Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospun Magnetic Fibers (EMFs) Processing
2.3. Electrospun Magnetic Fibers (EMFs) Characterization
2.4. Electrospun Magnetic Fibers (EMFs) Release Evaluation
3. Results and Discussion
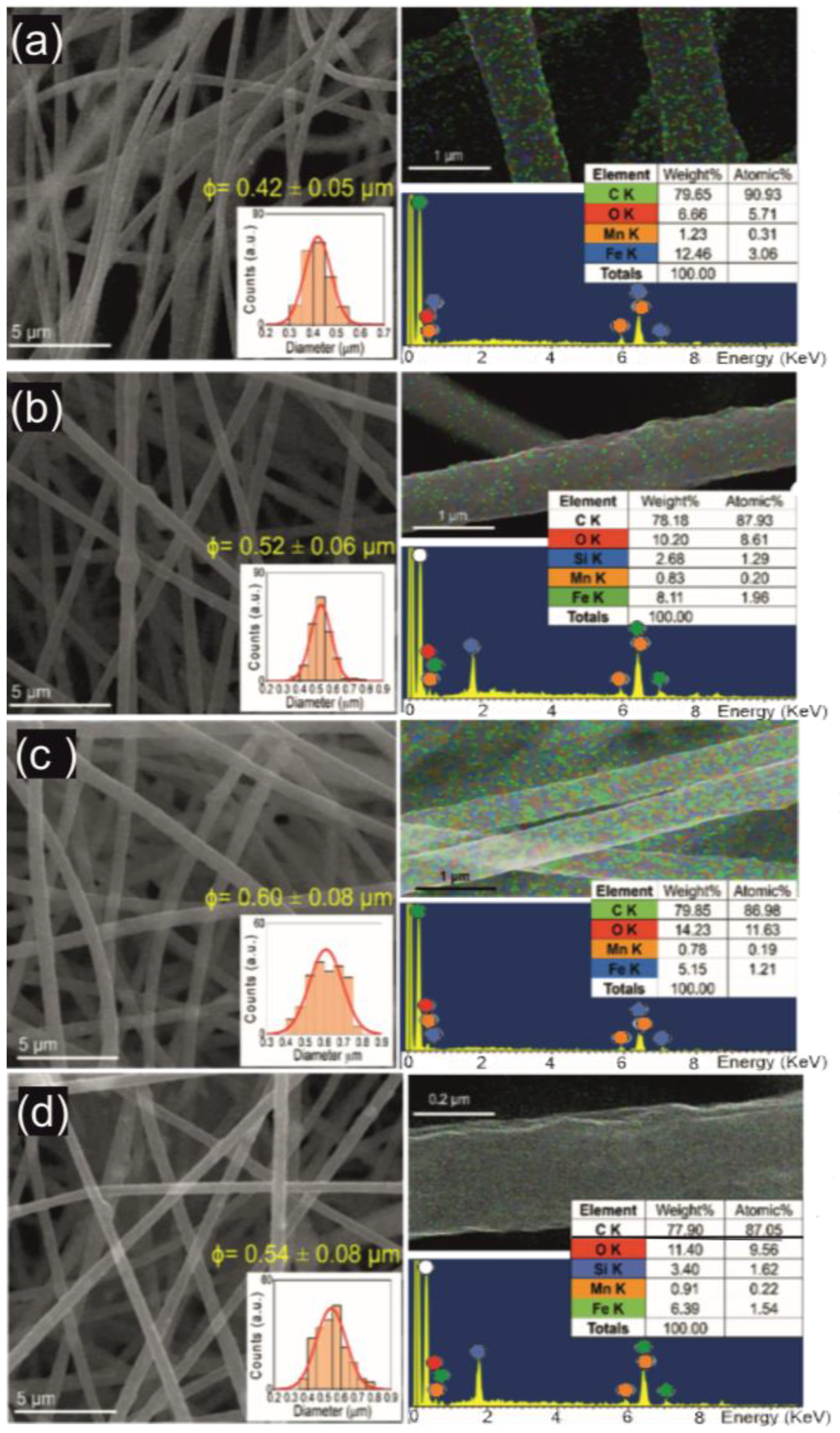
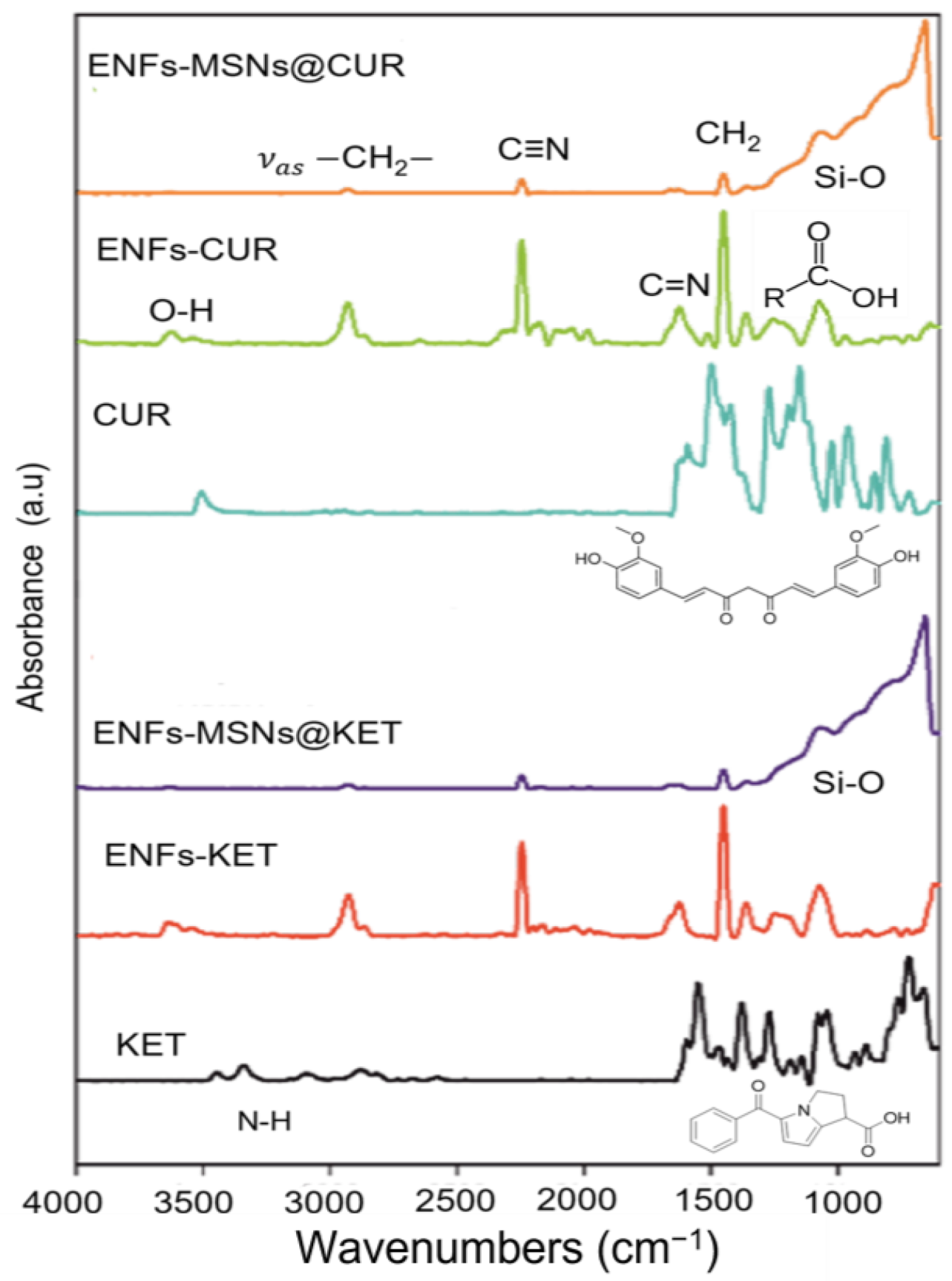
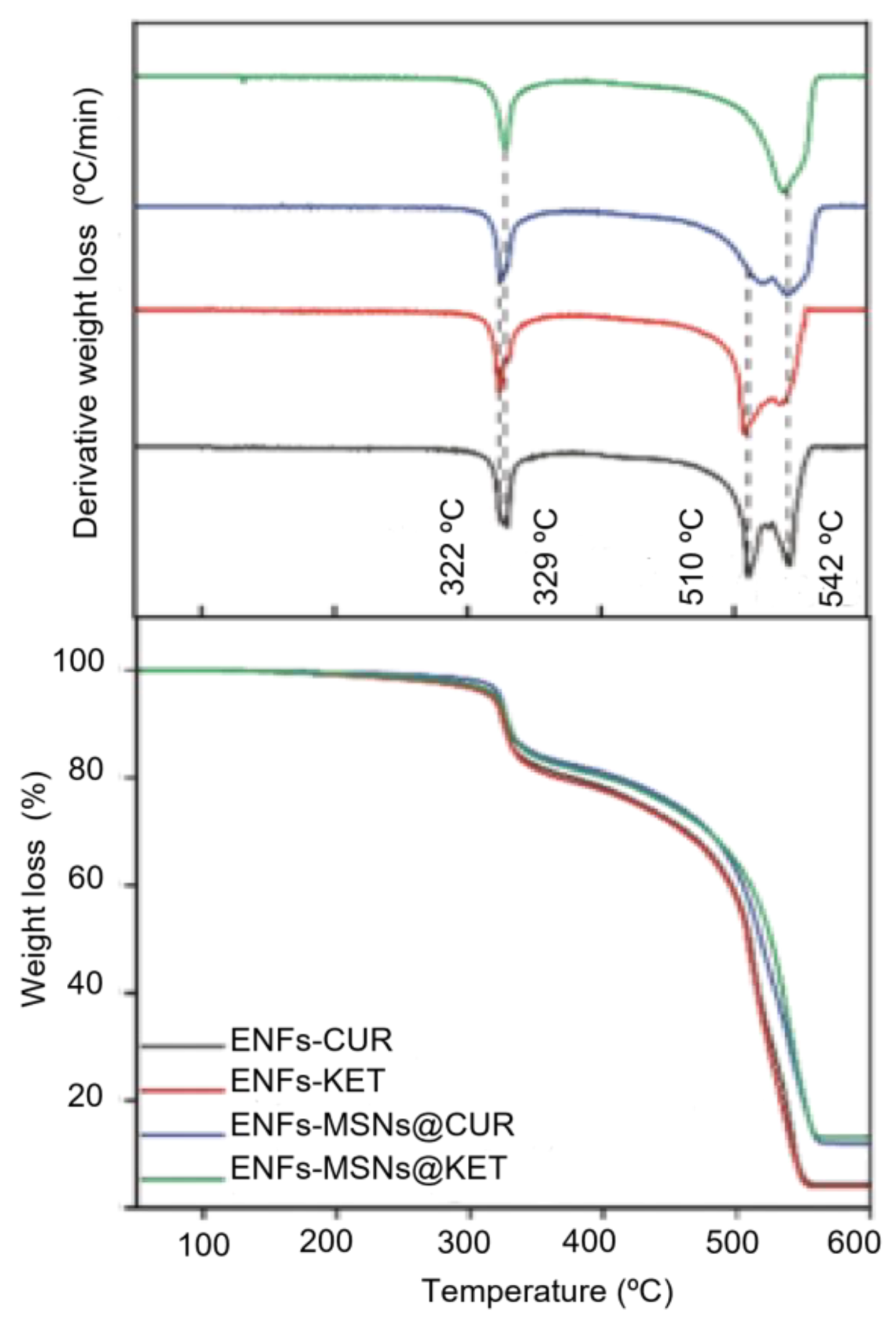
3.1. Materials Properties
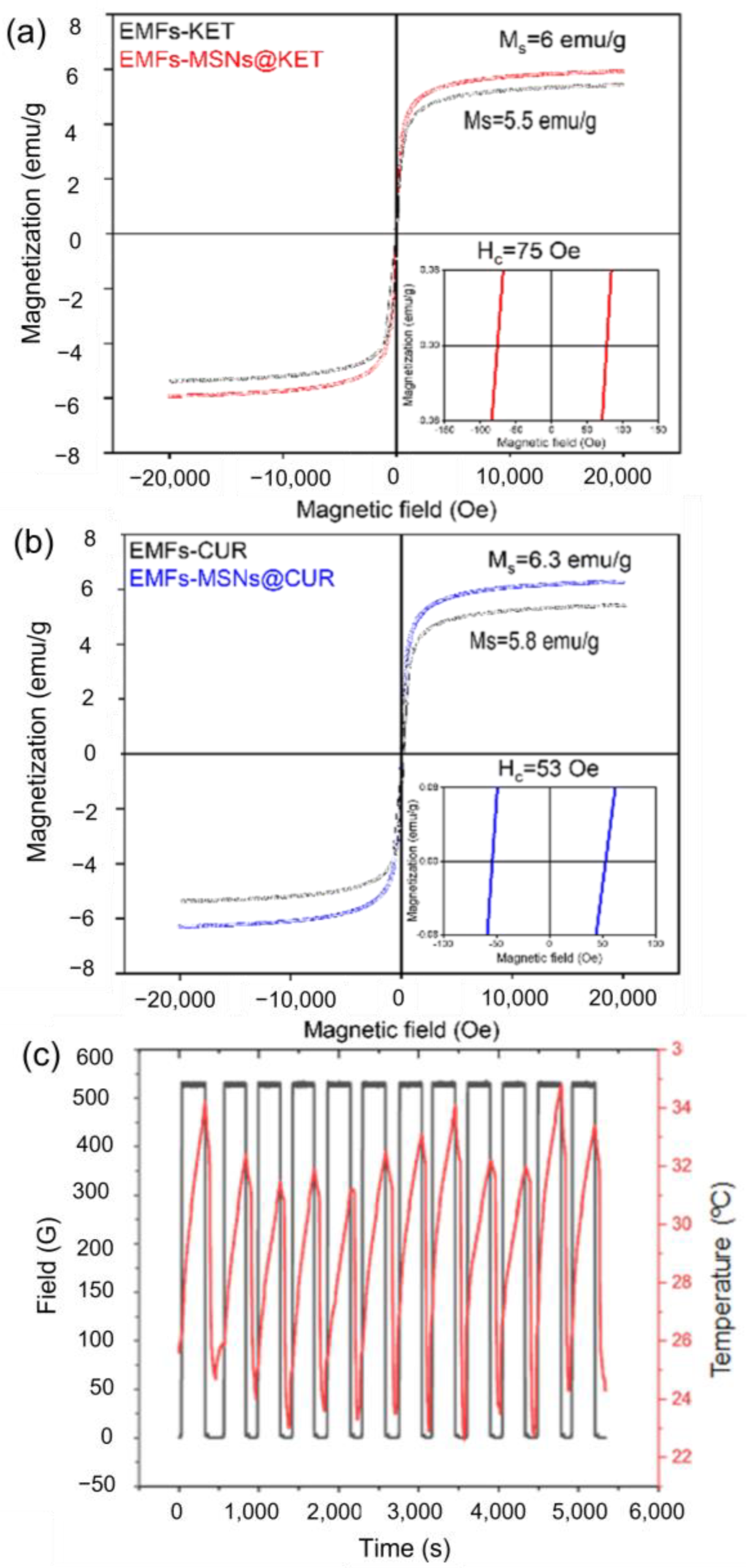
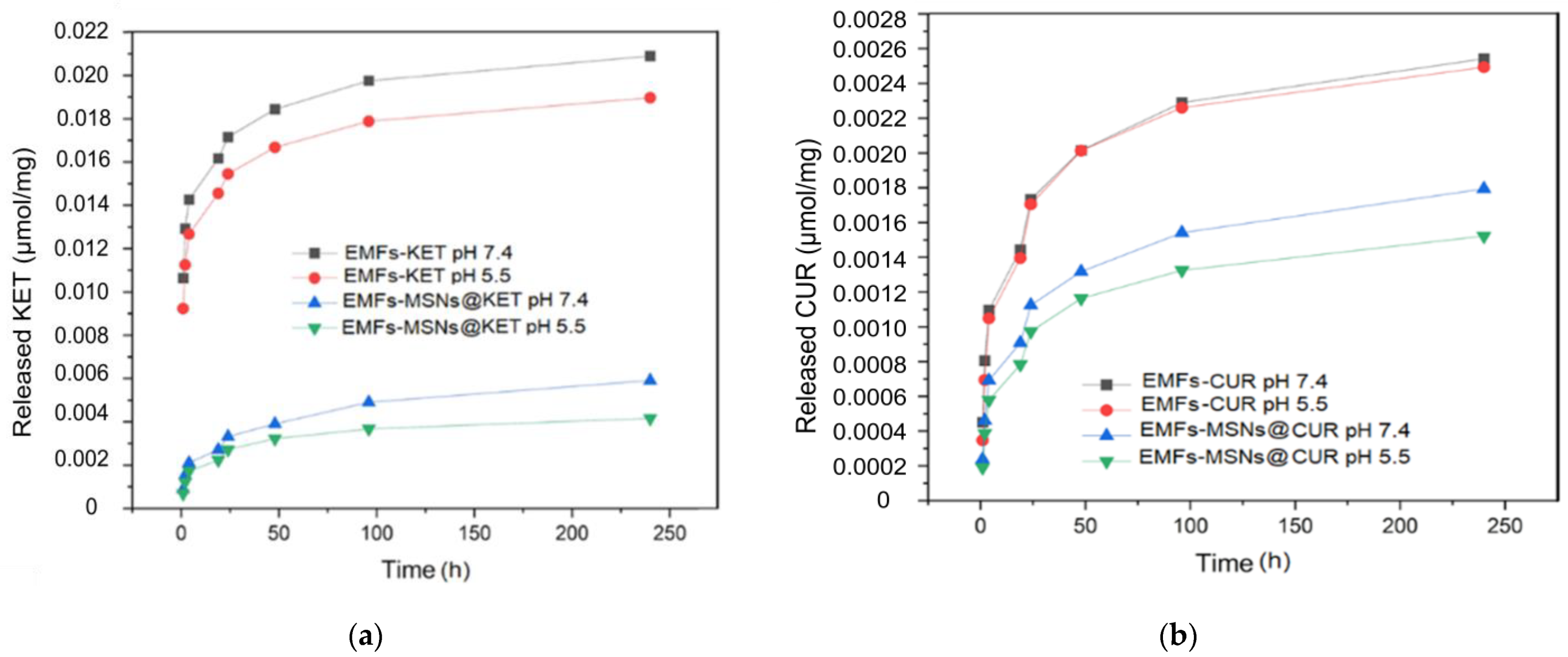
3.2. Drug Release Platform Responses
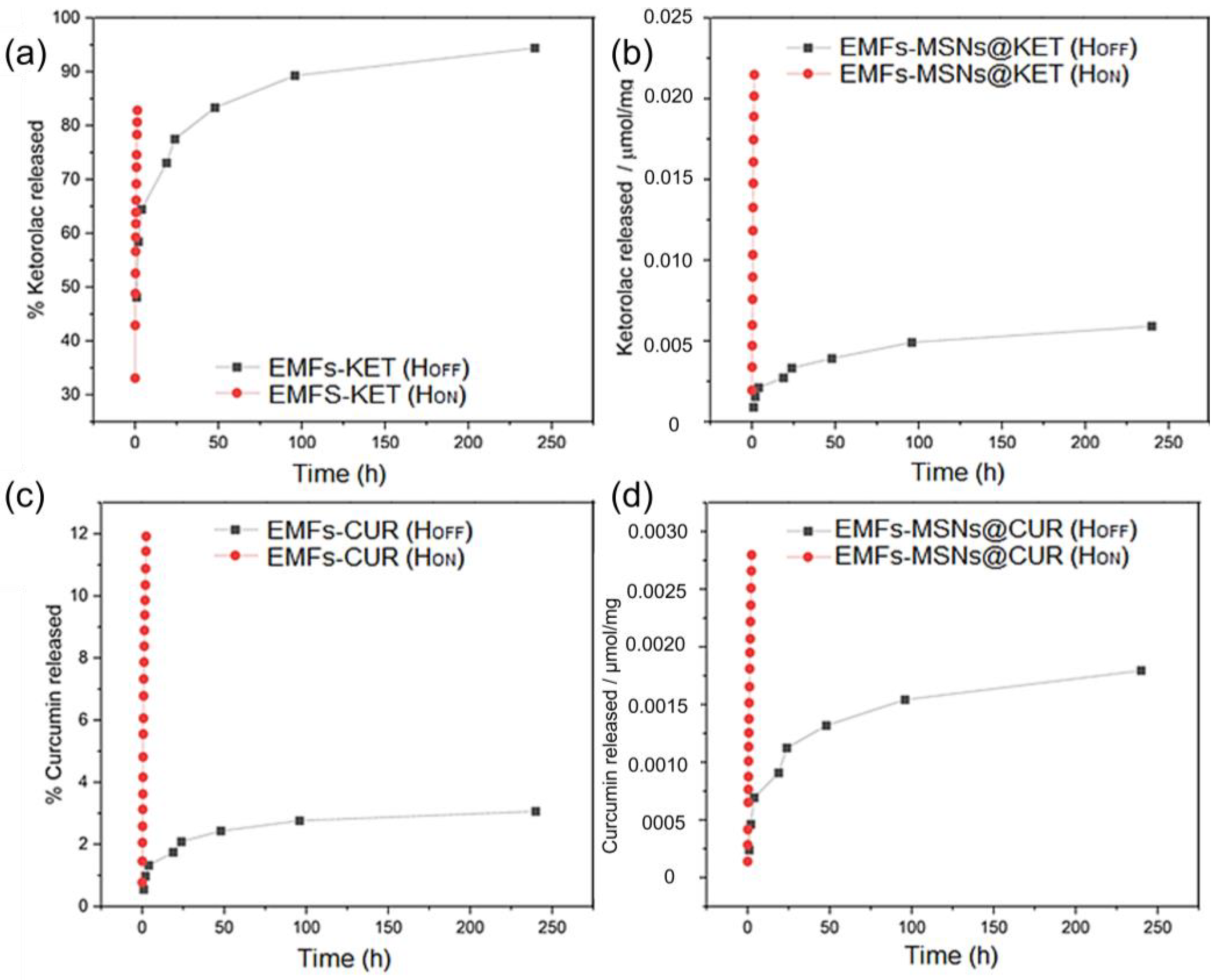
3.2.1. Alternating Magnetic Field OFF
3.2.2. Alternating Magnetic Field ON
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahoui, N.; Jiang, B.; Taloub, N.; Huang, Y.D. Spatio-temporal control strategy of drug delivery systems based nano structures. J. Control. Release 2017, 255, 176–201. [Google Scholar] [CrossRef] [PubMed]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent advances in electrospun nanofiber-mediated drug delivery strategies for localized cancer chemotherapy. J. Biomed. Mater. Res. Part A 2020, 108, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic drug delivery: Where the field is going. Front. Chem. 2018, 6, 424355. [Google Scholar] [CrossRef]
