Cotton Fiber Micronaire and Relations to Fiber HVI and AFIS Qualities Between Deltapine® and PhytoGen Upland Varieties
Abstract
Highlights
- Fiber MIC [EE1] development enhanced some conventional fiber qualities but also increased certain undesirable qualities.
- The MIC values were positively and significantly correlated with the algorithmic MIR values of the ATR FT-IR spectra.
- MIC impacts the market price of cotton fiber and influences fiber quality characteristics; therefore, assessing fiber MIC, even before the application of harvesting aids, is essential for ensuring premium fiber quality, [EE2].
- Due to its ability to sample as little as 0.5 mg of fiber directly without needing to first remove the cotton seeds, the ATR FT-IR method, when used in combination with the MIR approach, possesses the potential for the rapid, non-destructive, and indirect estimation of fiber MIC at any stage in remote or breeding locations.
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Cotton Samples
2.2. Seed Cotton Ginning
2.3. Fiber Quality Measurement
2.4. Fiber ATR FT-IR Spectral Collection and Analysis
2.5. Data Analysis
3. Results and Discussion
3.1. HVI and AFIS Qualities, Lint Turnout, and ATR FT-IR Characteristics Variability Between Deltapine® and PhytoGen Cotton Fibers
3.2. Correlations of HVI MIC vs. HVI and AFIS Qualities, Lint Turnout, and ATR FT-IR Characteristics Between Two Cotton Varieties
3.3. Correlations of HVI Qualities vs. Relevant AFIS Qualities Between Two Cotton Varieties
3.4. Implication of Fiber HVI MIC Assessment at Early Stages
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFIS | advanced fiber information system |
| Area% | percentage area |
| ATR FT-IR | attenuated total reflection Fourier transform infrared |
| +b | yellowness |
| CIIR | infrared crystallinity |
| ELO | elongation |
| HVI | high volume instrument |
| IFC | immature fiber content |
| MIC | micronaire |
| MIR | infrared maturity |
| MR | maturity ratio |
