Abstract
Increasing economic and environmental concerns arising from the extensive exploration and dependence on fossil fuel-based materials have encouraged the search for eco-friendly alternatives. Fibers based on biomass-derived materials have been attracting growing interest. Among other features, the mechanical performance of bio-based fibers needs to be improved to effectively compete with their counterparts and emerge as viable substitutes. This review presents scientific advancements in the development of naturally derived fibers, and strategies for their production with tailored mechanical properties. The potential of natural precursor-based fibers for their conversion into high-performance carbon fibers is also emphasized. Studies reporting the mechanical properties of bio-based fibers developed by wet spinning are identified, analyzed, and discussed. These studies show that cellulose is the most studied material, while Ioncell technology is identified as the most suitable method for producing cellulose-based fibers with the highest tensile strength. Studies have also demonstrated that silk fibroin exhibits tensile strength and elongation at break ranging from 300 to 600 MPa and 30 to 50%. Although several novel processes have been explored, there are still challenges that need to be addressed for bio-based fibers to become feasible options, and to boost their usage across industries.
Keywords:
wet spinning; mechanical properties; biopolymer; bio-based fibers; cellulose; silk fibroin; alginate 1. Introduction and Global Scenario
Natural fibers derived either from plants (cotton, hemp, and flax) or animals (wool and silk) were once predominant in the textile sector. They are mainly known for their comfort and environmentally friendly characteristics [1]. However, the rising costs associated with land usage and growing consumer demand have led to significant scientific and technological advancements in the production of synthetic fibers [2]. In fact, the global market size for synthetic fibers was valued at USD 66.11 billion in 2022, with projections indicating a compound annual growth rate (CAGR) of 7.4% from 2023 to 2030 [3]. While synthetic fibers possess excellent chemical, mechanical, and physical properties, their lower sustainability poses a serious threat to the environment as they rely on fossil fuel sources [1].
Bio-based synthetic fibers boast a low carbon footprint during synthesis, since their raw materials are sourced from biomass. Furthermore, many of them, in particular regenerated fibers (cellulose, protein, and chitosan), exhibit exceptional biodegradability, allowing for their natural decomposition, or with the aid of some microorganisms, under various conditions, including composting [4]. Biopolymers, often referred to as the “building blocks of nature”, are biodegradable materials composed by repeating units, such as nucleotides, amino acids, or monosaccharides [5,6]. Derived from a variety of living organisms, these organic compounds possess significant potential for producing environmentally friendly bio-based products [7].
Despite the different manufacturing technologies available, wet spinning, shown in Figure 1a, has garnered significant attention for designing and developing bio-based fibers. This process typically comprises four main stages: (1) dissolution, (2) extrusion, (3) coagulation, and (4) collection.
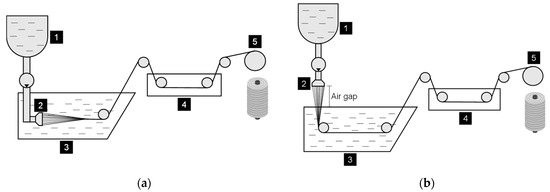
Figure 1.
General schematic representation of both (a) wet spinning and (b) dry-jet wet spinning processes: (1) spinning dope container, (2) spinneret/nozzle, (3) coagulation bath, (4) washing/stretching bath, and (5) rotating spool.
A spinning dope, consisting of a polymer dissolved in a suitable solvent, is extruded directly into a coagulation bath containing a “poor solvent”, a “non-solvent”, or a mixture of both via a spinneret [8]. Upon contact with the bath, the polymer within the filament undergoes coagulation and solidification, facilitated by the solvent/non-solvent exchange-induced phase inversion. Continuous fibers are produced and collected on a rotating spool, after passing through a sequence of rinsing and stabilizing baths, as well as heated rollers (drying drawing rollers) [8]. The dry-jet wet spinning, shown in Figure 1b, is a variation of the wet spinning process, which involves the extrusion of the spinning solution through an air gap before its immersion in the coagulation bath. The air gap promotes polymer stretching and orientation of the polymeric chain upon entry into the coagulation bath [9], which strongly influences the morphology and mechanical properties of the final fibers.
Moreover, other key parameters play a crucial role in both the thermodynamic and kinetic properties of the polymer coagulation, and ultimately, the fiber properties. These encompass polymer concentration and molecular weight distribution, the solvent/non-solvent system, the additives present in both the spinning dope and coagulation bath, processing temperature, extrusion rate, spinneret/nozzle diameter, and winding speed [8]. In fact, coagulation is a diffusion-controlled phase separation process responsible for filament formation, in which the solidification rate and final filament morphology are dictated by the exchange dynamics between the solvent and non-solvent. In addition, the coagulation bath temperature is a pivotal factor in regulating the mutual diffusion between the solvent and coagulant. While elevated processing temperatures tend to accelerate coagulation, leading to the formation of micropores on the filament surface, lower processing temperatures result in a slower mutual diffusion, yielding softer and less compact filament structures [8]. Furthermore, wet spinning enables drawing or applying tension to the fibers either upon entry into the bath, during rinsing or drying, or at a later stage in the spinning line through post-drawing [8]. This last step improves the mechanical performance by refining the molecular alignment and orientation of the fibers.
An underlying objective of this review was to spotlight potential natural-based precursors having adequate properties for developing precursor-based fibers and their conversion into carbon fibers (CFs). CFs are required for highly demanding applications, since they exhibit extraordinary in-plan mechanical, electrical, and thermal properties [10]. CFs have a carbon content higher than 90%, and currently they are predominantly manufactured from petroleum/coal-derived precursor fibers [11,12]. However, the main drawback of CFs is the high cost of their precursors, contributing more than 50% of the global costs in CF manufacturing [11]. On the other hand, the unprecedented demand for large-volume, low-cost, and sustainable materials has spurred the search for novel approaches to obtain suitable eco-friendly precursors for CFs [11]. In this context, based on the findings and insights compiled in this review, the potential candidates for CF precursors will be presented.
This review aims to provide an overview of the literature regarding the latest advancements in the development of sustainable and naturally derived wet spun fibers, production technologies either by wet spinning or dry-jet wet spinning techniques, and structure–property relationships with special emphasis on tailored mechanical properties. Specific criteria were refined to address the question: “Which bio-based fibers, produced through wet spinning technology, exhibit better mechanical properties?” Even though certain biopolymers are known for producing mostly fibers with poor mechanical performance, no specific constraints were imposed. The focus was on identifying and discussing potential effects of spinning strategies and parameters on the developed morphology and mechanical properties of the fibers within each polymer.
Despite the increasing abundance of raw-material sources, the on-going optimization of the production processes, and continuous improvements in the performance of bio-based fibers, it remains crucial to address several key challenges and shortcomings. These include the following: (i) ensuring the scalability of production to meet global demand, (ii) improving cost-competitiveness with conventional counterparts, and (iii) overcoming technical limitations related to specific application requirements. This review highlights that the transition towards bio-based synthetic fibers requires continued efforts to enhance the environmental benefits by further reducing energy consumption and waste generation in the production processes, as well as by developing efficient recycling methodologies for end-of-life fibers, both pre- and post-consumer.
2. Methods
2.1. Protocol
This systematic review was conducted in accordance with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 [13].
2.2. Eligibility Criteria
Inclusion criteria for this systematic literature review were as follows: (a) studies reporting the production of fibers either through wet spinning or dry-jet wet spinning processes using bio-based polymers as raw materials (or their combination with other natural or synthetic polymers). For those cases, the percentage of biomaterial in the dope/spinning solution was set at 50 wt.% as minimum; (b) studies presenting legible experimental data on the mechanical properties (at least two of the following properties: tensile strength (s), elongation at break (eb), and/or Young’s modulus (E)); (c) English language studies; and (d) those published as full manuscripts in peer-reviewed journals.
The exclusion criteria were as follows: (a) studies not involving natural raw materials (or when combined with synthetic polymers at concentrations of the biomaterial lower than 50 wt.%); (b) research not specifically focused on either wet spinning or dry-jet wet spinning (e.g., coaxial wet spinning or combination of wet spinning with other techniques, including electrospinning and centrifugal spinning); (c) studies lacking empirical data or not presenting clear methodological descriptions (e.g., polymer concentration(s), polymer/additive ratio(s), spinning parameters, mechanical properties, among others); and (d) review articles or book chapters. In addition, studies in which a full-text version was not available (e.g., conference abstracts), as well as those outside the scope of this literature review, including studies focused on scaffolds and/or meshes, were also excluded.
2.3. Research Strategy
The literature research was performed from database inception to November 20th, 2023, in three electronic databases: (a) Web of Science, (b) Scopus, and (c) PubMed. Manuscripts not indexed in these databases or published after 20 November 2023, were not considered. The research terms and keywords were fine-tuned to find the most promising bio-based polymer(s) (or combinations with other natural or synthetic polymers), and experimental spinning conditions to produce wet spun fibers with enhanced mechanical properties. In this contextfibers-12-00075, scientific manuscripts were selected from databases using relevant keywords and their combinations via the following strings: string set 1: (‘fiber’ OR ‘filament’); string set 2: (‘wet spinning’ OR ‘wet spun’); string set 3: (‘mechanical’ OR ‘tensile’ OR ‘strength’ OR ‘Young’s modulus’ OR ‘strain’ OR ‘elongation’); string set 4: (‘natural’ OR ‘biomaterial’ OR ‘biopolymer’ OR ‘biomass’ OR ‘bio-based’ OR ‘renewable’ OR ‘eco-friendly’); string set 5: (‘coagulation’ OR ‘precipitation’ OR ‘regeneration’ OR ‘gelation’). More detailed information is displayed in Table 1. The collected research papers were inspected thoroughly for further analysis.

Table 1.
Database search results up to 20 November 2023.
2.4. Selection Process
The retrieved records were exported to Zotero 6.0.36 software, where duplicates were automatically removed. Two independent researchers (C.P. and T.V.P.) screened records, applying the eligibility criteria to both titles and abstracts. Potentially eligible records were read in full to determine final inclusion and, subsequently, the full texts were assessed. Any disagreements between authors were solved through discussion and, if necessary, by consulting the remaining authors.
2.5. Data Extraction and Analysis
Data were extracted from each article by C.P. and T.V.P., and collected on a Microsoft® 365 Excel® 2019 version 2108 spreadsheet. The extracted data includes the following: (1) raw material(s) and additive(s); (2) dope features (e.g., material(s) concentration(s), solvent(s), material(s)/additive(s) ratio); (3) spinning technique and (4) parameters; (5) mechanical properties (strength, elongation at break, Young’s modulus); and (6) other properties and/or authors’ observations/conclusions. Data not provided were identified as “not reported”. The same team of reviewers analyzed the records for scientific relevance and rigor, assessed the validity of the conclusions drawn by the authors, and identified any limitations or potential errors in the data. Disagreements in the data collection process were solved by either a consensus process or consulting a third author.
3. Results of Data Collection
This systematic literature review was designed to identify promising bio-based raw materials to develop fibers through wet spinning or dry-jet wet spinning with tailored mechanical properties. After applying a set of specific strings in three databases, the electronic search yielded 370 articles (Web of Science = 192, Scopus = 92, and PubMed = 86), as described in Table 1. A total of 64 duplicate records (17%) were removed, and from the remaining 306 records, 125 irrelevant articles were excluded based on title and abstract. Afterwards, from the 181 reports sought for retrieval, 170 full-text articles were screened for eligibility and 71 were removed based on the fourth criterion (i.e., biopolymer ≤ 50.0 wt.%, not wet spinning, incomplete data, or others), leaving 99 articles for inclusion in the review (27%) (Figure 2 and Figure 3a). Notice that 179 records were considered outside of the scope based on two criteria: the second criterion (not natural, not wet spinning, or others), and the fourth criterion. These records account for 48% of the total manuscripts initially considered.
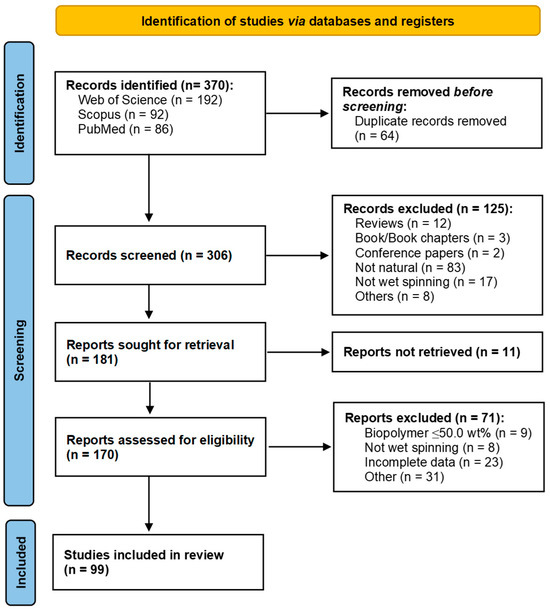
Figure 2.
PRISMA flow chart. Adapted from Ref. [13].
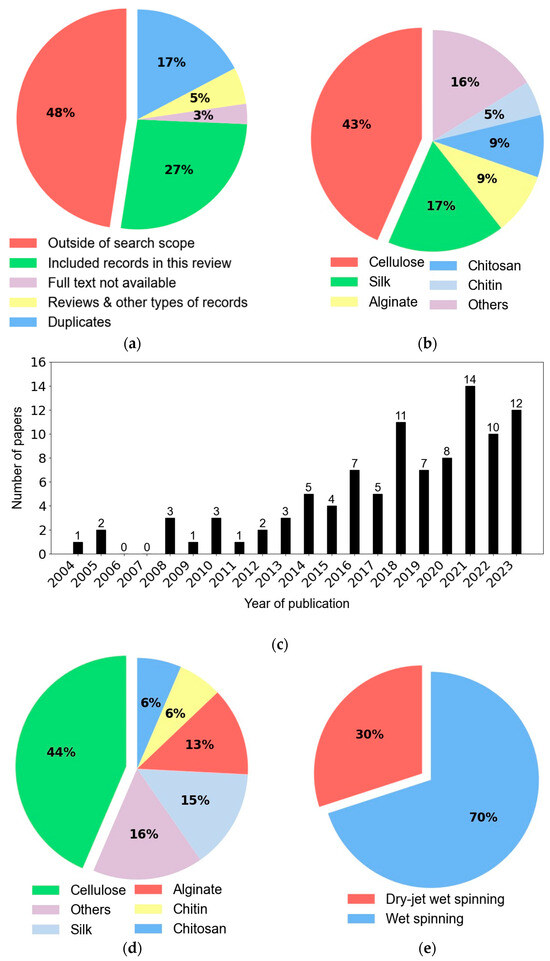
Figure 3.
Graphical illustration of the screening process and selected manuscripts. (a) General overview of the screening process (abstract/title and full text analysis). Graphical illustration of the included records per (b) polymer, (c) year of publication, (d) per polymer between 2018 and 2023, and (e) spinning process.
Excluded records comprised reviews (12; 3%), books chapters (3; 1%), conference papers or proceedings (2; 1%), and manuscripts not written in English (3; 1%), or not having the full text available (11; 3%). Notice that 48% (176) of the studies did not meet the inclusion criteria. The included records (99; 27%) were finally fully analyzed and important information regarding the type of raw materials and additives, dope solution characteristics, spinning process and parameters, mechanical properties, and other relevant information were extracted, and the most promising results are summarized in Table 2. Detailed information is provided in the Supplementary File (Table S1). Among the selected records for inclusion, the majority, dated from 2018 to 2023 (62; 63%), are shown in Figure 3c, and 2021 had the highest number of publications (14/62). In general, cellulose (27; 44%) was the selected raw material for producing bio-based fibers, followed by silk (9; 15%), alginate (8; 13%), chitin (4; 6%), chitosan (4; 6%), and other natural polymers, namely α-1,3-glucan (2; 3%), carrageenan (2; 3%), agar (1; 2%), collagen (1; 2%), gelatin (1; 2%), keratin (1; 2%), lignin (1; 2%), and ulvan (1; 2%) (Figure 3d). Overall, the wet spinning technique was widely preferred for producing bio-based fibers; 70% of the included studies reported using the wet spinning process, while 30% used dry-jet wet spinning (Figure 3e).

