65] | |
| Thermoplastic polyurethane and gelatin -Solvent: HFIP | 0.31 ± 0.06 μm | -Biocompatible and semi-permeable; blocks immune cell infiltration -Goat model showed stable allogeneic cartilage regeneration without immune rejection | [67] | |
| -PCL and chitosan -Solvents: HFIP, formic acid, and acetone, respectively | siRNAGP/CSPCL scaffold ± 0.06 μm | -Controlled COX-2 siRNA release, reducing fibroblast proliferation -Reduced inflammation and better healing in a tendon model | [71] | |
| -PCL/Cellulose acetate -Solvent: TFE | 600–1000 nm | -Supported hMSC proliferation and tendon gene expression. -Promoted tendon healing and functional recovery in a tendon model | [69] | |
| -PCL/PGS -Solvent: acetic acid | 1.01 ± 0.44 μm. | -Supported amniotic epithelial stem cells adhesion, growth, and teno-differentiation | [70] | |
| Cardiac and vascular | -GT/PCL -Solvent: Chloroform | 1.02 ± 0.13 μm | -High mechanical integrity enhanced endothelial cell viability -Good hemostatic performance and patency in the swine pulmonary artery model | [58] |
| -SF/fibroin -Solvent: Formic acid | SF/fibroin (25:75) 424 ± 11 nm | -Enhanced mechanical strength and MSC proliferation -Faster degradation and tissue regeneration in the rat model | [81] | |
| -PLGA core and PCL shell -Solvent: HFIP | HCS1508 scaffold (best tensile properties): 2.05 ± 0.60 μm | -Strong mechanical properties, improved degradation in vitro -Stable endothelialization, low inflammation, and no calcification at 12 months in rat aorta | [59] | |
| -PCL and placental extracellular matrix (pECM), -Solvent: HFIP | PCL-epECM/H-IL-4 7.4 ± 1.3 μm | -Promoted macrophage anti-inflammatory polarization and endothelial cell proliferation -Enhanced endothelialization and smooth muscle regeneration in rat artery model | [63] | |
| -5% Carbothane™ 3575A with 10% GT -Solvent: HFIP | 0.39 ± 0.11 µm | -Improved cell adhesion and endothelial compatibility -Superior patency and compatibility compared to expanded polytetrafluoroethylene grafts in the Wistar rat aorta model | [64] | |
| -PCL/Polydioxanone, PLCL, PLGA -Solvent: Chloroform and HFIP | PLCL/Polydioxanone (best-performing scaffold): 1.76 µm (prior to stent expansion) | -High stretchability and hemocompatibility except for polydioxanone -PLGA failed in 1 day; PCL and PLCL exhibited good biocompatibility and biodegradation in the rat aorta model | [82] | |
| -PCL, SF, CNTs with brown adipose-derived stem cell sheets -Solvent: HFIP | PCL/SF 1.156 ± 0.296 μm | -Promoted cardiomyogenic differentiation and electrical conductivity -Enhanced angiogenesis, reduced inflammation, and supported cardiomyocyte regeneration in rat myocardial infarction model | [60] | |
| -PU with salvianolic acid A -Solvent: HFIP | 1.33 ± 0.174 μm. | -Anti-inflammatory, angiogenic gene expression, reduced inflammatory cell chemotaxis -Improved myocardial recovery and re-endothelialization in myocardial infarction and arterial repair rat models | [70] | |
| -PLA, PLGA loaded with dexamethasone -Solvent: TFE and chloroform | PLA: 317.38 nm ± 82.57 nm. PCL: 342.72 nm ± 56.27 nm | -Sustained dexamethasone release over 50 days, promoting cell adhesion | [83] | |
| -PCL with ascorbic acid (AA) -Solvent: HFIP | PCL—0.3%AA: 1.88 ± 0.14 μm | -Reduced reactive oxygen species and oxidative stress -High cytocompatibility with human umbilical vein endothelial cells | [62] | |
| Nerve | -PLLA with PPy and PDA -Solvent: HFIP | PPy/PDA/PLLA: 3.60 ± 0.20 μm, PDA/PLLA: 3.46 ± 0.29 μm, PLLA: 2.81 ± 0.15 μm | -Enhanced nerve cell attachment and axonal alignment -Rat sciatic nerve model showed improved myelination and functional recovery | [72] |
| -PCL and porcine-derived nerve ECM -Solvent: TFE | 886.09 ± 185.43 nm | -Supported Schwann cell adhesion and proliferation -Rat sciatic nerve model demonstrated biocompatibility, improved nerve regeneration, and functional recovery | [74] | |
| -PLLA coated with DSS and pDOPA -Solvent: Chloroform | -Uncoated PLLA: 1.98 ± 0.38 μm -2pDOPA coated: 1.96 ± 0.20 μm | -Enhanced Schwann cell NT-3 secretion with the pDOPA functionalized scaffold -Supported increased neurite extension from dorsal root ganglia | [75] | |