| NIR | near infrared |
| Rd | reflectance |
| SCW | secondary cell wall |
| SFC(w) | short fiber content by weight |
| SFC(n) | short fiber content by number |
| SFI | short fiber index |
| STR | strength |
| UHML | upper-half mean length |
| UI | uniformity index |
| UQL(w) | upper quartile length by weight |
References
- Robertson, W.; Roberts, B. Integrated crop management for cotton production in the 21st century. In Cotton: Technology for the 21st Century; First International Cotton Advisory Committee: Washington, DC, USA, 2010; pp. 63–98. [Google Scholar]
- Bradow, J.M.; Davidonis, G.H. Quantitation of fiber quality and the cotton production-processing interface: A physiologist’s perspective. J. Cotton Sci. 2000, 4, 34–64. [Google Scholar]
- Brecke, B.J.; Banks, J.C.; Cothren, J.T. Harvest-aid treatments: Products and application timing. In Cotton Harvest Management: Use and Influence of Harvest Aids; Supak, J.R., Snipes, C.E., Eds.; The Cotton Foundation: Memphis, TN, USA, 2001; pp. 119–142. [Google Scholar]
- Delhom, C.D.; Knowlton, J.; Martin, V.B.; Blake, C. The classification of cotton. J. Cotton Sci. 2020, 24, 189–196. [Google Scholar]
- Delhom, C.D.; Kelly, B.; Martin, V. Physical properties of cotton fiber and their measurement. In Cotton Fiber: Physics, Chemistry and Biology; Fang, D.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 41–73. [Google Scholar]
- Kelly, B.; Abidi, N.; Ethridge, D.; Hequet, E.F. Fiber to fabric. In Cotton, 2nd ed.; Fang, D.D., Percy, R.G., Eds.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 2015; pp. 665–744. [Google Scholar]
- Kelly, C.M.; Hequet, E.F.; Dever, J.K. Interpretation of AFIS and HVI fiber property measurements in breeding for cotton fiber quality improvement. J. Cotton Sci. 2012, 16, 1–16. [Google Scholar]
- Paudel, D.; Hequet, E.; Abidi, N. Evaluation of cotton fiber maturity measurements. Ind. Crop Prod. 2013, 45, 435–441. [Google Scholar]