- Mertz, D.; Sandre, O.; Bégin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zheng, H.; Chang, M.W.; Ahmad, Z.; Li, J.S. Hollow polycaprolactone composite fibers for controlled magnetic responsive antifungal drug release. Colloids Surf. B Biointerfaces 2016, 145, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Perera, A.S.; Zhang, S.; Homer-Vanniasinkam, S.; Coppens, M.O.; Edirisinghe, M. Polymer-Magnetic Composite Fibers for Remote-Controlled Drug Release. ACS Appl. Mater. Interfaces 2018, 10, 15524–15531. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Jang, B.; Issadore, D.; Tsourkas, A. Use of magnetic fields and nanoparticles to trigger drug release and improve tumor targeting. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1571. [Google Scholar] [CrossRef] [PubMed]
- Suneet, K.; De, T.; Rangarajan, A.; Jain, S. Magnetic nanofibers based bandage for skin cancer treatment: A non-invasive hyperthermia therapy. Cancer Rep. 2020, 3, e1281. [Google Scholar] [CrossRef]
- Garciá, L.; Garaio, E.; López-Ortega, A.; Galarreta-Rodriguez, I.; Cervera-Gabalda, L.; Cruz-Quesada, G.; Cornejo, A.; Garrido, J.J.; Gómez-Polo, C.; Pérez-Landazábal, J.I. Fe3O4-SiO2 Mesoporous Core/Shell Nanoparticles for Magnetic Field-Induced Ibuprofen-Controlled Release. Langmuir 2023, 39, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sasikala, A.R.K.; Unnithan, A.R.; Yun, Y.H.; Park, C.H.; Kim, C.S. An implantable smart magnetic nanofiber device for endoscopic hyperthermia treatment and tumor-triggered controlled drug release. Acta Biomater. 