| -PLA-PCL outer layer, porcine-derived nerve ECM inner layer -Solvent: Acetic acid | -PLA-PCL layer thickness: 0.121 mm ± 0.010 -ECM layer: 0.104 mm ± 0.008 -Scaffold inner diameter: 1.342 mm ± 0.025 | -High biocompatibility, cell affinity, retention of bioactive molecules (collagen, laminin, and fibronectin, etc.) -Rat sciatic nerve gap model showed superior nerve regeneration compared to PLA-PCL alone | [77] | |
| -PPy, PDA, PLLA -Solvent: TFE | ~5 μm | -MSC differentiation was promoted into Schwann-like cells, as indicated by increased markers -Enhanced nerve regeneration, myelination, and functional recovery in rat sciatic nerve model | [73] | |
| -PLA with lithium -Solvent: Chloroform and DMF (4:1) | ~550 nm | -Scaffold-enhanced Schwann cell marker expressions and differentiation of human adipose-derived mesenchymal stem cells -Lithium-loaded scaffold facilitated Schwann cell differentiation and improved sensory and motor functions in vivo, with well-organized myelinated axons | [84] | |
| -PCL/GT with heparin and growth factors -Solvent: HFIP | -Inner side fiber diameter:1.49 ± 0.51 μm and -Outer: 1.89 ± 0.70 μm | -Heparin-immobilized PCL/gel with neural growth factor and basic fibroblast growth factor retained bioactivity and facilitated nerve regeneration, reducing inflammatory infiltration in vivo | [85] |
| Method | Materials | Scaffold Characteristics | Application and Findings | Refs. |
|---|---|---|---|---|
| Fused Deposition Modeling | PLA with 30% HA powder | Cubic, cylindrical, and hexagonal porous structures with pore sizes of 0.8 and 1.2 µm, compressive strength 5.5–7.5 MPa | Cubic structure had maximum permeability; hexagonal structure enhanced apatite formation, promoting bioactivity | [89] |
| Pure PLA filament | Gyroid structure with unit cell sizes of 2, 2.5, and 3 mm, pore sizes 1.3, 1.7, and 2 mm, porosity 86–90%, compressive strength up to 180 MPa | Gyroid structure exhibited high mechanical strength and anisotropy, making it suitable for bone tissue applications | [90] | |
| Stereolithography | Elastic resin with lidocaine hydrochloride | Hollow and solid elastic devices; 0.5 mm shell for hollow, flexible for bladder retention | Hollow devices released lidocaine over 4 days; solid devices extended release up to 14 days; high flexibility suitable for bladder retention | [91] |
| Poly-trimethylene carbonate (PTMC) with HA nanoparticles | Gyroid structure, 70% porosity, minimum pore size of 600 µm, HA-enriched surface | HA-enriched surfaces promoted cell attachment, differentiation, and mineralization; PTMC/HA scaffolds showed improved bone ingrowth and integration | [92] | |
| Ti6Al4V alloy (20–50 µm powder) | Hierarchical micro/submicron texture created by acid etching; dimensions: 10 × 10 × 2 mm (in vitro), 2 × 3 mm cylinders (in vivo); rougher than SLA surface | Osteogenic application: in vitro MSCs adhesion, proliferation, differentiation) and in vivo (osseointegration in rat femoral condyle) | [93] | |
| Selective Laser Sintering | PCL with HA | Circular discs, 15 mm diameter, 1 mm thickness, interconnected pores, HA distributed within PCL matrix | Optimized SLS conditions improve scaffold stability; HA promoted cell adhesion and apatite formation | [94] |
| PCL powder | Square pyramid unit cells, 85% porosity, micropores of 40–100 µm, tensile stiffness 0.43 MPa, compressive stiffness 345 kPa | Scaffold architecture supports cardiac cell colonization and differentiation, maintaining viability over 21 days | [95] | |
| PVA powder | Tetragonal structure with interconnected pores, 67.9% porosity, and pore size controlled via SLS settings | Stable, porous scaffolds for nutrient and waste exchange; MG-63 cells adhered and proliferated, confirming biocompatibility | [96] | |
| Digital Light Processing | HA powder with photopolymer resin and liquid sodium polyacrylate | 300–600 µm pore size, 49.8% porosity, compressive strength 15.25 MPa | Supported cell adhesion, proliferation, and differentiation; high precision and biocompatibility for bone TE | [97] |
| Zirconia (ZrO₂) and HA composite in photosensitive resin | Porosity 54.6%, shrinkage 23–28% post-sintering, compressive strength 20 MPa | HA addition improved bioactivity; optimal mechanical properties at 10 wt% HA for bone TE | [98] | |