- Lord, E. Air flow through plugs of textile fibers part II. The micronaire test for cotton. J. Text. Inst. 1956, 47, T16–T47. [Google Scholar]
- Montalvo, J.G. Relationships between micronaire, fineness and maturity. Part I. Fundamentals. J. Cotton Sci. 2005, 9, 81–88. [Google Scholar]
- Gordon, S.; Rodgers, J. Cotton fiber cross-section properties. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 65–86. [Google Scholar]
- Hequet, E.; Wyatt, B.; Abidi, N.; Thibodeaux, D.P. Creation of a set of reference material for cotton fiber maturity measurements. Text. Res. J. 2006, 76, 576–586. [Google Scholar]
- Liu, Y.; Thibodeaux, D.; Gamble, G. Development of FTIR spectroscopy in direct, non-destructive, and rapid determination of cotton Fiber maturity. Text. Res. J. 2011, 81, 1559–1567. [Google Scholar]
- Liu, Y.; Thibodeaux, D.; Gamble, G.; Bauer, P.; VanDerveer, D. Comparative investigation of Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD) in the determination of cotton fiber crystallinity. Appl. Spectrosc. 2012, 66, 983–986. [Google Scholar]
- Rodgers, J.; Montalvo, J.; Davidonis, G.; VonHoven, T. Near infrared measurement of cotton fiber micronaire, maturity and finesses—A comparative investigation. Text. Res. J. 2010, 80, 780–793. [Google Scholar]
- Rodgers, J.; Zumb, J.; Fortier, C. Measurement comparison of cotton fiber micronaire and its components by portable near infrared spectroscopy instruments. Text. Res. J. 2017, 87, 57–69. [Google Scholar] [CrossRef]
- Zumba, J.; Rodgers, J.; Indest, M. Fiber micronaire, fineness, and maturity predictions using NIR spectroscopy instruments on seed cotton and cotton fiber, in and outside the laboratory. J. Cotton Sci. 2017, 21, 247–258. [Google Scholar] [CrossRef]
- Long, R.L.; Bange, M.P. Measuring the maturity of unopened cotton bolls with near infrared spectroscopy. J. Near Infrared Spectrosc. 2020, 28, 204–213. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, M.; Zhao, Z.; Qiu, X.; Zhang, R. Optical properties and Monte Carlo simulation of cotton fibers with different micronaire. Ind. Crop Prod. 2025, 223, 120254. [Google Scholar] [CrossRef]