Table 2.
A brief summary of studies involving naturally sourced polymers, selected for inclusion.
A brief summary of the main findings for each selected manuscript is presented in Table 2. Table S1 of the Supplementary Materials contains all the studies included in this review.
4. Results Discussion
4.1. Cellulose and Its Derivatives
4.1.1. Sources and Properties
Cellulose (CE) is the most abundant renewable polymer, and has attracted attention owing to its outstanding properties, such as biocompatibility, biodegradability, thermal and chemical stabilities, and recyclability [1,32,33]. CE can be extracted from both wood and non-wood sources. While wood has traditionally been the predominant source of CE for industries, other non-wood resources, including agricultural waste, fibers (e.g., cotton, flax, jute, among others), marine animals, algae, and bacteria, are gaining increased interest [34,35]. In particular, agricultural waste stands out as a significant non-wood source, accounting for over 85% of cellulose, hemicellulose, and lignin content [36]. CE is a linear homopolymer composed by β-D-anhydroglucopyranose monomeric units linked by β-(1→4)-glycosidic bonds, with cellobiose as the repeating stereoregular unit, Figure 4 [32,36,37].
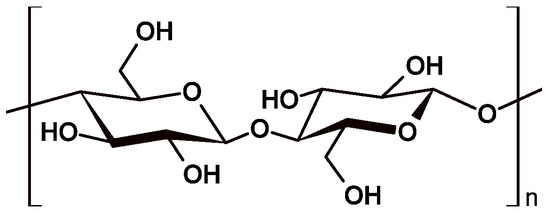
Figure 4.
Schematic representation of cellulose structure composed by β-D-anhydroglucopyranose monomeric units linked by β-(1→4)-glycosidic bonds, with cellobiose as the repeating stereoregular unit.
Due to the strong inter- and intra-molecular hydrogen bonds and significant van der Waals forces, CE molecules are organized into fibrils, possessing an amorphous-crystalline structure that afford the unique properties necessary, for instance, for the construction of plant cells [32,33,36,37]. However, the strong molecular bonds and extensive chain length of CE make its dissolution in water and common organic solvents challenging [1] and, therefore, significant efforts have been made to find suitable solvent systems.
Different forms of CE, including microcrystalline cellulose (MCC), microfibrillated cellulose (MFC), cellulose nanofibers (CNFs), cellulose nanocrystals (CNCs), and bacterial cellulose (BC) have also been investigated as promising sources for designing and developing novel fibers [33]. Among them, MCC obtained from wood pulp and purified cotton linters has been used in the pharmaceutical, food, and cosmetic sectors [38]. There has been increased interest in nanocellulose, such as CNFs, CNCs, and BC, and it has recently emerged as one of the most prominent green materials of modern times, owing to its high crystallinity, and special chemical and physical properties. For instance, CNFs possess high mechanical properties, thermal stability, easy functionalization via chemical reactions, and a low coefficient of thermal expansion (CTE). Correspondingly, it has been regarded as a latent matrix for numerous applications, including drug delivery, energy storage, optoelectronic conversion, and filtration [36]. CNFs are obtained through 2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPO)-mediated oxidation, which is a selective reaction at the C6 hydroxyl groups located on the microfibril surface [39]. CNFs are not soluble, although they are highly dispersible in water. Thus, a CNF suspension in water has a cellulose solution-like viscosity and non-flocculated uniformity, suggesting that these suspensions could be suitable for fiber spinning.
4.1.2. Technologies for the Production of Cellulose-Based Fibers
The growing demand for environmentally friendly or ‘green’ renewable fibers has underscored the interest in regenerated cellulose (rCE) fibers as potential alternatives to their synthetic counterparts. rCE fibers, produced from coagulated wet spun cellulose solutions, retain most of the natural characteristics of cellulose. However, depending on the manufacturing processes and additives incorporated, rCE fibers may exhibit additional properties, such as increased tensile strength or ductility [40,41]. The current applications of rCE fibers are vast, which include textile, medical, agriculture, food, and packaging industries [2,42]. In the textile sector, rCE fibers are used to produce a variety of fabrics, namely rayon, viscose, and lyocell, due to their softness, moisture absorbency, breathability, and ease of dyeing [42]. In the healthcare and medical field, these fibers are applied in drug delivery, wound dressing, and tissue engineering, taking full advantage of their biocompatibility, and ability to be absorbed by the body [2]. In the food industry, rCE fibers are used as thickening and stabilizing agents, while in the packaging industry, they are used in cardboard and biodegradable packaging [43].
Viscose and Cuprammonium Technologies
Viscose technology is the most widely used worldwide for producing rCE fibers, also known as viscose rayon fibers. It involves the dissolution of CE (e.g., wood pulp) in concentrate NaOH solution and CS2, and then, its regeneration in an acid/salt bath (e.g., sulfuric acid and salts as sodium and zinc sulphate) [1,9,44,45]. Developed in 1891, the viscose process is complex, time-consuming, and energy-demanding, generating a significant amount of toxic and harmful wastes, which is not in line with sustainable development goals [1,44,45,46]. The cuprammonium process, widely used by the textile industry since 1890, entails dissolving the CE in an aqueous solution of cuprammonium hydroxide (Cu(NH3)4(OH)2) followed by its extrusion into a coagulation bath containing dilute acid, alcohol, and a concentrated cresol solution [1,37,44]. Similar to the previous method, the cuprammonium process has gradually fallen into disuse due to its environmental and human concerns, and high production costs [1]. Another well-known cellulose solvent system is LiCl in DMAc or its cyclic form, 1-methyl-2-pyrrolidinone (NMP), which allows the dissolution of CE up to 10–15 wt.%, with a solubility proportional to LiCl concentration (1–10 wt.%) [44,47]. Although the LiCl/DMAc system offers several benefits, including its mild processing that prevents thermal runaway, the absence of additives or special equipment, and the ability to recover solvents, it requires the prior activation of the CE and poses significant challenges due to its high toxicity and volatility. These drawbacks impact the overall feasibility of this approach and its industrial implementation [1].
Lyocell Technology
Lyocell was the first successful technology applied for the manufacturing of CE fibers through direct dissolution, by employing a green solvent, N-methyl-morphine-N-oxide (NMMO), which can be fully recovered and reused [48].
This process consists of extruding a viscous CE/NMMO/water solution through an air gap into a water coagulation bath [1,9,44], resulting in fibers with remarkable properties and making it a competitor to the Viscose process. The Lyocell process presents several limitations, including side oxidation reactions, thermal instability, relatively high temperature requirements for dissolving CE (T > 90 °C), and uncontrolled fibrillation. Additionally, the strong oxidizing ability of NMMO leads to the formation of by-products, which may trigger side reactions that adversely affect the rCE fibers (e.g., degradation of the CE in the spinning solution, discoloration, and reduced mechanical properties) [1,44]. To minimize these limitations and improve the performance of fibers, new techniques and optimizations, such as the addition of an alkaline/antioxidant combination (NaOH/isopropyl gallate), cross-linking reactions, treatment with bio-enzymes, fiber crease reduction, and pre-singeing before and after dyeing, have been exploited [1]. Currently, Lyocell fibers are widely used in the textile sector (e.g., house and apparel textiles, such as mattresses, bed covers, denim, and underwear) and non-textile materials (e.g., wipes, diapers, and hygiene products), composite materials, and special paper [9,44].
Ioncell Technology
Ioncell is an innovative and sustainable technology that has been explored for developing high-quality and -tenacity bio-based fibers from ionic liquid solutions [49,50,51]. After a decade of development, this technology entered the pilot phase in 2021, emerging as an alternative to viscose and NMMO-based Lyoncell processes. It also has the potential to revolutionize textile waste recycling [52,53].
In 2002, Swatloski et al. [54] reported the ability of 1-butyl-3-methylimidazolium chloride (BMIMCl) and other hydrophilic ionic liquids (ILs) to dissolve and regenerate CE, without activation or pretreatments. Due to their thermal and chemical stabilities, low flammability, and miscibility with several solvent systems [32,44], ILs provide a sustainable approach for chemical modification and the processing of CE [55]. They consist of large organic cations combined with predominantly inorganic anions, forming salts that exhibit liquid properties at low temperatures, typically with melting points below 100 °C [32,37,44].
In the past decade, several ILs have been investigated to dissolve CE for fiber spinning, namely BMIMCl [56,57], 1-allyl-3-methylimidazolium chloride (AMIMCl) [15,58,59], 1-ethyl-3-methylimidazolium diethyl phosphate (EMIMDEP) [32,44], 1,5-diazabicyclo [4.3.0]non-5-enium acetate ([DBNH][OAc]) [32,37,44], 1-ethyl-3-methylimidazolium dimethylphosphate ([C2mim][(MeO)2PO2]) [60], among others [32,37]. Jiang et al. [59] tried to improve the spinning efficiency by studying the influence of extrusion velocity (ve) on a CE/BMIMCl solution, and it was observed that as the ve increased from 7.7 to 12.3 m/min, the tenacity and elastic modulus of rCE fibers also increased, rising from 3.5 to 4.2 cN/dtex and from 63.8 to 72.5 cN/dtex, respectively. According to Zhang et al. [14], the spinnability of CE/IL solutions is strongly influenced by variations in room temperature (RT) and relative humidity (RH), when air gap is used. The study revealed that temperatures around 22 °C and a RH of about 30% resulted in the increased spinnability of CE/BMIMCl solutions.
Moreover, the crystallite orientation, birefringence, and molecular orientation are improved with increasing draw ratios (DR). Regenerated CE fibers, with a tensile strength (sb) of ~1 GPa, a Young’s modulus exceeding 36 GPa, and an elongation at break (eb) of 6.7%, were prepared from a 12 wt.% CE/BMIMCl solution using a high Mn dissolving pulp and DR of 78. Moreover, Zhou et al. [15] also investigated the effect of DRs in three baths (coagulation, stretch, and washing) on the mechanical properties of rCE fibers. Wood pulp cellulose at 8.0 wt.% dissolved in EMIMDEP was extruded at 4 m/min via a 30 mm air gap into a water coagulation bath, and as-spun fibers were then stretched (DR, from 1.0 to 1.8) and washed in water baths. As the DR increased, the diameters and the elongation at break of the fibers decreased, while the strength at break increased, mainly due to a higher crystallinity degree and chain orientation with the longitudinal axes parallel to the deformation direction. The highest sb ~ 1 GPa was obtained for fibers drawn in both stretch and washing baths with DRs of 1.8.
Additional studies were developed by Wang et al. [58], focused on the effects of coagulation bath composition. Wood pulp (DP of 300) at 7.5 wt.% was dissolved in EMIMDEP and after passing through an air gap of 20 mm, the CE fibers were regenerated in a RT coagulation bath. As-spun fibers were molded, washed, and drawn at 1.5 in a water bath, and dried. The composition of the coagulation bath varied from 100% water to 100% absolute ethanol, including mixtures of both, where distinct water/ethanol proportions were investigated (10 wt.% of EMIMDEP was added to the water/ethanol mixture). The results pointed to different coagulation bath compositions that affect the internal structure of the fibers. Both crystallinity and orientation increased with the increase in water content from 41.6 to 59.7% and from 0.78 to 0.98, respectively, whereas eb decreased from 7.5 to 2.7%. This behavior is promoted by the different diffusion rates between solvents during the fiber forming process, and the addition of EMIMDEP to the coagulation bath slightly improved the sb from 386 (100% absolute ethanol) to 403 MPa (90/10% ethanol/EMIMDEP).
Although Ioncell is a promising technology aligned with sustainable development goals, enabling a closed-loop circular process by using non-toxic and recyclable ionic liquids, reducing waste generation, and reusing waste materials, it still faces challenges related to scalability and cost-effectiveness. This technology is expected to be ready for full commercial deployment in the coming years after successful operations in an operating environment [52].
Dissolution Process with Alkali/Urea Systems
An economically viable, non-toxic, and environmentally friendly strategy to dissolve CE is through the usage of alkali/urea aqueous solution systems (e.g., NaOH/urea or LiOH/urea) [61,62,63,64,65,66,67]. Moreover, this system has been also explored for recycling post-consumer products and regenerating value-added CE fibers via wet spinning [68]. In 1984, Kamida et al. [69] showed the viability of dissolving low MW non-crystalline CE (~1.9 × 104) in an aqueous solution of 10 wt.% NaOH at 4 °C, by separating the polymeric chains and disrupting hydrogen bonds. Nevertheless, this solvent can only dissolve low Mw CE, and the resulting solution is usually unstable and tending to easily form a gel. Later, Zhou et al. [70] discovered that by adding 2–4 wt.% of urea into 6–8 wt.% of NaOH aqueous solution, CE with a higher Mw (~6.7 × 104) could be dissolved, and the stability of the solution was improved.
The effects of the CE Mw, the stability of the spinning dope, ve, the coagulation bath, the DR on the structure, and the mechanical properties of the rCE fibers, prepared using alkali/urea aqueous solutions as the dope solvent, have been studied. Among these parameters, the coagulation bath composition has the greatest influence on these properties. Yang et al. [63] prepared rCE fibers by dissolving 7.0 wt.% of cotton linters in an NaOH/thiourea/urea aqueous solution at −2 °C using a twin-screw extruder and wet spinning process. The solution was extruded into a coagulation bath of 6–10 wt.% of H2SO4 and 10–15 wt.% of Na2SO4 at 10–30 °C. The jet stretch ratio varied from ~0.7 to 1.1, and the draw ratios in the second (DR1) and third (DR2) baths ranged from 1.0 to ~1.4 and from 0 to ~1.0, respectively. The tensile strength of the fibers increased from 0.79 to 1.06 cN/dtex as the jet stretch ratio increased from ~0.7 to 0.9, and then decreased at higher ratios. Low coagulation temperatures slowed the counter-diffusion rate, leading to insufficient solidification and poor tensile properties. Higher temperatures accelerated regeneration, causing defects and decreased tensile strength. Optimal H2SO4 concentrations facilitated coagulation, although excessive amounts increased fiber porosity and decomposition. Higher Na2SO4 concentrations increased tensile strength and decreased eb. The best fiber properties were achieved with 7.6 wt.% H2SO4 and 15.2 wt.% Na2SO4 at 15 °C, with a total draw ratio of 1.15 (jet stretch ratio × DR1 × DR2), resulting in a tensile strength of 2.22 cN/dtex and an eb of 9.47%. Tu et al. [67] reported CE filaments with a maximum tensile strength of 2.92 cN/dtex. A spinning solution consisting of 7.5 wt.% wood pulp in an aqueous NaOH/urea/ZnO system was extruded into a cold coagulation bath containing 15 wt.% citric acid, 5 wt.% trisodium citrate, and 40 wt.% glycol. The fibers were then drawn in 5% H2SO4 baths. ZnO improved CE solubility and stability by forming strong hydrogen bonds with cellulose. The slow solvent/non-solvent exchange due to the large molecular weight of citric acid, low coagulation bath temperature (5 °C), and the presence of trisodium citrate and glycol allowed CE chains to maintain high mobility and alignment. The complete breaking of the alkali/urea inclusion complex led to the formation of nanofibers (30–50 nm) through extensive hydrogen bonding. Stretching in the second H2SO4 bath further enhanced filament strength. The tensile strength of dry CE fibers increased significantly from 2.04 to 2.92 cN/dtex as the DR increased from 1.0 to 1.8.