2016, 31, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, V.; Ausanio, G.; Ferretti, A.M.; Babar, Z.U.D.; Guarino, V.; Ambrosio, L.; Lanotte, L. Magnetic Response of Nano/Microparticles into Elastomeric Electrospun Fibers. J. Funct. Biomater. 2023, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ico, G.; Bai, Y.; Yang, S.; Lee, J.H.; Yin, Y.; Myung, N.V.; Nam, J. Utilization of a magnetic field-driven microscopic motion for piezoelectric energy harvesting. Nanoscale 2019, 11, 20527–20533. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Li, W.; Chen, H.; Wang, L.; Shao, S.; Zhou, S. Remotely actuated shape memory effect of electrospun composite nanofibers. Acta Biomater. 2012, 8, 1248–1259. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ebara, M.; Aoyagi, T. A Smart Hyperthermia Nanofiber with Switchable Drug Release for Inducing Cancer Apoptosis. Adv. Funct. Mater. 2013, 23, 5753–5761. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.F.; Besenbacher, F. Electrospun Nanofibers-Mediated On-Demand Drug Release. Adv. Healthc. Mater. 2014, 3, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, S.; Babazadeh, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y.; Mousazadeh, H. An implantable smart hyperthermia nanofiber with switchable, controlled and sustained drug release: Possible application in prevention of cancer local recurrence. Mater. Sci. Eng. C 2021, 118, 111384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jung, K.; Li, A.; Liu, J.; Boyer, C. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Prog. Polym. Sci. 2019, 99, 101164. [Google Scholar] [CrossRef]