- Burns, A.; Ciurczak, E.W. (Eds.) Handbook of Near-Infrared Analysis; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef]
- Wang, H.; Cao, H.; Yang, L. Machine learning-driven multidomain nanomaterial design: From bibliometric analysis to applications. ACS Appl. Nano Mater. 2024, 7, 26579–26600. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Leng, D.; Feng, S.; Yang, Y.; Du, Y. Machine learning applications in nanomaterials: Recent advances and future perspectives. Chem. Eng. J. 2024, 500, 15668. [Google Scholar] [CrossRef]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2018, 88, 719–730. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fibers. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- He, Z.; Nam, S.; Fang, D.D.; Cheng, H.N.; He, J. Surface and thermal characterization of cotton fibers of phenotypes differing in fiber length. Polymers 2021, 13, 994. [Google Scholar] [CrossRef]
- Kim, H.-J.; Liu, Y.; Zeng, L. Fourier transform infrared (FT-IR) spectroscopy and simple algorithm analysis for rapid and non-destructive assessment of cotton fiber maturity and crystallinity for plant mapping. Sensors 2024, 24, 2888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Delhom, C.D. Investigation of fiber maturity and crystallinity information in Upland seed cottons by Fourier transform infrared spectroscopy. Text. Res. J. 2023, 93, 2507–2519. [Google Scholar]
- Liu, Y.; Delhom, C.D. Investigation of Fourier transform infrared spectroscopy potential in cotton fiber micronaire measurement and distribution. Text. Res. J. 2024, 94, 2178–2188. [Google Scholar] [CrossRef]
- Santiago, C.M.; Hinchliffe, D.J. FT-IR examination of the development of secondary cell wall in cotton fibers. Fibers 2015, 3, 30–40. [Google Scholar] [CrossRef]