4.1.3. Strategies for the Production of Cellulose-Based Fibers with Tailored Mechanical Properties
Different strategies have been investigated for the production of cellulose-based fibers with tailored mechanical properties. The incorporation of carbon-based nanomaterials having different geometric features, such as two-dimensional (2-D) graphene and its derivatives, one-dimensional (1-D) carbon nanotubes, curcumin [71], betulin [72], and antarctic krill protein [73], among others, into CE systems offers the possibility of imparting new functionalities and enhancing the mechanical performance of wet spun fibers [74,75,76]. For example, Tian et al. [77] reported the successful production of rCE/graphene oxide (GO) fibers through a pilot-scale wet spinning line, with a sb of 360 MPa and E of 2.3 GPa, corresponding to an improvement of 50 and 25% in comparison with rCE fibers. The same authors showed enhanced thermal stability and dynamic heat transfer performance with an addition of only 0.2 wt.% of GO. Jiang et al. [78] studied the influence of functionalized multiwalled carbon nanotubes (f-MWCNTs) into CE/TBAA/DMSO solutions on the mechanical properties of fibers obtained by dry-jet wet spinning. The E increased from 0.8 to 3.1 GPa through the incorporation of 5.0 wt.% of f-MWCNTs.
On the other hand, cellulose nanofibrils (CNFs) have been investigated for developing high-performance and multifunctional macroscopic materials by establishing synergetic interactions between building blocks. They have also been used as dispersant agents to improve the dispersion level and stability of other nanomaterials. Z. Wan et al. [79] successfully produced wet spun filaments through CNF composites with SWCNTs composed by axially oriented building blocks with CNF networks wrapping to SWCNTs. The authors reported a high σ up to 472.2 MPa and a strain of 11.8%, exceeding the results reported so far based on CNF/SWNT composites.
Another suitable strategy to improve the mechanical properties of CE-based fibers is through the incorporation of polymers [34,80]. In particular, Geng et al. [80] studied the influence of polyamide-epichlorohydrin (PAE) in the performance of CNF suspensions at different ratios (0.5:10 and 1.0:10) in an acetone coagulation bath. It was found that both the tensile strength and modulus of the produced filaments increased from 268.7 to 369.8 MPa and from 22.8 to 28.9 GPa, respectively, for the formulation CNFs/PAE ratio of 10:1. Marsano et al. [81] produced wet spun fibers of CE (DP of 390) and silk fibroin, a fibrous protein extracted from silk, at different compositions by using NMMO (50 wt.% in water; containing 0.7 wt.% of isopropyl gallate) as the solvent and ethanol as the coagulant. Composite fibers were obtained in different drawing conditions and the amount of silk fibroin did not influence the spinnability of the solutions. Although the CE and silk fibroin were immiscible and showed phase separation in all samples, the fibers containing 75% of the cellulose showed better mechanical properties than pure CE fibers: a Young’s modulus of about 23 GPa and strength at break of 307 MPa.
4.1.4. Strategies for the Production of Cellulose-Precursor Fibers for Their Conversion into CFs
Cellulose and its composites have been extensively researched as alternative precursors of PAN for CF manufacturing. In fact, PAN precursors represent more than 50% of the global costs in CF manufacturing, restricting their spread across industries, with a special focus on mobility and renewable energy. Therefore, Zahra et al. [11] investigated the use of a chitosan (CS) as a natural charring agent, aiming at enhancing the carbon yield of CE-derived CFs. The [DBNH][OAc] was used for the direct dissolution of 12.0 wt.% wood pulp (~263 × 103 g/mol) and CS to spin CE/CS blended fibers through a dry-jet wet spinning process. CS with different molecular weights (30 × 103 and 250 × 103 g/mol) was used, and its content varied from 0 to 25 wt.%, resulting in blends with distinct rheological behavior. At 25 wt.%, CS induced a slight reduction in the crystallinity degree and crystallite size, and consequently, a decrease in tenacity (~3.6 cN/dtex). However, synergistic interactions between CE and CS were observed during the pyrolysis stage for the conversion of CE/CS blended fibers into CFs, leading to a higher carbon yield and preserving the structural and mechanical properties of the final fibers. This discovery opens up new possibilities for the development of bio-based CFs, offering viable alternatives to those based on non-renewable polymers.
There has been extensive research focused on developing affordable and environmentally sustainable CF precursors. Bengtsson et al. [82] reported a cold aqueous NaOH/ZnO system used to prepare CE/lignin fibers, by wet spinning, through solutions containing kraft pulp and softwood kraft lignin. The lignin content varied from 0 to 30 wt.%, and solutions were successfully spun using two different coagulation bath compositions. The results showed that the H2SO4/Na2SO4 system provided precursors with a striated surface and irregular cross-section, while the H3PO4/NH4H2PO4 system allowed the production of fibers with a smoother surface and circular cross-section. The precursor fibers were then converted into CFs via batchwise and continuous conversion. The precursor and conversion conditions had a significant effect on the conversion yield (12–44 wt.%), Young’s modulus (33–77 GPa), and tensile strength (0.48–1.17 GPa), while the precursor morphology was preserved.
4.2. Silk
4.2.1. Sources, Properties, and Applications
Silk is a lightweight structural material, produced in nature by silkworms and spiders [83,84]. It is composed of two major proteins—fibroin (70%) and sericin (25%)— and minor impurities (5%) [84]. The silk structure mainly comprises two silk fibroin (SF) fibers covered by a sericin layer containing small non-protein impurities [84]. Sericin is a water-soluble protein with a disordered spatial structure, which promotes lubrication, protection, and adhesion in cocoon silk [84]. SF is a fibrous protein composed of a heavy chain (H-chain) (390 kDa), light chain (L-chain) (26 kDa), and glycoprotein P25 (30 kDa) at a molar ratio of 6:6:1 [84,85]. The larger H-chain is glycine (Gly) rich, and most of its amino acid composition consists of Gly, alanine (Ala), and serine (Ser), Figure 5.
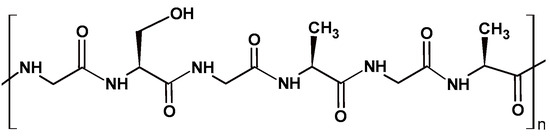
Figure 5.
Schematic representation of silk fibroin protein structure, with amino acid composition consisting of glycine (Gly), alanine (Ala), and serine (Ser).
SF can be converted into water-soluble rSF protein through a series of treatments, although its molecular weight distribution and structure vary depending on the extraction method used [84], impacting its mechanical properties, degradability, biocompatibility, thermal stability, and processability. Regenerated SF solutions can be utilized to create films, sponges, microspheres, gels, and nanofibers for different applications, which include biomedicine (drug delivery carriers, wound dressings), tissue adhesion, and engineering (scaffolds). Moreover, they find utility in food processing, such as food additives, packaging, and biosensing applications (flexible wearable sensors and human–machine interaction devices) [84].
The extraction process of rSF comprises four steps, including (1) degumming, (2) dissolution, (3) dialysis, and (4) centrifugation [84]. Several methods have been reported for the degumming of raw silk, including alkaline [17,18,83,84,86,87,88,89,90,91], acid [92,93], biological [92,93,94], and physical degumming [95,96,97]. In particular, sodium carbonate (Na2CO3) is the most common degumming agent with advantages related to the simple operation and degumming rate. The dissolution step converts insoluble SF into soluble rSF through the interaction between solvent molecules and SF, facilitating subsequent processing. Lithium bromide (LiBr) has been extensively used as a solvent to dissolve SF [18,19,83,86,87,88,89,90], where the presence of Li salts disrupts the intermolecular hydrogen bonds and van der Waals forces within SF, due to the strong polarity of the ions. The traditional method consists of dissolving silk fiber in concentrated aqueous LiBr, and then dialyzing the resulting solution against deionized water that yields a dilute fibroin solution (4.0–5.0%) unsuitable for wet spinning because of its low viscosity [86,92]. To overcome this drawback, researchers have lyophilized dilute aqueous rSF solutions and dissolved them in other solvents, including hexafluoroisopropanol (HFIP) [97], hexafluoroacetone [98], or trifluoroacetic acid [99], to increase the protein concentration and, consequently, meet the requirements for wet spinning. Nevertheless, the spinning dope preparation process is quite complex, and the solvents involved are costly and toxic [86]. Therefore, reverse dialysis has been applied to prepare concentrated aqueous rSF solutions [19,83,86,87,88,89,100], in combination with specific solvent systems (NMMO/water [96], CaCl2/ethanol/water [101], CaCl2/formic acid [17], and formic acid [92]).
4.2.2. Strategies for the Production of Silk-Based Fibers with Tailored Mechanical Properties
The production of rSF fibers with acceptable mechanical performance is challenging because alcohol-based coagulants, usually methanol or ethanol, induce rapid β-sheet formation of silk molecules [83], avoiding the alignment of the β-sheet crystals, as evidenced by the poor mechanical properties. In addition to solidifying the silk solution in an alcohol bath, post-drawing is an essential step to enhance the strength of silk fibers, although their extensibility is generally low, leading to lower overall toughness compared with native silk fibers. Marsano et al. [96] prepared wet spun rSF fibers by using NMMO/water as the solvent and ethanol as the coagulant. Previous to dope preparation, SF fibers were purified from silk cocoons by degumming with water at 120 °C for 15 min, followed by extensive rinsing with warm water to completely remove sericin. Afterwards, SF fibers were dissolved in saturated aqueous LiBr (9–10 M) at 60 °C for 3 h, filtrated, dialyzed until the complete removal of the salt, and freeze-dried. The resulting sponge-like SF fibers were dispersed in an aqueous solution of NMMO containing 0.7 wt.% of isopropyl gallate. The dope solution was extruded through an air gap of 5 mm into an ethanol coagulation bath. rSF fibers were collected at different draw ratios (1.0–2.7) after which they were further washed in water and dried under a vacuum. The resulting rSF fibers exhibited a maximum sb of 120 MPa and eb of 35% (DRTotal of 40.5). In addition, Ling et al. [87] investigated the effect of several coagulants on the wet spinning of 20 wt.% SF dope solution into rSF fibers, and the results revealed that glycerol provided fibers with higher mechanical properties (stress and strain at break of 221 MPa and 30%, respectively). This behavior can be explained by the β-sheet growth after the nucleation step, which allowed a greater efficiency of the molecular segment’s alignment during spinning process. Yazawa et al. [83] prepared predominantly amorphous silk fibers with a trace amount of β-sheet structures, and then, post-drew to align the β-sheet crystals. The combined process allowed for the attainment of fibers with higher ductility and toughness than natural B. mori silk fibers. At DR of 3.0, the resulting rSF fibers exhibited a tensile strength, E, eb, and toughness of ~160 MPa, ~2.2 GPa, ~75%, and ~110 MJ/m3, respectively. Remarkable results were reported by Yao et al. [18] who used silk nanofibers (SNFs) directly fibrillated from degummed B. mori silk fibers as an additive. rSF fibers containing 0.06 wt.% of SNFs exhibited a strain at break of ≈100% and strength at break of ~491 MPa. These results were attributed to SNF-induced templated and enhanced nanocrystallite formation in the early stages of the spinning process, which was further organized through shear stresses during the drawing process. Moreover, Wöltje et al. [97] prepared a spinning dope consisting of 4.0% (w/v) of freeze-dried rSF dissolved in HFIP, which was spun through an air gap of 2 mm into an ethanol (80% (v/v), ammonium acetate (0.6 M), and PEG (1% (w/v)) bath. After drawing (DR of 3.1), the rSF fibers were washed. The tensile strength reached was 256.8 MPa, with an average strain at break of 13.9% and E of 13.2 GPa. According to the authors, the tensile strength of these wet spun rSF fibers per percentage of fibroin is higher than that of all continuous spinning approaches applied to regenerated and native silk fibroin published up to now. Zhang et al. [17] reported a simple, efficient, and environmentally compatible process to prepare high-quality rSF fibers based on 15.0 wt.% of SF dissolved in CaCl2/formic acid solution and water in the coagulation bath. The preserved silk nanofibrils assembled into fibers due to physical shear and water-induced coagulation, which was accompanied by structural transitions from an amorphous state to silk II during this process. After drawing (DR between 2 and 4), the resulting fibers exhibited enhanced mechanical properties (at DR of 4, tensile strength and E of 470 MPa and 6.9 GPa, respectively) compared with natural fibers, owing to the more highly oriented fibroin molecules, β-sheet crystal, nanofibrous structure, and reduced fiber diameter. Recently, Yazawa et al. [102] used an IL to dissolve the cocoon waste of wild silkworms, Antheraea yamamai, and produce rSF. The spinning solution composed of 10.0 wt.% of freeze-dried SF in BMIMCl was extruded through a 100 mm air gap into a methanol coagulation bath. The mechanical properties of the resulting fibers were improved by increasing the DR (from 1 to 4), reaching a maximum tensile strength, E and eb at DR of 4 (350 MPa, 7.0 GPa and 55%, respectively), due to the molecular alignment of the silk chains. The effect of CNCs and post-drawing on the structural characteristics, and thermal and mechanical properties of rSF/CNC fibers were investigated by Liu et al. [19]. Different contents of CNCs were added into a 17.0 wt.% SF in 9.0 M LiBr, and the dope solutions were post-drawn (DR between 1 and 3) after being extruded into a methanol coagulation bath. The CNCs were uniformly dispersed into an SF matrix and aligned along the fiber axis, being beneficial to the formation of more ordered structure through intermolecular hydrogen bonding interactions. The crystallinity and overall molecular orientation of rSF/CNC fibers increased by increasing the DR and CNC contents. The rSF/CNC fibers composed of 5.0 wt.% CNCs and drawn at 3 showed values of tensile strength, Young’s modulus, and strain at break of 728.5 MPa, 28.8 GPa, and 23.1%, respectively, suggesting that the CNCs could substantially enhance the mechanical properties of SF.
Alternatively, biomimetic spinning systems have been developed based on recombinant silkworm silk or spider silk-like protein solutions as raw materials [103,104,105]. While the tensile strength of biomimetic silk fibers has improved, achieving high extensibility remains challenging, mainly due to the difficulty in precisely controlling pH changes and ion compositions, along with the shear forces employed during spinning [83]. Zhang et al. [103] dissolved purified spider silk protein (spidroin) in HFIP and spun this solution into an aqueous methanol coagulation bath. The resulting stable continuous fibers were then subjected to a secondary stretching in both air and methanol to investigate how these steps affected the physical and chemical properties of the fibers [103]. Lower concentrations of methanol favor the production of continuous, thinner fibers with higher tensile strength (for instance, using a coagulation bath composed of 2.0% methanol, the tensile strength was 30.4 MPa). Furthermore, it was found that two-step stretching, specifically stretching in air and in 0.1% methanol, resulted in a higher strain at break of the fiber. Weatherbee-Martin et al. [105] reported a wet spinning method using the W3 AcSp1 construct as a raw material for reproducible high-throughput aciniform silk fiber formation. Native aciniform silk protein (spidroin), AcSp1, from Argiope trifasciata consists of a core domain with at least 14 consecutive 200 amino acid repeat units (referred as “W” units herein) with identical primary structure that comprise > 95% of the protein sequence. Furthermore, 8 wt.% of recombinant W3 aciniform spidroin was solubilized in HFIP/water mixture (7:3). The dope solution was extruded into an aqueous ethanol solution (95%) and post-stretched in a water bath. The post-spin stretching of the resulting wet spun fibers in water significantly improved fiber strength, enriched β-sheet conformation without complete α-helix depletion, and enhanced birefringence. These steps enabled the consistent formation of aciniform silk fibers, albeit with reduced extensibility compared with native silk (2.6%), requiring different conditions and approaches from those previously documented for other silk proteins. In addition, Cheng et al. [104] presented a novel approach for simultaneously functionalizing and reinforcing recombinant spider silk fibers through the in situ formation of CuS and gold (Au) nanoparticles (NPs). A mimic silk protein (N16C) of spider Trichonephila clavipes was recombinantly produced and wet spun into fibers. Drawing the as-spun fibers in water led to post-drawn fibers more suitable for the templated synthesis of NPs with uniform distribution throughout the synthetic fibers. The in situ-formed NPs also served as a template to induce the formation of more β-sheet structures from the silk protein within the fibers and thus significantly improved the mechanical properties of the functionalized fibers. Furthermore, the confined synthesis of Au NPs through a redox reaction was shown to improve the ultraviolet-protective effect and both tensile strength and Young’s modulus of 214.7 MPa and 4.9 GPa, respectively.