| Calcium phosphate powders with monoalcohol ethoxylate phosphate in photosensitive resin | Three designs (cube, octet-truss, inverse fcc); inverse fcc has the highest porosity and compressive strength | Macropore geometry influenced osteogenic properties; inverse fcc showed enhanced osteoinduction | [99] | |
| Selective Laser Melting | Commercially pure titanium | Gyroid design, 68–73% porosity, unit cell sizes of 2, 2.5, and 3 mm; compressive strength 44.9 to 56.5 MPa | High compatibility with bone elastic modulus; ductility up to 50% strain; optimized roughness for bone integration | [100] |
| Titanium alloy (Ti6Al4V) | Gyroid microarchitecture, 50–90% porosity, unit cell sizes 4 mm and 6 mm; wall thicknesses 0.25, 0.5, and 1.0 mm | Improved fatigue resistance with thicker walls, optimal laser parameters, and enhanced integrity for bone repair | [101] | |
| Ti6Al4V alloy with chitosan-wollastonite nanoparticles. | Porous structure, 48–52% porosity; 10 wt% WS-NPs showed the highest compressive strength of 420 MPa | Chitosan–wollastonite improved bioactivity and mechanical strength, has low cytotoxicity, and is promising for orthopedic use | [102] | |
| Laminated Object Manufacturing | Biodegradable poly(lactic) acid (PLA) | Laminated object manufacturing technique; infill patterns (linear, triangular, honeycomb); infill densities (50–90%); disc thickness (3.4–5.6 mm) | Intended for marine and structural engineering applications; best performance observed with honeycomb infill pattern at 70% density, achieving a compressive strength of 42.47 MPa; highlighted the significance of the infill pattern and number of discs in enhancing mechanical properties | [103] |
| Melt-electrowriting | Polycaprolactone (PCL), gelatin methacryloyl (GelMA), chondroitin sulfate methacryloyl (ChsMA) | Anisotropic scaffolds made by MEW combined with bioactive hydrogels | Intended for pediatric heart valve tissue engineering; improved hemocompatibility and endothelialization; reduced immune reaction and calcification in vivo | [104] |
| PCL | A bilayered scaffold made by MEW mimics conjunctival stromal and epithelial layers | Used for ocular surface reconstruction; supported growth of conjunctival stromal and epithelial cells; no in vivo assessments mentioned | [105] | |
| PCL, HA, and Roxithromycin (ROX) | Composite scaffold combining MEW and SES techniques contains microfibers and nanofibers with embedded HAP and ROX | Aimed at bone tissue engineering, enhanced osteogenic differentiation, and antibacterial properties; significant bone formation observed in vivo | [106] | |
| Low-molecular-weight PCL, and HA nanoparticles | The scaffold was made using melt-electrowriting with high HA concentration | Designed for bone tissue regeneration, higher metabolic activity, and osteogenic markers; no in vivo results were reported | [107] |
| Approach | Materials and Fabrication Process | Applications | Key Findings | Refs. |
|---|---|---|---|---|
| Electrospinning onto 3D-printed scaffolds | PLA printed scaffold coated with PVA/HLA electrospun fibers | Cartilage TE | Enhanced elastic modulus and tensile strength; increased cell proliferation with chondrocytes. | [133] |
| PCL printed scaffold layered with BG/Zn and PCL-OST electrospun membrane | Nasal cartilage and bone TE | Improved chondrocyte viability, collagen type II secretion, and cell proliferation. | [134] | |
| 3D-printed PCL grid scaffold with electrospun PCL/gelatin/ε-PL nanofibers | Wound healing and skin TE | Enhanced cell alignment, strong antibacterial properties, and skin-like mechanical properties. | [136] | |
| 3D-printed silicone scaffold with aligned PLGA electrospun fibers | Cardiovascular TE | Improved hiPSC-CM alignment, calcium handling, and mechanical anisotropy for myocardium mimicry. | [137] | |
| Extrusion-printed gelMA scaffold reinforced with electrospun PCL/PLCL nanofibers | Cardiovascular TE | High cell viability, compliance suitable for muscular and elastic arteries, high burst pressure. | [138] | |
| 3D-printed PCL membrane with electrospun PVA/CS/SC mat | Wound healing and skin TE | Accelerated wound closure, enhanced collagen deposition, and favorable hydrophilicity. | [139] | |