- Alves, D.; Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Localized Therapeutic Approaches Based on Micro/Nanofibers for Cancer Treatment. Molecules 2023, 28, 3053. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fujisawa, N.; Takanohashi, M.; Najmina, M.; Uto, K.; Ebara, M. A smart hyperthermia nanofiber-platform-enabled sustained release of doxorubicin and 17aag for synergistic cancer therapy. Int. J. Mol. Sci. 2021, 22, 2542. [Google Scholar] [CrossRef] [PubMed]
- Soares, P.I.P.; Borges, J.P. Recent advances in magnetic electrospun nanofibers for cancer theranostics application. Prog. Nat. Sci. Mater. Int. 2021, 31, 835–844. [Google Scholar] [CrossRef]
- Kamali, M.; Dewil, R.; Appels, L.; Aminabhavi, T.M. Nanostructured materials via green sonochemical routes—Sustainability aspects. Chemosphere 2021, 276, 130146. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G. How sonochemistry contributes to green chemistry? Ultrason. Sonochem. 2018, 40, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-García, J.A.; Alavarse, A.C.; Maldonado, A.C.M.; Toro-Córdova, A.; Ibarra, M.R.; Goya, G.F. Simple Sonochemical Method to Optimize the Heating Efficiency of Magnetic Nanoparticles for Magnetic Fluid Hyperthermia. ACS Omega 2020, 5, 26357–26364. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, C.; Lu, X. Nanofibrous Materials. In Electrospinning: Nanofabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 53–92. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, X.; Ren, Z.; Mao, C.; Han, G.; Fu, Y.; Li, X.; Ren, Z.; Han, G.; Mao, C. Multifunctional Electrospun Nanofibers for Enhancing Localized Cancer Treatment. Small 2018, 14, 1801183. [Google Scholar] [CrossRef] [PubMed]
- Khunová, V.; Pavliňák, D.; Šafařík, I.; Škrátek, M.; Ondreáš, F. Multifunctional Electrospun Nanofibers Based on Biopolymer Blends and Magnetic Tubular Halloysite for Medical Applications. Polymers 2021, 13, 3870. [Google Scholar] [CrossRef]
- Lu, L.; Yang, B.; Liu, J. Flexible multifunctional graphite nanosheet/electrospun-polyamide 66 nanocomposite sensor for ECG, strain, temperature and gas measurements. Chem. Eng. J. 2020, 400, 125928. [Google Scholar] [CrossRef]
- Bavatharani, C.; Muthusankar, E.; Wabaidur, S.M.; Alothman, Z.A.; Alsheetan, K.M.; AL-Anazy, M.M.; Ragupathy, D. Electrospinning technique for production of polyaniline nanocomposites/nanofibres for multi-functional applications: A review. Synth. Met. 2021, 271, 116609. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Kadavil, H.; Zagho, M.; Elzatahry, A.; Altahtamouni, T. Sputtering of Electrospun Polymer-Based Nanofibers for Biomedical Applications: A Perspective. Nanomaterials 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Van Dijck, A.