4.3. Alginate
4.3.1. Sources, Properties, and Applications
Alginate (ALG), a naturally occurring polysaccharide extracted from brown algae and seaweed species, or produced by bacterial microorganisms, has emerged as a highly versatile biomaterial, distinguished by its biocompatibility, ease of gelation, non-toxicity, regenerability, and cost-effectiveness [106,107]. This unique combination of properties positions ALG as a promising material for several applications and sectors [106,108].
Commercial alginate is predominantly extracted from the brown algae Laminaria, Macrocystis, and Ascophyllum [109]. The procedure for ALG extraction from algae, Figure 6, is divided into three main steps: (1) pre-extraction, (2) neutralization, and (3) precipitation.
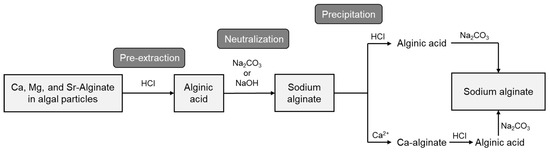
Figure 6.
Schematic representation of extraction of alginate from algal material. Adapted from Ref. [109].
During pre-extraction, a strong acid is employed to remove salts from alginic acid (e.g., Na+, Mg2+, etc.) through ion exchange with protons. Neutralization is carried out with an alkaline solution, transforming the insoluble alginic acid into water-soluble sodium alginate (SA). This stage is followed by sodium alginate precipitation in a strong acid, calcium chloride, or alcohol, and finally, drying and milling [110].
This biomaterial is a copolymer with an anionic charge formed by 1,4-glycosidic bonds between the monomers β-D-mannuronic acid (M) and α-L-guluronic acid (G) [106,111]. These two acids differ stereochemically at carbon C5 and, depending on the natural source and extraction process, they are arranged in the linear alginate chain in consecutive homopolymeric blocks (MM and GG) or heteropolymeric blocks (MG), Figure 7 [112,113].
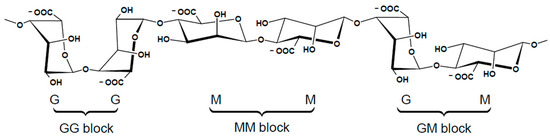
Figure 7.
Schematic representation of alginate structure, with the chair conformation of GG block, MM block, and GM block. Adapted from Ref. [109].
The MM blocks link di-equatorially at carbon C1 and C4 forming a relatively straight polymer, while the GG blocks link from di-axial groups at the same carbons, resulting in a large hindered rotation around the glycosidic bond, creating buckled polymers [109,112]. The chemical and physical properties of alginates are significantly influenced by their composition, specifically by the sequence and ratio of M and G monomers. A high proportion of G in the ALG demonstrates remarkable gelation properties in the presence of divalent cations, strongly influencing the mechanical characteristics of the resulting alginate gel [109,114,115]. Alginate, characterized by a higher content of M, reveals increased flexibility and better biocompatibility relative to G-rich alginate [112,114,115]. It is conventionally accepted that G blocks, exhibiting a greater affinity for divalent cations, are solely responsible for the cross-linking and subsequent gelation of ALG, while the contribution of M blocks is negligible due to their low stereospecificity [20,112]. However, a more in-depth study of the behavior of M blocks in the presence of ions is required for a better understanding of their influence on the cross-linking process [112].
Sodium alginate stands out for its water solubility and ease of application in different formats [20,112,116]. Substituting sodium with a cation in SA leads to its precipitation, and being able to be cross-linked through several mechanisms, with cation cross-linking as the most frequently investigated [20,112,116]. Cross-linkers are used to replace inter-hydrogen bonds between carboxy and hydroxy groups on the G segments within alginate chains, generating an insoluble “egg-box” cross-linked structure, enclosing the cations [116,117]. The hydrogel-forming ability of ALG salts in the presence of divalent cations has led to the exploitation of this biopolymer in several physical forms [106,118,119]. The research and focus on ALG fibers has been steadily growing in the field of textile and biomedical fields [115]. The most widely employed technique for the production of alginate fibers is wet spinning, wherein divalent cations have been used in the coagulation bath to cross-link with the carboxyl groups of alginates [112,115].
4.3.2. Production of Alginate-Based Fibers
Alginate-based fibers produced by spinning technologies typically exhibit insufficient mechanical properties [22,106,116], since they are strongly influenced by various stages of the process, beginning with the selection of raw material, extending to dope characteristics, and culminating in coagulation conditions [20,120].
In the raw material selection, several features, namely molecular weight and the G/M ratio, must be considered [119]. In 2023, Silva et al. [121] prepared ALG fibers by using two distinct brown algae species for alginate extraction, Saccharina latissimi (SAC) and Laminaria digitata (LAM), where each one of them contained distinct proportions of M and G monomers. Enhanced E of 2.4 GPa and a tensile strength of 32.8 MPa were obtained in fibers produced from SAC, being related to its lower M/G ratio. In terms of eb, fibers derived from LAM alginate demonstrated superior performance (12.6%), which is related to the higher content of the M blocks. ALG fibers, exclusively composed by M blocks, were produced by Aneem and co-workers [112], showing excellent eb (43%), although with very poor E (88 MPa) and strength (1.8 MPa). This underscores the effective cross-linking potential of M blocks, despite contributing to strength weakness. However, this approach reveals interesting results for its application in therapeutic encapsulation [112]. Comparing the tensile strength reported by Silva et al. [121] (26.4–32.8 MPa) with the values reported by Aneem et al. [112] (with a maximum of 1.8 MPa), it can be proved that G monomers play a significant role for enhancing this property. Moreover, M monomers contribute to increasing fiber elasticity, as evidenced by the eb being three times higher for fibers produced from SA with exclusively M blocks [22,106,112,116,121].
Dope characteristics are strongly influenced by ALG concentration, where high concentrations weaken the biopolymer’s solubility and decrease the fluidity of the solution, which compromises the spinnability and, consequently, the mechanical properties of the fibers [20,122]. In highly viscous solutions, the interactions between the polymer chains are intensified, making alignment during extrusion challenging. Dope degassing becomes more difficult and time-consuming, potentially resulting in spinneret blockage [122]. Therefore, decreasing ALG concentration or increasing both temperature and pH are effective strategies for controlling and optimizing the dope viscosity and processability. However, lower values of dope concentration led to weaker fiber properties, similar to the other two strategies, as they involve SA chain breakage. It is therefore necessary to find a good compromise between the viscosity of the dope solution and the physical properties of the ALG fibers [120,122].
Moreover, during the coagulation step, the type and amount of the cross-linking agent [123], along with coagulation time and temperature [20], are key elements in the fiber formation process. The most commonly explored coagulation bath is a calcium salt solution owing to its high solubility in aqueous media and the abundant availability of Ca2+ ions [20,21,116,118,121] (Supplementary File). To boost the application of ALG fibers, investigations have been conducted into combinations of Ca2+ ions with other metallic ions and coagulants [112,123]. Alternative metal ions with enhanced gelling ability have also been explored aiming to increase the affinity with alginate [113]. The order of divalent cations’ exchange rate with ALG decreases as follows: Pb2+, Cu2+, Cd2+, Ba2+, Sr2+, Ca2+, Co2+ ~ Ni2+ ~ Zn2+, and Mn2+ [115]. Aneem et al. [112] studied the impact of two different types of cross-linking agents in the coagulation bath, ionic and covalent, combined with different coagulants (DMSO, DMF, and THF) on alginate fibers. While both bivalent (Ca2+, Ba2+) and trivalent (Al3+) ions were used as ionic cross-linkers, citric acid was used as a covalent cross-linker. Calcium ions, in combination with the coagulant DMSO, proved to be the most advantageous coagulation system for enhancing the mechanical properties of ALG fibers. This system yielded the highest tensile modulus and strength of the fibers with 88 MPa and 1.82 MPa, respectively. Less promising findings were attained through the AL3+/DMSO system mainly due to the smaller ionic radius of AL3+, enabling the establishment of interchain hydrogen bonds of M blocks, and leading to a more densely packed Al3+ cross-linking structure in the fibers, making them more brittle. Concerning coagulants, DMSO yielded more elastic fibers with ionic cross-linkers, achieving a higher strain for Ca2+ ions (43%). For all coagulants, the following trend was observed: > Ca2+ > Ba2+, and >AL3+ [112]. Moreover, the most common solvent used for dissolving alginate is water, rendering the manufactured fibers more environmentally friendly, and thereby attracting considerable interest [112,116,117,121]. Nonetheless, Yan et al. [20] developed ALG fibers with enhanced mechanical properties by using a LiOH/urea aqueous solution to dissolve SA at low temperatures and under optimal coagulation conditions. In the coagulation process, the diffusion of multiple ions in a specific order, with Ca2+ preferentially exchanging with Li+ and subsequently with Na+, led to the establishment of Ca2+ cross-linking points within the SA fibers. From this approach [20], it can be concluded that coagulation conditions play a significant influence on the mechanical properties. By increasing the coagulation time to 15 min, the temperature to 35 °C, and the concentration of calcium chloride to 2 wt.%, fibers with the highest sb (4.0 cN/dtex) and eb (11.4%) were obtained, mainly promoted by the double diffusion enhancement during coagulation and by an increase in the cross-linking degree [20,112,116,117,121].
4.3.3. Strategies for the Production of Alginate-Based Fibers with Tailored Mechanical Properties
Different strategies employed to enhance the mechanical performance of ALG and its derivatives include the incorporation of modifiers/fillers into dope solution, such as proteins and other polysaccharides, and modifications of the type and amount of divalent cations in the coagulation bath [22,106,123]. However, it is important to note that some additives, even at low concentrations, can also contribute to an increase in dope solution viscosity, affecting the spinning capacity [122].
In 2022, Li and co-workers [122] demonstrated the effectiveness of the polyelectrolyte sodium polyacrylate (PAAS) in lowering the viscosity of SA polymer solutions for wet spinning technologies, achieved by providing counterions in the SA aqueous solution. Notably, for PAAS surpassing 10% loading content, this polyelectrolyte acted as an antiplasticizer, reducing the dope viscosity and enhancing the mechanical properties of the fibers, especially at higher PAAS contents (40%) [122]. In addition, Wang et al. [21] successfully produced SA/gelatin blended fibers by incorporating varying amounts of gelatin and oxidized starch into the SA dope. The fiber formation involved the cross-linking of SA by Ca2+ and gelatin by oxidized starch through the wet spinning process. The optimal amount of gelatin, corresponding to a mass ratio of SA/gelatin of 10:2, enhanced the mechanical properties and swelling properties, as well as the water absorption and retention properties of the alginate/gelatin blended hydrogel fibers. When comparing the mechanical properties of SA/gelatin (10:2) in the blended fibers reported by Wang et al. [21] to the values described by Li et al. [122] for SA fibers with 10–40% of PAAS loading, it is noteworthy that the previous exhibit higher strength (1.3 cN/dtex [21]) but lower strain (4.4% [21]) than the latter [122], although SA raw material is also different in the M/G ratio, being much lower for fibers blended with gelatin. This difference may be the explanation for the better tensile strength and lower eb of SA/GEL fibers.
Wet spun alginate/chitosan derivative composite fibers were produced by Zhao et al. [22] using a quaternary ammonium chitosan (QAC) polysaccharide polyelectrolyte complex (PEC) system to blend with SA. The resulting composite fibers not only exhibited interesting mechanical performance, with a tensile strength of 2.4 cN/dtex but also demonstrated commendable antibacterial and hydrophobic properties [22]. Other researchers have used SA modification as a strategy to improve the antimicrobial properties of fibers. ALG fibers doped with Eucalyptus essential oil (EEO) were prepared by Khajavi et al. [117] with the aim of creating a naturally antibacterial fiber, taking advantage of EEO’s anti-inflammatory, antiseptic, and antibacterial properties. However, the addition of EEO decreased the eb and E modulus compared with neat ALG fiber. Notably, no significant difference in tenacity was observed between the two types of fibers [117].
While the majority of manufactured ALG fibers are purposed for biomedical fields, there is interest in exploring alternative applications for them. In this context, Zhao et al. [116] prepared fluorescent textile fibers capable of detecting trace amounts of Hg2+ ions over other metallic ions by incorporating CdTe nanocrystals (NCs) into SA using thioglycolic acid (ThGA) as a surface ligand. It was found that the mechanical performance was improved by incorporating CdTe nanocrystals (NCs), attributed to the effective dispersion of nanocrystals and their strong adhesion to the calcium alginate matrix [116].
4.4. Chitin and Chitosan
4.4.1. Sources, Properties, and Applications
Chitin, a poly(1→4)-2-acetamido-2-deoxy-β-d-glucose, is the second most prevalent naturally occurring polymer after cellulose, and it is widely distributed in the cell walls of fungi and the exoskeletons of crustaceans, including crabs, lobsters, and shrimps [23,124]. The conventional process of chitin extraction typically involves acidic reactions to remove calcium carbonate, followed by alkali treatments to solubilize proteins [124]. Alternative methods have been investigated to mitigate the environmental impact associated with the conventional approaches. Enzyme-assisted extraction stands out as a promising solution for extracting chitin from crustaceans, demonstrating its effective separation from proteins and minerals. Moreover, this method ensures the molecular integrity of chitin, preventing undesired phytochemical changes [125].
Chitosan (CS) predominantly emerges as a derivative of partially deacetylated chitin. Nevertheless, this polymer is also presented in certain biomass varieties, particularly within fungal classes, such as Zygomycetes (Mucor, Absidia, Benjaminiella, Cunninghamella, Gongronella, and Rhizopus genera) [126]. The production of CS through chitin involves deacetylation processes, which can be achieved via enzymatic hydrolysis or alkaline treatments [127]. An overview of the literature reveals various definitions for chitin and chitosan, although the prevalent criterion is the degree of deacetylation (DD) of at least 50% for chitosan [125]. The acetylated groups within CS may exhibit block configurations along the polymer chain or display a more regular distribution, resulting in diverse chitosan materials with distinct characteristics and molecular weight.
The chemical structure of these two natural polymers consists of a random distribution unit of two monosaccharides, N-acetyl-D-glucosamine and D-glucosamine, linked by β-1,4-glycoside bonds [106,126]. Chitin is mainly composed of N-acetyl-D-glucosamine units, while CS predominantly comprises D-glucosamine units [106,126]. This difference arises from the partial deacetylation of the monomer N-acetyl-D-glucosamine to D-glucosamine during the transformation of chitin into CS, Figure 8 [126].
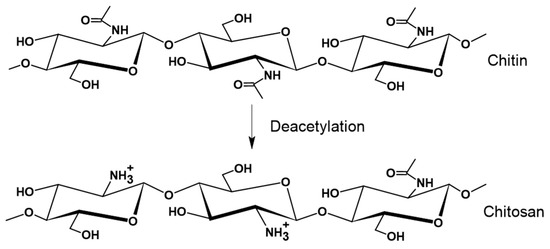
Figure 8.