| 3D Printing onto electrospun fibers | 3D printing PCL/Gel/nano-HA onto an electrospun layer of PCL/gelatin | Periodontal defect repair | In vitro: Enhanced fibroblast proliferation on the membrane; BMSCs showed osteogenic differentiation with high BMP-2 expression (p < 0.001). In vivo: After 20 weeks, defects treated with a hybrid scaffold had the highest bone volume-to-total volume (BV/TV) ratio (p < 0.01). | [140] |
| 3D printing onto electrospun PCL fibers | Large-scale skin wound repair | In vitro: Antibacterial activity effective against E. coli and S. aureus; HaCaT cells exhibited increased proliferation. In vivo: Higher wound closure rate (89.60 ± 0.83%) in rats by day 14; enhanced collagen deposition and neovascularization. | [141] | |
| 3D printing of a PLA mesh onto an electrospun PCL/gelatin | TE applications requiring enhanced mechanical properties | In vitro: Tensile strength increased to 1001 Â ± 302 kPa with 6 mm PLA mesh reinforcement (13-fold increase). The elastic modulus for a 6 mm mesh was 501 ± 197 kPa (p < 0.0001). In vivo: Biocompatibility was confirmed, with no immune response in the rat cranial defect model after 20 weeks. | [143] | |
| Alternate use of 3D printing and electrospinning | Alternate 3D printing and electrospinning; PCL helical 3D-printed layers and electrospun PCL/PLA/collagen fibers | Small-diameter blood vessel TE | In vivo: 80% patency rate, endothelialization, ECM deposition, normal blood flow (RI = 0.61, PSV = 39.9 cm/s). | [144] |
| Alternate 3D printing and electrospinning; PU top layer, 3D-printed F127-QCS-AgNO3 middle, electrospun F127-Mup/Pec-Kr bottom | Skin TE | In vitro: High cell viability, antibacterial, controlled Ag and mupirocin release; In vivo: 94% wound closure rate by day 12. | [146] | |
| Alternate 3D printing and electrospinning; PLLA-TMC/TPU base layer with electrospun PLGA/GT and bioprinted GelMA/GT | Uterine tissue regeneration | In vitro: Shape-morphing, high cell viability, pH-sensitive drug release. | [145] | |
| Alternate 3D printing and electrospinning; PAM-LDH/PCL mats glued between 3D-printed PCL grids | Bone TE | In vitro: Enhanced osteoconductive markers (ALP activity 18.5% increase). | [147] | |
| Decorating/Infusing 3D-Printed Scaffolds with Electrospun Nanofiber Segments | Electrospun PLCL embedded with bFGF attached to a 3D-printed PCL/HA layer through high-temperature treatment | Skull base reconstruction | In vitro: Slow bFGF release and enhanced fibroblast proliferation and collagen deposition.In vivo: Enhanced bone volume. | [148] |
| Decorating 3D-printed GelMA hydrogel infused with ciprofloxacin with electrospun PCL-collagen fibers | TE with antibacterial properties | In vitro: High fibroblast viability (102%) and effective antibacterial properties against E. coli, S. aureus, and P. aeruginosa. Mechanical strength: 24.46 kPa, suitable for biomedical applications. | [149] | |
| Electrospun fibers as inks for 3D printing | Electrospun fibers were dispersed in a bio-ink composed of polyethylene oxide to produce inks | Osteochondral regeneration and tumor ablation | In vitro: High BMSC viability and enhanced osteogenic and chondrogenic differentiation (e.g., COL1A1 and RUNX2 expression). In vivo: Tumor ablation in GCTB nude mouse model and significant cartilage and bone regeneration in the rabbit model. | [151] |
| Electrospun GT/PLGA fibers are processed into short fragments to serve as inks for 3D printing | Cartilage regeneration | In vitro: High chondrocyte viability and ECM production in scaffold layers after six weeks. In vivo: Supported cartilage formation and maintained shape post-implantation, forming thicker cartilage than the control in the rabbit model. | [152] | |
| Silica nanofibers were processed into short segments and combined with sodium alginate to create a bio-ink | Bone TE, particularly for cranial bone defect repair | In vitro: Mechanical properties showed compressive stress of 566.6 ± 128.0 kPa and modulus of 196.0 ± 22.4 kPa; high ALP activity and BMSC proliferation with osteogenic marker expression. In vivo: Promoted significant new bone formation in a rat cranial defect model, with the highest bone volume and mineral density among tested scaffolds. | [153] | |