- Averett, L.A.; Griffiths, P.A.; Nishikida, K. Effective path length in attenuated total reflection spectroscopy. Anal. Chem. 2008, 80, 3045–3049. [Google Scholar]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 27–54. [Google Scholar]
- Van der Sluijs, M.H.J. Cotton appearance. In Cotton Fibers: Characteristics, Uses and Performance; Gordon, S., Abidi, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 135–158. [Google Scholar]
- Santiago, C.M.; Von Hoven, T.; Hinchliffe, D.J.; Hron, R. Examination of cotton maturity and maturity distribution using infrared focal plane array imaging system. AATCC J. Res. 2021, 8, 14–24. [Google Scholar]
- Mathangadeera, R.W.; Hequet, E.F.; Kelly, B.; Dever, J.K.; Kelly, C.M. Importance of cotton fiber elongation in fiber processing. Ind. Crop Prod. 2020, 147, 112217. [Google Scholar]
- Liu, Y. Cotton fiber strength measurement and its relation to structural properties from Fourier transform infrared spectroscopic characterization. Textiles 2024, 4, 126–137. [Google Scholar] [CrossRef]



| Fiber Properties | Deltapine® Varieties | PhytoGen Varieties | |||
|---|---|---|---|---|---|
| Range | Mean ± SD (CV, %) | Range | Mean ± SD (CV, %) | ||
| HVI | MIC | 2.52–5.45 | 4.12 ± 1.30 (31.6) | 3.44–4.66 | 4.06 ± 0.42 (10.3) |
| UHML, in | 1.16–1.25 | 1.22 ± 0.04 (3.3) | 1.13–1.26 | 1.19 ± 0.05 (4.2) | |
| UI, % | 81.5–85.8 | 83.45 ± 1.77 (2.1) | 82.0–85.0 | 83.62 ± 0.87 (1.0) | |
| STR, g/tex | 28.22–32.88 | 30.63 ± 2.03 (6.6) b | 31.28–33.94 | 32.83 ± 0.90 (2.7) a | |
| ELO, % | 5.80–6.10 | 5.95 ± 0.13 (2.2) a | 4.60–5.70 | 5.28 ± 0.33 (6.3) b | |
| Rd, % | 70.2–76.6 | 73.4 ± 2.7 (3.7) | 64.1–78.5 | 74.3 ± 4.1 (5.5) | |
| +b | 5.90–9.10 | 7.89 ± 1.42 (18.0) | 5.90–9.40 | 7.89 ± 1.05 (13.3) | |
| SFI, % | 4.70–7.90 | 6.28 ± 1.31 (20.9) | 4.00–7.20 | 6.05 ± 0.92 (15.2) | |
| AFIS | Neps, gm | 103–539 | 270 ± 217 (80.4) | 115–365 | 217 ± 70 (35.0) |
| L(w), in | 1.04–1.10 | 1.07 ± 0.02 (1.9) | 1.00–1.16 | 1.06 ± 0.05 (4.7) | |