Schematic representation of chitin and chitosan structures.
Chitin exhibits chemical structural similarities to cellulose, differing predominantly in the presence of nitrogen atoms [127]. Despite this distinction, both chitin and cellulose serve a common function of providing structural integrity and protection to the organisms in which they are found [126]. Depending on the orientation of the layers of polysaccharides sheets, three crystalline forms have been reported for chitin (α, β, and γ), exhibiting varied structural characteristics that influence its solubility and reactivity. The predominant α-chitin, found in the cell walls of crustaceans, insects, fungi, and yeast, features strong inter- and intra-molecular hydrogen bonding interactions among chitin chains. These create highly ordered crystalline structures, rendering α-chitin insoluble in most common solvents. On the other hand, β-chitin is characterized by chitin sheets arranged in parallel with weaker intermolecular forces, improving its affinity to solvents and reactivity compared with α-chitin. The third crystalline form, γ-chitin, is less prevalent, and it is regarded as a hybrid or intermediate state between α- and β-chitin [126].
The numerous advantages inherent to chitin and CS, including biocompatibility, biodegradability, and low toxicity, have led to increasing interest in a large variety of fields, with special emphasis on medical applications (wound dressings, drug delivery systems, and tissue engineering) [126,128].
4.4.2. Production of Chitin- and Chitosan-Based Fibers
Beyond the materials developed from chitin and CS, the exploration of high-performance fibers has emerged as a research focus [24,126,129]. These fibers, in addition to their application in wound dressings, can be useful as sutures [127], showcasing the diverse and promising potential of chitin and CS-based materials in the field of advanced biomaterials [126], and wet spinning has been employed in the manufacture of chitin- and CS-based fibers [127,130]. Nevertheless, the processability of chitin for fiber production is largely restricted [127,128], mainly attributable to chitin’s stable crystalline structure, which induces poor solubility in different media [24].
To address this limitation, and subsequently enhance filament-forming ability, a prevalent approach employing strong solvent systems, such as formic acid, dichloroacetic acid, and HFIP, is used [23,128]. However, this strategy results in weaker mechanical performance since the crystalline structure is negatively affected, as well as environmental concerns [23,24]. Hence, the search for eco-friendly alternative strategies that preserve the crystalline structure of chitin, while concurrently achieving improved solubility, remains a pivotal goal in the ongoing development of chitin-based fibers. For example, Nguyen et al. [23] reported that chitin solutions with a concentration of 2.0 wt.% could be obtained by dissolving chitin extracted from crab shell waste in DMAc/5% LiCl at RT. The dissolved chitin was regenerated into filaments in a water coagulation bath at different temperatures (5–60 °C), and the results showed that fibers with superior tensile strength (182 MPa) and eb (33%) were produced at a higher coagulation temperature (60 °C) [23]. This improvement may be related to the increased diffusivity of water molecules during the coagulation step, resulting in a denser and smoother cross-sectional morphology with fewer microvoids and, consequently, better mechanical performance [23]. In 2021, Huang et al. [128] used an aqueous solution of KOH/urea to dissolve chitin, yielding a stable spinning dope. The chitin solution was regenerated in a coagulation bath comprising 80% ethanol and 10 wt.% aqueous potassium acetate anhydride (KAc). Chitin/CS multifilaments were obtained through the deacetylation of chitin, with superior mechanical properties at a higher degree of deacetylation (83%). In addition, Chen et al. [24] developed a new approach to produce mechanically strong chitin fibers (tensile strength of ~251 MPa) by preserving their crystalline structure. This approach involved the alkaline gelation of β-chitin nanofibers (β-ChNF) in an aqueous NaOH solution, followed by neutralization in a bath containing a 2.0 wt.% acetic acid solution, to produce all-chitin filaments.
Although there are current approaches for generating chitin fibers using solvents that yield stable spinning solutions, achieving the large-scale and continuous production of chitin fibers using environmentally friendly and cost-effective methods remains a challenge [128]. Therefore, researchers have increasingly turned their attention to chitosan materials, widely known for their polycationic nature arising from numerous amine groups—a key distinction from chitin [24,106,131]—that enable the creation of several CS derivatives with enhanced functional properties, as the positive charge of chitosan makes its involvement in chemical reactions easier for modification purposes [106,131]. By leveraging the advantages inherent in chitosan, such as its modified chemical reactivity, CS fibers have been produced by wet spinning.
The prevalent solvent employed for CS dissolution is a diluted acetic acid solution (1.0–3.0 wt.%) [27,28,131,132,133], which allows the solubilization of CS by protonating the amino groups in the chitosan molecule and destroying their hydrogen bonds, rendering it soluble in acidic solutions [131]. Nonetheless, alternative acids, such as adipic acid (AD) and lactic acid (LA), have demonstrated their suitability for CS dissolution [26]. In a study conducted by Perrin et al. [26], the optimal acid for preparing CS fibers sourced from fungi and shrimp was investigated, instead of commercially available CS derived from chitin deacetylation. The research focused on the influence of both high and low molecular weight sources on the properties of the resulting fibers. The formed fibers were regenerated in a coagulation bath containing NaOH/10 wt.% ethanol (1:1), and subsequently, stretched vertically on a board. The highest mechanical tensile strength and Young’s modulus, measuring 308.0 MPa and 22.7 GPa, respectively, were achieved with fibers produced from high molecular weight shrimp CS (407,000 g/mol) at a dope concentration of 3.0 wt.%, utilizing adipic acid as the solvent. The type of acidic solvent proved to have a significant impact on the fibers produced from shrimp sources, resulting in an improvement of tensile strength from 242.7 MPa (LA) to 308.0 MPa (AD), which may be attributed to chitosan cross-linking facilitated by the presence of the two carboxylic acid groups in AD, as opposed to the single carboxylic group of lactic acid, leading to the formation of stronger fibers [26]. Li et al. [131] explored the utilization of formic acid as solvent, with the aim of improving the elasticity of CS fibers, and broadening the potential applications of chitosan fibers in biomedical fields, particularly in bandages or wound dressings, where softness and flexibility are important requirements. In contrast to diluted acetic acid, the concentrated formic acid employed in this approach not only serves as a solvent but also reacts with the amino groups in CS when subjected to heat (50 °C). This dual functionality had a more pronounced impact on disrupting the crystal structure of chitosan, inducing a decrease in strength and an increase in the elongation at break of CS fibers. Formic acid demonstrated its efficacy as a modifier, rendering CS chains more extendable and resulting in fibers with an enhanced elongation at break of 21.7%, surpassing the maximum elongation observed with the acetic acid solvent (approximately 9%) [131].
Moreover, ionic liquids have also been employed as alternative solvents for CS-based fibers, leveraging their excellent properties that provide environmental and safety benefits compared with conventional acidic solvent systems [129,134]. In 2013, a binary ionic liquid solvent containing glycine hydrochloride (Gly·HCl) along with BMIMCl was used for CS dissolution by Ma et al. [134]. The dissolved chitosan was extruded through a coagulation bath of ethanol, employing two distinct spinning techniques: wet spinning and dry–wet spinning. During coagulation, ethanol replaces BMIMCl in the forming fiber, contributing to the partial deprotonation of NH3+, forming the primary fibers. An additional neutralization bath is required for the complete regeneration of the primary fibers, since the residual NH3+ is completely deprotonated in the presence of NaOH. The dry–wet spinning method resulted in regenerated fibers characterized by a smooth surface and irregular shape, accompanied by high tenacity (2.1 cN/dtex), while yielded fibers with a striated surface and a round shape resulted from wet spinning, exhibiting lower tenacity in comparison (1.5 cN/dtex). The presence of an air gap in the dry–wet spinning enables an uniform extrusion of spinning drops, leading to a homogeneous structure without striations and exhibiting fewer flaws, which results in fibers with better mechanical performance [134]. In 2021, Kuznik and co-workers [129] obtained CS fibers via the wet spinning method using a different IL, 1-butyl-3-methylimidazolium acetate (BmimOAc), for dissolving three commercially available CS variants sourced from different suppliers, where each of them had varying molecular weights. The chitosan monofilaments exhibited a significantly lower tensile strength and Young’s modulus when compared with the binary ionic liquid solvent (Gly·HCl)-BMIMCl approach [134], with values ranging from 0.4 to 0.6 cN/dtex and 2.57 to 4.56 GPa, respectively [129]. However, Kuznik et al. [129] produced stretchy CS fibers, achieving a maximum elongation of 33.4% [129] compared with the 11.9% obtained with the binary IL solvent approach [134]. This approach is distinguished from the many others due to the utilization of a water coagulation bath and the absence of additional chemicals for solvent neutralization, with this alternative being more environmentally friendly [129]. In addition to Kuznik et al.’s [129] approach, several studies have shown that the mechanical properties of the resulting fibers are directly influenced by the molecular weight of the chitosan raw material [26]. Bentley et al. [135] introduced low molecular weight chitosan to a spinning dope containing a high molecular weight CS solution, and the resulting wet spun fibers showed a decrease in both tenacity and elongation at break. The tenacity was deeply affected by the addition of 30% short CS chains to the dope solution, decreasing from 2.2 to 1.4 cN/dtex for the same draw ratio (DR of 1.3), owing to a reduction in entanglement density, and consequently, a decrease in chain orientation between these physical cross-links within the amorphous phase in the solid state [135]. As described for other fibers, the chitosan concentration in the spinning dope significantly impacts the mechanical properties of fibers [129]. Higher concentrations lead to an enlarged number of molecules and chemical bonds among them, thus resulting in better tensile strength [129]. Kuznik et al. [129] demonstrated this phenomenon with two different commercial chitosans, where an increase in the concentration from 4 to 6 and from 6 to 8 improved the tenacity of the fibers from 4.4 to 6.1 cN/dtex (HMW chitosan) and from 4.5 to 5.1 cN/dtex (LMW chitosan), respectively. Furthermore, during the wet spinning process, drawing also stands out as the most widely used physical treatment for improving the strength of wet spun fibers. As shown in the study conducted by Bentley et al. [135], the authors evidenced an improvement in the tenacity from ~1.8 to 2.2 cN/dtex with the increase in the DR from 1.3 to 1.9.
4.4.3. Strategies for the Production of Chitin- and Chitosan-Based Fibers with Tailored Mechanical Properties
For addressing the insufficient mechanical properties of chitin fibers, Shamshina et al. [136] incorporated poly(lactic acid) (PLA) into the chitin dope solution using 1-ethyl-3-methylimidazolium acetate solvent prior to the dry-jet wet spinning. PLA was chosen owing to its favorable mechanical properties, contingent upon its molecular weight and degree of crystallinity. The resulting homogeneous blend of chitin/PLA fibers, with a chitin to PLA weight ratio of 1:0.3, exhibited elevated sb and eb, reaching 112 MPa and 8.8%, respectively. However, it is important to note that, when compared with other approaches, these improvements are still insufficient. Chitin fibers with higher tensile strength and elongation at break were achieved by using DMAc/LiCl as a solvent without the use of any additives in the dope solution [23]. However, a notable challenge associated to this solvent lies in the removal and recovery of lithium from fibers due to strong Lewis acid–base interactions between the lithium ions and the chitin chains [128].
In 2013, Wawro et al. [25] improved the fibers tenacity by producing highly porous dibutyrylchitin (DCB) fibers incorporating tri-calcium phosphate (β-TCP) or nano-hydroxyapatite (nano HAp). The porosity of the fibers was affected by the concentration of ethanol solvent in the dope solution and the pH of the coagulation bath (water or 5% ethanol), with higher porosity achieved at elevated ethanol concentrations and lower pH. The maximum porosity was attained at a pH of 2.0, resulting in a tenacity of 0.6 cN/dtex and an eb of approximately 8%. Moreover, the tenacity of highly porous DCB fibers ranged from 0.4 to 1.0 cN/dtex, depending on the ethanol concentration in the dope solution, the content of β-TCP or nano HAp, and the spinning conditions [25]. Thus, these versatile fibers are positioned as an attractive option for a wide range of applications.
With the aim of enhancing the mechanical performance of CS fibers obtained through wet spinning for applications involving weaving or knitting in biomedical contexts, solutions involving both physical and chemical treatments have been investigated. Concerning chemical treatment, techniques such as blending CS with suitable polymers, cross-linking, and the coating of fibers have been employed to solve the brittleness of CS fibers [131,134]. For example, SNFs were successfully blended with CS using 1.0 vol% acetic acid prior to wet spinning by Xiao et al. [28]. The abundant presence of polar amino acids on the surface of SNFs allows them to create a uniform dispersion within the CS solution. The tensile strength and elastic modulus of the prepared fibers were significantly higher in the nanocomposite than in the control CS fiber while elongation at break decreased. An outstanding improvement (approximately 293%) in the tensile strength of CS/SNF nanocomposite fibers was obtained for a mass ratio of 100/100 [28].
Granero et al. [27] produced polyion complex (PIC) fibers through wet spinning by employing two oppositely charged biopolymers, chitosan and gellan gum. The dope solution represented a polyelectrolyte solution, and the coagulation bath consisted of a polyelectrolyte solution with an opposite charge. In addition to the processing conditions (pH of the polyelectrolyte solution), the order of polymer addition, specifically the injection of gellan gum into CS or vice versa, showed a significant influence on the mechanical properties of the reinforced fibers. The strongest fibers were obtained when spinning dope gellan gum was injected into a CS coagulation bath. The post-processing treatment of the dried fibers further enhanced their mechanical performance. The tensile strength and Young’s modulus were particularly improved by spinning gellan gum into CS and applying post-treatment, while the strain at break remained essentially unchanged. The resulting fibers exhibited a tensile strength of 300 MPa, Young’s modulus of 11.3 GPa, and a strain at break of 6.6% [27].
Owing to the outstanding cell adhesive properties of CS resulting from its positive charge, Funakoshi et al. [133] suggested a hyaluronan (HA) coating for CS fibers for application as a scaffold in ligament tissue engineering, as HA has demonstrated its efficacy for enhancing healing in various tissues. The incorporation of a 0.1 mass fraction of HA in the third bath of the wet spinning setup resulted in the better mechanical properties of the hybrid polymer fibers, with a tensile strength of 217.6 MPa and an elastic modulus of 11.8 GPa. This represents a remarkable improvement of 70% and 71%, respectively, compared with neat CS fibers. The strain at failure increased with the addition of 0.05 wt.% of HA to 6.1% and then decreased with 0.1 wt.% of HA to 3.2%, these values being very small compared with the control group, polyglactin 910, which is a suture material [133]. In the biomedical applications domain, Wu et al. [132] prepared wet spun fibers comprising chitosan-polylactide/alginate for neural tissue engineering purposes. The core material consisted of CS/PLA fibers, with nerve growth factor (NGF) loaded onto these fibers through ALG coating layers. NGF is recognized as a crucial stimulator for promoting neuron survival, branching, and outgrowth. Both CS/PLA and CS/PLA/ALG fibers exhibited an enhancement in the mechanical properties compared with neat CS fibers [132].
4.5. Other Natural Polymers
In addition to the biopolymers discussed in the previous sections, there are other natural polymers with significant potential for wet spun fiber production, despite having received comparatively less attention in the scientific literature. Among these less explored materials are agar [137] and agarose [138], carrageenan [29,139,140], collagen [30,141,142], gelatin [143], keratin [31,144], and lignin [145,146], among others. These polysaccharides and proteins have intrinsic characteristics that make them promising for the fibers’ fabrication with desirable properties.