| Fabrication of electrospun scaffolds on 3D-printed collectors/templates | Fabrication of multilayered vascular grafts using 3D printing and electrospinning | Vascular grafts for cardiovascular diseases | Successful fabrication demonstrates biocompatibility and mechanical properties suitable for vascular applications. | [154] |
| Conformal fabrication of nanofibers on 3D ear cartilage-shaped hydrogel collectors | Ear cartilage reconstruction | Enhanced precision in fiber deposition with improved coverage and uniformity on complex shapes. | [155] | |
| Electrospinning of PCL/Cefuroxime on various 3D-printed collector geometries | Tissue regeneration with antibiotic delivery | Wide slot collectors in ribbed configurations enhanced cell adhesion and viability, demonstrating the impact of collector geometry on scaffold performance. | [156] | |
| High-resolution deposition of electrospun fibers on patterned dielectric elastomers | Biosensors, drug delivery systems, and tissue engineering | Patterned dielectric elastomers significantly enhance the precision and control of fiber deposition for medical applications. | [158] | |
| Melt electrowriting on dissolvable PVA molds for tubular scaffolds with patient-specific geometry | TE of vascular and/or anatomically relevant tubular tissues | PVA molds preserved scaffold morphology better than PLA molds, allowing non-destructive removal and maintaining microarchitectural details. | [159] | |
| Platforms combining 3D printing and electrospinning techniques | Platform setup included a bioprinting unit with a syringe extruder for 3D printing and an electrospinning nozzle | TE, focusing on wound healing | In vitro: Human dermal fibroblasts showed 75% activity at 24 hrs, increasing to 91.8% at 48 hrs and 106.8% at 72 hrs. Cell mortality remained low (~10%). | [160] |
| Set up that integrates far-field jet writing with 3D printing by equipping the platform with electrostatic lenses | Tissue scaffolding, especially in applications requiring precise fiber arrangement | In vitro: The system achieved 200 μm precision in fiber placement. | [161] | |
| Hybrid 3D-printing and electrospinning platform | Bone TE specifically to induce bone differentiation | In vitro: Significant increase in ALP activity by day 7; calcium deposition up to day 14. Gene expression of ALP and osteopontin markers is upregulated. | [162] |
| Patent Title | Summary | Assessments and Findings | Combination Technique | Patent No. |
|---|---|---|---|---|
| Near-Field Direct-Writing Electrospinning 3D Bionic Tendon-Bone Repair Scaffold and Preparation Method | Development of a 3D bionic scaffold for tendon-bone interface repair. Combines 3D printing and near-field direct-writing electrospinning to enhance mechanical properties and biocompatibility. | The final product is a scaffold that can enable stem cells to differentiate to promote tendon and bone repair. In vitro assays showed enhanced cell proliferation. | Alternate use of 3D printing and electrospinning | CN115845136A [163] |
| Composite 3D-Printing Forming System, Forming Method, and Intravascular Stent | Fabrication of vascular stents integrating 3D printing and electrospinning for improved flexibility and strength, suitable for vascular tissue engineering. | The final product is a hollow fibrous stent with branching morphology, mimicking blood vessel structure.The only evaluations presented are visual/photographic fabrication outcomes. | Alternate use of 3D printing and electrospinning | CN106584836A [164] |
| Method of Manufacturing a Medical Device Using 3D Printing and Electrospinning | Fabrication of a multilayered medical device, particularly a scaffold, using a combination of 3D printing and electrospinning | This patent defines a manufacturing method for multilayered medical scaffolds/devices using 3D printing and electrospinning. | Alternate use of 3D printing and electrospinning | WO2023170557A1 [165] |
| A biodegradable meniscus scaffold and its manufacturing method | Development of a scaffold for meniscal cartilage repair using 3D printing and electrospinning. | In vitro, biocompatibility assays demonstrated enhanced cell adhesion and proliferation. | Alternate use of 3D printing and electrospinning | KR20220040773A [166] |