| UQL(w)), in | 1.25–1.32 | 1.29 ± 0.03 (2.3) | 1.22–1.38 | 1.28 ± 0.06 (4.7) | |
| SFC(w), % | 4.63–9.57 | 6.91 ± 2.50 (36.2) | 4.70–8.90 | 6.83 ± 1.36 (19.9) | |
| L(n), in | 0.80–0.93 | 0.86 ± 0.06 (7.0) | 0.78–0.96 | 0.85 ± 0.05 (5.9) | |
| L5%(n), in | 1.40–1.49 | 1.46 ± 0.04 (2.7) | 1.35–1.57 | 1.43 ± 0.07 (4.9) | |
| SFC(n), % | 17.27–28.57 | 23.18 ± 5.90 (25.5) | 18.30–28.47 | 23.33 ± 3.34 (14.3) | |
| Fineness, mTex | 131.0–185.0 | 157.0 ± 23.8 (15.2) | 149.0–174.0 | 159.1 ± 8.6 (5.4) | |
| IFC, % | 5.27–9.33 | 7.46 ± 1.77 (23.7) | 5.67–7.57 | 6.79 ± 0.56 (8.2) | |
| MR | 0.79–0.94 | 0.86 ± 0.06 (7.0) | 0.85–0.91 | 0.88 ± 0.02 (2.3) | |
| Lint turnout, % | 41.3–44.1 | 42.5 ± 1.3 (3.1) | 38.5–44.9 | 41.4 ± 2.2 (5.4) | |
| FT-IR | MIR | 0.58–0.93 | 0.80 ± 0.15 (18.8) | 0.72–0.90 | 0.81 ± 0.06 (7.4) |
| CIIR | 63.5–100.0 | 82.1 ± 15.2 (18.5) | 73.4–90.8 | 81.3 ± 5.4 (6.6) | |
| MIC vs. HVI Quality | Adjusted Slope | R2 | ANCOVA | |
|---|---|---|---|---|
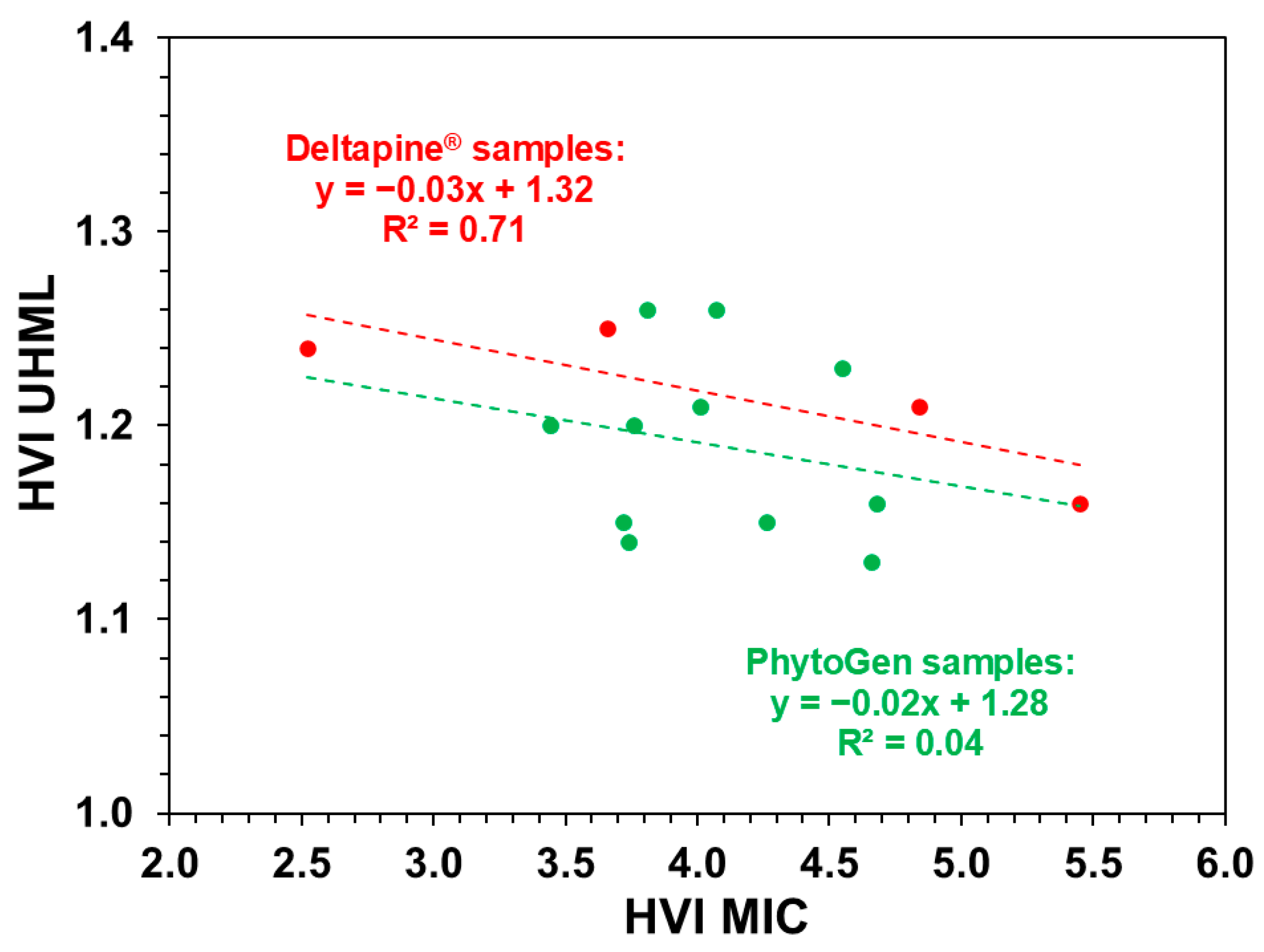
| MIC vs. UHML | Deltapine® fibers | −0.01 | 0.71 | 0.92 (Slope) |
| PhytoGen fibers | −0.01 | 0.04 | 0.32 (Intercept) | |
| MIC vs. UI | Deltapine® fibers | 0.02 | 0.43 | 0.81 (Slope) |
| PhytoGen fibers | 0.02 | 0.29 | 0.70 (Intercept) | |
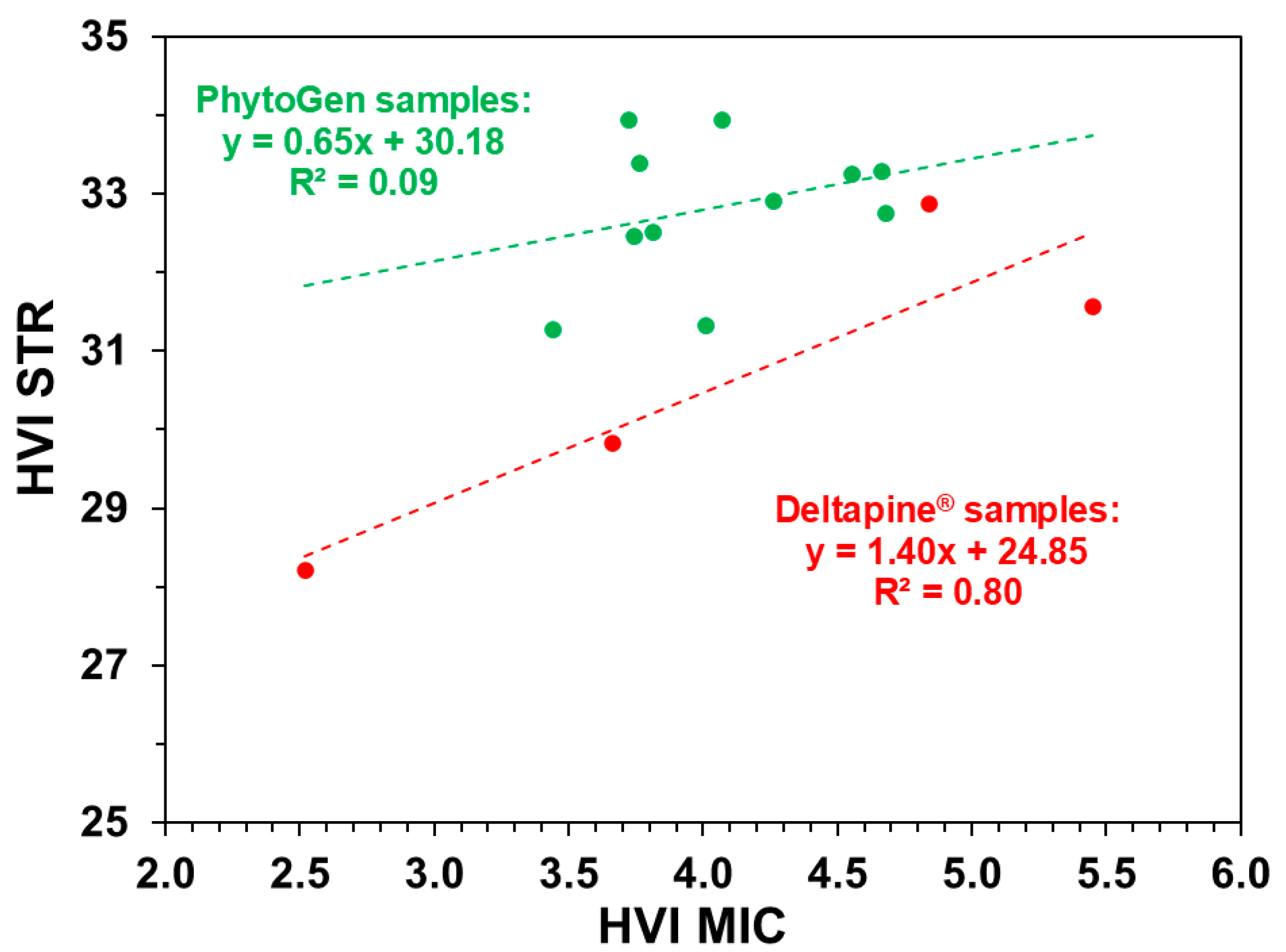
| MIC vs. STR | Deltapine® fibers | 0.08 | 0.8 | 0.38 (Slope) |
| PhytoGen fibers | 0.04 | 0.09 | <0.01 (Intercept) | |
| MIC vs. ELO | Deltapine® fibers | −0.01 | 0.57 | 0.76 (Slope) |
| PhytoGen fibers | 0 | 0.01 | <0.01 (Intercept) | |
| MIC vs. Rd | Deltapine® fibers | −0.02 | 0.15 | 0.29 (Slope) |
| PhytoGen fibers | −0.11 | 0.21 | 0.78 (Intercept) | |
| MIC vs. +b | Deltapine® fibers | 0.17 | 0.84 | 1.00 (Slope) |
| PhytoGen fibers | 0.17 | 0.16 | 0.92 (Intercept) | |
| MIC vs. SFI | Deltapine® fibers | −0.13 | 0.46 | 0.71 (Slope) |
| PhytoGen fibers | −0.07 | 0.02 | 0.66 (Intercept) | |
| HVI MIC vs. AFIS Quality | Adjusted Slope | R2 | ANCOVA | |
|---|---|---|---|---|