The great gel properties of agar make this material excellent for wet spinning, leveraging agar’s ability to form cold- and salt-setting reversible gels [137]. Fibers obtained with this polysaccharide showed significant flexibility, achieving a maximum eb of 104.9%, according to Liu et al. [137]. In 2023, Chen et al. [143], inspired by the salting-out effect utilized in Chinese tofu production, harnessed the fast and controllable gelation of gelatin to fabricate wet spun fibers, exhibiting an exceptional eb of 658.3%, and a high toughness of 4.4 MJ/m3. Moreover, carrageenan fibers have been successfully produced through wet spinning due to their inherent gelling properties using an aqueous solution of NaOH for dissolving this water-soluble polysaccharide [29,139,140]. In a recent study conducted by Foroughi et al. [147], ulvan, a sulfated polysaccharide known for its gelling properties, was employed with the purpose of enhancing the biological and mechanical characteristics of wet spun fibers, where a remarkable elongation at break of approximately 55% was achieved. This significant elongation underscores the promising potential of these fibers for several biomedical applications, including wound dressings and drug delivery systems.
Animal proteins, including collagen and keratin, have gathered attention from researchers due to their peculiar properties. Collagen, renowned for imparting mechanical stability, strength, and elasticity to native tissues, has found prominent applications in biomedicine, particularly in neural tissue engineering and guided bone regeneration [30,142]. In a study conducted by Deng et al. [142], bionic collagen fibers were prepared, aiming to mimic the ordered fibrillar arrays derived from the self-assembly of liquid crystalline collagen in natural tendons. The study harnessed the synergistic effects between the pre-orientation of liquid crystalline collagen and shearing orientation, aiming to achieve longitudinally aligned collagen fibrils. This approach resulted in collagen fibers with excellent wound suture performance, approaching the tensile strength and E observed in tendons [30,142].
In 2021, Togo et al. [148,149] developed regenerated fibers from α-1,3-glucan through the dry-jet wet spinning technique. This polysaccharide, known for its linear and crystalline structure, is enzymatically polymerized in vitro from sucrose [148]. The post-treatments involving solvent substitution with water and stretching resulted in the conversion of the anhydrous crystalline form of regenerated α-1,3-glucan fibers, initially coagulated in ethanol, into a hydrate form [148]. The subsequent drying of the fibers at 105 °C facilitated the reversion of the hydrated form to an anhydrous state [148]. These post-treatments played a pivotal role for enhancing the overall performance of α-1,3-glucan fibers, with a particular improvement in the Young’s modulus increasing from 3.5 GPa [149] to 11.0 GPa [148].
Despite the growing interest and utilization of several natural polymers for developing fibers, by using wet spinning technology, it is crucial to recognize that there is still significant work ahead to refine processes and optimize production conditions. A major limitation faced by these bio-based fibers is their mechanical weakness in comparison with synthetic-based counterparts. Therefore, ongoing efforts in research and development are imperative to overcome this drawback and strengthen the applicability of these innovative materials in a wide range of applications.
5. Brief Overview
This systematic literature review encapsulates the recent advancements in wet spun bio-based fibers, providing an overview of the significant progress in this domain. This review delves into the main challenges faced and highlights interesting strategies, with a particular emphasis on the improvement of mechanical properties.
Cellulose is the most used biopolymer for the production of naturally derived wet spun fibers. Regenerated CE fibers, through Ioncell technology, exhibited the highest tensile strength (1055 MPa; ~7.3 cN/dtex) [15], followed by the Lyocell process (4.6 cN/dtex) [16], and the alkali/urea system (3.4 cN/dtex) [150]. Cellulose-based fibers with improved multifunctionality and tailored mechanical properties have been successfully produced either by wet spinning or dry-jet wet spinning through the modification of CE with (nano)fillers (composites), and other polymers (blends) [151,152].
Regenerated silk fibers with a tensile strength up 615 MPa and strain of 57% were produced by a simple and eco-friendly methodology, which consisted of adding ultralow contents of directly exfoliated silk nanofibers (0.06 wt.%) from degummed Bombyx mori silk fibers in comparison with rSFs, promoted by a high degree of crystal orientation that mimics natural silk [61]. Despite these promising findings, the most effective strategy reported up to now for rSFs was through the incorporation of cellulose nanocrystals and combining with post-drawing, aiming at biomimicking the β-sheet crystallites present in natural silk, and giving rise to rSF fibers with the highest tensile strength (728 MPa) [64].
Other biopolymers, such as agar, alginate, chitin, chitosan, carrageenan, collagen, and gelatin-based fibers have shown significant potential for biomedical applications, where elongation at break is the most relevant mechanical property. Gelatin and agar fibers, for example, exhibited unusual and remarkable elongation at break. Alginate, chitin, and chitosan fibers, with tensile strength values of 2–4 cN/dtex, 1–2 cN/dtex, and 2 cN/dtex, respectively, have been investigated for applications.
6. Concluding Remarks
The potential effects of spinning solutions, spinning techniques, and parameters on the morphology and mechanical properties of bio-based fibers were identified and discussed. Some key factors should be considered in the wet spinning of these fibers, which include the following:
- The selection of the raw material, in which molecular weight is a crucial parameter;
- The conditions for dissolving the biopolymer (e.g., concentration and spinnability of the dope solution); specifically, the viscosity of the dope solution strongly affects the spinnability, and the mechanical performance of the resulting fibers;
- The spinning parameters (e.g., the extrusion speed, spinning temperature, air gap length);
- The composition and coagulation conditions.
Strategies, such as polymer blending, the incorporation of (nano)fillers, cross-linking in the coagulation bath, and the utilization of post-treatments (e.g., drawing and coating), have been explored, with the aim of producing bio-based fibers with enhanced mechanical properties.
Cellulose has emerged as the preferred material for bio-based fiber production through wet spinning technology. The exceptional tensile strength of cellulose-based fibers, produced by wet spinning, makes them highly attractive for several applications, such as multifunctional wearable sensors and energy devices, or for their conversion into CFs, among others. Cellulose, either alone or in combination with lignin, proves to be the most promising bio-based candidate for precursors in the production of sustainable CFs. Despite significant scientific and technologic advances, several efforts are still required to achieve the mechanical performance of PAN-based CFs (E ranging from 260 to 290 GPa, and s from 1700 to 2700 MPa for non-aerospace grades).
SF fibers rank as the second most employed biopolymer, aiming to mimic the structures found in natural spider silk. While challenges persist in achieving tensile strength and toughness comparable to dragline spider silk, significant strides have been made in the wet spinning production of these bio-based fibers. Potential high-performance SF fibers with high tensile strength, reaching 300–600 MPa, and elongation at break between 30 and 50% has already obtained. The usage of other biopolymers in wet spun fibers has also been investigated, although their current applications are still very limited due to their poor mechanical properties.
In the pursuit of sustainable and eco-friendly alternatives, this summary highlights the crucial contribution of wet spun natural fibers in shaping the future landscape of material science. Given the considerable research dedicated to advancing natural fibers for several applications, it is reasonable to anticipate ongoing notable progress in this domain.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fib12090075/s1, Table S1: Studies involving naturally sourced polymers, selected for inclusion.
Author Contributions
Ideation and implementation of the systematic review protocol, C.P. and T.V.P.; collection, analysis, and interpretation of data, C.P. and T.V.P.; writing—original draft preparation, C.P. and T.V.P.; writing—review and editing, R.M.S. and N.C.; funding acquisition: R.M.S. and N.C.; supervision: R.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Recovery and Resilience Plan (PRR) and European Union Funds Next Generation EU, through the CircularTech project (02/C05-i01.02/2022.PC644968678-00000054). We would like to express our gratitude to the PRR for their financial support and to the involved institutions for their collaboration and support. The authors acknowledge Fundação para a Ciência e a Tecnologia (FCT) for its financial support via the project UIDB/50022/2020 (LAETA Base Funding).
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shen, H.; Sun, T.; Zhou, J. Recent Progress in Regenerated Cellulose Fibers by Wet Spinning. Macromol. Mater. Eng. 2023, 308, 2300089. [Google Scholar] [CrossRef]
- Nocca, G.; Arcovito, A.; Elkasabgy, N.A.; Basha, M.; Giacon, N.; Mazzinelli, E.; Abdel-Maksoud, M.S.; Kamel, R. Cellulosic Textiles-An Appealing Trend for Different Pharmaceutical Applications. Pharmaceutics 2023, 15, 2738. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research, Inc. Synthetic Fibers Market Size, Share & Trends Analysis Report by Type (Acrylics, Polyester, Nylon), by Application (Clothing, Home Furnishing, Filtration), by Region, and Segment Forecasts, 2023–2030; Grand View Research, Inc.: San Francisco, CA, USA, 2018; p. 145. [Google Scholar]
- Tian, W.; Huang, K.; Zhu, C.; Sun, Z.; Shao, L.; Hu, M.; Feng, X. Recent Progress in Biobased Synthetic Textile Fibers. Front. Mater. 2022, 9, 1098590. [Google Scholar] [CrossRef]
- Levison, P.R. Ion Exchange|Isolation of Biopolymers. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Oxford, UK, 2005; pp. 481–484. [Google Scholar] [CrossRef]
- Deb, P.K.; Kokaz, S.F.; Abed, S.N.; Paradkar, A.; Tekade, R.K. Chapter 6—Pharmaceutical and Biomedical Applications of Polymers. In Basic Fundamentals of Drug Delivery: Advances in Pharmaceutical Product Development and Research; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 203–267. [Google Scholar] [CrossRef]
- Das, A.; Ringu, T.; Ghosh, S.; Pramanik, N. A Comprehensive Review on Recent Advances in Preparation, Physicochemical Characterization, and Bioengineering Applications of Biopolymers. Polym. Bull. 2023, 80, 7247–7312. [Google Scholar] [CrossRef] [PubMed]
- Rohani Shirvan, A.; Nouri, A.; Sutti, A. A Perspective on the Wet Spinning Process and its Advancements in Biomedical Sciences. Eur. Polym. J. 2022, 181, 111681. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated Cellulose by the Lyocell Process, a Brief Review of the Process and Properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- Li, K.; Ni, X.; Wu, Q.; Yuan, C.; Li, C.; Li, D.; Chen, H.; Lv, Y.; Ju, A. Carbon-Based Fibers: Fabrication, Characterization and Application. Adv. Fiber Mater. 2022, 4, 631–682. [Google Scholar] [CrossRef]
- Zahra, H.; Sawada, D.; Guizani, C.; Ma, Y.; Kumagai, S.; Yoshioka, T.; Sixta, H.; Hummel, M. Close Packing of Cellulose and Chitosan in Regenerated Cellulose Fibers Improves Carbon Yield and Structural Properties of Respective Carbon Fibers. Biomacromolecules 2020, 21, 4326–4335. [Google Scholar] [CrossRef]
- Lengsfeld, H.; Mainka, H.; Altstädt, V. Carbon Fibers. In Carbon Fibers; Springer: Berlin/Heidelberg, Germany, 2020; pp. 17–73. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Tominaga, K.; Gotoh, Y. High-strength Regenerated Cellulose Fibers Spun from 1-butyl-3-methylimidazolium Chloride Solutions. J. Appl. Polym. Sci. 2017, 134, 45551. [Google Scholar] [CrossRef]
- Zhou, L.; Kang, Z.; Nie, Y.; Li, L. Fabrication of Regenerated Cellulose Fibers with Good Strength and Biocompatibility from Green Spinning Process of Ionic Liquid. Macromol. Mater. Eng. 2021, 306, 2000741. [Google Scholar] [CrossRef]
- Elsayed, S.; Hummel, M.; Sawada, D.; Guizani, C.; Rissanen, M.; Sixta, H. Superbase-based Protic Ionic Liquids for Cellulose Filament Spinning. Cellulose 2021, 28, 533–547. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Q.; Yue, X.; Zuo, B.; Qin, M.; Li, F.; Kaplan, D.L.; Zhang, X. Regeneration of High-quality Silk Fibroin Fiber by Wet Spinning from CaCl2-formic Acid Solvent. Acta Biomater. 2015, 12, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Allardyce, B.J.; Rajkhowa, R.; Hegh, D.; Qin, S.; Usman, K.A.S.; Mota-Santiago, P.; Zhang, J.; Lynch, P.; Wang, X.; et al. Toughening Wet-Spun Silk Fibers by Silk Nanofiber Templating. Macromol. Rapid Commun. 2022, 43, e2100891. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, X.; Yu, H.; Ma, C.; Yao, J. Biomimicking the Structure of Silk Fibers via Cellulose Nanocrystal as β-Sheet Crystallite. RSC Adv. 2014, 4, 14304–14313. [Google Scholar] [CrossRef]
- Yan, M.; Shi, J.; Liu, L.; Zhu, H.; Tang, S.; Zhou, G.; Zeng, J.; Zhang, H.; Yu, Y.; Guo, J. Preparation of High-strength and High-toughness Sodium Alginate Fibers Based on the Study of Multi-ion Diffusion Kinetics in a Low Temperature Dissolution System. New J. Chem. 2021, 45, 5981–5991. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhang, C.; Zhu, P. Alginate/gelatin Blended Hydrogel Fibers Cross-linked by Ca2+ and Oxidized Starch: Preparation and Properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Zhao, T.; Li, X.; Gong, Y.; Guo, Y.; Quan, F.; Shi, Q. Study on Polysaccharide Polyelectrolyte Complex and Fabrication of Alginate/chitosan Derivative Composite Fibers. Int. J. Biol. Macromol. 2021, 184, 181–187. [Google Scholar] [CrossRef]