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of drugs in an amorphous state into electrospun nanofibers composed of a water-insoluble, nonbiodegradable polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Zhang, L.; Xing, F.; Lin, H. Controlled synthesis of monodispersed mesoporous silica nanoparticles: Particle size tuning and formation mechanism investigation. Microporous Mesoporous Mater. 2016, 225, 238–244. [Google Scholar] [CrossRef]
- Amul, B.; Muthu, S.; Raja, M.; Sevvanthi, S. Molecular structure, spectroscopic (FT-IR, FT-Raman, NMR, UV-VIS), chemical reactivity and biological examinations of Ketorolac. J. Mol. Struct. 2020, 1210, 128040. [Google Scholar] [CrossRef]
- Perera, K.D.; Weragoda, G.K.; Haputhanthri, R.; Rodrigo, S.K. Study of concentration dependent curcumin interaction with serum biomolecules using ATR-FTIR spectroscopy combined with Principal Component Analysis (PCA) and Partial Least Square Regression (PLS-R). Vib. Spectrosc. 2021, 116, 103288. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Sanz, B.; Mallada, R.; Ibarra, M.R.; Goya, G.F. Magnetic Nanofibers for Remotely Triggered Catalytic Activity Applied to the Degradation of Organic Pollutants. Mater. Des. 2023, 226, 111615. [Google Scholar] [CrossRef]
- Fuentes-García, J.A.; Alavarse, A.C.; de Castro, C.E.; Giacomelli, F.C.; Ibarra, M.R.; Bonvent, J.J.; Goya, G.F. Sonochemical route for mesoporous silica-coated magnetic nanoparticles towards pH-triggered drug delivery system. J. Mater. Res. Technol. 2021, 15, 52–67. [Google Scholar] [CrossRef]