| MIC vs. neps | Deltapine® fibers | −1.11 | 0.97 | 0.56 (Slope) |
| PhytoGen fibers | −1.15 | 0.59 | 0.04 (Intercept) | |
| MIC vs. L(w) | Deltapine® fibers | 0.01 | 0.53 | 0.92 (Slope) |
| PhytoGen fibers | 0.01 | 0.01 | 0.70 (Intercept) | |
| MIC vs. UQL(w) | Deltapine® fibers | −0.01 | 0.93 | 0.77 (Slope) |
| PhytoGen fibers | −0.01 | 0.06 | 0.59 (Intercept) | |
| MIC vs. SFC(w) | Deltapine® fibers | −0.33 | 0.91 | 1.00 (Slope) |
| PhytoGen fibers | −0.35 | 0.35 | 0.78 (Intercept) | |
| MIC vs. L(n) | Deltapine® fibers | 0.02 | 0.75 | 1.00 (Slope) |
| PhytoGen fibers | 0.02 | 0.11 | 0.84 (Intercept) | |
| MIC vs. L5%(n) | Deltapine® fibers | −0.01 | 0.8 | 0.75 (Slope) |
| PhytoGen fibers | −0.02 | 0.08 | 0.37 (Intercept) | |
| MIC vs. SFC(n) | Deltapine® fibers | −0.3 | 0.46 | 0.92 (Slope) |
| PhytoGen fibers | −0.32 | 0.02 | 1.00 (Intercept) | |
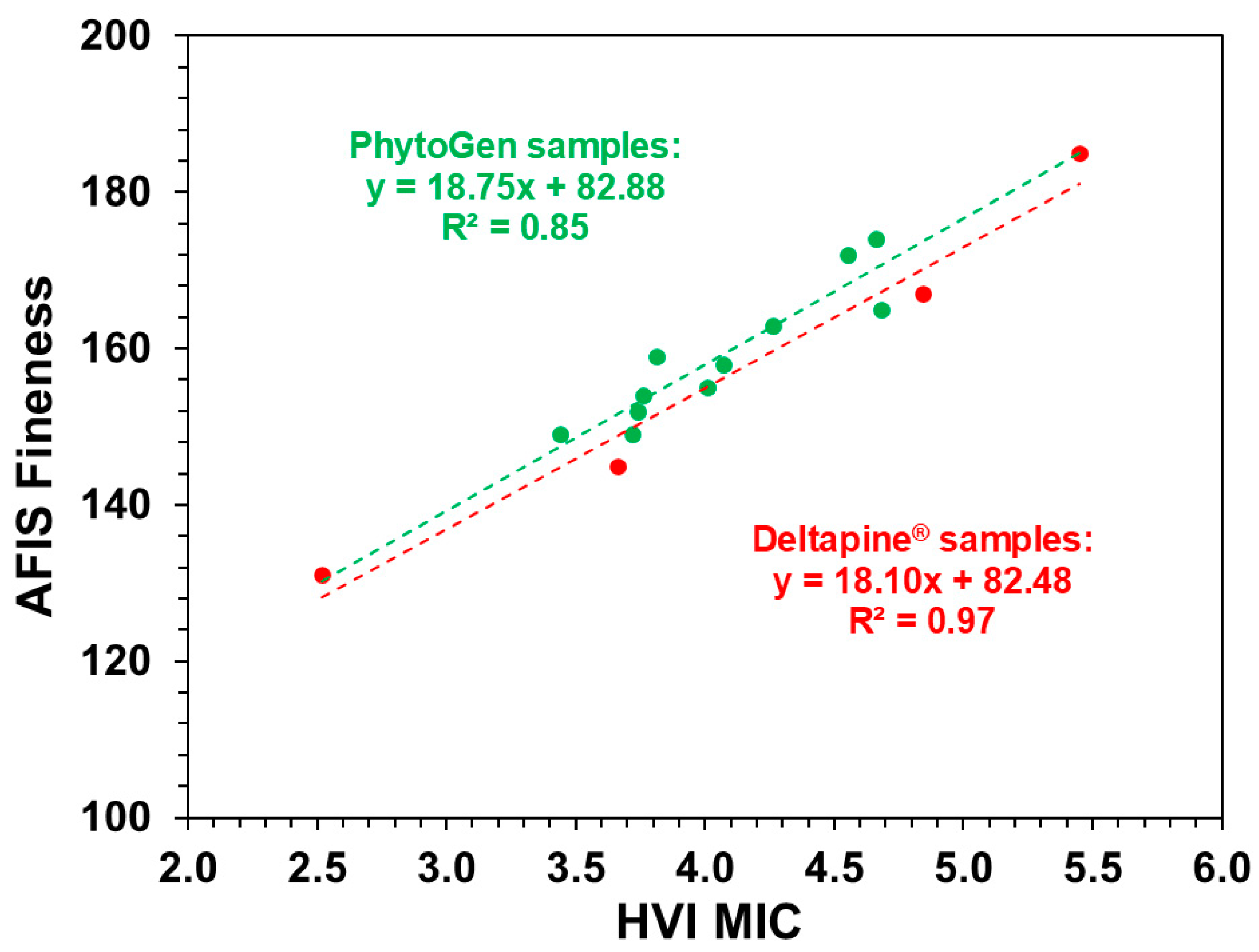
| MIC vs. fineness | Deltapine® fibers | 0.22 | 0.97 | 0.84 (Slope) |
| PhytoGen fibers | 0.23 | 0.85 | 0.17 (Intercept) | |
| MIC vs. IFC | Deltapine® fibers | −1.33 | 0.95 | 0.57 (Slope) |
| PhytoGen fibers | −1.12 | 0.72 | <0.01 (Intercept) | |
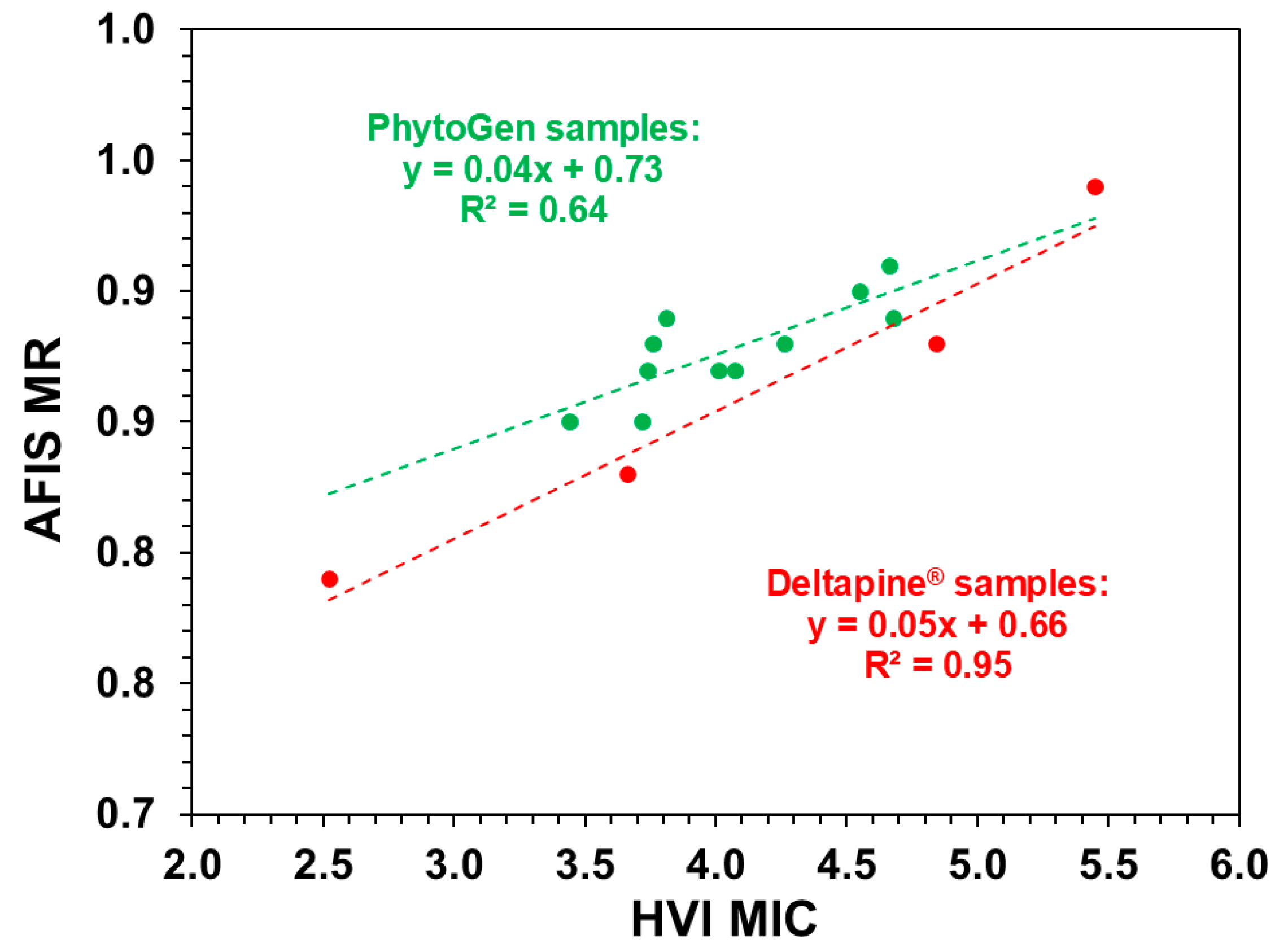
| MIC vs. MR | Deltapine® fibers | 0.02 | 0.95 | 0.28 (Slope) |
| PhytoGen fibers | 0.01 | 0.64 | 0.02 (Intercept) | |
| HVI MIC vs. | Adjusted Slope | R2 | ANCOVA | |
|---|---|---|---|---|
| Lint Turnout and FT-IR Analysis | ||||
| MIC vs. Lint turnout | Deltapine® fibers | −0.03 | 0.04 | 0.66 (Slope) |
| PhytoGen fibers | 0.01 | 0.02 | 0.38 (Intercept) | |
| MIC vs. MIR | Deltapine® fibers | 0.06 | 0.91 | 0.92 (Slope) |
| PhytoGen fibers | 0.05 | 0.75 | 0.63 (Intercept) | |
| MIC vs. CIIR | Deltapine® fibers | 0.26 | 0.89 | 0.84 (Slope) |
| PhytoGen fibers | 0.24 | 0.66 | 0.92 (Intercept) | |
| HVI or AFIS vs. AFIS Qualities | Adjusted Slope | R2 | ANCOVA | |
|---|---|---|---|---|
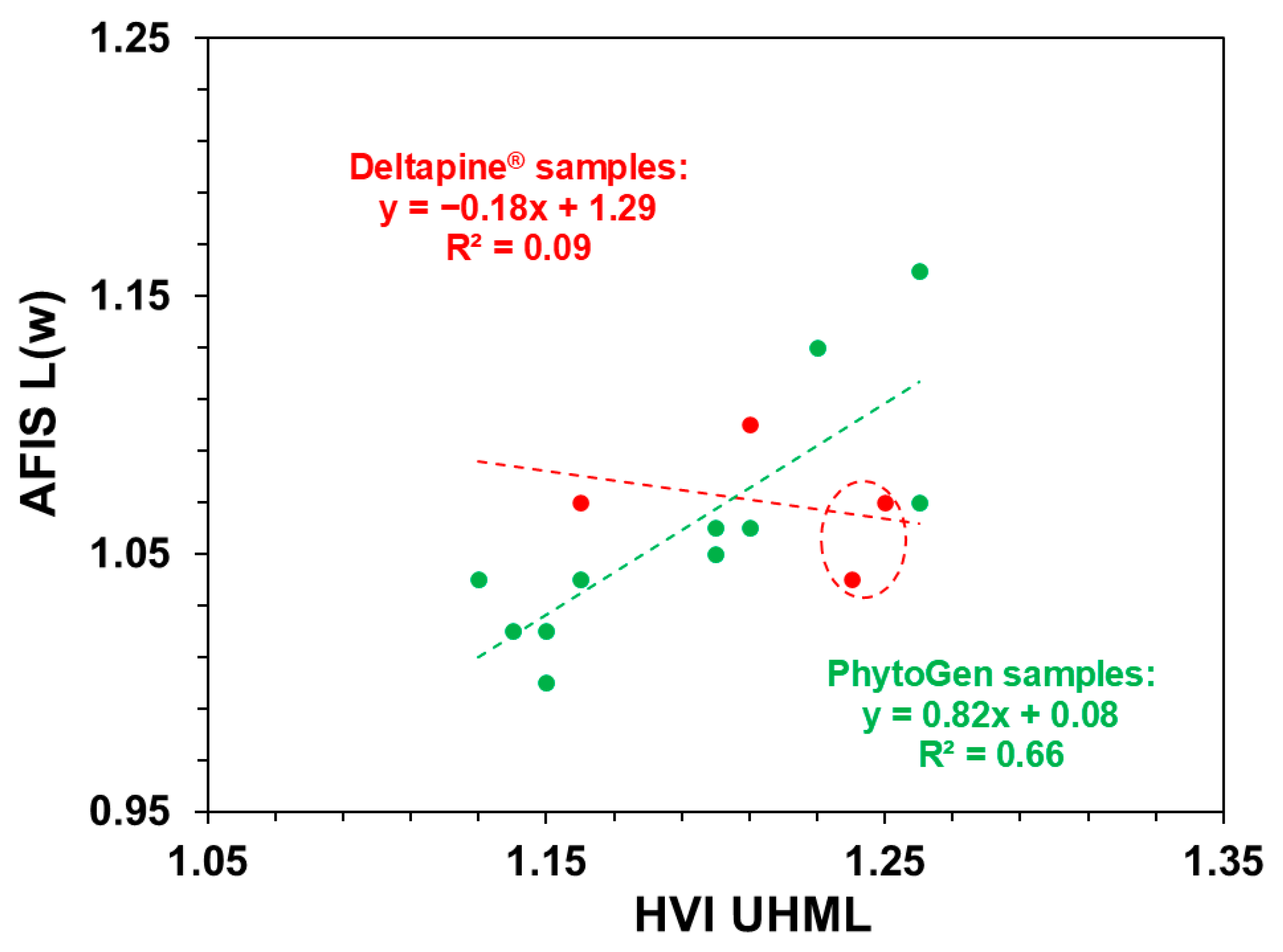
| UHML vs. L(w) | Deltapine® fibers | −0.16 | 0.09 | 0.06 (Slope) |