- Nguyen, K.D. Temperature Effect of Water Coagulation Bath on Chitin Fiber Prepared through Wet-Spinning Process. Polymers 2021, 13, 1909. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Q.; Zao, Y.; Ma, J.; Wan, Z.; Li, S.; Li, D.; Jin, Y. Mechanically Strong All-chitin Filaments: Wet-spinning of β-chitin Nanofibers in Aqueous NaOH. Int. J. Biol. Macromol. 2022, 222, 3243–3249. [Google Scholar] [CrossRef]
- Wawro, D.; Steplewski, W.; Komisarczyk, A.; Krucinska, I. Formation and Properties of Highly Porous Dibutyrylchitin Fibres Containing Nanoparticles. Fibres Text. East. Eur. 2013, 21, 31–37. [Google Scholar]
- Perrin, N.; Mohammadkhani, G.; Homayouni Moghadam, F.; Delattre, C.; Zamani, A. Biocompatible Fibers from Fungal and Shrimp Chitosans for Suture Application. Curr. Res. Biotechnol. 2022, 4, 530–536. [Google Scholar] [CrossRef]
- Granero, A.J.; Razal, J.M.; Wallace, G.G.; in het Panhuis, M. Mechanical Reinforcement of Continuous Flow Spun Polyelectrolyte Complex Fibers. Macromol. Biosci. 2009, 9, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, L.; You, H.; Zhou, S.; Feng, Y.; You, R. Silk Nanofibrils/chitosan Composite Fibers with Enhanced Mechanical Properties. Polym. Eng. Sci. 2023, 63, 379–386. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, Z.; Yan, M.; Liu, J.; Xia, Y. Effect of Epichlorohydrin on the Wet Spinning of Carrageenan Fibers under Optimal Parameter Conditions. Carbohydr. Polym. 2016, 150, 232–240. [Google Scholar] [CrossRef]
- Siriwardane, M.L.; DeRosa, K.; Collins, G.; Pfister, B.J. Controlled Formation of Cross-linked Collagen Fibers for Neural Tissue Engineering Applications. Biofabrication 2014, 6, 015012. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, N.; Li, S.; Zhang, L.; Tong, X.; Shao, Y.; Shen, C.; Wen, Y.; Jian, M.; Shao, Y.; et al. Reinforced Wool Keratin Fibers via Dithiol Chain Re-bonding. Adv. Funct. Mater. 2023, 33, 2213644. [Google Scholar] [CrossRef]
- Wang, H.; Gurau, G.; Rogers, R.D. Ionic Liquid Processing of Cellulose. Chem. Soc. Rev. 2012, 41, 1519–1537. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Zou, Y.; Mao, J.; Wang, L. Turning Industrial Waste-flax Noil into Regenerated Cellulose Fiber Electrodes for Eco-friendly Supercapacitors. Ind. Crops Prod. 2022, 176, 114377. [Google Scholar] [CrossRef]
- Jiang, Z.; Miao, J.; Yongqi, Y.; Zhang, L. Effective Preparation of Bamboo Cellulose Fibers in Quaternary Ammonium/DMSO Solvent. BioResources 2016, 11, 4536–4549. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Banitaba, S.N.; Khademolqorani, S.; Kamika, I.; Jadhav, V.V. Overlooked Promising Green Features of Electrospun Cellulose-Based Fibers in Lithium-Ion Batteries. ACS Omega 2023, 8, 43388–43407. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.S.F.; Prates, A.; Evtuguin, D.V. Production of Rayon Fibres from Cellulosic Pulps: State of the Art and Current Developments. Carbohydr. Polym. 2021, 273, 118466. [Google Scholar] [CrossRef] [PubMed]
- Ohwoavworhua, F.O.; Adelakun, T.A. Non-wood Fibre Production of Microcrystalline Cellulose from Sorghum caudatum: Characterisation and Tableting Properties. Indian J. Pharm. Sci. 2010, 72, 295–301. [Google Scholar] [CrossRef]
- Iwamoto, S.; Isogai, A.; Iwata, T. Structure and Mechanical Properties of Wet-Spun Fibers Made from Natural Cellulose Nanofibers. Biomacromolecules 2011, 12, 831–836. [Google Scholar] [CrossRef]
- Chen, J.; Guan, Y.; Wang, K.; Zhang, X.; Xu, F.; Sun, R. Combined Effects of Raw Materials and Solvent Systems on the Preparation and Properties of Regenerated Cellulose Fibers. Carbohydr. Polym. 2015, 128, 147–153. [Google Scholar] [CrossRef]
- Zhu, C.; Koutsomitopoulou, A.F.; Eichhorn, S.J.; van Duijneveldt, J.S.; Richardson, R.M.; Nigmatullin, R.; Potter, K.D. High Stiffness Cellulose Fibers from Low Molecular Weight Microcrystalline Cellulose Solutions Using DMSO as Co-Solvent with Ionic Liquid. Macromol. Mater. Eng. 2018, 303, 1800029. [Google Scholar] [CrossRef]
- Rana, S.; Pichandi, S.; Parveen, S.; Fangueiro, R. Regenerated Cellulosic Fibers and Their Implications on Sustainability. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Muthu, S.S., Ed.; Springer: Singapore, 2014; pp. 239–276. [Google Scholar] [CrossRef]
- Khan, G.M.A.; Hasan, M.S.; Rahaman, M.H.; Aydid, A.; Rahman, M.M.; Hasanuzzaman, M.; Jahan, R.; Jannat-Al-Foisal, M. Cellulose and Its Composites in Textiles and Food Industry. In Regenerated Cellulose and Composites: Morphology-Property Relationship; Shabbir, M., Ed.; Springer: Singapore, 2023; pp. 223–264. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A Critical Review of Manufacturing Processes Used in Regenerated Cellulosic Fibres: Viscose, Cellulose Acetate, Cuprammonium, LiCl/DMAc, Ionic Liquids, and NMMO Based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Shaikh, T.; Chaudhari, S.; Varma, A. Viscose Rayon: A Legendary Development in the Manmade Textile. Int. J. Eng. Res. Appl. 2012, 2, 675–680. [Google Scholar]
- Tu, H.; Li, X.; Liu, Y.; Luo, L.; Duan, B.; Zhang, R. Recent Progress in Regenerated Cellulose-based Fibers from Alkali/urea System via Spinning Process. Carbohydr. Polym. 2022, 296, 119942. [Google Scholar] [CrossRef]
- Gross, A.S.; Bell, A.T.; Chu, J.W. Preferential interactions between lithium chloride and glucan chains in N,N-dimethylacetamide drive cellulose dissolution. J. Phys. Chem. B 2013, 117, 3280–3286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tominaga, K.; Yamagishi, N.; Gotoh, Y. Comparison of Regenerated Cellulose Fibers Spun from Ionic Liquid Solutions with Lyocell Fiber. J. Fiber Sci. Technol. 2020, 76, 257–266. [Google Scholar] [CrossRef]
- Asaadi, S.; Kakko, T.; King, A.W.T.; Kilpeläinen, I.; Hummel, M.; Sixta, H. High-Performance Acetylated Ioncell-F Fibers with Low Degree of Substitution. ACS Sustain. Chem. Eng. 2018, 6, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Stepan, A.M.; Michud, A.; Helisten, S.; Hummel, M.; Sixta, H. IONCELL-P&F: Pulp Fractionation and Fiber Spinning with Ionic Liquids. Ind. Eng. Chem. Res. 2016, 55, 8225–8233. [Google Scholar] [CrossRef]
- Vocht, M.P.; Beyer, R.; Thomasic, P.; Müller, A.; Ota, A.; Hermanutz, F.; Buchmeiser, M.R. High-performance Cellulosic Filament Fibers Prepared via Dry-jet Wet Spinning from Ionic Liquids. Cellulose 2021, 28, 3055–3067. [Google Scholar] [CrossRef]
- Ma, Y.; You, X.; Nieminen, K.; Sawada, D.; Sixta, H. Influence of DP and MMD of the pulps used in the Ioncell® process on processability and fiber properties. RSC Sustain. 2023, 1, 1497–1510. [Google Scholar] [CrossRef]
- Rissanen, M.; Schlapp-Hackl, I.; Sawada, D.; Raiskio, S.; Ojha, K.; Smith, E.; Sixta, H. Chemical Recycling of Hemp Waste Textiles via the Ionic Liquid based Dry-jet-wet Spinning Technology. Text. Res. J. 2022, 93, 2545–2557. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Nypelö, T.; Asaadi, S.; Kneidinger, G.; Sixta, H.; Konnerth, J. Conversion of Wood-biopolymers into Macrofibers with Tunable Surface Energy via Dry-jet Wet-spinning. Cellulose 2018, 25, 5297–5307. [Google Scholar] [CrossRef]
- Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength Enhancement of Regenerated Cellulose Fibers by Adjustment of Hydrogen Bond Distribution in Ionic Liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Zhang, J.; He, J. 1-Allyl-3-methylimidazolium Chloride Room Temperature Ionic Liquid: A New and Powerful Nonderivatizing Solvent for Cellulose. Macromolecules 2005, 38, 8272–8277. [Google Scholar] [CrossRef]
- Wang, B.; Nie, Y.; Kang, Z.; Liu, X. Effects of Coagulating Conditions on the Crystallinity, Orientation and Mechanical Properties of Regenerated Cellulose Fibers. Int. J. Biol. Macromol. 2023, 225, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yuan, Y.; Wang, B.; Yin, X.; Mukuze, K.S.; Huang, W.; Zhang, Y.; Wang, H. Analysis of Regenerated Cellulose Fibers with Ionic Liquids as a Solvent as Spinning Speed is Increased. Cellulose 2012, 19, 1075–1083. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose Dissolution with Polar Ionic Liquids under Mild Conditions: Required Factors for Anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Li, R.; Chang, C.; Zhou, J.; Zhang, L.; Gu, W.; Li, C.; Liu, S.; Kuga, S. Primarily Industrialized Trial of Novel Fibers Spun from Cellulose Dope in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 11380–11384. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, L.; Cai, J.; Zhou, J.; Kondo, T. Effects of Coagulation Conditions on Properties of Multifilament Fibers Based on Dissolution of Cellulose in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2008, 47, 8676–8683. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Dawelbeit, A.; Deng, Y.; Lang, Y.; Yu, M. Structure and Properties of Regenerated Cellulose Fibers from Aqueous NaOH/thiourea/urea Solution. Cellulose 2017, 24, 4123–4137. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Lang, Y.; Yu, M. Structure Development in the Condensed State of Cellulose Fiber Regenerated from Alkali Complex Solution. Cellulose 2018, 25, 1555–1569. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Zhang, S.; Li, F.; Yu, J.; Lin, J. Structure and Properties of Novel Regenerated Cellulose Fibers Prepared in NaOH Complex Solution. Carbohydr. Polym. 2013, 98, 1031–1038. [Google Scholar] [CrossRef]
- Ruan, D.; Zhang, L.N.; Zhou, J.P.; Jin, H.M.; Chen, H. Structure and Properties of Novel Fibers Spun from Cellulose in NaOH/thiourea Aqueous Solution. Macromol. Biosci. 2004, 4, 1105–1112. [Google Scholar] [CrossRef]
- Tu, H.; Xie, K.; Ying, D.; Luo, L.; Liu, X.; Chen, F.; Duan, B.; Fu, Q.; Zhang, L. Green and Economical Strategy for Spinning Robust Cellulose Filaments. ACS Sustain. Chem. Eng. 2020, 8, 14927–14937. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-friendly Post-consumer Cotton Waste Recycling for Regenerated Cellulose Fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kamida, K.; Okajima, K.; Matsui, T.; Kowsaka, K. Study on the Solubility of Cellulose in Aqueous Alkali Solution by Deuteration IR and 13C NMR. Polym. J. 1984, 16, 857–866. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L. Solubility of Cellulose in NaOH/Urea Aqueous Solution. Polym. J. 2000, 32, 866–870. [Google Scholar] [CrossRef]
- Coscia, M.G.; Bhardwaj, J.; Singh, N.; Santonicola, M.G.; Richardson, R.; Thakur, V.K.; Rahatekar, S. Manufacturing & Characterization of Regenerated Cellulose/curcumin Based Sustainable Composites Fibers Spun from Environmentally Benign Solvents. Ind. Crops Prod. 2018, 111, 536–543. [Google Scholar] [CrossRef]
- Makarov, I.; Vinogradov, M.; Gromovykh, T.; Lutsenko, S.; Feldman, N.; Shambilova, G.; Sadykova, V. Antifungal composite fibers based on cellulose and betulin. Fibers 2018, 6, 23. [Google Scholar] [CrossRef]
- Ma, Y.; Guo, J.; Zhao, M.; Gong, Y.; Qiao, B. Effect of Coagulation Bath Temperature on Mechanical, Morphological, and Thermal Properties of Cellulose/Antarctic Krill Protein Composite Fibers. Langmuir 2020, 36, 5647–5653. [Google Scholar] [CrossRef]
- Kim, H.C.; Panicker, P.S.; Kim, D.; Adil, S.; Kim, J. High-strength Cellulose Nanofiber/graphene Oxide Hybrid Filament Made by Continuous Processing and Its Humidity Monitoring. Sci. Rep. 2021, 11, 13611. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Yu, H.-Y.; Abdalkarim, S.Y.H.; Zhou, J.; Yao, J.; Zhang, L. Continuous Meter-Scale Wet-Spinning of Cornlike Composite Fibers for Eco-Friendly Multifunctional Electronics. ACS Appl. Mater. Interfaces 2021, 13, 40953–40963. [Google Scholar] [CrossRef]
- Biswas, M.C.; Bush, B.; Ford, E. Glucaric Acid Additives for the Antiplasticization of Fibers Wet Spun from Cellulose Acetate/acetic acid/water. Carbohydr. Polym. 2020, 245, 116510. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Chen, D.; Yu, Y.; Miao, J.; Liu, Y.; Zhang, L. Composite Fibers Prepared from Multi-walled Carbon Nanotubes/cellulose Dispersed/dissolved in Ammonium/dimethyl Sulfoxide Mixed Solvent. RSC Adv. 2017, 7, 2186–2192. [Google Scholar] [CrossRef]
- Wan, Z.; Chen, C.; Meng, T.; Mojtaba, M.; Teng, Y.; Feng, Q.; Li, D. Multifunctional Wet-Spun Filaments through Robust Nanocellulose Networks Wrapping to Single-Walled Carbon Nanotubes. ACS Appl. Mater. Interfaces 2019, 11, 42808–42817. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Chen, B.; Peng, X.; Kuang, T. Strength and Modulus Improvement of Wet-spun Cellulose I Filaments by Sequential Physical and Chemical Cross-linking. Mater. Des. 2017, 136, 45–53. [Google Scholar] [CrossRef]
- Marsano, E.; Corsini, P.; Canetti, M.; Freddi, G. Regenerated Cellulose-silk Fibroin Blends Fibers. Int. J. Biol. Macromol. 2008, 43, 106–114. [Google Scholar] [CrossRef]
- Bengtsson, A.; Landmer, A.; Norberg, L.; Yu, S.; Ek, M.; Brannvall, E.; Sedin, M. Carbon Fibers from Wet-Spun Cellulose-Lignin Precursors Using the Cold Alkali Process. Fibers 2022, 10, 108. [Google Scholar] [CrossRef]
- Yazawa, K.; Malay, A.D.; Ifuku, N.; Ishii, T.; Masunaga, H.; Hikima, T.; Numata, K. Combination of Amorphous Silk Fiber Spinning and Postspinning Crystallization for Tough Regenerated Silk Fibers. Biomacromolecules 2018, 19, 2227–2237. [Google Scholar] [CrossRef]
- Huang, L.; Shi, J.; Zhou, W.; Zhang, Q. Advances in Preparation and Properties of Regenerated Silk Fibroin. Int. J. Mol. Sci. 2023, 24, 13153. [Google Scholar] [CrossRef]
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-chain, L-chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528. [Google Scholar] [CrossRef]
- Yan, J.; Zhou, G.; Knight, D.P.; Shao, Z.; Chen, X. Wet-Spinning of Regenerated Silk Fiber from Aqueous Silk Fibroin Solution: Discussion of Spinning Parameters. Biomacromolecules 2010, 11, 1–5. [Google Scholar] [CrossRef]
- Ling, S.; Zhou, L.; Zhou, W.; Shao, Z.; Chen, X. Conformation Transition Kinetics and Spinnability of Regenerated Silk Fibroin with Glycol, Glycerol and Polyethylene Glycol. Mater. Lett. 2012, 81, 13–15. [Google Scholar] [CrossRef]