| Multi-Channel Peripheral Nerve Conduit and Preparation Method | Fabrication of a multi-channel nerve conduit for peripheral nerve repair, integrating 3D printing and electrospinning to guide axonal growth and promote regeneration. | Scaffold showed suitable morphology for nerve repair. In vitro assays cells demonstrated cell adhesion and biocompatibility. | Alternate use of 3D printing and electrospinning | CN109172036A [167] |
| Method for Preparing Tendon Scaffold Using 3D-printing and Electrospinning Techniques | Developing a tendon scaffold integrating 3D printing and electrospinning to mimic the natural tendon structure with enhanced mechanical properties and biocompatibility. | Mechanical testing showed suitable tensile strength; in vitro assays demonstrated tendon cell adhesion and proliferation. | Decorating/infusing 3D-printed scaffolds with electrospun nanofiber segments | CN108404213A [168] |
| Method for promoting biological printing myocardial tissue morphological induction by utilizing three-dimensional nanofiber constraint and application of method | Developing a method that enables in situ morphological induction of myocardial tissue during bioprinting by using a 3D aligned nanofiber scaffold that offers topographical cues. | Scaffold promoted cell viability and aligned growth along the fiber orientation. Also, co-printing with endothelial cells enabled the formation of a vascularized engineered myocardial tissue. | 3D-bioprinting onto electrospun fibers. | CN118048298A [169] |
| Method for Preparing Conductive Biological Scaffolds by Melt Jet 3D Printing Driven by Self-Excited Electrostatic Field | A novel hybrid fabrication technique combining melt-jet 3D printing with electrohydrodynamic principles (akin to electrospinning), driven by a self-excited electrostatic field to fabricate a scaffold for TE applications. | High-resolution scaffold structures with controlled porosity and microscale features were achieved | Platforms combining 3D-printing and electrospinning techniques | CN112157906A [170] |
| Systems and methods for optimized patient specific tissue engineering vascular grafts | Fabrication of patient-specific, multi-scale scaffolds by combining 3D printing and electrospinning. The system allows precise placement of nanofiber mats onto anatomical 3D-printed forms, offering great potential for customized biomedical devices | Patient-specific polymeric vascular grafts were created and it was shown that nanofibers can enhance surface area, mimic ECM, or provide bioactivity. | Fabrication of Electrospun Scaffolds on 3D-Printed Collectors/Templates | US11541149B2 [171] |
| Micro-nano composite dual-layer skin ramework and manufacturing method thereof | A composite bilayer scaffold for skin tissue, integrating micro and nanolayers to support skin tissue regeneration with enhanced structural mimicry. | The nanofibrous scaffold provide suitable surface morphology for cell adhesion and growth, thereby facilitating cell adhesion and growth; Scaffold provides a microenvironment for cell growth cells, and facilitates cell infiltration. | Alternate use of 3D printing and electrospinning | CN106110401A [172] |
| Preparation Method Of Multilayer Composite Nano-Micron Fiber Topological Morphology Support With Imitated ECM Structure | Development of a multilayer composite scaffold mimicking ECM structure using 3D printing, electrospinning, or electrostatic spraying, enhancing porosity, fiber diameter control, and tissue support. | SEM and optical images show fiber morphology and layered structures. Fiber diameter (200 nm–3.83 μm), layer thickness (0.4–0.57 mm), pore size (~80 μm), contact angle (114.77°–117.45°) reported. | Alternate use of 3D printing and electrospinning | CN112121232A [173] |
| Artificial blood vessel and the manufacturing method thereof | Artificial blood vessels produced by combining 3D printing and electrospinning in a unified system to combine biocompatibility and mechanical strenght. | An artificial blood vessel was fabricated by combining electrospinning and 3D-printing in a single system. It consists of an inner electrospun microfiber layer, a middle support 3D-printed layer to enhance mechanical strength, and an outer layer of electrospun nanofibers. | Alternate use of 3D printing and electrospinning | KR102467263B1 [174] |