- Gazmeh, M.; Khajenoori, M.; Yousefi-Nasab, S. Effect of temperature on curcumin solubility in a pressurized hot water solvent: Experimental and molecular dynamics simulation. Int. Commun. Heat Mass Transf. 2023, 146, 106918. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, A.K.; Agrahari, A.K.; Sharma, K.; Singh, A.S.; Gupta, M.K.; Prakash, P. Making of water soluble curcumin to potentiate conventional antimicrobials by inducing apoptosis-like phenomena among drug-resistant bacteria. Sci. Rep. 2020, 10, 14204. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Madan, S.; Majumdar, D.K.; Maitra, A. Ketorolac entrapped in polymeric micelles: Preparation, characterisation and ocular anti-inflammatory studies. Int. J. Pharm. 2000, 209, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Filosa, R.; Peduto, A.; De Caprariis, P.; Rizza, L.; Bonina, F.; Blasi, P. Evaluation of alternative strategies to optimize ketorolac transdermal delivery. Aaps Pharmscitech 2006, 7, E61–E69. [Google Scholar] [CrossRef] [PubMed]
- Alsarra, I.A.; Bosela, A.A.; Ahmed, S.M.; Mahrous, G.M. Proniosomes as a drug carrier for transdermal delivery of ketorolac. Eur. J. Pharm. Biopharm. 