| PhytoGen fibers | 0.73 | 0.66 | 0.81 (Intercept) | |
| UHML vs. UQL(w) | Deltapine® fibers | 0.61 | 0.99 | 0.21 (Slope) |
| PhytoGen fibers | 0.92 | 0.89 | 0.32 (Intercept) | |
| UHML vs. L(n) | Deltapine® fibers | −0.96 | 0.44 | 0.06 (Slope) |
| PhytoGen fibers | 0.55 | 0.29 | 1.00 (Intercept) | |
| UHML vs. L5%(n) | Deltapine® fibers | 0.78 | 0.99 | 0.46 (Slope) |
| PhytoGen fibers | 1.03 | 0.86 | 0.92 (Intercept) | |
| L(w) vs. L(n) | Deltapine® fibers | 2.25 | 0.77 | 0.07 (Slope) |
| PhytoGen fibers | 0.97 | 0.82 | 0.81 (Intercept) | |
| UQL(w) vs. L5%(n) | Deltapine® fibers | 1.09 | 0.98 | 0.49 (Slope) |
| PhytoGen fibers | 0.97 | 0.97 | 0.09 (Intercept) | |
| SFI vs. SFC(w) | Deltapine® fibers | 0.22 | 0.56 | 0.87 (Slope) |
| PhytoGen fibers | 0.2 | 0.4 | 0.78 (Intercept) | |
| SFI vs. SFC(n) | Deltapine® fibers | 0.22 | 0.52 | 0.89 (Slope) |
| PhytoGen fibers | 0.2 | 0.38 | 0.89 (Intercept) | |
| SFC(w) vs. SFC(n) | Deltapine® fibers | 0.16 | 0.98 | 0.72 (Slope) |
| PhytoGen fibers | 0.16 | 0.97 | 0.41 (Intercept) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Hinchliffe, D.J. Cotton Fiber Micronaire and Relations to Fiber HVI and AFIS Qualities Between Deltapine® and PhytoGen Upland Varieties. Fibers 2025, 13, 41. https://doi.org/10.3390/fib13040041
Liu Y, Hinchliffe DJ. Cotton Fiber Micronaire and Relations to Fiber HVI and AFIS Qualities Between Deltapine® and PhytoGen Upland Varieties. Fibers. 2025; 13(4):41. https://doi.org/10.3390/fib13040041
Chicago/Turabian StyleLiu, Yongliang, and Doug J. Hinchliffe. 2025. "Cotton Fiber Micronaire and Relations to Fiber HVI and AFIS Qualities Between Deltapine® and PhytoGen Upland Varieties" Fibers 13, no. 4: 41. https://doi.org/10.3390/fib13040041
APA StyleLiu, Y., & Hinchliffe, D. J. (2025). Cotton Fiber Micronaire and Relations to Fiber HVI and AFIS Qualities Between Deltapine® and PhytoGen Upland Varieties. Fibers, 13(4), 41. https://doi.org/10.3390/fib13040041