| Preparation of coaxial electrospun-containing multilayered cartilage complex by electrospinning 3D printing | Design of hybrid scaffolds for ligament repair combining 3D printing for structural support with electrospinning for enhanced ligament mimicry and elasticity. | In vitro assays indicated a strong biological effect, shown as an enhanced cell proliferation, and increased collagen production. | Alternate use of 3D printing and electrospinning | CN109701079A [175] |
| Bionic bone tissue engineering scaffold material and preparation method thereof | Multi-layered bone scaffold fabricated via 3D printing, surface biofunctionalization, and bioactive gel infusion with antimicrobial peptides. | Mechanical testing indicated compression strengths comparable to human cortical bone; antibaterial assays showed inhibition rates up to 99%; graudal release of BMP-2 and antimicrobial peptides. | Decorating./infusing 3D-printed scaffolds with electrospun nanofiber segments | CN115054728A [176] |
| Bio-Tubular Scaffold for Fabricating Artificial Vascular and the Fabricating Method Thereof | A method for creating a tubular scaffold for artificial blood vessels thet uses electrospinning for the inner layer and 3D printing for the outer layer to enhance mechanical strength and biocompatibility. | The final product is an scaffold with suitable mechanical strength for vascular applictions. Water uptake, contact angle and chemical characerization are reported. | Decorating/infusing 3D-printed scaffolds with electrospun nanofiber segments | KR101751986B1 [177] |
| Artificial Esophagus Scaffold and Manufacturing Method Thereof | A method for fabricating an artificial esophageal scaffold combining electrospinning and 3D printing to mimic the ECM, providing flexibility and mechanical integrity. | Mechanical testing demonstrated suitable properties for esophageal support; in vitro and in vivo tests showed cell adhesion, with animal studies indicating successful integration. | Alternate use of 3D printing and electrospinning | KR102347096B1 [178] |
| Regeneration bone scaffold forming system and method based on comprehensive 3D printing formation | System and method for fabricating regenerative bone scaffolds using 3D printing, electrospinning, and freeze-drying to enhance porosity and stability for bone regeneration. | This scaffold system enables multi-material integration in a continuous proces to allow patient-specific cutomization and support cell proliferation | Platforms combining 3D-printing and electrospinning techniques | CN103341989A [179] |
| Composite biological 3D printing device and printing method thereof | A method for preparing a biocompatible scaffold for TE made of a 3D-printed PCL base, modified with gelatin nanofibers fabricated by electrospinning. | In vitro assays showed enhanced cell adhesion and good cytocompatibility. | Electrospinning onto 3D bio-printed scaffolds | CN106827496A [180] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juarez-Navarro, K.J.; Guarino, V.; Alvarez-Perez, M.A. Converging Electrospinning and 3D-Printing Technologies: From Innovative Design for Tissue Engineering to Global Patent Trends and Technology Transfer. Fibers 2025, 13, 83. https://doi.org/10.3390/fib13060083
Juarez-Navarro KJ, Guarino V, Alvarez-Perez MA. Converging Electrospinning and 3D-Printing Technologies: From Innovative Design for Tissue Engineering to Global Patent Trends and Technology Transfer. Fibers. 2025; 13(6):83. https://doi.org/10.3390/fib13060083
Chicago/Turabian StyleJuarez-Navarro, Karen J., Vincenzo Guarino, and Marco A. Alvarez-Perez. 2025. "Converging Electrospinning and 3D-Printing Technologies: From Innovative Design for Tissue Engineering to Global Patent Trends and Technology Transfer" Fibers 13, no. 6: 83. https://doi.org/10.3390/fib13060083
APA StyleJuarez-Navarro, K. J., Guarino, V., & Alvarez-Perez, M. A. (2025). Converging Electrospinning and 3D-Printing Technologies: From Innovative Design for Tissue Engineering to Global Patent Trends and Technology Transfer. Fibers, 13(6), 83. https://doi.org/10.3390/fib13060083