2005, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Sohn, Y.T.; Seo, H.O. Crystal forms of ketorolac. Arch. Pharmacal Res. 2004, 27, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Cao, Y.; Kumeria, T.; Blaskovich MA, T.; Falconer, J.R.; Popat, A. Engineering Mesoporous Silica Nanoparticles towards Oral Delivery of Vancomycin. J. Mater. Chem. B 2021, 9, 7145–7166. [Google Scholar] [CrossRef] [PubMed]
- Gessner, I.; Krakor, E.; Jurewicz, A.; Wulff, V.; Kling, L.; Christiansen, S.; Brodusch, N.; Gauvin, R.; Wortmann, L.; Wolke, M.; et al. Hollow Silica Capsules for Amphiphilic Transport and Sustained Delivery of Antibiotic and Anticancer Drugs. RSC Adv. 2018, 8, 24883–24892. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing KS, W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziegler, R.; Ilyas, S.; Mathur, S.; Goya, G.F.; Fuentes-García, J.A. Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers. Fibers 2024, 12, 48. https://doi.org/10.3390/fib12060048
Ziegler R, Ilyas S, Mathur S, Goya GF, Fuentes-García JA. Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers. Fibers. 2024; 12(6):48. https://doi.org/10.3390/fib12060048
Chicago/Turabian StyleZiegler, Richard, Shaista Ilyas, Sanjay Mathur, Gerardo F. Goya, and Jesús Antonio Fuentes-García. 2024. "Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers" Fibers 12, no. 6: 48. https://doi.org/10.3390/fib12060048
APA StyleZiegler, R., Ilyas, S., Mathur, S., Goya, G. F., & Fuentes-García, J. A. (2024). Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers. Fibers, 12(6), 48. https://doi.org/10.3390/fib12060048