- Wang, H.; Dong, Q.; Yao, J.; Shao, Z.; Ma, J.; Chen, X. Colorless Silk/Copper Sulfide Hybrid Fiber and Fabric with Spontaneous Heating Property under Sunlight. Biomacromolecules 2020, 21, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Zheng, Z.; Yao, J.; Chen, M.; Tang, Y.; Zhong, J.; Qi, Z.; Li, Z.; Shao, Z.; Chen, X. Tough Protein-carbon Nanotube Hybrid Fibers Comparable to Natural Spider Silks. J. Mater. Chem. B 2015, 3, 3940–3947. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Cai, J.; Wang, H.; Chen, S.; Liu, Y.; Yao, J.; Shao, Z.; Chen, X. Artificial Ligament Made from Silk Protein/Laponite Hybrid Fibers. Acta Biomater. 2020, 106, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, W.; Zou, H.; Ke, G.; Li, W.; Ouyang, C. Feasibility of Wet Spinning of Silk-Inspired Polyurethane Elastic Biofiber. Mater. Lett. 2008, 62, 1949–1952. [Google Scholar] [CrossRef]
- Li, X.; Ming, J.; Ning, X. Wet-spun Conductive Silk Fibroin-polyaniline Filaments Prepared from a Formic Acid-shell Solution. J. Appl. Polym. Sci. 2019, 136, 47127. [Google Scholar] [CrossRef]
- Gulrajanid, M.L.; Sethi, S.; Gupta, S. Some Studies in Degumming of Silk with Organic Acids. J. Soc. Dye. Colour. 1992, 108, 79–86. [Google Scholar] [CrossRef]
- Prathumpai, W.; Promboon, A.; Werapan, B.; Nutaratat, P.; Chim-Anek, P.; Ninpetch, U. Pilot-Scale Protease Production by Bacillus sp. C4 for Silk Degumming Processes. J. Nat. Fibers 2022, 19, 1055–1068. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Y.; Gong, K.; Zhou, Q.; Zhang, T.; Li, Q. A Comparative Study of Ultrasonic Degumming of Silk Sericin using Citric Acid, Sodium Carbonate and Papain. Color. Technol. 2019, 135, 195–201. [Google Scholar] [CrossRef]
- Marsano, E.; Corsini, P.; Arosio, C.; Boschi, A.; Mormino, M.; Freddi, G. Wet Spinning of Bombyx mori Silk Fibroin Dissolved in N-methyl Morpholine N-oxide and Properties of Regenerated Fibres. Int. J. Biol. Macromol. 2005, 37, 179–188. [Google Scholar] [CrossRef]
- Wöltje, M.; Isenberg, K.L.; Cherif, C.; Aibibu, D. Continuous Wet Spinning of Regenerated Silk Fibers from Spinning Dopes Containing 4% Fibroin Protein. Int. J. Mol. Sci. 2023, 24, 13492. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Masuda, H.; Zhao, C.; Asakura, T. Artificial Spinning and Characterization of Silk Fiber from Bombyx mori Silk Fibroin in Hexafluoroacetone Hydrate. Macromolecules 2002, 35, 6–9. [Google Scholar] [CrossRef]
- Ha, S.-W.; Tonelli, A.E.; Hudson, S.M. Structural Studies of Bombyx mori Silk Fibroin during Regeneration from Solutions and Wet Fiber Spinning. Biomacromolecules 2005, 6, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, H.; Zhang, M.; Hou, Z.; Li, S.; Wang, H.; Wu, X.-E.; Zhang, Y. In Situ Mineralizing Spinning of Strong and Tough Silk Fibers for Optical Waveguides. ACS Nano 2023, 17, 5905–5912. [Google Scholar] [CrossRef]
- Zhang, M.; Weng, Y.-J.; Zhang, Y.-Q. Accelerated Desalting and Purification of Silk Fibroin in a CaCl2-EtOH-H2O Ternary System by Excess Isopropanol Extraction. J. Chem. Technol. Biotechnol. 2021, 96, 1176–1186. [Google Scholar] [CrossRef]
- Yazawa, K.; Iwata, S.; Gotoh, Y. Wild Silkworm Cocoon Waste Conversion into Tough Regenerated Silk Fibers by Solution Spinning. Biomacromolecules 2023, 24, 1700–1708. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, M.; Meng, Q. Wet Spinning is Employed to Produce Spider Silk with High Elasticity. APL Mater. 2023, 11, 081110. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, C.-F.; Gan, C.-Y.; Xia, X.-X.; Qian, Z.-G. Functionalization and Reinforcement of Recombinant Spider Dragline Silk Fibers by Confined Nanoparticle Formation. ACS Biomater. Sci. Eng. 2022, 8, 3299–3309. [Google Scholar] [CrossRef]
- Weatherbee-Martin, N.; Xu, L.; Hupe, A.; Kreplak, L.; Fudge, D.S.; Liu, X.-Q.; Rainey, J.K. Identification of Wet-Spinning and Post-Spin Stretching Methods Amenable to Recombinant Spider Aciniform Silk. Biomacromolecules 2016, 17, 2737–2746. [Google Scholar] [CrossRef]
- Ainul Hafiza, A.H.; Khairunnisa-Atiqah, M.K.; Mazlan, N.S.N.; Salleh, K.M.; Zakaria, S. Chapter 14—Biocompatible and Biodegradable Materials in Medical Applications. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, D., Altalhi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 331–358. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, Q.; Li, W.; Xu, W.; Yang, H. Preparation of Wet-spun Polysaccharide Fibers from Chinese Medicinal Bletilla striata. Mater. Lett. 2014, 117, 208–210. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, W.; Ni, B.-J. Chapter 3—Algae-based Alginate Biomaterial: Production and Applications. In Biomass, Biofuels, and Biochemicals; Ngo, H., Guo, W., Pandey, A., Chang, J.-S., Lee, D.-J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 37–66. [Google Scholar] [CrossRef]
- Skjåk-Bræk, G.; Draget, K.I. 10.10—Alginates: Properties and Applications. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 213–220. [Google Scholar] [CrossRef]
- Alistair, M.; Stephen, G.O.P. Food Polysaccharides and Their Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Schoukens, G. 5—Bioactive Dressings to Promote Wound Healing. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 135–167. [Google Scholar] [CrossRef]
- Aneem, T.H.; Wong, S.Y.; Afrin, H.; Nurunnabi, M.; Li, X.; Arafat, M.T. Investigation of Coagulation Process of Wet-spun Sodium Alginate Polymannuronate Fibers with Varied Functionality using Organic Coagulants and Cross-linkers. Mater. Today Chem. 2021, 22, 100580. [Google Scholar] [CrossRef]
- Dodero, A.; Alberti, S.; Gaggero, G.; Ferretti, M.; Botter, R.; Vicini, S.; Castellano, M. An Up-to-Date Review on Alginate Nanoparticles and Nanofibers for Biomedical and Pharmaceutical Applications. Adv. Mater. Interfaces 2021, 8, 2100809. [Google Scholar] [CrossRef]
- Inoue, A. Chapter Sixteen—Characterization of PL-7 Family Alginate Lyases From Marine Organisms and Their Applications. In Methods in Enzymology; Moore, B.S., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 605, pp. 499–524. [Google Scholar]
- Zhang, X.; Wang, X.; Fan, W.; Liu, Y.; Wang, Q.; Weng, L. Fabrication, Property and Application of Calcium Alginate Fiber: A Review. Polymers 2022, 14, 3227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Geng, C.; Zhao, X.; Xue, Z.; Quan, F.; Xia, Y. Preparation of CdTe/Alginate Textile Fibres with Controllable Fluorescence Emission through a Wet-Spinning Process and Application in the Trace Detection of Hg2+ Ions. Nanomaterials 2019, 9, 570. [Google Scholar] [CrossRef] [PubMed]
- Khajavi, R.; Abbasipour, M.; Barzi, M.G.; Rashidi, A.; Rahimi, M.K.; Mirzababa, H.H. Eucalyptus Essential Oil-Doped Alginate Fibers as a Potent Antibacterial Wound Dressing. Adv. Polym. Technol. 2014, 33, 21408. [Google Scholar] [CrossRef]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Thomas, S.; Balakrishnan, P.; Sreekala, S.M. Fundamental Biomaterials. In Fundamental Biomaterials: Polymers; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Frent, O.D.; Vicas, L.G.; Duteanu, N.; Morgovan, C.M.; Jurca, T.; Pallag, A.; Muresan, M.E.; Filip, S.M.; Lucaciu, R.; Marian, E. Physico-Chemical Properties of Sodium Alginate. Available online: https://encyclopedia.pub/entry/29966 (accessed on 11 February 2024).
- Silva, M.P.; Badruddin, I.J.; Tonon, T.; Rahatekar, S.; Gomez, L.D. Environmentally Benign Alginate Extraction and Fibres Spinning from Different European Brown Algae Species. Int. J. Biol. Macromol. 2023, 226, 434–442. [Google Scholar] [CrossRef]
- Li, S.; Chandra Biswas, M.; Ford, E. Dual Roles of Sodium Polyacrylate in Alginate Fiber Wet-spinning: Modify the Solution Rheology and Strengthen the Fiber. Carbohydr. Polym. 2022, 297, 120001. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Liu, Y.; Zhang, G.; Zhu, P. Characterization and Functional Assessment of Alginate Fibers Prepared by Metal-calcium Ion Complex Coagulation Bath. Carbohydr. Polym. 2020, 232, 115693. [Google Scholar] [CrossRef]
- Inamuddin; Ahamed, M.I.; Boddula, R.; Altalhi, T. Polysaccharides—Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Kuddus, M.; Aguilar, C.N. Value-Addition in Food Products and Processing through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Van Den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan—Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Mather, R.R.; Wardman, R.H. Chapter 2—Cellulosic Fibres. In Chemistry of Textile Fibres, 2nd ed.; Royal Society of Chemistry: London, UK, 2015; pp. 25–66. [Google Scholar]
- Huang, J.; Zhong, Y.; Zhang, X.; Xu, H.; Zhu, C.; Cai, J. Continuous Pilot-Scale Wet-Spinning of Biocompatible Chitin/Chitosan Multifilaments from an Aqueous KOH/Urea Solution. Macromol. Rapid Commun. 2021, 42, 2100252. [Google Scholar] [CrossRef]
- Kuznik, I.; Kruppke, I.; Cherif, C. Pure Chitosan-Based Fibers Manufactured by a Wet Spinning Lab-Scale Process Using Ionic Liquids. Polymers 2022, 14, 477. [Google Scholar] [CrossRef] [PubMed]
- Jafri, N.F.; Mohd Salleh, K.; Ahmad Ghazali, N.; Nyak Mazlan, N.S.; Ab Halim, N.H.; Zakaria, S. Effects of Carboxymethyl Cellulose Fiber Formations with Chitosan Incorporation via Coating and Mixing Processes. Int. J. Biol. Macromol. 2023, 253, 126971. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Hu, C.; Sun, F.; Gustave, W.; Tian, H.; Yang, S. Flexible Fibers Wet-spun from Formic Acid Modified Chitosan. Carbohydr. Polym. 2016, 136, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Liu, J.; Fang, Q.; Xiao, B.; Wan, Y. Establishment of Nerve Growth Factor Gradients on Aligned Chitosan-polylactide/alginate Fibers for Neural Tissue Engineering Applications. Colloids Surf. B 2017, 160, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Majima, T.; Iwasaki, N.; Yamane, S.; Masuko, T.; Minami, A.; Harada, K.; Tamura, H.; Tokura, S.; Nishimura, S.-I. Novel Chitosan-based Hyaluronan Hybrid Polymer Fibers as a Scaffold in Ligament Tissue Engineering. J. Biomed. Mater. Res. A 2005, 74, 338–346. [Google Scholar] [CrossRef]
- Ma, B.; Qin, A.; Li, X.; He, C. High Tenacity Regenerated Chitosan Fibers Prepared by Using the Binary Ionic Liquid Solvent (Gly•HCl)-[Bmim]Cl. Carbohydr. Polym. 2013, 97, 300–305. [Google Scholar] [CrossRef]
- Bentley, F.E.; Passieux, R.; David, L.; Osorio-Madrazo, A. Pure Chitosan Biomedical Textile Fibers from Mixtures of Low- and High-Molecular Weight Bidisperse Polymer Solutions: Processing and Understanding of Microstructure-Mechanical Properties’ Relationship. Int. J. Mol. Sci. 2022, 23, 4767. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Zavgorodnya, O.; Berton, P.; Chhotaray, P.K.; Choudhary, H.; Rogers, R.D. Ionic Liquid Platform for Spinning Composite Chitin-Poly(lactic acid) Fibers. ACS Sustain. Chem. Eng. 2018, 6, 10241–10251. [Google Scholar] [CrossRef]
- Liu, J.; Xue, Z.; Zhang, W.; Yan, M.; Xia, Y. Preparation and Properties of Wet-spun Agar Fibers. Carbohydr. Polym. 2018, 181, 760–767. [Google Scholar] [CrossRef]
- Bao, X.; Hayashi, K.; Li, Y.; Teramoto, A.; Abe, K. Novel Agarose and Agar Fibers: Fabrication and Characterization. Mater. Lett. 2010, 64, 2435–2437. [Google Scholar] [CrossRef]
- Dong, M.; Xue, Z.; Liu, J.; Yan, M.; Xia, Y.; Wang, B. Preparation of Carrageenan Fibers with Extraction of Chondrus via Wet Spinning Process. Carbohydr. Polym. 2018, 194, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wu, Y.; Zhang, Q.; Xue, Z.; Geng, C.; Xia, Y. Study on the Mechanical and Flame Retardant Properties of a Novel Carrageenan Fiber-CAF-K,Al. Eur. Polym. J. 2021, 150, 110408. [Google Scholar] [CrossRef]
- Arafat, M.T.; Tronci, G.; Yin, J.; Wood, D.J.; Russell, S.J. Biomimetic Wet-stable Fibres via Wet Spinning and Diacid-based Crosslinking of Collagen Triple Helices. Polymer 2015, 77, 102–112. [Google Scholar] [CrossRef]
- Deng, F.; Dang, Y.; Tang, L.; Hu, T.; Ding, C.; Hu, X.; Wu, H.; Chen, L.; Huang, L.; Ni, Y.; et al. Tendon-inspired Fibers from Liquid Crystalline Collagen as The Pre-oriented Bioink. Int. J. Biol. Macromol. 2021, 185, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, S.; Qiao, D.; Wang, J.; Zhou, Q.; Wu, C.; Li, X.; Neisiany, R.E.; Sun, L.; Liu, Y.; et al. Chinese Tofu-Inspired Biomimetic Conductive and Transparent Fibers for Biomedical Applications. Small Methods 2023, 7, 2201604. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Qiao, X.; Hou, X.; Yang, Y. Pure Keratin Membrane and Fibers from Chicken Feather. Int. J. Biol. Macromol. 2016, 89, 614–621. [Google Scholar] [CrossRef]
- Yang, S.M.; Shaffer, M.S.P.; Brandt-Talbot, A. High Lignin Content Carbon Fiber Precursors Wet-Spun from Low-Cost Ionic Liquid Water Mixtures. ACS Sustain. Chem. Eng. 2023, 11, 8800–8811. [Google Scholar] [CrossRef]
- Wang, L.; Ago, M.; Borghei, M.; Ishaq, A.; Papageorgiou, A.C.; Lundahl, M.; Rojas, O.J. Conductive Carbon Microfibers Derived from Wet-Spun Lignin/Nanocellulose Hydrogels. ACS Sustain. Chem. Eng. 2019, 7, 6013–6022. [Google Scholar] [CrossRef]
- Foroughi, J.; Ruhparwar, A.; Aloko, S.; Wang, C.H. Manufacturing Ulvan Biopolymer for Wound Dressings. Macromol. Mater. Eng. 2024, 309, 2300268. [Google Scholar] [CrossRef]
- Togo, A.; Suzuki, S.; Kimura, S.; Iwata, T. High Tensile Strength Regenerated α-1,3-Glucan Fiber and Crystal Transition. ACS Omega 2021, 6, 20361–20368. [Google Scholar] [CrossRef]
- Togo, A.; Suzuki, S.; Kimura, S.; Iwata, T. Wet Spinning and Structure Analysis of α-1,3-Glucan Regenerated Fibers. ACS Appl. Polym. Mater. 2021, 3, 2063–2069. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, K.; Zhou, X.; Luo, L.; Zeng, J.; Huang, R.; Lu, A.; Liu, X.; Chen, F.; Zhang, L.; et al. Influences of Coagulation Conditions on the Structure and Properties of Regenerated Cellulose Filaments via Wet-Spinning in LiOH/Urea Solvent. ACS Sustain. Chem. Eng. 2018, 6, 4056–4067. [Google Scholar] [CrossRef]
- Kammiovirta, K.; Jääskeläinen, A.-S.; Kuutti, L.; Holopainen-Mantila, U.; Paananen, A.; Suurnäkki, A.; Orelma, H. Keratin-reinforced Cellulose Filaments from Ionic Liquid Solutions. RSC Adv. 2016, 6, 88797–88806. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, L.; Gao, X.; Zhang, W.; Ma, J.; Zhang, L. Solvent Regulation Approach for Preparing Cellulose-Nanocrystal-Reinforced Regenerated Cellulose Fibers and Their Properties. ACS Omega 2019, 4, 2001–2008. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).